Abstract
Background
Overweight and obesity pose a big challenge to pregnancy as they are associated with adverse maternal and perinatal outcome. Evidence of lifestyle intervention resulting in improved pregnancy outcome is conflicting. Hence the objective of this study is to determine the efficacy of antenatal dietary, activity, behaviour or lifestyle interventions in overweight and obese pregnant women to improve maternal and perinatal outcomes.
Methods
A systematic review and meta-analyses of randomised and non-randomised clinical trials following prior registration (CRD420111122 http://www.crd.york.ac.uk/PROSPERO) and PRISMA guidelines was employed. A search of the Cochrane Library, EMBASE, MEDLINE, CINAHL, Maternity and Infant care and eight other databases for studies published prior to January 2012 was undertaken. Electronic literature searches, study selection, methodology and quality appraisal were performed independently by two authors. Methodological quality of the studies was assessed according to Cochrane risk of bias tool. All appropriate randomised and non-randomised clinical trials were included while exclusions consisted of interventions in pregnant women who were not overweight or obese, had pre-existing diabetes or polycystic ovarian syndrome, and systematic reviews. Maternal outcome measures, including maternal gestational weight gain, gestational diabetes and Caesarean section, were documented. Fetal outcomes, including large for gestational age and macrosomia (birth weight > 4 kg), were also documented.
Results
Thirteen randomised and six non-randomised clinical trials were identified and included in the meta-analysis. The evidence suggests antenatal dietary and lifestyle intervention in obese pregnant women reduces maternal pregnancy weight gain (10 randomised clinical trials; n = 1228; -2.21 kg (95% confidence interval -2.86 kg to -1.59 kg)) and a trend towards a reduction in the prevalence of gestational diabetes (six randomised clinical trials; n = 1,011; odds ratio 0.80 (95% confidence interval 0.58 to 1.10)). There were no clear differences reported for other outcomes such as Caesarean delivery, large for gestational age, birth weight or macrosomia. All available studies were assessed to be of low to medium quality.
Conclusion
Antenatal lifestyle intervention is associated with restricted gestational weight gain and a trend towards a reduced prevalence of gestational diabetes in the overweight and obese population. These findings need to be interpreted with caution as the available studies were of poor to medium quality.
Background
Both developed and developing countries are experiencing a rapid increase in the prevalence of obesity [1-3]. In the UK, 24% of women of reproductive age are now obese (body mass index (BMI) equal or greater than 30 kg/m2) and the prevalence appears to be increasing [4]. Studies in UK women show that the rates of obesity in pregnancy have almost doubled in the last two decades [5,6]. Recent estimates suggest the prevalence of obesity in pregnancy in the UK is at least 20% with 5% having severe or morbid obesity [7,8].
Observational study data has linked obesity in pregnancy with adverse maternal and infant outcomes [7-10]. Obesity increases the risks of gestational diabetes [8,10-12], hypertensive disease (including pre-eclampsia) [8,13,14], thromboembolism [15,16], infection [14,17], Caesarean section [8,18], congenital fetal anomalies [19], macrosomia [13], induction [20], stillbirth [12], shoulder dystocia [14] and preterm delivery [21]. Moreover, maternal obesity may impact on long-term outcomes such as the increasing weight of the child in infancy and the severity of obesity in future generations [10,22,23].
As most of the adverse outcomes of obese pregnancies show strong associations with pre-pregnancy BMI, it is reasonable to assume that the ideal intervention would be to reduce obesity prior to pregnancy [24]. However, this is difficult to achieve because 50% of pregnancies in the UK are unplanned and a recent study concluded that only a small proportion of women planning pregnancy follow nutrition and lifestyle recommendations [25]. As such, an intervention pre-pregnancy may reach only a small proportion of the intended women.
Alternatively, pregnancy itself may represent an ideal opportunity to target lifestyle change as women have increased motivation to maximise their own health and that of their unborn child [25]. However, evidence of benefit from published intervention studies appears limited and inconsistent [26-44]. We therefore sought to determine the efficacy of combined dietary activity and behaviour support interventions in overweight and obese pregnant women by undertaking a systematic review and meta-analysis according to PRISMA (Transparent Reporting of Systematic Reviews and Meta-analyses) criteria for maternal clinical outcomes of weight gain, gestational diabetes and Caesarean section and infant outcomes, such as large for gestational age and macrosomia. Our aim was to generate data of the highest statistical power and sensitivity. Hence, in comparison with previous similar themed systematic reviews [45-48], we chose to interrogate multiple databases (not restricted to English) and also separately meta-analyse randomised clinical trials (RCTs) and non-RCTs evaluating relevant clinical outcomes, including gestational diabetes and Caesarean section, which had not been attempted in prior meta-analyses.
Methods
Eligibility criteria
The eligible studies included RCTs and non-RCTs that evaluated antenatal dietary and lifestyle interventions in obese and overweight pregnant women whose outcome measures included quantitative maternal and fetal health outcomes. Systematic reviews and trials of women with existing gestational diabetes, or trials of pre-conception or postpartum interventions, were not included. Inclusion of trials was not restricted by language, publication date or country. Systematic reviews and observational studies were excluded.
Information sources
Literature searches were performed using five mainstream electronic databases (Cochrane Library, MEDLINE, EMBASE, CINAHL, Maternity and Infant care), and eight other databases (PsyclINFO via OVID SP, PyscLNFO via OVID SP, Science Citation Index via Web of Science, Social Science Citation Index via Web of Science, Global Health, Popline, Medcarib, Nutrition database).
