Abstract
Telomerase activity is readily detectable in extracts from human hematopoietic stem and progenitor cells, but appears unable to maintain telomere length with proliferation in vitro and with age in vivo. We performed a detailed study of the telomere length by flow FISH analysis in leukocytes from 835 healthy individuals and 60 individuals with reduced telomerase activity. Healthy individuals showed a broad range in average telomere length in granulocytes and lymphocytes at any given age. The average telomere length declined with age at a rate that differed between age-specific breakpoints and between cell types. Gender differences between leukocyte telomere lengths were observed for all cell subsets studied; interestingly, this trend could already be detected at birth. Heterozygous carriers for mutations in either the telomerase reverse transcriptase (hTERT) or the telomerase RNA template (hTERC) gene displayed striking and comparable telomere length deficits. Further, non-carrier relatives of such heterozygous individuals had somewhat shorter leukocyte telomere lengths than expected; this difference was most profound for granulocytes. Failure to maintain telomere homeostasis as a result of partial telomerase deficiency is thought to trigger cell senescence or cell death, eventually causing tissue failure syndromes. Our data are consistent with these statements and suggest that the likelihood of similar processes occurring in normal individuals increases with age. Our work highlights the essential role of telomerase in the hematopoietic system and supports the notion that telomerase levels in hematopoietic cells, while limiting and unable to prevent overall telomere shortening, are nevertheless crucial to maintain telomere homeostasis with age.
Author Summary
Human blood cells all originate from a common precursor, the hematopoietic stem cell. Telomerase, the enzyme responsible for adding telomere repeats to chromosome ends, is active in human hematopoietic stem cells but appears unable to maintain a constant telomere length with age. We first document the telomere length of different blood cell subsets from 835 healthy individuals between birth and 100 years, to delineate the normal rate of telomere attrition with age. Telomere lengths of blood cells were found to be slightly longer in women than in men, from birth and throughout life. We then compared this reference data to the telomere length in similar blood cell subsets from individuals with reduced telomerase activity as a result of a mutation in one of the genes encoding telomerase and from their direct relatives. Strikingly short telomeres were found in telomerase-deficient individuals, consistent with their cellular pathology and disease susceptibility, and somewhat shorter telomeres than expected were found in cells of relatives with normal telomerase maintenance. Our data can be used as a reference for blood cell telomere length in studies of normal and accelerated aging.
Introduction
At least a few hundred nucleotides of telomere repeats must “cap” each chromosome end in order to suppress DNA damage signals and avoid the activation of DNA repair pathways [1]–[3]. Critically short or “uncapped” telomeres may be repaired by the enzyme telomerase [4] or by recombination [5]. However, the capacity of these telomere repair processes appears limited in most human somatic cells [6]. Apoptosis or cellular senescence is triggered when too many “uncapped” telomeres accumulate [7], posing a barrier to tumor growth, but also contributing to loss of cells with age [8].
Despite increasing evidence that telomere homeostasis is important in human aging, cancer and disease states, detailed and comparative information regarding the telomere length in different human cell subtypes of healthy individuals in relation to their age is surprisingly modest. Apart from being technically challenging [9] such studies are complicated because at birth and throughout life, telomere length is highly variable between chromosomes [10], [11], between cells [12], [13] and between individuals. Studies of identical twins have shown that individual differences in average telomere length appear to be largely genetically determined [14], [15].
In most somatic cells the telomere length declines with age and with cell division in culture, albeit at different rates [13], [16]. For example, in humans and baboons, lymphocytes show a more pronounced telomere loss with age than granulocytes [14], [17]. These two cell types represent the two major branches of the hematopoietic system, which can be further subdivided into distinct cell populations based on their phenotype and function. Within the hematopoietic hierarchy, the most primitive cells, hematopoietic stem cells (HSC), have the longest telomeres [18], [19]. HSC differentiate to produce progenitor cells of both the myeloid and lymphoid lineage that proliferate prior to differentiation into mature “end” cells. Unlike most immune cells most differentiated myeloid cells such as granulocytes are incapable of further cell divisions.
The precise role of telomerase in hematopoietic stem and progenitor cells and in lymphocytes remains poorly understood. Telomerase expression is readily detected in hematopoietic cells [20]–[22]; however, this activity appears unable to prevent telomere loss with age or proliferation. It is often assumed that telomerase is required to maintain the telomere length in various stem cells. With the exception of embryonic stem cells and abnormal tumor (stem) cells this assumption is not supported by data. Studies on the role of telomeres and telomerase in HSC from healthy individuals are challenging because HSC are very rare cells that typically reside in bone marrow. In contrast, the various nucleated blood cells that are derived from HSC are easily accessible for study. The average telomere length in granulocytes can be used as a surrogate marker for the telomere length in HSC [23], if one assumes that the number of cell divisions between HSC and granulocytes is relatively constant [18]. Individual carriers of heterozygous mutations for either the telomerase RNA gene (hTERC) or the telomerase reverse transcriptase gene (hTERT) can present with a wide spectrum of diseases [24] including dyskeratosis congenita [25], [26], bone marrow failure [24] and pulmonary fibrosis [27]. Heritable telomerase deficiencies provide an excellent model to study the role of telomerase in human hematopoietic cells.
Here we report our data on the median telomere length (MTL) in five distinct leukocyte subpopulations of over 800 healthy individuals between birth and 100 years of age as well as 60 individuals that are heterozygous for one of the telomerase genes, hTERC or hTERT. The telomere length in leukocytes from healthy individuals was found to vary over a broad range at any given age and the rate of telomere attrition also varied with age and with cell type. Strikingly, the telomeres in cells from individuals with telomerase deficiency were found to be very short in all cell types, and this deficit was found to be comparable for most cell subtypes for hTERC or hTERT deficiency. The largest (age adjusted) differences in telomere length deficits between hTERC or hTERT were seen in “naïve” T cells for hTERC deficient individuals and in NK/differentiated T cells for hTERT deficient individuals. These results demonstrate that normal telomerase levels are essential to maintain normal telomere homeostasis in HSC and lymphocytes. Our results provide valuable reference data for further studies of telomere biology in health and disease and point to a crucial rate-limiting role for telomerase in HSC and immune cells.
Results
Lymphocyte and granulocyte telomere length dynamics with age
We measured the telomere length in lymphocytes and granulocytes of 835 healthy individuals using automated multicolor flow FISH (Figure 1). On average, 7 to 8 individuals were tested for each age-year. Various best-fit models were tested to model the overall decline in telomere length with age. In view of the very rapid decline in telomere length in the first years of life in humans [14] as well as non-human primates [28], we divided the telomere length decline over three age segments. The first is between birth and one year of age when the growth rate of bones and weight in infants shows a marked deceleration (for reference curves, see http://www.cdc.gov/growthcharts/clinical_charts.htm). A second arbitrary cut-off was set at 18 years of age because the decline in telomere length in all leukocytes appeared to drop notably after puberty. Telomere length data within the three selected age segments: below 1 year (yr), 1–18 yrs and 19 yr and higher are shown in Figure 1 and Table 1. The overall age-related telomere length decline was most pronounced in lymphocytes with significant losses ranging from 1190 base pairs (bp) per year between birth and 1 year of age to 126 bp per year during childhood and 43 bp per year in adulthood. In contrast, the age-related telomere length decline in granulocytes and by extension in HSC was more modest during early life (485 bp per year), childhood (74 bp per year) and adulthood (28 bp per year).
