Abstract
Hypothesis
To determine whether intracochlearly applied dexamethasone will lead to better control of drug levels, higher peak concentrations and lower base-to apex concentration gradients in scala tympani (ST) of the guinea pig than after intratympanic (round window, RW) application.
Background
Local application of drugs to the RW results in substantial variation of intracochlear drug levels and significant base-to apex concentration gradients in ST.
Methods
Two μL of dexamethasone-phosphate (10 mg/mL) were injected into ST either through the RW membrane which was covered with 1% sodium hyaluronate gel or through a cochleostomy with a fluid tight seal of the micropipette. Perilymph was sequentially sampled from the apex at a single time point for each animal, at 20, 80, or 200 min after the injection ended. Results were mathematically interpreted by the means of an established computer model and compared with prior experiments performed by our group with the same experimental techniques but using intratympanic applications.
Results
Single intracochlear injections over 20 min resulted in approximately ten times higher peak concentrations (on average) than 2-3 hours of intratympanic application to the round window niche. Intracochlear drug levels were less variable and could be measured for at least up to 220 min. Concentration gradients along scala tympani were less pronounced. The remaining variability in intracochlear drug levels was attributable to perilymph and drug leak from the injection site.
Conclusion
With significantly higher, less variable drug levels and smaller base-to apex concentration gradients, intracochlear applications have advantages to intratympanic injections. For further development of this technique, it is of importance to control leaks of perilymph and drug from the injection site and to evaluate its clinical feasibility and associated risks.
Keywords: cochlea, drug delivery, dexamethasone, glucocorticoids, guinea pig, intratympanic, perilymph, pharmacokinetics, round window membrane, steroids
Introduction
Local applications of drugs to the inner ear are increasingly being used in the therapy of inner ear disorders. At present, the two main application strategies are 1) the extracochlear delivery of drug to the intact but diseased inner ear with the aim of protection, rescue or regeneration and 2) the intracochlear drug delivery in combination with an auditory prosthesis in order to improve their safety and performance. There has been a marked increase in interest in the use of intratympanic applications of glucocorticoids for the treatment of sudden hearing loss (1). Application protocols, however, are mostly empirical and drug distribution in the inner after local delivery is still being explored experimentally (2). Animal experiments have demonstrated that intratympanic, extracochlear drug application to the round window membrane (RWM) resulted in poor control of intracochlear drug levels and distribution in the inner ear. A high variability of the absolute drug levels in the perilymph was found in animal and human pharmacokinetic studies (3-10). In addition, after drug application to the round window (RW) niche substantial, but variable, base-to-apex concentration gradients in scala tympani were measured for dexamethasone and dexamethasone-phosphate (10) and for various other substances (11-15). The direct intracochlear administration of drugs to the perilymph using single shot injections through the round window membrane has been shown to be a promising alternative approach to deliver drugs quantitatively to the perilymph of the scala tympani (16).
In our study we analyzed the pharmacokinetics following single shot injections of dexamethasone-phosphate (Dex-P) through the round window membrane or through a cochleostomy with a fluid tight seal of the micropipette in the guinea pig model. Based on prior experiments with ion markers (16), intracochlear application of DexP should 1) increase the amount of drug in the cochlear fluids, 2) reduce the variability of intracochlear drug concentrations, and 3) increase the time the drug is detectable in the cochlear perilymph.