Search strategy
The following MeSH terms, words and combinations of words, were used in constructing the systematic search: overweight OR obesity; pregnancy OR pregnancy complications OR pregnancy outcome OR prenatal care, prenatal, antenatal, intervention, randomised controlled trial, life style, "early intervention (education)", health education, education, patient education handout, patient education, exercise, exercise therapy, health promotion, diet, carbohydrate-restricted, diet, fat-restricted, diet, reducing, diet therapy, weight loss. Full details of the search strategy are shown in Table 1. The searches were unlimited by time up to January 2012 and limited to human studies and clinical trials. The systematic search was undertaken in the mainstream databases and targeted searches were conducted in the other databases.
Table 1.
Search strategy utilised for MEDLINE 1946 to January 2012
| Batch | Search term (MESH) | Combination | Result |
|---|---|---|---|
| 1 | Pregnancy Complications/OR Pregnancy/OR Pregnancy Outcome/OR Pregnancy, High Risk/ | 646,055 | |
| 2 | Prenatal Care/OR Pregnancy/OR Pregnancy Complications | 647,726 | |
| 3 | Antenatal.mp. | 18,393 | |
| 4 | Gestation intervention.mp. | 4 | |
| 5 | 1 OR 2 OR 3 OR 4 | 651,321 | |
| 6 | Overweight.mp. OR Obesity/OR Overweight/OR Body Weight/ | 249,097 | |
| 7 | Obesity/OR Obesity, Morbid/or Obesity.mp. | 145,882 | |
| 8 | Body Weight/OR Obesity/OR Body Mass Index/or BMI.mp. OR Overweight/ | 293,584 | |
| 9 | 6 OR 7 OR 8 | 328,089 | |
| 10 | 5 AND 9 | 21,583 | |
| 11 | Diet, Fat-Restricted/OR Diet/OR Diet, Protein-Restricted/OR Diet, Carbohydrate-Restricted/OR Diet.mp. OR Diet, Reducing/OR Diet Therapy/ | 255,985 | |
| 12 | Life Style/ | 36,837 | |
| 13 | Health Education/ | 48,625 | |
| 14 | Patient Education as Topic/ | 63,238 | |
| 15 | Exercise.mp. OR Exercise/OR Exercise, Therapy/ | 192,937 | |
| 16 | Health Promotion/ | 43,967 | |
| 17 | Weight Loss/ | 19,434 | |
| 18 | 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 | 601,919 | |
| 19 | 10 AND 18 | 3,769 | |
| 20 | LIMIT 19 TO ((female or humans or pregnancy) and (clinical trial, all OR clinical trial, phase i OR clinical trial, phase ii OR clinical trial, phase iii OR clinical trial, phase iv OR clinical trial OR controlled clinical trial OR randomized controlled trial)) | 154 |
Study selection
Electronic literature searches, study selection, methodology, appropriateness for inclusion and quality appraisal were performed independently and in duplicate by two authors (E-ON and RV). Disagreements between reviewers were resolved by consensus. Included studies were divided into two groups (RCTs and non-RCTs) and separately meta-analysed.
Data collection process
Two independent reviewers extracted the data. As a first step, each paper was screened using the title and the abstract. In the next round, studies were assessed for methodological quality and appropriateness for inclusion by two reviewers working independently from the full text of the manuscript. This was done without consideration of the results.
Data items
For each included trial, data was extracted on maternal gestational weight gain; gestational diabetes; Caesarean section; large for gestational age baby (> 4 kg); and birth weight. The included studies have been summarised in Tables 2 and 3.
Table 2.
A summary of the studies that met the criteria of the systematic review on lifestyle interventions in overweight and obese pregnant women: randomised trials
| Author (year) | Ethnic group/Country | Participant/setting | Sample size | Intervention | Outcome measure(s) | Conclusion |
|---|---|---|---|---|---|---|
| Polley et al. (2002) [32] | 31% black and 61% white/USA | Recruited before 20 weeks of pregnancy (normal BMI > 19.5 to 24.9; overweight BMI ≥ 25 to < 30 kg/m2)/Hospital based | 120, including 49 overweight 59 in control arm; 61 in intervention arm |
Exercise and nutrition information (oral and newsletter) Personalised graphs and behavioural counselling. | Gestational weight gain; gestational diabetes; Caesarean section; birthweight | No statistically significant reduction in gestational weight, prevalence of gestational diabetes, Caesarean section, or large for gestational age baby |
| Hui et al. (2006) [33] | Predominantly Caucasian/Canada | Less than 26 weeks pregnant (community based and antenatal clinics). All BMI categories. Mean BMI of non-intervention arm = 25.7 (SD = 6.3) and for intervention arm = 23.4(SD = 3.9) | 45 21 in non-intervention arm; 24 in intervention arm |
Physical exercise (group-sessions home-based exercise) Individualized nutrition plans |
Gestational weight gain | No statistically significant reduction in gestational weight gain |
| Wolff et al., 2008 [30] | 100% Caucasian/Denmark | Obese (BMI ≥ 30 kg/m2) women enrolled at 15 weeks' gestation | 50 analysed 23 in control arm; 27 in intervention arm |
Intensive intervention with 10 one-hour visits with a dietician at each antenatal visit, dietary guidance provided | Gestational weight gain; gestational diabetes; Caesarean section; birthweight | Statistically significant reduction in gestational weight gain, no statistically significant reduction in prevalence of gestational diabetes or Caesarean section, or birthweight |
| Jeffries et al., 2009 [28] | > 90% Caucasian/Australia | Women at or below 14 weeks' gestation. All BMI categories included | 286 138 in control arm; 148 in intervention arm |
Personalised weight measurement card (based on Institute of Medicine guidelines). Control had only single measurement at enrolment | Gestational weight gain | No statistically significant reduction in gestational weight gain. |
| Ong et al., 2009 [42] | Predominantly Caucasian/Australia | Pregnant obese women recruited at 18 weeks' gestation | 12 six in control arm; six in intervention arm |
Personalised 10 weeks of home-based supervised exercise (three sessions per week) | Maternal aerobic fitness and gestational diabetes | No statistically significant difference in aerobic fitness or gestational diabetes |
| Barakat et al., 2011 [41] | 100% Caucasian/Spain | All BMI categories | 160 80 in control arm; 80 in intervention arm |
Three group-based sessions per week, light resistance and toning exercise from the second trimester | Gestational weight gain and birthweight | No statistically significant difference in gestational weight gain and birth weight. Exercise intervention might attenuate adverse consequences of maternal BMI on newborn birth size |
| Asbee et al., 2009 [27] | 26% African American/USA | Pregnant women recruited before 16 weeks' gestation. All BMI categories except those of BMI > 40 kg/m2 | 100 43 in control arm; 53 in intervention arm |
One session of dietetic counselling and activity | Gestational weight gain; pregnancy outcome | Statistically significant reduction in gestational weight gain. No effect on pregnancy outcome |