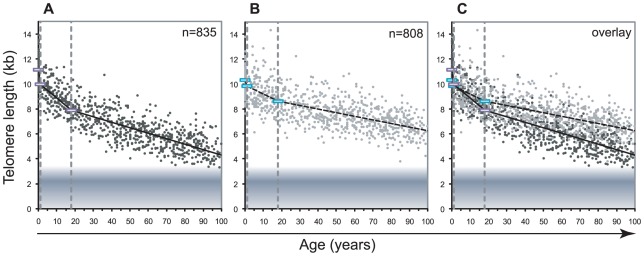
Figure 1. Decline in telomere length with age differs between lymphocytes and granulocytes.
The median telomere length in nucleated blood cells from 835 healthy individuals ranging from birth (umbilical cord blood) to 102 years of age were measured by flow FISH. The results were used to calculate the telomere attrition over time using linear regression in three age segments. A. Median telomere length in lymphocytes (black dots). B. Median telomere length in granulocytes (grey dots). Breakpoints in the piece-wise linear regression lines are marked by rectangles and the three age groups are marked by dotted vertical grey lines at 1 and 18 years. On average 8 individuals were tested per age-year. C. At any given age, a wide range of telomere length was observed and the decline in telomere length with age in lymphocytes was more pronounced than in granulocytes. The shaded area represents the estimated length of subtelomeric DNA. Note that in older individuals, on average only 1–2 kb of telomere repeats were present in lymphocytes.
Table 1. Telomere length distribution and age-related telomere length decline in healthy individuals.
| lymphocytes | granulocytes | CD20+ | CD45RA+ CD20− | CD45RA− | CD57+ | ||
| Slope (bp/yr) age range: | <1 | −1190 | −485 | −916 | −947 | −2073 | −2818 |
| 1–18 | −126 | −74 | −70 | −89 | −144 | −155 | |
| 19–102 | −43 | −28 | −27 | −51 | −32 | −39 | |
| MTL regression (kb) age: | birth | 11.2 | 10.3 | 10.9 | 11.1 | 11.4 | 12.4 |
| 1 | 10.0 | 9.9 | 9.9 | 10.1 | 9.3 | 9.6 | |
| 18 | 7.9 | 8.6 | 8.8 | 8.6 | 6.8 | 7.0 | |
| 102 | 4.3 | 6.2 | 6.5 | 4.3 | 4.1 | 3.7 | |
| MTL range (kb) | 1–99 percent | 4.34 | 4.83 | 4.67 | 5.02 | 4.09 | 5.19 |
| 10–90 percent | 2.45 | 2.67 | 2.37 | 2.80 | 2.28 | 2.89 |
The decline in telomere length with age varies between leukocyte subpopulations
Telomere length measurements versus age in granulocytes and lymphocyte subpopulations were used to determine the regression lines for telomere attrition in the three selected age ranges (regression estimates shown in Figure 2A; the complete data set can be accessed in Table S1). These regression lines were shifted according to data distribution (from the overall regression estimate) to represent the 99th, 90th, 10th and 1st percentile of the telomere length distribution in each age segment for each blood cell subset in healthy individuals. The rate of telomere length decline varied amongst the different lymphocyte subsets analyzed. The telomere length decline with age in B lymphocyte subset (CD45RA+ CD20+) was comparable to that in granulocytes. Memory T (CD45RA−CD20−) and mature NK/T (CD45RA+CD57+) lymphocyte subsets showed the sharpest decline in telomere length with age, particularly during childhood with slopes of −144 and −155 bp per year respectively. The CD45RA+CD20− T lymphocyte subset enriched for “naïve” T cells and the CD45RA+CD57+ mature NK/T lymphocyte subset displayed the widest distributions, 2.80 and 2.89 kilobase (kb) respectively between the 10th and 90th percentile of the normal distribution, throughout the age ranges. Unlike other subsets CD45RA+CD20− T lymphocytes showed only a modest difference in the telomere attrition rate between childhood and adulthood: 89 and 51 bp per year respectively. In contrast, the memory T lymphocyte subset (CD45RA−CD20−) displayed the narrowest range of telomere length distribution (2.28 kb between the 10th and 90th percentile of the normal distribution). Overall, the shortest telomere lengths were measured in memory T and mature NK/T lymphocytes from older individuals.
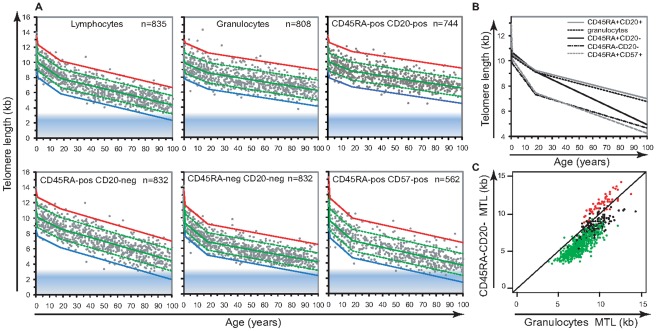
Figure 2. Cell type–specific differences in the range and attrition rate of leukocyte telomere length.
A. Cross-sectional median telomere length in nucleated cell types determined by flow FISH in 835 healthy individuals over the age range from birth to 102 years of age. The following nucleated blood cell subtypes were analyzed : lymphocytes, granulocytes, CD45RA positive CD20 positive B lymphocytes (CD20+), CD45RA positive CD20 negative lymphocytes (CD45RA+pos CD20−) “naïve” T cells, CD45RA negative CD20 negative lymphocytes (CD45RA−) memory T cells and CD45RA positive CD57 positive mature NK/T cells (CD57+). Data were analyzed using a piece-wise linear regression model in the age categories 0, 1 yr; 18 yrs and 102 years and for calculation and representation of the telomere length distribution range at any given age (expressed as a percentile): 99th (red), 90th (dashed green top), 50th (green), 10th (dashed green bottom) and 1st (blue). Some of the healthy subjects (n = 835) did not have sufficient cells for analysis of one or more of the cell subsets. B. Piece-wise linear regression analysis overlay representing the modeled estimate of telomere length per age for: B lymphocytes (grey), granulocytes (black dashed), “naïve” T lymphocytes (black), memory T lymphocytes (black interrupted dashed) and mature NK/T lymphocytes (grey dashed). Regression breakpoints were set at age 1 and age 18 years. C. Paired comparison of telomere length in granulocytes and memory T lymphocytes from the same individuals. Age groups were as follows: cord blood samples and below age 1 (n = 60, red), age 1 to 18 (n = 171, black), 19 and above 19 (n = 604, green).