Materials and Methods
Animal preparation and drug application
The methods for animal preparation, drug application and apical sampling of perilymph described in detail elsewhere (10;12;17). In brief, specific pathogen free guinea pigs (mean weight: 410 g (Hartmut); 312-533 g; Charles River, Kißlegg, Germany) were anesthetized by intraperitoneal injection of an initial dose of 8 mg/kg xylazine (Bayer, Leverkusen, Germany), and 140 mg/kg ketamine hydrochloride (Pharmacia & Upjohn, Erlangen, Germany). Anesthesia was maintained by repeated intramuscular injections of 1/4th to 1/3rd of the initial dose. Secretions were controlled by 0.3 mg/kg atropine sulphate (Braun, Melsungen, Germany). For later experiments a combination of fentanyl, midazolam, and medetomidin (0.025, 1.0, and 0.2 mg/kg, respectively) was used allowing for a more stable anaesthesia for longer durations. The trachea was cannulated and the body temperature was maintained at 37.5 °C with a temperature controlled heating blanket. The animal was mounted in a head-holder, the auditory bulla was exposed by a ventrolateral approach and was opened to expose the cochlea. The mucosa was removed from the bone at the apex of the cochlea and the bone was allowed to dry. The apex was coated with a thin layer of cyanoacrylate glue (Aesculap, Tuttlingen, Germany) subsequently covered by a two-part silicone elastomer sealant (KWIK-Cast, WPI, Sarasota, USA). To minimize leakage during injections, the RW niche was filled with 1% sodium-hyaluronate gel (Healon®, AMO, Karlsruhe, Germany) as described earlier (16). Twenty μg of dexamethasone-21-dihydrogen-phosphate-disodium-salt (2 μl of 10 mg/mL Fortecortin® Inject; Merck, Darmstadt, Germany) was injected through the RWM at a rate of 100 nL/min for 20 min using a 10 μL gas tight syringe (#1701, Hamilton, Reno, NV). The syringe was coupled to a glass micropipette and was mounted on an Ultrapump (WPI, Sarasota, FL) held in a micromanipulator. After the termination of the injection, the pipette was slowly removed from the cochlea.
In a variant of this intracochlear application, drug solution was injected through a cochleostomy in the bony wall of the basal turn of scala tympani. After thinning the bone overlying the base of ST with a flap knife, thin layers of cyanoacrylate then Kwik-Cast silicone (as above) were applied to the dry bone. A small fenestra (20-30 μm diameter) was made through both the adhesives and the bony wall, through which the injection pipette was inserted. The pipette was sealed in place by wicking the fluid droplet from the silicone surface and immediately applying cyanoacrylate to the fenestration site. This technique prevents all perilymph leakage from around the micropipette.
Sequential apical sampling and quantitative analysis
Each animal was sampled at a single time point, 40, 100 or 220 min after the start of the injection for round window injections (i.e. after a 20, 80 or 200 min delay after the end of drug application) and 40 min after the start of injection through the cochlear wall (20 min delay). The silicone-coated apex of the cochlea (see above) was carefully perforated and samples collected by touching a glass capillary to the emerging fluid. Ten nominally 1 μL samples of perilymph were collected from each animal over an approximately 10 minute period (1 minute per sample). Sample volumes were determined by measuring the sample length in the collection capillaries. Each sample was diluted into 10 μL sterile filtered water. After completion of ten sequential apical samples, the animal was sacrificed. The number of animals used for the four experimental groups were n = 7 for the 40 min, n = 7 for the 100 min, and n = 3 for the 220 min RW healon group, respectively, and n = 4 for the cochleostomy (sealed in ST wall) group. The chromatographic separation and quantification of dexamethasone-21-dihydrogen-phosphate using HPLC analysis is described in detail elsewhere (9;10). Limits of detection (LOD) and quantification (LOQ) were 100 ng/ml and 195 ng/ml for Dex-P and 500 ng/ml and 1000 ng/ml for Dex, respectively. Results of one animal study from the “cochlear wall group” were excluded due to HPLC measurement problems including two samples being lost. The animal experiments were approved by the animal studies committee of the University of Tübingen.
Interpretation of sequential sample data
Experimental data were interpreted using a publically available finite element model (http://oto.wustl.edu/cochlea/; version 1.7) considering the experimental design (application site and time, sampling times and sample size), fluid movements, drug diffusion and volume accumulation. The sample concentrations were fitted by adjusting rates of clearance, perilymph flow (before sampling), and accessibility to compartments parallel to ST such as spiral ligament and modiolus. The analysis also allowed for the presence of leaks at the injection site, i.e. at the location where the glass pipette perforated the round window membrane. With this analysis, it was possible to derive the concentration profile along ST immediately before sampling, which best accounted for the sample values (12;18;19).