| Thornton et al., 2009 [29] | 41% African American/USA | Obese pregnant women (BMI ≥ 30 kg/m2) recruited between 12 and 28 weeks' gestation | 257 randomised. 25 lost to follow up. 116 in control arm; 116 in intervention arm |
Nutritional regime for gestational diabetes | Gestational weight gain; gestational diabetes; Caesarean section; pregnancy outcome | Statistically significant reduction in gestational weight gain, no statistically significant reduction in prevalence of gestational diabetes, Caesarean section or birthweight |
| Guelinckx et al., 2010 [26] | 100% Caucasian/Belgium | Obese (BMI > 30 kg/m2) women enrolled at 15 weeks' gestation. | 195 randomised 85 analysed 65 in control arm; 65 in passive arm, 65 in intervention arm |
Three arms: group sessions with a dietician; written brochures; and standard care Dietary and physical activity guidance provided by dietician and in written brochures |
Nutritional habits; gestational weight gain; gestational diabetes; Caesarean section; birthweight | Improved nutritional habits; no statistically significant reduction in gestational weight gain, prevalence of gestational diabetes, Caesarean section or birthweight. |
| Phelan et al., 2011 [34] | 67% White/USA | Pregnant women BMI between 19.8 and 40 kg/m2 recruited between 10 and 16 weeks' gestation | 401 randomised. 201 in non-intervention arm; 200 in intervention arm |
Exercise and nutrition information (oral and newsletter) Personalised graphs and behavioural counselling | Gestational weight gain; gestational diabetes; Caesarean section; pregnancy outcome | Significant reduction in gestational weight gain; no statistically significant reduction in prevalence of gestational diabetes, Caesarean section or birthweight |
| Quinlivan et al., 2011 [59] | 73% white, 19% Asian/Australia | Pregnant women: overweight (BMI 25 to 29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) | 132 randomised. 65 in non-intervention arm; 67 in intervention arm |
Attended a study- specific antenatal clinic providing continuity of care, weighing on arrival, brief dietary intervention by food technologist and psychological assessment and intervention if indicated | Gestational weight gain; gestational diabetes; birthweight | Statistically significant reduction in gestational weight gain and prevalence of gestational weight gain. No statistically significant reduction in birthweight. |
| Luoto et al., 2011 [43] | Predominantly white/Finland | Pregnant women at risk of gestational diabetes. All BMI ranges | 399 cluster randomised. 219 in non-intervention arm; 180 in intervention arm |
Attended a study-specific individual antenatal lifestyle counselling clinic including group exercise | Gestational diabetes; gestational weight gain; birthweight | Statistically significant reduction in birthweight and macrosomia but no statistically significant difference in gestational diabetes |
| Nascimento et al., 2011 [44] | Predominantly white/Brazil | Pregnant women of all BMI categories | 82 randomised. 42 in non-intervention arm; 40 in intervention arm |
Attended a group-based exercise under supervision and received a home exercise counselling | Gestational weight gain; raised blood pressure; perinatal outcome | No statistically significant difference in gestational weight gain in terms of gestational weight gain, raised blood pressure or perinatal outcome |
BMI: body mass index; SD: standard deviation
Table 3.
Summary of the studies that met the criteria of the systematic review on lifestyle interventions in overweight and obese pregnant women: non-randomised trials
| Author (year) | Ethnic group/country | Participants/setting | Sample size | Intervention | Outcome measure(s) | Conclusion |
|---|---|---|---|---|---|---|
| Gray-Donald et al. (2000) [38] | Native Americans/Canada | Recruited before the 26th week of pregnancy, non-parallel recruitment of control and intervention arms. Mean BMI = 29.6 kg/m2 (SD = 6.45) in non-intervention arm and mean BMI = 30.8 kg/m2 (SD = 6.85) in intervention arm at baseline. |
219 107 in non-intervention arm; 112 in intervention arm |
Dietary and weight counselling Exercise groups provided |
Gestational weight gain; gestational diabetes; Caesarean section; birthweight; postpartum weight retention |
No statistically significant difference in gestational weight gain, prevalence of gestational diabetes, Caesarean section or large for gestational age baby |
| Olson et al. (2004) [51] | 96% white/USA | Recruited before third trimester. Hospital and clinic setting BMI range: 19.8 to 29 kg/m2 |
498 381 in non-intervention arm; 117 in the intervention arm |
Used the Institute of Medicine recommended guidelines on weight gain; 'health book' used to record diet and exercise and contained healthy eating and exercise information | Gestational weight gain; birthweight | No statistically significant reduction in gestational weight gain or prevalence of large for gestational age baby |
| Claesson et al. (2007) [36] | Not stated. Predominantly Caucasian/Sweden | Obese and registered at antenatal care clinic. BMI ≥ 30 kg/m2 |
348 193 in non-intervention arm; 155 in intervention arm |
Nutritional habits interview, weekly counselling and aqua aerobic sessions | Gestational weight gain; Caesarean section. | Statistically significant reduction in gestational weight gain; no difference in prevalence of Caesarean section |
| Kinnunen et al. (2007) [37] | Over 90% Caucasian/Finland | First-time pregnant women who were obese (BMI ≥ 30 kg/m2) | 196 95 in non-intervention arm; 101 in intervention arm |
Individual counselling at each antenatal visits. Dietary guidance and optional activity sessions. | Gestational weight gain; diet change; birthweight | No statistically significant reduction in gestational weight gain or prevalence of large for gestational age baby. Statistically significant reduction in dietary glycaemic load. |
| Shirazian et al., 2010 [39] | 33% blacks; 67% Latino/USA | Singleton obese (≥ 30 kg/m2) pregnant women recruited in the first trimester. Historical non-intervention group. | 54 28 in non-parallel control arm; 28 in intervention arm) |
One-to-one counselling; six structured seminars on healthy living (healthy eating and walking) | Gestational weight gain; gestational diabetes; Caesarean section | Statistically significant reduction in gestational weight gain; no difference in prevalence of gestational diabetes |
| Mottola et al., (2010) [35] | Not stated/Canada | Overweight (BMI ≥ 25 to 29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) pregnant women recruited before 16 weeks' gestation; historical non-intervention group. | 65 matched non-parallel control of 260 | Individualised nutrition plan; exercise consisted of walking (three to four times per week, used pedometers) | Gestational weight gain; Caesarean section; birthweight; peripartum weight retention | Possible reduction in gestational weight gain; no difference in prevalence of Caesarean section or large for gestational age baby; minimal effect on peripartum weight retention |
BMI: body mass index; SD: standard deviation.