Comparisons of telomere length in different leukocyte subsets
From our cross-sectional data, we determined the average telomere length decline with age for the different leukocyte subpopulations (Figure 2B). During childhood, granulocytes, CD45RA+CD20− “naïve” T lymphocytes and CD20+ B lymphocytes all showed a very similar decline in telomere length, whereas the rate of decline in memory T cells was much higher. Paired MTL values in different blood cell subsets from the same individual revealed that around one year of age the telomere length values in memory T lymphocytes drop below those of granulocytes (Figure 2C). In contrast, telomere length values in B lymphocytes remained comparable to those in granulocytes over the entire age range. One caveat in our measurement of telomere length in “naïve” (CD45RA+CD20−) T lymphocytes is that terminally differentiated effector lymphocytes re-expressing CD45RA are likely to represent an increased proportion within this cell population in older individuals. As a consequence, measurements within the subset of “naïve” T cells are variably skewed in older individuals (as illustrated by the direct comparison of MTL between “naïve” T lymphocytes and other cell subtypes from the same individual over 4 distinct age groups in Figure S2A and Table S2).
Gender differences in leukocyte telomere length measurements
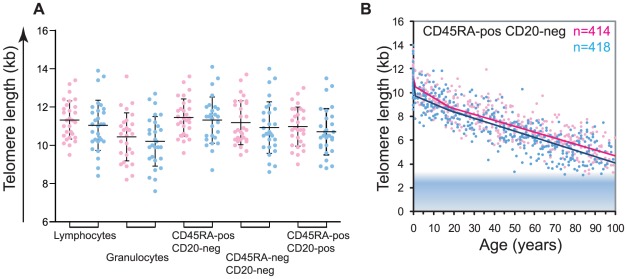
Measurements from cord blood samples provided the earliest opportunity to assess the telomere lengths in cells from healthy individuals. Interestingly, of all the cell types measured from cord blood, granulocytes showed the shortest telomeres at birth: differences were significant for granulocytes versus CD45−CD20− memory T lymphocytes and CD45+CD20− “naïve” T lymphocytes but not for granulocytes versus B lymphocytes. Comparisons were tested by one-way ANOVA (n = 58): F(5,265) = 5.7; P = 0.0002, Table S3, followed by Tukey's multiple comparison test, see details in Table S4). Interestingly, female newborns appeared to have longer telomeres than males (Figure 3A); however this trend did not reach statistical significance. Further comparisons of telomere length in leukocyte subsets as a function of gender showed highly significant differences between males and females in the CD45RA+CD20− “naïve” T lymphocyte subset over the entire age range (F(4,825) = 9.05; P = 3.7×10−7, ANOVA test result comparing regression fits; Figure 3B and Table S5). Significant differences were also seen for other leukocyte subsets in each age segment with the exception of granulocytes and memory T lymphocytes, which displayed similar, average telomere lengths after 18 years of age (Figure S3 and Table S5).
Figure 3. Gender differences in leukocyte telomere length.
A. Telomere length measurements in leukocyte subsets from female (pink) versus male (blue) healthy newborns (n = 58; females n = 29, males n = 29). The following white blood cell subsets were tested lymphocytes, granulocytes, “naïve” T lymphocytes (CD45RA+CD20−), memory T lymphocytes (CD45RA−CD20−) and B lymphocytes (CD20+). Each dot represents an individual sample, mean (horizontal bar) and standard deviation (vertical bar) for each cell type are shown. All subsets display a trend for longer average telomere lengths in females; however this trend does not reach statistical significance (Student's t test, data not shown). B. Piece-wise linear regression analysis representing the calculated telomere length per age for females (pink) versus males (blue) “naïve” T lymphocytes (CD45RA+CD20−). Breakpoints were at 1 year of age and at 18 years. Analysis of variance for females versus males (statistical model in R) showed significance (F(4,825) = 9.05; P = 3.7×10−7, ANOVA test result comparing regression fits). For data on other subsets and details of statistical analysis, see Figure S3 and Table S5.
Telomerase is essential to maintain leukocyte telomere length homeostasis
To study the role of telomerase in hematopoietic cells, we analyzed the telomere length in leukocyte subpopulations of individuals carrying a mutation in either hTERT or hTERC (n = 60) in comparison to non-carrier relatives (n = 37). The results, plotted on the telomere length versus age distribution curves derived from healthy individuals (Figure 2A) are shown in Figure 4 and Table 1. Strikingly, telomerase heterozygous individuals showed very short telomeres (typically below the 1st percentile of the normal distribution) at all ages and for all blood cell subsets tested (ANOVA test P<2.2×10−16, for full details of analyses see Table S5). The shortest telomeres were measured in mature NK/T cells (mean of 3.7±0.7 kb for all telomerase heterozygous individuals not adjusted for age, Figure 4A) and the CD45RA+CD20− “naive” lymphocyte subset appeared the most severely impacted by telomerase deficiency with an average difference to the normal distribution (adjusted for age) of Δtel: 3.2 kb. Differential analysis of leukocyte telomere lengths in leukocytes from hTERT vs. hTERC heterozygous individuals showed a similar effect on most blood cell subtypes (Figure 4B and Table 2). Exceptions were an increased telomere loss in the CD45RA+CD57+ mature NK/T cells (difference of 0.4 Kb) of hTERT deficient individuals (n = 37) and a slightly increased effect (difference of 0.2 Kb) on the CD45RA+CD20− “naive” lymphocyte subset for hTERC deficient individuals (n = 23).
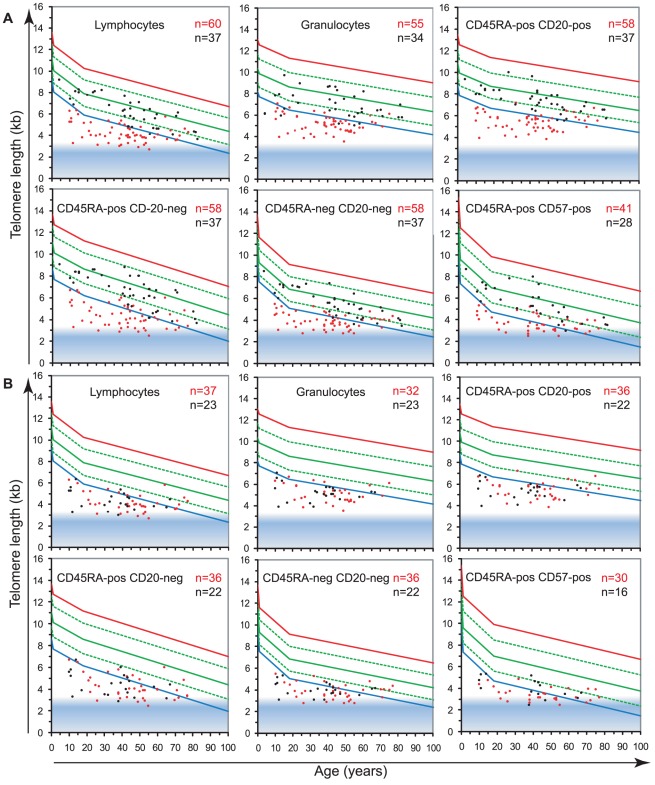
Figure 4. Telomerase is essential to maintain telomere length in leukocytes.