Results
Absolute intracochlear concentrations
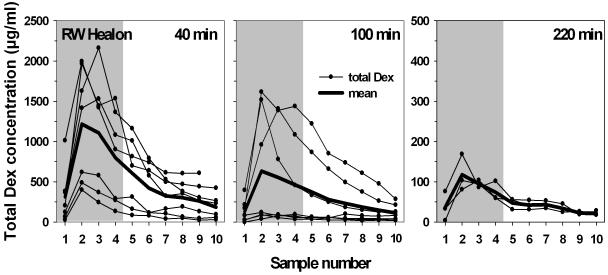
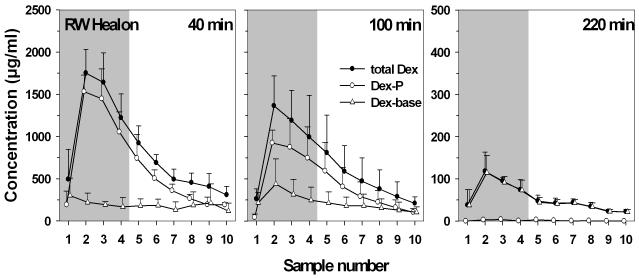
Figure 1 shows the sample concentrations for total dexamethasone, i.e. the sum of dexamethasone-phosphate and dexamethasone base (Dex-P + Dex) in scala tympani perilymph at 40, 100 and 220 min after start of injection through the RWM. The drug persisted in scala tympani for at least 220 min postinjection. Maximum concentrations of total dexamethasone were most often found in the second sample, originating from the upper basal/lower second turn regions of ST. The mean peak value was highest at 40 min compared to 100 and 220 min, although this difference was not statistically significant. Peak values in sample 2 varied from 399.6 to 1996.0 μg/ml at 40 min (mean: 1216.1 μg/ml; SD: ±699.0) from 38.2 to 1616.1 μg/ml at 100 min (mean: 633.1 μg/ml; SD: ±715.0) and from 81.0 to 168.6 μg/ml (mean: 117.6 μg/ml, SD: ±45,5) at 220 min. The measured curves appeared to fall into two different groups, a high concentration and a low concentration group in the 40 min and in the 100 min experiments, respectively. Although sodium-hyaluronate gel was placed in the RW niche before perforating the membrane with the injection pipette, leakage from the injection site could not be excluded. Such leakage would result in lower intracochlear concentrations of the applied substance (16) and could thus account for the low concentration groups in figure 1.
Fig. 1.
Total dexamethasone (Dex + Dex-P) concentrations in scala tympani in sequential samples taken from the cochlear apex at various times after injection through the RWM. The RW niche was filled with 1% sodium hyaluronate gel to help control fluid leaks. Samples 1 to 4 (shaded area) contain perilymph from scala tympani. From samples 5 on the aspirates contain mainly CSF entering scala tympani through the cochlear aqueduct. The low concentration groups in the 40 and the 100 min experiments are likely due to leakage of drug at the injection site despite covering of the RW niche with sodium-hyaluronate gel. Mean peak concentrations were most often reached in the second sample (originating from the upper basal/lower second turn regions of ST). Drug was still clearly detectable after 220 min. (Note scale difference in right panel.).
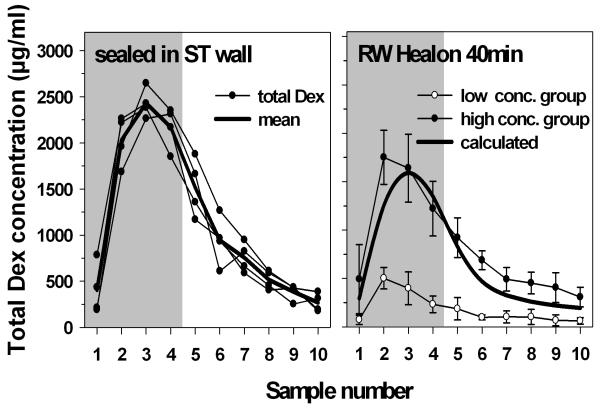
In order to provide a more reliable seal of the injection pipette with the perilymphatic space, in some experiments the injection pipette was inserted through a cochleostomy into the base of scala tympani and reliably sealed as described above. The intracochlear peak concentrations reached in this experimental subgroup were larger then after injection through the RWM covered with sodium-hyaluronate gel (figure 2, left panel).
Fig. 2.