Risk of bias in individual studies
The quality of studies was assessed based on how the studies had minimised bias and error in their methods. We categorised the studies according to criteria based on PRISMA guidelines [49] and the Cochrane Library [50]. For example, high quality trials reported study aims; control comparison similar to the intervention group; relevant population demographics pre- and post-intervention; and data on each outcome. These study characteristics are tabulated in Tables 4 and 5. A final assessment categorised the studies as high, medium or low quality.
Table 4.
Assessment of the quality of the included trials: non-randomised trials
| Author (year) | Population representativeness | Adequacy of sequence generation | Masking/selection bias | Incomplete outcome data | Contamination | Sample size | Grade of quality |
|---|---|---|---|---|---|---|---|
| Gray-Donald et al. (2000) [38] | Yes: Registered from clinic | No | No | No | No: non-parallel control | 219 | Low |
| Olson et al. (2004) [51] | Yes | No | No | No | No: non-parallel control | 560 | Low |
| Claesson et al. (2007) [36] | Yes: Registered from clinic | No | No | Yes | No: selected from nearby city | 315 | Low |
| Kinnunen et al. (2007) [37] | Yes | No | No | No | Yes | 55 | Low |
| Shirazian et al., (2010) [39] | Yes | No | No | Yes | No: non-parallel control | 28 | Low |
| Mottola et al., (2010) [35] | Yes | No | No | Yes | No: non-parallel control | 65 | Low |
Table 5.
Assessment quality of included trials: randomised trials
| Author (year) | Population representativeness | Adequacy of sequence generation | Masking/selection bias | Intention to treat | Incomplete outcome data | Loss to follow up | Sample size | Grade of quality |
|---|---|---|---|---|---|---|---|---|
| Polley et al. 2002 [32] | Yes | Yes: | No | Not reported | No | Yes | 120 | Low |
| Hui et al. (2006) [33] | Yes: from clinic | Exact method not described | No | Not reported | No | Yes | 52 | Low |
| Wolff et al., 2008 [30] | Yes | Yes: computer generated | No | Not reported | Yes | Yes | 50 | Low |
| Jeffries et al., 2009 [28] | Yes | Yes: Opaque envelope | Yes | Not reported | Yes | Yes | 286 | Low |
| Ong et al., 2009 [42] | Yes | Exact method not described | No | Not reported | No | No | 12 | Low |
| Barakat et al., 2011 [41] | Yes | Yes | Yes | Yes | Yes | Yes | 160 | Medium |
| Asbee et al. 2009 [27] | Yes | Yes | No | Not reported | Yes | No | 100 | Low |
| Thornton et al., 2009 [29] | Yes | Yes | Yes | Not reported | Yes | Yes | 257 | Medium |
| Guelinckx et al., 2010 [26] | Not reported | Randomised but not reported how | Not reported | Not reported | Yes | Not reported | 99 | Low |
| Phelan et al., 2011 [34] | Yes | Yes: Opaque envelope | Yes | Yes | Yes | Yes | 401 | Medium, |
| Quinlivan et al., 2011 [59] | Yes | Yes: Opaque envelope | Yes | Yes | Yes | Yes | 124 | Medium |
| Luoto et al., 2011 [43] | Yes | Yes | Yes | Yes | Yes | Yes | 399 | Medium |
| Nascimento et al., 2011 [44] | Yes | Yes: Opaque envelope | Yes | Yes | Yes | Yes | 82 | Low |
Summary and analysis of studies that meet the criteria
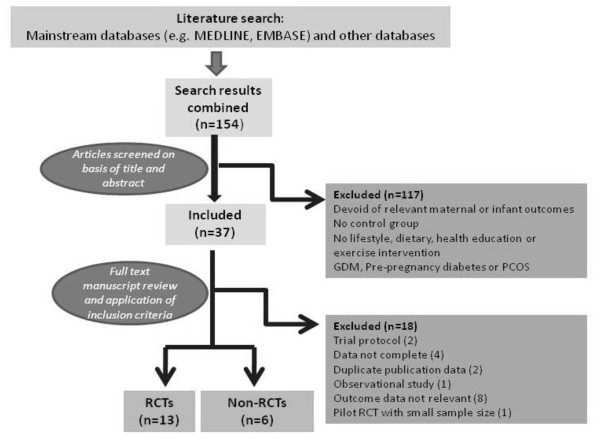
This is shown in Figure 1 and in a tabulated format contained within Table 2 and 3.