The telomere length distribution in healthy individuals (Figure 2) was used to plot the median telomere length values of leukocyte subsets obtained with: A. leukocytes from 60 telomerase deficient patients (red) and 37 non-carrier relatives (black). B. leukocytes from 37 hTERT mutation heterozygous individuals (red) and 23 hTERC mutation heterozygous carriers (black).
Table 2. Telomere loss corrected for age (Δtel) in telomerase heterozygous individuals and unaffected relatives.
| Δtel (kb)§ | lymphocytes | granulocytes | CD20+ | CD45RA+ CD20− | CD45RA− | CD57+ |
| Tel +/− individuals (60) | −2.7 | −2.9 | −2.8 | −3.2 | −2.3 | −2.4 |
| TERT +/− individuals (37) | −2.6 | −2.9 | −2.7 | −3.1 | −2.3 | −2.5 |
| TERC +/− individuals (23) | −2.8 | −2.9 | −2.9 | −3.3 | −2.3 | −2.1 |
| Tel +/+ relatives (36) | −0.6 | −0.9 | −0.6 | −0.7 | −0.6 | −0.6 |
| Tel +/+ parent (7) | −0.9 | −0.8 | −0.8 | −1.1 | −0.8 | −0.6 |
| Tel +/+ sibling (8) | −0.7 | −0.7 | −0.8 | −0.9 | −0.8 | −0.6 |
Summary of telomere loss adjusted for age (Δtel) in telomerase heterozygous individuals considered as a group (Tel), considered as TERT or TERC heterozygous individuals separately, and unaffected relatives of telomerase heterozygous individuals considered as a group (Tel), or separating parents or siblings.
Difference between the median telomere length and the regression estimate of the telomere length distribution in healthy individuals of the same age (Δtel).
Tel: hTERC or hTERT gene.
Non-carrier relatives of telomerase deficient individuals (hTERT and hTERC considered together), despite having intact telomerase genes, also showed somewhat shorter median telomere lengths in all leukocytes compared to the control population. The largest difference was measured in the granulocyte subset of non-carrier relatives considered together, with Δtel: 0.9 kb, which may be indicative of HSC deficit and warrants further investigation (Figure 4A; ANOVA test: F(3,837) = 8.1; P = 2.63×10−5, for full details of analyses see Table S5). Both parents and siblings of heterozygous individuals were found to have slightly shorter telomere lengths for age (Table 2 and Figure S4). Differential analysis of parents (n = 6) and siblings (n = 4) of hTERT deficient individuals was also performed and showed comparable telomere length deficits for all cell subsets tested (Figure S4 and data not shown) in this relatively small group.
Discussion
In this report, we show telomere length data for five distinct leukocyte subpopulations from over 800 healthy individuals, representing a comprehensive and representative cross-sectional analysis of telomere length in leukocyte subpopulations over the entire human life span. The value of this data is illustrated by our analysis of individuals with heritable telomerase deficiencies. Leukocyte telomere length was found to clearly distinguish between relatives with and without mutations in hTERT or hTERC supporting telomere length measurements as a screen for mutations in “telomere maintenance” genes. Our results confirm and extend earlier reports of telomere loss in leukocytes with age [13], [14] and document a crucial role for telomerase in controlling leukocyte telomere length.
Telomerase expression is readily detected in most hematopoietic cells [20]–[22], yet this activity appears unable to prevent the overall loss of telomeric DNA with age or proliferation. Most likely, telomerase is primarily required to directly act on chromosome ends in hematopoietic cells themselves, however secondary, indirect effects of telomerase via cells that support cell proliferation [29] or possible effects of the TERT protein on transcription in stem cells [30] are difficult to exclude.
Heterozygosity for one of the telomerase genes, expected to reduce telomerase levels by half, results in a striking telomere deficit (Figure 4). How can this finding be explained? One possibility is that the primary function of telomerase in somatic cells is the repair [8] or protection [31] of critically short telomeres. Failure to properly “cap” all chromosome ends with telomere repeats results in activation of a DNA damage response [1], [32]. Detrimental consequences for HSCs and lymphocytes could result when DNA damage signals from uncapped telomeres persist or reach a certain threshold and cause apoptosis of such cells. Impaired “capping” of telomeres in cells with reduced telomerase could affect telomere length directly and indirectly. Direct effects on telomere length could result from normal replication of telomeric DNA [8] and damage caused by reactive oxygen species [33]–[35]. Indirect effect on telomere length would result from the additional cell division required to compensate for the increased cell losses. Compensatory cell divisions in cells from telomerase deficient individuals could be particularly taxing as more short telomeres are expected to emerge with each extra cell division. The resulting feed-forward loop could exhaust the stem cell compartment in infants and children explaining the marrow failure typically seen in pediatric telomerase deficient patients. In cases where sufficient stem cells survived till adulthood, the same unproductive feed-forward loop could exhaust cells of the immune system. This possibility is in line with the observation that after puberty telomere attrition in more mature subsets of T and NK cells is notably higher than in granulocytes as a surrogate marker for stem cells (Figure 2). We speculate that the balance between end cells such as granulocytes and macrophages on the one hand and various other immune cell types on the other is perturbed in older telomerase deficient patients. Such an imbalance could result in failure to clear pathogens and immunogens and create pro-fibrotic conditions or result in failure to remove senescent cells [36].
Apart from cell turnover and telomerase levels, the telomere length in parental chromosomes at fertilization is another probable variable in the disease manifestations of telomerase deficient patients. This variable will determine when critically short telomeres, requiring repair or capping by telomerase, will appear: during development or during adult life. This notion is in line with the age-related onset of symptoms or “anticipation” in multi-generation telomerase deficiency disorders [24], [37] and our observation that telomeres in cells from unaffected children and parents of telomerase heterozygous individuals are somewhat shorter than expected (Figure 4 and Table 2).
As was shown previously for human lymphocytes and granulocytes [14], [38] and confirmed in longitudinal studies of non-human primates [28], leukocyte telomere length shortens most dramatically very early in life. This rapid decline can be explained by steady proliferation of stem cells and immune cells after birth. After one year of age we observed a rapid deceleration in telomere loss most likely reflecting an intrinsic, ontogeny-related change in stem cell turnover and function [39], which has also been observed in postnatal mice [40], [41]. Our observations with human and primate cells suggest that each HSC cell division in these species is “counted” by the loss of telomeric DNA. Why a relative modest decline in telomerase activity in humans results in a wide spectrum of diseases whereas complete loss of telomerase is typically tolerated for several generations in yeast, plants, worms and mice remains incompletely understood [42].