Left) Total dexamethasone (Dex + Dex-P) concentrations in scala tympani in sequential apical samples taken from the cochlear apex 40 min after start of the injection. In these experiments a glass micropipette was tightly sealed into the cochlear wall of the basal turn of ST with cyanoacrylate and silicone (no leak). Peak concentrations were higher then in the experiments where drug was injected through the RWM covered with sodium-hyaluronate gel. Right) Full and open symbols: mean sample concentration of high concentration (low leak) and low concentration (high leak) groups from figure 1 (40 min), respectively. Full line: Computer simulation of expected sample concentrations based on pharmacokinetic parameters derived for the low concentration group but with mean leak value from high concentration group.
The influence of fluids leaks at the injection site (RWM) on intracochlear drug concentration was further demonstrated using computer simulations to interpret the experiments. In this analysis the rate of fluid leakage required to account for the low- and high-concentration groups in Figures 1 (left panel) and 2 (right panel) differed by a factor of approximately 14 (0,11 μL/min and 0,008 μL/min, repectively). The heavy line in Figure 2 (right panel) shows calculated sample concentrations based on pharmacokinetic parameters derived for the low concentration group, but with mean leak rate reduced to that of the high concentration group. The line showed good correspondence with measured sample concentrations in these animals, confirming that a higher rate of fluid leakage from the injection site could account for the low concentration group. The higher drug levels in the group with injection through the ST wall could be attributed to the absence of leakage at the injection site but are also influenced by the ST injection site being more apical and further from the cochlear aqueduct than for RW injections, resulting in less drug being displaced through the cochlear aqueduct into CSF for the ST wall injection group.
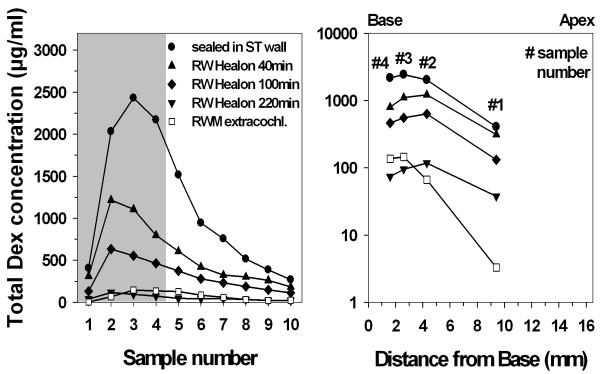
Intracochlear concentration gradients
Figure 3 summarizes the mean total dexamethasone (Dex + Dex-P) concentrations in scala tympani at different sampling time points and using different sealing procedures for the application pipette. Sample 2 was usually the highest for the RW injection studies (see also figure 1). This concentration gradient between the most apical (sample number 1) and the more basal second sample appeared to be stable throughout all postinjection sampling time points (figure 3 right panel). In the experiments where Dex-P was applied through the pipette sealed in the cochlear wall, samples 3-4 were the highest. Despite the limited spatial resolution based on the 4 samples, the plots in figure 3 (right panel) clearly show lower basal-apical concentration gradient gradients, i.e. a more uniform distribution, in the experiments using intracochlear injections compared to prior experiments done by our group with the same experimental techniques but using extracochlear RW irrigation (10).
Fig. 3.
Left) Mean total dexamethasone (Dex + Dex-P) concentrations in scala tympani in sequential apical samples taken from ST at different sampling time points and using different sealing procedures for the application pipette. As a comparison the mean total Dex concentrations after continuous (extracochlear) irrigation of the RWM for 2-3 hours are shown with open squares (historical data from a previous study of our group [figure 5 in Plontke et al. 2008]). Right) Mean concentrations in the first 4 samples for the different experimental groups. The distance along ST plotted is that of the midpoint of the estimated region of origin for 1 μL samples (half the sample volume apical and half the volume basal to the location). (Note logarithmic scale on right panel).