Figure 1.
Flow diagram of study selection.
Summary measures and data synthesis
The main measure of effect of the meta-analysis was the odds ratio or standardised mean difference. The data syntheses were conducted according to the Cochrane methodology [50]. First, we used statistical meta-analysis techniques to assess the efficacy of the interventions of controlled trials. Chi-square statistics tests were used to test for heterogeneity (Q statistics) between controlled trials. When there was no significant heterogeneity, we combined effect sizes in a fixed effect statistical meta-analysis using Review Manager (RevMan; Version 5.0, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). The meta-analyses were performed by calculating the odds ratios (for proportion data) or standardised mean differences (for scale data) using a fixed effects model. Quantitative analysis was performed on an intention-to-treat basis focused on data derived from the period of follow-up. There was heterogeneity between studies because of the smaller sample size of some of the studies (poor quality), variation of the study population and the intensity and duration of the interventional strategies being evaluated. A random effects model was used to adjust for heterogeneity.
Results
Study characteristics
The review process is outlined in Figure 1 and the selected papers summarised in Tables 2 and 3.
Fifteen trials met the inclusion criteria: 13 RCTs [26-34] and six non-RCTs [35,36,38,39,51,52]. All 19 trials were performed in developed countries: five in the USA, three in Canada, three in Australia, two in Finland and one in Denmark, Netherlands, Sweden, Spain, Brazil and Belgium (Tables 2 and 3). Five RCTs were judged to be of medium quality [27,29,34]. The rest were deemed low quality (Tables 4 and 5).
The pooled RCTs included a total of 1,228 participants and the pooled non-RCTs included 1,534 participants. Participants were predominantly white except in the studies by Asbee et al. [27], Gray-Donald et al. [38] and Hui et al. [33]. In the Asbee et al. study, the majority were described as being of Hispanic ethnicity [27].
For all included RCTs, the control group received no intervention or standard care. In the non-RCTs, most used non-parallel controls [35,38,39,51] or controls from another centre [36]. The outcomes investigated in the trials were gestational weight gain, gestational diabetes, Caesarean section delivery, large for gestational age baby and birth weight.
Effects of the intervention on outcomes
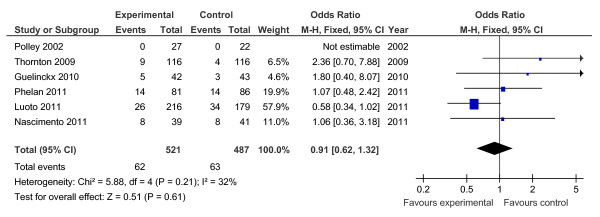
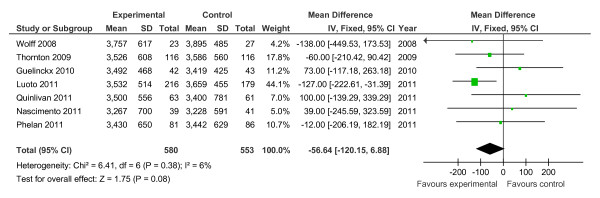
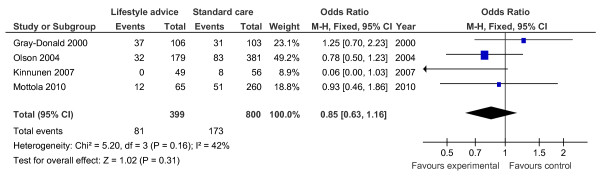
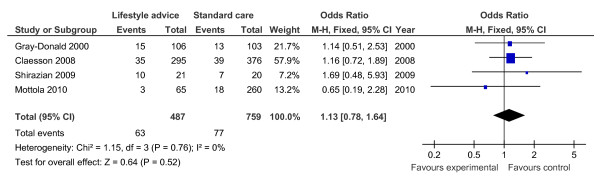
Of the 19 controlled trials, 16 measured gestational weight gain (10 randomised, 6 non-randomised); 8 recorded gestational diabetes (6 randomised, 2 non-randomised); 10 recorded Caesarean delivery (6 randomised, 4 non-randomised); 10 measured large for gestational age (6 randomised, 4 non-randomised); and 7 measured birth weight (7 randomised). Meta-analyses for the different outcomes are shown in Tables 6 and 7, and Figures 2, 3, 4, 5, 6, 7, 8, 9 and 10.
Table 6.
Effect estimates for randomised trials of lifestyle advice versus standard care
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
|---|---|---|---|---|
| Large for gestational age | 6 | 1,008 | Odds ratio (Fixed, 95% CI) | 0.91 (0.62, 1.32) |
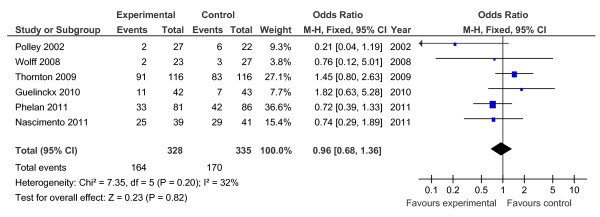
| Caesarean delivery | 6 | 663 | Odds ratio (Fixed, 95% CI) | 0.96 (0.68, 1.36) |
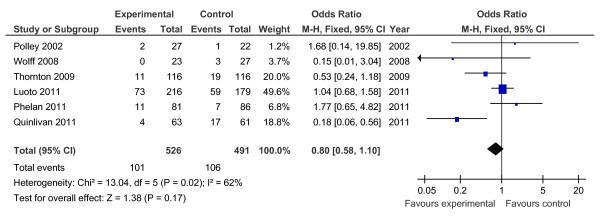
| Gestational diabetes | 6 | 1,017 | Odds ratio (M-H, Fixed, 95% CI) | 0.80 (0.58, 1.10)a |
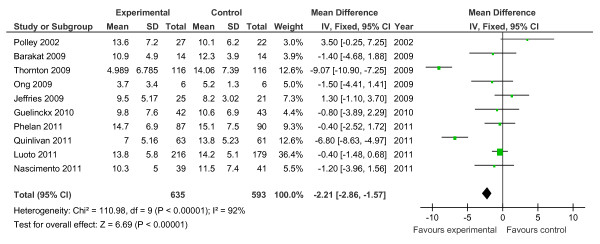
| Gestational weight gain (kg) | 10 | 1,228 | Mean difference (Fixed, 95% CI) | -2.21 (-2.86, -1.57)a |
| Birth weight (g) | 7 | 1,133 | Mean difference (Fixed, 95% CI) | -56.64 (-120.15, 6.88) |
aStatistically significant pooled estimates. CI: confidence interval
Table 7.