The shortest overall telomere lengths were measured in the mature NK/T cell subsets of older healthy individuals and of hTERT telomerase-deficient individuals. These results suggest that one of the primary consequences of telomere attrition and telomerase deficiencies could be the loss of NK immune function. This notion is compatible with the reported age-related decline in the number and function of these cells [43]. Of note, in some individuals the estimated telomere length in mature NK/T cells was near the predicted minimal telomere length (represented as a shaded area in Figure 1 and Figure 2) meaning that on average each chromosome end in those cells has fewer than 1 kb of telomere repeats.
Despite the finding that at birth telomeres in lymphocytes are longer than in granulocytes and despite the selective expression of telomerase in cells of the lymphoid lineage upon activation [22], [44], T lymphocytes displayed a sharp decline in telomere length with age. The steady decline in the telomere length in T cells likely contributes to compromised adaptive immunity in the elderly [45] and in individuals with telomerase deficiencies [46]. Interestingly, the narrowest telomere length distribution in leukocyte subsets from healthy individuals was observed in the memory T cell compartment, pointing to a possible role of telomere length in shaping the T cell repertoire and immune memory.
Our study of gender specific differences in telomere length confirmed previous observations that telomere lengths on average appear to be somewhat longer in females than in males [47]. The fact that this trend is already seen at birth raises questions that warrant further investigation: do females have fewer HSC at birth? Do female HSC have a higher replicative potential because of longer telomeres? Do stem cells in females have higher telomerase activity, possibly influenced by levels of sex hormones [48] or do other factors explain the longer telomeres in female leukocytes?
Epidemiological studies have been conducted to examine the potential validity of using relative leukocyte telomere length as a disease or aging associated biomarker. Interest in this area has greatly increased following recent reports of associations of shorter leukocyte telomere lengths with morbidity (such as cardiovascular disease or diabetes reviewed in [49]) and in response to external factors such as chronic stress [50]. More data is needed to confirm these findings and establish whether shorter leukocyte telomere lengths are associated with overall increased mortality in older adults [51], [52] and whether the increased risk of infection such as pneumonia in elderly individuals differs significantly in relation to their telomere lengths.
In conclusion, the data presented here contribute valuable base-line information regarding the telomere length in subpopulations of leukocytes during normal human ageing. This information will be a useful reference in studies of a variety of health conditions. Our data show that suppression of half of telomerase levels over a lifetime can severely compromise the telomere homeostasis of granulocytes as a surrogate marker for HSCs, and of immune cells. This likely is a dominant factor in the serious impairment of cell function and proliferative capacity that has been documented in telomerase deficient individuals. It seems possible that more effective short-term inhibition of telomerase could compromise the function of hematopoietic cells more acutely. Most likely, limitations imposed by progressive telomere loss act as a tumor suppressor mechanism in long-lived animals [42]. If so, caution is also needed for strategies that aim to rejuvenate older cells by reactivation of telomerase. The telomere length data described in this paper provide reference data for therapeutic strategies that target telomerase and for further studies on the role of telomeres and telomerase in normal aging and a variety of pathological conditions.
Materials and Methods
Ethical statement
All subjects enrolled in this study in Vancouver signed informed consent forms that were approved by the University of British Columbia (BC) and BC Cancer Agency Research Ethics Board. All samples from patients outside Vancouver were obtained with informed consent and approval of local ethical review boards in accordance with the Declaration of Helsinki.
Healthy donors, telomerase-deficient individuals, and non-carrier relatives
Anonymous cord blood samples were obtained from healthy full term births with parental informed consent. Since no associate information was available for these samples, gender testing was performed by FISH as described below.
Anonymous peripheral blood samples were obtained from 835 healthy individuals between the ages of 6 months to 102 years of age screened for clotting disorders; samples where no clotting disorders were found were made available for study; only gender and age information were provided.
Samples from 60 individuals with confirmed telomerase deficiencies due to heterozygous mutations for either the telomerase reverse transcriptase (hTERT) or the RNA template (hTERC) gene and their 37 (non-carrier) relatives were included in our analysis and were described previously, (mean ages for both groups were 41 and 45 years respectively [25], [53]–[59]; all 97 participants or their guardians provided written informed consent in accordance with the Declaration of Helsinki.
Cord blood gender determination
X and Y chromosome specific FISH was preformed as previously described [60]. Briefly, nucleated cord blood cells were fixed with methanol–acetic acid then dropped onto slides. Slides were fixed with formaldehyde, treated with pepsin, and dehydrated with ethanol. The hybridization mix containing fluorescently labeled peptide nucleic acid (PNA) probes specific for centromere repeats of respectively the X chromosome and the long arm of the Y chromosome were added to the slides. Following denaturation of DNA at 80°C for 3 minutes slides were incubated at room temperature for 30 minutes, washed, counterstained with DAPI and mounted using DABCO anti-fading reagent (Sigma Aldrich). Images were acquired and analyzed as previously described [60].
Mutational analysis
hTERT and hTERC genotyping was performed as described previously [25], [53]–[59].
Telomere length measurements by flow FISH
Telomere length measurements using automated multicolor flow-fluorescence in situ hybridization (flow FISH) was performed as described [61]. Briefly, white blood cells (WBCs) were isolated by osmotic lysis of erythrocytes in whole blood using NH4Cl. The WBCs were then mixed with bovine thymocytes of known telomere length (which serve as an internal control), denatured in formamide at 87°C, and hybridized with a fluorescein-conjugated (CCCTAA)3 peptide nucleic acid (PNA) probe specific for telomere repeats and counterstained with LDS751 DNA dye. The fluorescence intensity in, granulocytes, total lymphocytes and lymphocyte subsets defined by labeled antibodies specific for CD20, CD45RA and CD57 relative to internal control cells and unstained controls was measured on a FACSCalibur instrument (Becton Dickinson) to calculate the median telomere length from duplicate measurements. Further details regarding telomere length measurements and data sets are described in Online Supplementary Material as well as depicted in Figure S1.
Some of the total 835 healthy subjects did not have sufficient cells for analyses of one or more of the cell subsets tested: granulocytes, B lymphocytes, or mature NK/T cells. Specific improvements were developed during the 8 year of the healthy donor study allowing for the testing of additional cell subsets (B and mature NK/T [62]) explaining why fewer measurements are reported for these subsets. In addition, a slight modification was made to the cell lysis protocol: from a semi-automated small volume lysis in a 96 well format [63]) to the current larger volume individual sample lysis [61]. The data obtained during these two experimental periods were first analyzed separately to test for differences between the first and second data sets. Briefly, both data sets were found to have comparable telomere length distributions over age, with a small but notable decrease in the calculated granulocyte telomere length together with a decrease in the range of granulocyte telomere length values in the second data set (Figure S1). These differences may be explained by the protocol improvements in the second set that resulted in a better resolution of signal and a reduction in the background fluorescence observed in cell types with large volumes of cytoplasm. Since the ranges lower limits were similar between the two data sets and since the majority of the data for both sets falls within the same 10th to 90th percentile range, the two sets were merged and analyzed together for curve fitting models and reference comparisons.