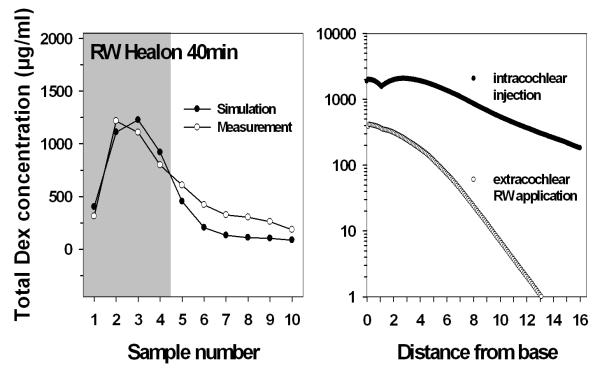
Mean sample concentrations of total Dex at 40 min after start of injection were compared with concentrations calculated by the means of a computer model. Calculated values were generally in good agreement with the experimental data (figure 4, left). However, the computer model calculated Cmax for sample 3 whereas the experiments showed Cmax for sample 2. It might be speculated that additional disturbance of perilymph caused by penetration of the RWM or withdrawal of the pipette caused a decrease of dexamethasone in basal regions (samples 3 and 4) of scala tympani. The calculated mean concentration profile of total dexamethasone in ST after intracochlear injection is plotted against the calculated mean concentration profile after continuous RW application for 2-3 hours based on previous experimental data in figure 4 (right panel).
Fig. 4.
Left: Comparison of experimental and calculated sample concentrations using a finite element computer model (Washington University Cochlear fluids simulator, version 1.7). Right: The calculated concentration gradient of total dexamethasone (Dex + Dex-P) 40 min after start of an intracochlear injection (2 μL Dex-P over 20 min) through the RWM (filled symbols) is much lower than after a 2-3 hours irrigation of the RW membrane with the same drug and concentration (open symbols, historical data from Plontke et al. 2008). (Note logarithmic scale on right panel).
Conversion of the prodrug Dex-P to the active moiety Dex-base
The prodrug dexamethasone-21-dihydrogen-phosphate-disodium salt (Dex-P) and its active moiety Dex-base, both were detectable in almost all perilymph samples at all time points (figure 5). At 220 min postinjection nearly all of the prodrug had been converted to Dex-base and Dex-P was below LOD in all samples in two of three experiments.
Fig. 5.
Concentrations of Dex-base, Dex-P and total Dex in sequential apical samples taken from ST after different time intervals after intracochlear injection through the RWM. At 3-4 hours after injection almost all of the prodrug Dex-P had been converted into its active moiety Dex-base. (Note lower scale in right panel. Left and middle panel: high concentration/ low leakage groups from figure 1 only).
Discussion
It is important to establish effective, well controlled and safe application methods of local drug delivery to the inner ear if they are to be widely accepted as a strategy for treating and preventing inner ear disorders. In current clinical practice, single or repeated intratympanic (extracochlear) injections of drug solutions are mainly used (1). Experimental studies in animals, however, have demonstrated that after RW irrigation or single intratympanic injections into the bulla tympanica, only a low percentage of the applied drug enters ST. In addition, intracochlear drug concentrations were found to be highly variable (summarized in (7)). A high degree of variability has also been reported in the human (3;4). Our results show that better control of drug levels in the ear can be achieved with intracochlear injections. At present, however, no studies have shown that this can be performed safely in humans so it would not be realistic to treat mild or moderate sudden hearing loss with glucocorticoids injected though the RW membrane. Nevertheless, as safe procedures for direct intracochlear injection are developed, they may be initially applicable to other therapeutics such as peptides, proteins, (si)RNA, or cells which would be less efficiently delivered by extracochlear applications.
The presented study showed that for the same applied solution (Dex-P, 10 mg/mL) delivered as a single injection intracochlearly over 20 min, the peak concentrations in scala tympani perilymph were significantly higher (approximately 10 times on average) than after 2 – 3 hours of intratympanic (extracochlear) application to the round window niche as measured in a previous study (10). Of the applied drug concentration a mean maximum percentage of 12.2% was measured in ST after intracochlear injection through the RWM compared to 2.9% (9), 1.4% (10), 0.05% (20), 0.04% (5), 0.04% (7), 0.005% (21), and 0.0013%(8) following extracochlear application. When comparing the percentage intracochlear concentrations it has to be noted, however, that they are also influenced by the fact that some studies used regional measurements at different locations in ST (9;10) while others used average concentrations of entire ST perilymph with considerable sample dilution with CSF due to large samples from the RWM (5;7;8;19-22). Nevertheless, even after considering differences in experimental designs with respect to drug application, sampling procedures and differences in analytical methods, our study, not unexpectedly, clearly demonstrated a more efficient delivery for intracochlear application with significantly higher concentrations of drug measured in the inner ear fluids with far less total amount of drug applied.