Effect estimates for non-randomised trials of lifestyle advice versus standard care
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
|---|---|---|---|---|
| Large for gestational age | 4 | 1,199 | Odds ratio (Fixed, 95% CI) | 0.85 (0.63, 1.16) |
| Caesarean delivery | 4 | 1,246 | Odds ratio (Fixed, 95% CI) | 1.13 (0.78, 1.64) |
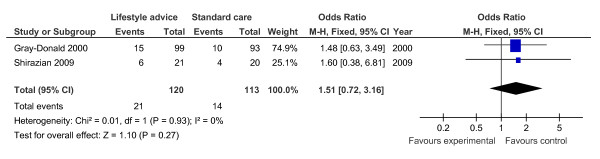
| Gestational diabetes | 2 | 233 | Odds ratio (Fixed, 95% CI) | 1.51 (0.72, 3.16) |
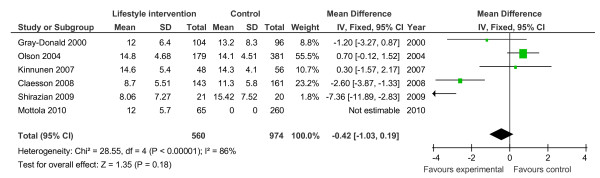
| Gestational weight gain (kg) | 6 | 1,534 | Mean difference (Fixed, 95% CI) | -0.42 (-1.03, 0.19) |
CI: confidence interval
Figure 2.
Forest plot of randomised trials investigating the effect of lifestyle advice versus standard care on gestational weight gain (kg).
Figure 3.
Forest plot of randomised trials investigating the effect of lifestyle advice versus standard care on risk of gestational diabetes.
Figure 4.
Forest plot of randomised trials investigating the effect of lifestyle advice versus standard care on risk of Caesarean delivery.
Figure 5.
Forest plot of randomised trials investigating the effect of lifestyle advice versus standard care on risk of large for gestational age baby.
Figure 6.
Forest plot of randomised trials investigating the effect of lifestyle advice versus standard care on birthweight.
Figure 7.
Forest plot of non-randomised trials investigating the effect of lifestyle advice versus standard care on risk of large for gestational age baby.
Figure 8.
Forest plot of non-randomised trials investigating the effect of lifestyle advice versus standard care on risk of Caesarean section.
Figure 9.
Forest plot of non-randomised trials investigating the effect of lifestyle advice versus standard care on risk of gestational diabetes.
Figure 10.
Forest plot of non-randomised trials investigating the effect of lifestyle advice versus standard care on gestational weight gain (kg).
Meta-analysis of RCTs showed that combined antenatal lifestyle, dietary and activity intervention restricts gestational weight gain (Table 6 and Figure 2) and there was a trend towards reduction in the prevalence of gestational diabetes in overweight and obese women (Table 6 and Figure 3). However, meta-analysis of non-RCTs only showed weak evidence that lifestyle intervention reduces gestational weight gain (Table 7 and Figure 7) and there was no evidence for a reduction in prevalence of gestational diabetes (Table 7 and Figure 8). There was no robust evidence that lifestyle intervention is associated with a lower prevalence of Caesarean delivery or macrosomia or any alteration in birth weight (Tables 6 and 7, Figures 4,5, 6, 8, 9 and 10).
Intervention characteristics
The nature of the interventions varied widely between studies and some of the key features of the interventions are outlined in Tables 2 and 3. In summary, for the six non-RCTs, three of the interventions comprised individual and group or seminar components [36,38,39,53], two were individual [35,52] and one was unclear [51]. Of the 13 RCTs, one comprised individual and group components [33], eight were individual [27-30,32,34] and three were group-based [26]. Where there were individual and group components, the latter were usually physical activity sessions. All of the non-RCTs included dietary and physical activity guidance, as did the majority of the randomised studies. Exceptions were two studies which included only nutritional guidance [29,30] and one which included guidelines about weight gain and weight monitoring only [28]. The majority of studies included dietary or physical activity guidance, with one of the non-RCTs [35] and three of the RCTs [29,32-34] specifying that guidance was personalised.
Discussion
Summary of main findings
Antenatal lifestyle, dietary and activity advice for overweight and obese pregnant women restricts maternal weight gain during pregnancy and lowers the prevalence of gestational diabetes in women who are overweight or obese. However, the quality of the study designs was generally poor. The reduction in gestational weight gain was observed to be statistically significant in the meta-analysis of randomised trials (10 RCTs; n = 1,228; -2.21 kg (95% CI, -2.86 to -1.57 kg)) but non-significant in the meta-analysis of non-randomised trials (six non-RCTs; n = 1,534). No effects of antenatal lifestyle interventions were identified in obese and overweight pregnant women in relation to Caesarean delivery, large for gestational age, birth weight and macrosomia (> 4 kg).