Statistical analyses
Analyses were performed using Microsoft Excel (Microsoft Office 2007), GraphPad Prism (version 4) and R (version 2.6.1, 2007, The R Foundation for Statistical Computing); t-Tests were two-tailed and performed on data with a normal distribution (KS test). Linear modeling (lm function in the R language) was used to carry out the regression analysis and estimate the piecewise linear curves with breakpoints hinged at 1 and 18 years of age. We found that this gave the best fit with the least mean square error and more consistent error distribution across the age ranges as compared to using a number of polynomial fits (linear, quadratic, cubic or quartic). The 99th, 90th, 10th and 1st percentile curves were obtained by vertical shift of the estimated regression curve to span the desired number of data points in the primary data set of healthy subjects (n = 835) that were representative for this segment of distribution. To compare the telomere lengths of one population against another, the ANOVA function in the R language was employed to test if the data was from the same or 2 different model fits [64].
Online supplementary material
Figure S1 depicts data from two consecutive experimental periods separately for lymphocytes and granulocytes respectively, and displays the previous quadratic curve fitting model (first ∼400 data points) compared to the current three piece-wise linear regression model (for which first and second data sets were combined) used in Figure 2, Figure 3, and Figure 4.
Figure S2 complements Figure 2 and highlights the telomere length skewing specifically seen in CD45RA+ CD20− lymphocytes of older individuals, where a higher proportion of terminally differentiated lymphocytes are likely present within the cell population with this phenotype. Further, Figure S2 depicts the skewing observed in telomerase heterozygous individuals, comparable to that seen in healthy older individuals (over 75 years of age).
Figure S3 complements Figure 3 and depicts gender segregated telomere length data measured by flow FISH for all leukocyte cell subsets tested, together with the model fit statistical test results from these analyses.
Figure S4 complements Figure 4 and depicts further analysis of leukocyte telomere length data from direct relatives, siblings or parents of telomerase heterozygous individuals. From this relatively small group, although a trend towards shorter MTLs is observed, no statistical difference between the groups and no statistically significant difference compared to healthy individuals was detected (Table S5).
Table S1 displays the complete telomere length data sets. Duplicate leukocyte telomere length data were collected over an eight year period and analyzed on two occasions (first analysis after 391 samples and the present analysis after next 445 samples). For the first set of data, freshly isolated nucleated blood cells were used whereas for the second set, nucleated cells were frozen prior to flow FISH (see Materials and Methods and data set comparisons, Figure S1). No marked differences in the calculated telomere length between the two data sets were observed for lymphocytes or lymphocyte subsets. Although the granulocyte telomere length values were slightly lower and more narrowly distributed in the second data set, the overall results were pooled for the current analysis.
Table S2 displays telomere length cell subset correlations at different age ranges. This table complements Figure 2 and Figure S2. It displays the correlative r values between paired cell population telomere length values. The cell population chosen as a reference has a set value of 1.
Tables S3, S4 and S5 display the complete statistical ANOVA analysis results for comparing telomere lengths of leukocyte subsets in cord blood (at birth), and comparing the two linear models (an estimate of the entire population) and an estimate of where factor “X” (gender for example) was taken into consideration and showed a significant difference.
Supporting Information
Comparison between leukocyte telomere length data sets and between statistical models over the study period. A. In the first study period, median lymphocyte and granulocyte telomere length measurements determined by flow FISH in 391 healthy individuals from birth (umbilical cord blood) to 102 years of age (blue dots) with reference curves derived from a quadratic function which provided a better fit than linear regression models over the entire age-range. Quadratic model distribution curves represent the projected modeled percentiles of the data: 99th (red), 90th (green top), 50th (green centre), 10th (green bottom) and 1st (blue) percentile of telomere range distribution per age. B. Median lymphocyte and granulocyte telomere length measurements in 391 consecutive individuals after 96 well format red cell lysis (Data set 1, blue dots) and 444 other individuals (Data set 2, black dots) after large volume red cell lysis. C. In the combined data set, median lymphocyte and granulocyte telomere length measurements from the combined data sets with piece-wise linear model percentile estimates: 99th (red), 90th (dashed green top), 50th (green centre), 10th (dashed green bottom) and 1st (blue) percentile of the telomere range distribution at any given age. Three linear regression segments were connected at respectively one year and eighteen years of age, to give better representation of the data at younger and older age ranges; insets represent magnifications of the predicted telomere length distribution between birth and five years of age.
(EPS)
Comparison of median telomere length (MTL) in CD45RA+CD20− “Naïve” T lymphocytes and other nucleated blood cell types from the same individual over four distinct age groups. This table complements Figure 2 and Figure S2A. It displays the correlative r values between paired cell population telomere length values. Age-related changes in the relationship between the telomere length (in kb) in “naïve” T lymphocytes and the indicated cell types (granulocytes, memory T lymphocytes (CD45RA−CD20−) or B lymphocytes (CD45RA+CD20+). The cell population chosen as a reference has a set value of 1. Each point represents paired measurements for the two leukocyte subsets within the same healthy individual; individuals are coded within age group as follows: cord blood samples and below one year of age (n = 60, red), between one and eighteen years of age (n = 171, black), adults between nineteen and seventy five years of age (n = 431, green) and elderly, over seventy five years of age (n = 173, orange). Paired measurements are represented for: A. healthy individuals and B. telomerase heterozygous individuals.
(EPS)
Gender differences in telomere length between leukocyte subpopulations. Median telomere length values in leukocyte subpopulations from 835 healthy females and males ranging from newborns (umbilical cord blood) to hundred years of age determined by flow FISH. The following white blood cell subtypes were tested: lymphocytes, granulocytes, B lymphocytes (CD20+), “naïve” T lymphocytes (CD45RA−pos CD20+), memory T lymphocytes (CD45RA−) and mature NK/T cells (CD57+). Curves representing the 50th percentile curve of the modeled median telomere length per age for females (red) versus males (blue) are shown. Three linear segments connected at one year and eighteen years of age were used. ANOVA test P values for the difference between females and males for each gender specific subset were: lymphocytes P = 1.56×10−5, granulocytes P = 0.37, B lymphocytes P = 0.0018, “naïve” T lymphocytes P = 3.7×10−7, memory T lymphocytes P = 0.0012, mature NK/T cells P = 0.0028 respectively. (for complete analysis details, see Table S5).
(EPS)
Leukocyte telomere lengths of non-carrier siblings or parents of telomerase deficient individuals. The telomere length distribution in healthy individuals (Figure 2) was used to plot the median telomere length values obtained with cells from: leukocytes from 8 non-carrier siblings (cyan), 7 non-carrier parents (orange) of telomerase-deficient patients and 21 direct relatives of idiopathic pulmonary fibrosis telomerase-deficient patients (sibling or parent status unknown).
(EPS)
Complete telomere length data sets from healthy individuals. Leukocyte median telomere length data (MTL, kb) were collected over an eight year period and analyzed on two occasions (first analysis after 391 samples and the present analysis after another 445 samples). For the first set of data, freshly isolated nucleated blood cells were used whereas for the second set, nucleated cells were frozen prior to flow FISH (see Online Supplementary Material on flow FISH method for details and Figure S1 for data set comparisons). No marked differences in the calculated telomere length between the two data sets were observed for lymphocytes or lymphocyte subsets (not shown). Although the granulocyte telomere length values were slightly lower and more narrowly distributed in the second data set, the overall results were pooled for the current analysis.