In addition to higher peak concentrations drug levels in ST perilymph persisted longer then after applications to the RW niche and could be measured for up to 220 min postinjection This is most like due to higher starting concentrations in ST leading to detectable levels at later time points. Dexamethasone was also detectable in perilymph after volume stabilization in the middle ear (7). Actual persistence of drug levels in the inner ear is best facilitated by sustained drug application through controlled release drug delivery system like pumps and biodegradable sustained release biopolymers (20;23-33), o r b y considering the physicochemical properties of the drug delivered (34).
Concentration gradients along scala tympani after intracochlear injections were less pronounced when compared to intratympanic drug delivery (figures 3 and 4). This could be partially due to “washout” of drug from the basal turn by fluid leakage at the injection site, making apical drug levels relatively more pronounced. However, it has to be noted that the 1st (most apical) sample was considerably higher just 40 min after the start of intracochlear injection as compared to 2-3 hrs after extracochlear RW application (figure 3). In addition, the second and third sample concentrations were always highest while in previous experiments with identical intracochlear injection of an ion marker Cmax was observed highest in the third and fourth samples (16). We have currently no definitive explanation for this difference. It is likely related to very low rates of apically-directed perilymph volume flow which might be related to pressure changes during the injection procedure or possibly an effect of the high concentration of the applied dexamethasone-disodium-salt solution in some way affecting perilymph homeostasis.
Although the intracochlear drug levels reached by injection through the RWM covered with hyaluronic acid gel were less variable than after RW applications without gel (16), we found that with this method considerable variation remained. When leaks of perilymph fluid were controlled by tightly sealing the application pipette in the cochlear wall, the variability of intracochlear drug concentrations was found to be lower than after injections through the RWM (figures 2 and 3). In humans, leakage after injection through the RWM might not be such an extensive problem, since the communication with CSF is less than in the guinea pig, as the guinea pig has a wider cochlear aqueduct. However, for the development of future intracochlear drug injection techniques with quantitative delivery of specific amounts of drug to the inner ear, it will be of general importance to control fluid leaks and possibly drug diffusion out of the inner ear back to the middle ear if fluid is present in the RW niche.
In summary, the present data suggest that because intracochlear applications produce significantly higher, less variable drug levels and smaller base-to apex concentration gradients, in pharmacokinetic terms they may provide a promising alternative to the commonly used intratympanic injections. For further development of this technique, it is of high importance to control leakage of perilymph and drug from the injection site. As the drug levels become better controlled, both acute and chronic functional consequences of the procedure remain to be evaluated, its risk benefit ratio needs to be assessed and specific indications identified.
Acknowledgements
This work was supported by BMBF grant 0313844B (SKP) and by NIH/NIDCD grant DC01368 (AS).
List of abbreviations
- Dex Dexamethasone base (active drug)
Dex-base Dex-P Dexamethasone-21-dihydrogen phosphate disodium salt (prodrug)
- HPLC
High performance liquid chromatography
- LOD
Limit of detection
- LOQ
Limit of quantification
- RW
Round window
- RWM
Round window membrane
- ST
Scala tympani total
- Dex
Dex+Dex-P
Footnotes
Disclosures: Stefan K. Plontke is a consultant for Otonomy, Inc., San Diego, USA. Alec N. Salt is a member of the scientific advisory board of Otonomy, Inc and may receive income based on equity holdings. This work was not sponsored by Otonomy.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hu A, Parnes LS. Intratympanic steroids for inner ear disorders: a review. Audiol Neurootol. 2009;14:373–382. doi: 10.1159/000241894. [DOI] [PubMed] [Google Scholar]
- 2.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol. 2007;28:1124–1130. doi: 10.1097/MAO.0b013e31815aee21. [DOI] [PubMed] [Google Scholar]
- 4.Bird PA, Murray DP, Zhang M, et al. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011;32:933–936. doi: 10.1097/MAO.0b013e3182255933. [DOI] [PubMed] [Google Scholar]