Interpretation
There is evidence to suggest antenatal lifestyle interventions may restrict gestational weight gain and a trend towards a reduced prevalence of gestational diabetes, but there was no statistical effect on other important clinical outcomes, possibly due to inadequate power of the combined sample size. The effect on restricted weight gain and gestational diabetes was not consistent across all the trial populations and therefore cannot be generalised. There was also wide variation in the types of interventions evaluated in the studies. The majority were individual-based and most provided generic guidance comprising mainly dietary and physical activity information, with few tailoring guidelines. There was considerable heterogeneity in intervention design and no obvious patterns between intervention type and study outcomes. For the gestational weight gain and gestational diabetes outcomes, both the successful and non-successful studies included those which were personalised, combined physical activity and dietary guidance and were individual-based. Moreover, degrees of weight gain restriction achieved were modest overall. It is even harder to make conclusions regarding the specific behaviour change strategies included (for example, monitoring and goal setting) or theoretical basis of interventions since these were typically poorly reported.
Identifying specific components of successful interventions aids understanding of how interventions are having an effect and clear reporting of intervention design allows for easier replication [54]. Previous reviews have attempted to make conclusions regarding specific effective components of interventions. Suggestions that weight monitoring and setting weight goals could be useful [46] and also monitoring along with education counselling and physical activity sessions [51,55] have been made. Another review suggested that interventions should be based on the Theory of Planned Behaviour, but the rationale for using this model over others in this population was unclear [56]. None of these reviews examined intervention components systematically. A more recent review by Gardner et al. assessed interventions targeting gestational weight gain from a psychological perspective and specifically examined intervention content and delivery methods [57]. This review comprised 10 controlled trials, all included in the current review; only two of the studies reported basing interventions on theory and the studies used, on average, five behaviour change strategies (self-monitoring, feedback provision and setting behavioural goals were the most common), but no conclusions could be drawn as to their contribution to study outcomes. Broadly consistent with this were the four studies in the current review which were not included in the review by Gardner et al. [57]. Their review questioned the evidence supporting the benefits of weight monitoring, but tentatively suggested that information provision had been underused and that it might be of benefit to have a narrower focus of intervention targets [57].
Comparison with other systematic reviews and strengths
Our study adds to a growing body of evidence that aims to evaluate lifestyle intervention as a means to minimise the adverse outcome associated with obesity in pregnancy. In comparison to other published reviews [45,46,56], we have adopted an original approach by broadening the literature source (multiple data sources, no language restriction), focusing on relevant clinical outcomes (such as Caesarean section, gestational diabetes, macrosomia), and improving our sensitivity by meta-analysing both RCTs and non-RCTs. Furthermore, to minimise bias, the review methodology was registered a priori (Prospero number CRD420111122 http://www.crd.york.ac.uk/PROSPERO). We therefore believe our review provides a comprehensive and reliable analysis of the current evidence and for the first time highlights that lifestyle intervention in pregnancy may reduce the prevalence of gestational diabetes.
Limitations of this systematic review
The evidence summarised in this work comes from available studies of which most are of low quality, with only four studies fulfilling a medium quality score. Hence, the evidence base is weak and calls for more robust studies. Our trial population is relatively small, the intensity and duration of the interventions of trials varied and trials were predominantly USA in origin; a phenomenon common to many public health reviews, especially on obesity. Although our focus was on antenatal lifestyle intervention for obese and overweight pregnant women, our search yielded some studies that contained a mixed group of obese and normal weight women and we excluded all the non-obese participants from our analysis. Still, this may lead to inconsistencies in measuring the effect of the intervention as well as under- or overestimating the treatment effect. Furthermore, even though our search was systematic and rigorous, we could have missed eligible studies inadvertently.
Conclusions
This review reveals that lifestyle interventions for obese and overweight women during pregnancy restrict gestational weight gain and a trend was evident towards reducing the prevalence of gestational diabetes. However, the quality of the published studies is mainly poor. This then highlights a paradox. At a time when solutions to address adverse outcome associated with maternal overweight and obesity are identified as a public health priority, we find that most of the research evidence lacks robustness to inform future evidence-based lifestyle interventions for obese pregnant women. There is thus a research gap regarding the effectiveness of lifestyle intervention in pregnancy. It is unlikely that further meta-analysis will help to refine the quality of evidence because studies demonstrated significant heterogeneity in relation to demography, outcome measurement, follow-up and degree of intervention. Hence, we conclude that there is the need for a well-designed large-scale prospective trial which examines combined antenatal lifestyle interventions in obese pregnant women that is suitably powered and incorporates robust methodology in accordance with standards set by Medical Research Council's framework for evaluating complex interventions [58]. There are two such studies which are currently ongoing called LIMIT (ACTRN 12607000161426) and UPBEAT (ISRCTN89971375). Both of these studies are appropriately powered to show convincingly whether lifestyle intervention is most likely to improve pregnancy outcome or not.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The idea was conceived by PD and EO-N. The literature search and meta-analysis were by RV and EO-N and all contributed to the write up. All authors read and approved the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Eugene Oteng-Ntim, Email: Eugene.oteng-ntim@gstt.nhs.uk.
Rajesh Varma, Email: drbobbyvarma@gmail.com.
Helen Croker, Email: h.croker@ucl.ac.uk.
Lucilla Poston, Email: Lucilla.poston@kcl.ac.uk.
Pat Doyle, Email: pat.doyle@lshtm.ac.uk.