(XLS)
Telomere length cell subset correlations at different age ranges. This table complements Figure 2 and Figure S2. It displays the correlative r values between paired cell population telomere length values. The cell population chosen as a reference has a set value of 1.
(DOC)
ANOVA results summary table on the effect of gender on cord blood telomere length measurements. Effect of gender test of cord blood samples, in reference to Figure 3A; One way ANOVA with Tukey's multiple comparison test (Table S4).
(DOC)
ANOVA post test results summary table on the effect of gender on cord blood telomere length measurements. One way ANOVA with Tukey's multiple comparison test results of the effect of gender on cord blood telomere length measurements.
(DOC)
ANOVA results summary table on the effects of gender on telomere length measurements. This table summarizes the results comparing the two linear models (an estimate of the entire population) and an estimate of where factor “X” (gender for example) was taken into consideration and showed a significant difference. The following factors were tested: effect of gender (total population); effect of telomerase deficiency; effect for relatives of telomerase deficient individuals; effect of gene affected TERT or TERC for heterozygous individuals or relatives; effect of family relationship to heterozygous individual (sibling or parent). A linear model was applied to fit the telomere length variation with age to a piece-wise linear function with two age breakpoints (age 1and age 18). An ANOVA (F-test) of the model fit against a model that also takes into consideration the gender of the individual and was applied and showed that there was a significant difference in gender on telomere length (P = 3.7e-07). The ANOVA compares the two linear models (an estimate of the entire population (one linear fit) and an estimate where gender (or another factor) was taken into consideration (two linear fits to the data)) and showed a significance difference (i.e 2 fits are better than 1 fit, or differences in gender; which can be extended to say a difference in female gender or just as equally male gender.) With reference to the ANOVA tables, the first line shows the Residual degree of freedom (Res.Df) and Residual sum of squares (RSS) for the first model (no difference in gender). The second line (difference in gender model) shows in addition, the degree of freedom for the model, sum of squares, the F-value and the probability of the F-statistics (Pr (>F). If this value is less than 0.05, then there is a good chance that gender (for example) has an effect on telomere length.
(DOC)
Acknowledgments
The authors wish to thank the blood donors, telomerase heterozygous individuals, and their family members for their cooperation. We are very grateful to our collaborators Mary Armanios, Rodrigo Calado, Neal Young, Sharon Savage, Blanche Alter, Frederick Goldman, Cindy Toze, and Jeff Davis for providing clinical data and blood samples for studies of individuals with telomerase deficiencies and their relatives. We thank Elizabeth Chavez for excellent technical assistance and Steven McKinney for critical reading of the manuscript.
Footnotes
PML is a founding shareholder in Repeat Diagnostics, a company specializing in leukocyte telomere length measurements using flow FISH. All other authors declare no competing interests exist.
Work in the PML laboratory is supported by grants from the Canadian Institutes of Health Research (RMF-92093), the U.S. National Institutes of Health (R01GM094146), the Canadian Cancer Society, and the Terry Fox Foundation (Grants 018006 and 105265). GMB was supported by the Swiss National Science Foundation and the Bernese Cancer League. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 2.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. Embo J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britt-Compton B, Rowson J, Locke M, Mackenzie I, Kipling D, et al. Structural stability and chromosome-specific telomere length is governed by cis-acting determinants in humans. Hum Mol Genet. 2006;15:725–733. doi: 10.1093/hmg/ddi486. [DOI] [PubMed] [Google Scholar]
- 4.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 5.Muntoni A, Neumann AA, Hills M, Reddel RR. Telomere elongation involves intra-molecular DNA replication in cells utilizing alternative lengthening of telomeres. Hum Mol Genet. 2009;18:1017–1027. doi: 10.1093/hmg/ddn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansdorp PM. Major cutbacks at chromosome ends. Trends Biochem Sci. 2005;30:388–395. doi: 10.1016/j.tibs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 8.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 9.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-Caveats and a critical assessment of the available technologies and tools. Mutat Res. 2011;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, et al. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 11.Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, et al. Short telomeres on human chromosome 17p. Nat Genet. 1998;18:76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- 12.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 14.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 16.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 17.Baerlocher GM, Mak J, Roth A, Rice KS, Lansdorp PM. Telomere shortening in leukocyte subpopulations from baboons. J Leukoc Biol. 2003;73:289–296. doi: 10.1189/jlb.0702361. [DOI] [PubMed] [Google Scholar]
- 18.Hills M, Lucke K, Chavez EA, Eaves CJ, Lansdorp PM. Probing the mitotic history and developmental stage of hematopoietic cells using single telomere length analysis (STELA). Blood. 2009;113:5765–5775. doi: 10.1182/blood-2009-01-198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 22.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, et al. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd BE, Guttorp P, Lansdorp PM, Abkowitz JL. Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Exp Hematol. 2004;32:1040–1050. doi: 10.1016/j.exphem.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet. 2008;73:103–112. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 27.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 28.Baerlocher GM, Rice K, Vulto I, Lansdorp PM. Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell. 2007;6:121–123. doi: 10.1111/j.1474-9726.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 29.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 30.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Blackburn EH. Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol Cell. 2007;28:315–327. doi: 10.1016/j.molcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 33.Lansdorp PM. Repair of telomeric DNA prior to replicative senescence. Mech Ageing Dev. 2000;118:23–34. doi: 10.1016/s0047-6374(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 34.Rhee DB, Ghosh A, Lu J, Bohr VA, Liu Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst) 2011;10:34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vulliamy TJ, Dokal I. Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2008;90:122–130. doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lansdorp PM. Telomeres and disease. EMBO J. 2009;28:2532–2540. doi: 10.1038/emboj.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 44.Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Knudson M, Kulkarni S, Ballas ZK, Bessler M, Goldman F. Association of immune abnormalities with telomere shortening in autosomal-dominant dyskeratosis congenita. Blood. 2005;105:682–688. doi: 10.1182/blood-2004-04-1673. [DOI] [PubMed] [Google Scholar]
- 47.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, et al. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 48.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209:35–38. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 52.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calado RT, Regal JA, Kajigaya S, Young NS. Erosion of telomeric single-stranded overhang in patients with aplastic anaemia carrying telomerase complex mutations. Eur J Clin Invest. 2009;39:1025–1032. doi: 10.1111/j.1365-2362.2009.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldman FD, Aubert G, Klingelhutz AJ, Hills M, Cooper SR, et al. Characterization of primitive hematopoietic cells from patients with dyskeratosis congenita. Blood. 2008;111:4523–4531. doi: 10.1182/blood-2007-10-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, et al. Identification and functional characterization of two variant alleles of the telomerase RNA template gene (TERC) in a patient with Dyskeratosis Congenita. Blood. 2005;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 60.Taneja KL, Chavez EA, Coull J, Lansdorp PM. Multicolor fluorescence in situ hybridization with peptide nucleic acid probes for enumeration of specific chromosomes in human cells. Genes Chromosomes Cancer. 2001;30:57–63. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1054>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 61.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baerlocher GM, Lansdorp PM. Telomere length measurements in leukocyte subsets by automated multicolor flow-FISH. Cytometry A. 2003;55:1–6. doi: 10.1002/cyto.a.10064. [DOI] [PubMed] [Google Scholar]
- 64.Chambers JM, Hastie TJ. 1992. Statistical Models in S. Pacific Grove, CA: Wadsworth & Brooks/Cole.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison between leukocyte telomere length data sets and between statistical models over the study period. A. In the first study period, median lymphocyte and granulocyte telomere length measurements determined by flow FISH in 391 healthy individuals from birth (umbilical cord blood) to 102 years of age (blue dots) with reference curves derived from a quadratic function which provided a better fit than linear regression models over the entire age-range. Quadratic model distribution curves represent the projected modeled percentiles of the data: 99th (red), 90th (green top), 50th (green centre), 10th (green bottom) and 1st (blue) percentile of telomere range distribution per age. B. Median lymphocyte and granulocyte telomere length measurements in 391 consecutive individuals after 96 well format red cell lysis (Data set 1, blue dots) and 444 other individuals (Data set 2, black dots) after large volume red cell lysis. C. In the combined data set, median lymphocyte and granulocyte telomere length measurements from the combined data sets with piece-wise linear model percentile estimates: 99th (red), 90th (dashed green top), 50th (green centre), 10th (dashed green bottom) and 1st (blue) percentile of the telomere range distribution at any given age. Three linear regression segments were connected at respectively one year and eighteen years of age, to give better representation of the data at younger and older age ranges; insets represent magnifications of the predicted telomere length distribution between birth and five years of age.