- 5.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann G, Su J, Zumegen C, et al. [Permeability of the round window membrane for prednisolone-21-hydrogen succinate. Prednisolone content of the perilymph after local administration vs. systemic injection] HNO. 2001;49:538–542. doi: 10.1007/s001060170078. [DOI] [PubMed] [Google Scholar]
- 7.Borden RC, Saunders JE, Berryhill WE, et al. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol Neurootol. 2011;16:1–11. doi: 10.1159/000313506. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekhar SS, Rubinstein RY, Kwartler JA, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- 9.Hahn H, Kammerer B, DiMauro A, et al. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hear Res. 2006;212:236–244. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plontke SK, Biegner T, Kammerer B, et al. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol. 2003;4:176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mynatt R, Hale SA, Gill RM, et al. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7:182–193. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plontke SK, Mynatt R, Gill RM, et al. Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saijo S, Kimura RS. Distribution of HRP in the inner ear after injection into the middle ear cavity. Acta Otolaryngol. 1984;97:593–610. doi: 10.3109/00016488409132937. [DOI] [PubMed] [Google Scholar]
- 15.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 16.Salt AN, Sirjani DB, Hartsock JJ, et al. Marker retention in the cochlea following injections through the round window membrane. Hear Res. 2007;232:78–86. doi: 10.1016/j.heares.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006;153:121–129. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plontke SK, Wood AW, Salt AN. Analysis of gentamicin kinetics in fluids of the inner ear with round window administration. Otol Neurotol. 2002;23:967–974. doi: 10.1097/00129492-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Plontke SK, Salt AN. Quantitative interpretation of corticosteroid pharmacokinetics in inner fluids using computer simulations. Hear Res. 2003;182:34–42. doi: 10.1016/s0378-5955(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Fernandez R, Dellamary L, et al. Pharmacokinetics of dexamethasone solution following intratympanic injection in guinea pig and sheep. Audiol Neurootol. 2011;16:233–241. doi: 10.1159/000320611. [DOI] [PubMed] [Google Scholar]
- 21.Liu HJ, Dong MM, Chi FL. Dexamethasone pharmacokinetics in Guinea pig inner ear perilymph. ORL J Otorhinolaryngol Relat Spec. 2006;68:93–98. doi: 10.1159/000091210. [DOI] [PubMed] [Google Scholar]
- 22.Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hear Res. 2003;182:24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 23.Arnold W, Senn P, Hennig M, et al. Novel slow- and fast-type drug release round-window microimplants for local drug application to the cochlea: an experimental study in guinea pigs. Audiol Neurootol. 2005;10:53–63. doi: 10.1159/000082575. [DOI] [PubMed] [Google Scholar]
- 24.Brown JN, Miller JM, Altschuler RA, et al. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 25.Endo T, Nakagawa T, Kita T, et al. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–2020. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- 26.Pararas EE, Chen Z, Fiering J, et al. Kinetics of reciprocating drug delivery to the inner ear. J Control Release. 2011;152:270–277. doi: 10.1016/j.jconrel.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulson DP, Abuzeid W, Jiang H, et al. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–711. doi: 10.1097/MLG.0b013e31815f8e41. [DOI] [PubMed] [Google Scholar]
- 28.Piu F, Wang X, Fernandez R, et al. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32:171–179. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- 29.Praetorius M, Limberger A, Muller M, et al. A novel microperfusion system for the long-term local supply of drugs to the inner ear: implantation and function in the rat model. Audiol Neurootol. 2001;6:250–258. doi: 10.1159/000046130. [DOI] [PubMed] [Google Scholar]
- 30.Salt AN, Hartsock J, Plontke S, et al. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16:323–335. doi: 10.1159/000322504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura T, Kita T, Nakagawa T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Dellamary L, Fernandez R, et al. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393–401. doi: 10.1159/000241896. [DOI] [PubMed] [Google Scholar]
- 33.Kopke RD, Hoffer ME, Wester D, et al. Targeted topical steroid therapy in sudden sensorineural hearing loss. Otol Neurotol. 2001;22:475–479. doi: 10.1097/00129492-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Dellamary L, Fernandez R, et al. Principles of inner ear sustained release following intratympanic administration. Laryngoscope. 2011;121:385–391. doi: 10.1002/lary.21370. [DOI] [PubMed] [Google Scholar]