Acknowledgements
We acknowledge Lisa Xue, Royal College of Obstetricians and Gynaecologists Librarian, for her help in acquiring most of the manuscripts in time. We also acknowledge Guy's and St Thomas' charity for supporting E-ON. This manuscript presents independent research commissioned by the National Institute for Health Research (NIHR, UK) under the programme grants for Applied Research programme RP-0407-10452. The views expressed in this manuscript are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
References
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylott J, Brown I, Copeland R, Johnson D. Tackling obesity: the foresight report and implication for local government. 2008. http://www.idea.gov.uk/idk/aio/8268011
- Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow Maternity Hospital. BJOG. 2005;112:1431–1433. doi: 10.1111/j.1471-0528.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. Int J Obes (Lond) 2010;34:420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- Centre for Maternal and Child Enquiries. Maternal obesity in the United Kingdom: findings from a national project. 2010. http://www.oaa-anaes.ac.uk/assets/_managed/editor/File/CMACE/CMACE_Obesity_Report_2010_Final%20for%20printing.pdf
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- Poston L. Developmental programming and diabetes - the human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:541–552. doi: 10.1016/j.beem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14:77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Larsen TB, Sorensen HT, Gislum M, Johnsen SP. Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: a population-based nested case-control study. Thromb Res. 2007;120:505–509. doi: 10.1016/j.thromres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Knight M. Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG. 2008;115:453–461. doi: 10.1111/j.1471-0528.2007.01622.x. [DOI] [PubMed] [Google Scholar]
- Usha Kiran TS, Hemmadi S, Bethel J, Evans J. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768–772. doi: 10.1111/j.1471-0528.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Poobalan AS, Aucott LS, Precious E, Crombie IK, Smith WC. Weight loss interventions in young people (18 to 25 year olds): a systematic review. Obes Rev. 2010;11:580–592. doi: 10.1111/j.1467-789X.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114:343–348. doi: 10.1111/j.1471-0528.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007;97:157–162. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, Harthoorn LF, van der Beek EM. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res. 2011;69:175–180. doi: 10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- Inskip HM, Crozier SR, Godfrey KM, Borland SE, Cooper C, Robinson SM. Women's compliance with nutrition and lifestyle recommendations before pregnancy: general population cohort study. BMJ. 2009;338:b481. doi: 10.1136/bmj.b481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91:373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol. 2009;113:305–312. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust. 2009;191:429–433. doi: 10.5694/j.1326-5377.2009.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009;101:569–577. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- Yeo S. A randomized comparative trial of the efficacy and safety of exercise during pregnancy: design and methods. Contemp Clin Trials. 2006;27:531–540. doi: 10.1016/j.cct.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord. 2002;26:1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- Hui AL, Ludwig SM, Gardiner P, Sevenhuysen G, Murray R, Morris M, Shen GX. Community based excercise and dietary intervention during pregnancy: a pilot study. Canadian Journal of Diabetes. 2006;30:169–175. [Google Scholar]
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola MF, Giroux I, Gratton R, Hammond JA, Hanley A, Harris S, McManus R, Davenport MH, Sopper MM. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Med Sci Sports Exerc. 2010;42:265–272. doi: 10.1249/MSS.0b013e3181b5419a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson IM, Sydsjo G, Brynhildsen J, Cedergren M, Jeppsson A, Nystrom F, Sydsjö A, Josefsson A. Weight gain restriction for obese pregnant women: a case-control intervention study. BJOG. 2008;115:44–50. doi: 10.1111/j.1471-0528.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Aittasalo M, Koponen P, Ojala K, Mansikkamaki K, Weiderpass E, Fogelholm M, Luoto R. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC Pregnancy Childbirth. 2008;8:37. doi: 10.1186/1471-2393-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ. 2000;163:1247–1251. [PMC free article] [PubMed] [Google Scholar]
- Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification program decreases pregnancy weight gain in obese women. Am J Perinatol. 2010;27:411–414. doi: 10.1055/s-0029-1243368. [DOI] [PubMed] [Google Scholar]
- HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med. 2011. in press . [DOI] [PubMed]
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab. 2009;35:418–421. doi: 10.1016/j.diabet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, Mansikkamäki K, Lamberg S, Vasankari T, Komulainen T, Tulokas S. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLoS Med. 2011;8:e1001036. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento SL, Surita FG, Parpinelli MA, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomised clinical trial. BJOG. 2011;118:1455–1463. doi: 10.1111/j.1471-0528.2011.03084.x. [DOI] [PubMed] [Google Scholar]
- Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010;117:1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010;92:678–687. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11:81. doi: 10.1186/1471-2393-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Grivell RM, Dodd JM. Antenatal exercise to improve outcomes in overweight or obese women: a systematic review. Acta Obstet Gynecol Scand. 2012;91:538–545. doi: 10.1111/j.1600-0412.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions. 2011. http://www.cochrane.org/training/cochrane-handbook
- Olson CM. A call for intervention in pregnancy to prevent maternal and child obesity. Am J Prev Med. 2007;33:435–436. doi: 10.1016/j.amepre.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, Luoto R. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr. 2007;61:884–891. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- Claesson IM, Brynhildsen J, Cedergren M, Jeppsson A, Sydsjo A, Josefsson A. Weight gain restriction during pregnancy is safe for both the mother and neonate. Acta Obstet Gynecol Scand. 2009;88:1158–1162. doi: 10.1080/00016340903214916. [DOI] [PubMed] [Google Scholar]
- Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci. 2011;6:10. doi: 10.1186/1748-5908-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutr. 2008;28:411–423. doi: 10.1146/annurev.nutr.28.061807.155322. [DOI] [PubMed] [Google Scholar]
- Birdsall KM, Vyas S, Khazaezadeh N, Oteng-Ntim E. Maternal obesity: a review of interventions. Int J Clin Pract. 2009;63:494–507. doi: 10.1111/j.1742-1241.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obes Rev. 2011;12:e602–e620. doi: 10.1111/j.1467-789X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust NZJ Obstet Gynaecol. 2011;51:141–146. doi: 10.1111/j.1479-828X.2010.01268.x. [DOI] [PubMed] [Google Scholar]