(EPS)
Comparison of median telomere length (MTL) in CD45RA+CD20− “Naïve” T lymphocytes and other nucleated blood cell types from the same individual over four distinct age groups. This table complements Figure 2 and Figure S2A. It displays the correlative r values between paired cell population telomere length values. Age-related changes in the relationship between the telomere length (in kb) in “naïve” T lymphocytes and the indicated cell types (granulocytes, memory T lymphocytes (CD45RA−CD20−) or B lymphocytes (CD45RA+CD20+). The cell population chosen as a reference has a set value of 1. Each point represents paired measurements for the two leukocyte subsets within the same healthy individual; individuals are coded within age group as follows: cord blood samples and below one year of age (n = 60, red), between one and eighteen years of age (n = 171, black), adults between nineteen and seventy five years of age (n = 431, green) and elderly, over seventy five years of age (n = 173, orange). Paired measurements are represented for: A. healthy individuals and B. telomerase heterozygous individuals.
(EPS)
Gender differences in telomere length between leukocyte subpopulations. Median telomere length values in leukocyte subpopulations from 835 healthy females and males ranging from newborns (umbilical cord blood) to hundred years of age determined by flow FISH. The following white blood cell subtypes were tested: lymphocytes, granulocytes, B lymphocytes (CD20+), “naïve” T lymphocytes (CD45RA−pos CD20+), memory T lymphocytes (CD45RA−) and mature NK/T cells (CD57+). Curves representing the 50th percentile curve of the modeled median telomere length per age for females (red) versus males (blue) are shown. Three linear segments connected at one year and eighteen years of age were used. ANOVA test P values for the difference between females and males for each gender specific subset were: lymphocytes P = 1.56×10−5, granulocytes P = 0.37, B lymphocytes P = 0.0018, “naïve” T lymphocytes P = 3.7×10−7, memory T lymphocytes P = 0.0012, mature NK/T cells P = 0.0028 respectively. (for complete analysis details, see Table S5).
(EPS)
Leukocyte telomere lengths of non-carrier siblings or parents of telomerase deficient individuals. The telomere length distribution in healthy individuals (Figure 2) was used to plot the median telomere length values obtained with cells from: leukocytes from 8 non-carrier siblings (cyan), 7 non-carrier parents (orange) of telomerase-deficient patients and 21 direct relatives of idiopathic pulmonary fibrosis telomerase-deficient patients (sibling or parent status unknown).
(EPS)
Complete telomere length data sets from healthy individuals. Leukocyte median telomere length data (MTL, kb) were collected over an eight year period and analyzed on two occasions (first analysis after 391 samples and the present analysis after another 445 samples). For the first set of data, freshly isolated nucleated blood cells were used whereas for the second set, nucleated cells were frozen prior to flow FISH (see Online Supplementary Material on flow FISH method for details and Figure S1 for data set comparisons). No marked differences in the calculated telomere length between the two data sets were observed for lymphocytes or lymphocyte subsets (not shown). Although the granulocyte telomere length values were slightly lower and more narrowly distributed in the second data set, the overall results were pooled for the current analysis.
(XLS)
Telomere length cell subset correlations at different age ranges. This table complements Figure 2 and Figure S2. It displays the correlative r values between paired cell population telomere length values. The cell population chosen as a reference has a set value of 1.
(DOC)
ANOVA results summary table on the effect of gender on cord blood telomere length measurements. Effect of gender test of cord blood samples, in reference to Figure 3A; One way ANOVA with Tukey's multiple comparison test (Table S4).
(DOC)
ANOVA post test results summary table on the effect of gender on cord blood telomere length measurements. One way ANOVA with Tukey's multiple comparison test results of the effect of gender on cord blood telomere length measurements.
(DOC)
ANOVA results summary table on the effects of gender on telomere length measurements. This table summarizes the results comparing the two linear models (an estimate of the entire population) and an estimate of where factor “X” (gender for example) was taken into consideration and showed a significant difference. The following factors were tested: effect of gender (total population); effect of telomerase deficiency; effect for relatives of telomerase deficient individuals; effect of gene affected TERT or TERC for heterozygous individuals or relatives; effect of family relationship to heterozygous individual (sibling or parent). A linear model was applied to fit the telomere length variation with age to a piece-wise linear function with two age breakpoints (age 1and age 18). An ANOVA (F-test) of the model fit against a model that also takes into consideration the gender of the individual and was applied and showed that there was a significant difference in gender on telomere length (P = 3.7e-07). The ANOVA compares the two linear models (an estimate of the entire population (one linear fit) and an estimate where gender (or another factor) was taken into consideration (two linear fits to the data)) and showed a significance difference (i.e 2 fits are better than 1 fit, or differences in gender; which can be extended to say a difference in female gender or just as equally male gender.) With reference to the ANOVA tables, the first line shows the Residual degree of freedom (Res.Df) and Residual sum of squares (RSS) for the first model (no difference in gender). The second line (difference in gender model) shows in addition, the degree of freedom for the model, sum of squares, the F-value and the probability of the F-statistics (Pr (>F). If this value is less than 0.05, then there is a good chance that gender (for example) has an effect on telomere length.
(DOC)