Abstract
Recall of a studied item and retrieval of its encoding context (source memory) both depend upon recollection of qualitative information about the study episode. The present study investigated whether recall and source memory engage overlapping neural regions. Subjects (N=18) studied a series of words which were presented either to the left or right of fixation. fMRI data were collected during a subsequent test phase in which three-letter word stems were presented, two-thirds of which could be completed by a study item. Instructions were to use each stem as a cue to recall a studied word and, when recall was successful, to indicate the word’s study location. When recall failed, the stem was to be completed with the first word to come to mind. Relative to stems for which recall failed, word stems eliciting successful recall were associated with enhanced activity in a variety of cortical regions, including bilateral parietal, posterior midline, and parahippocampal cortex. Activity in these regions was enhanced when recall was accompanied by successful rather than unsuccessful source retrieval. It is proposed that the regions form part of a ‘recollection network’ in which activity is graded according to the amount of information retrieved about a study episode.
Keywords: fMRI, episodic memory, item memory, context memory, retrieval
Introduction
Since the advent of event-related fMRI, numerous studies have been conducted with the aim of identifying the neural correlates of successful episodic memory retrieval (recollection of qualitative information about a prior event). The dominant approach has been to contrast neural activity elicited by recollected and unrecollected recognition memory test items, and to dissociate these ‘retrieval success’ effects from effects associated with recognition memory based on an acontextual sense of familiarity (e.g., Eldridge et al., 2005; Henson et al., 1999; for reviews see Skinner and Fernandez, 2007; Spaniol et al., 2009; Kim, 2010). Through this approach, a number of regions have been identified where enhanced retrieval-related activity is selectively associated with recollection (although see Squire, Wixted, and Clark, 2007 for a different interpretation of findings for the medial temporal lobe (MTL)). Chief among these regions are the hippocampus and adjacent MTL cortex, posterior cingulate and retrosplenial cortex, medial prefrontal cortex, and posterior parietal cortex. These regions have been identified using at least two different methods for operationalizing recollection - ‘Remember/Know’ and source memory procedures (e.g., Wheeler and Buckner, 2004; Woodruff et al., 2005; Cansino et al., 2002; Duarte, Henson, and Graham, 2011) – and a variety of different stimulus materials. In light of these findings it has been proposed that the regions are candidates for belonging to a ‘core’ network that supports recollection regardless of the content of the retrieved information (Johnson and Rugg, 2007). In one of the regions belonging to this network – left inferior parietal cortex in the vicinity of the angular gyrus–retrieval-related activity has been reported to co-vary with the amount of information recollected (Vilberg and Rugg, 2007; 2009a; 2009b; Guerin and Miller, 2011), prompting the proposal that this region contributes to the maintenance or representation of recollected content (Vilberg and Rugg, 2008; Guerin and Miller, 2011).
Although fMRI studies have yielded a reasonably consistent picture of the neural correlates of recollection, almost without exception their findings have come from variants of tests of recognition memory. Recollection is held however to support both recognition and recall (Mandler, 1980; Yonelinas, 2002). Therefore, if the putative recollection network identified in studies of recognition memory is indeed central to the recovery and representation of episodic information, it should be engaged during successful item recall. As is discussed below, this issue has been little addressed.
The use of fMRI to investigate the neural correlates of free recall presents significant challenges, which are only just beginning to be addressed (e.g., Oztekin, Long, and Badre, 2010). Cued recall, however, is more amenable to investigation with event-related fMRI since, as with recognition memory, retrieval-related activity can be time-locked to the presentation of a specific stimulus event. Nonetheless, only a handful of fMRI studies of cued recall have been reported. Four of these investigated recall of paired associates (de Zubicaray et al., 2007; Meltzer and Constable, 2005; Henson et al., 2002; Habib and Nyberg, 2008). In these studies, word pairs were presented at study, and test items comprised one member of a studied pair with the instruction to recall its pairmate (in Habib and Nyberg, the recall test was followed by a test of associative recognition for the study pairs). Henson et al. (2002) did not report contrasts between cue words eliciting successful versus unsuccessful recall. In Meltzer and Constable (2005), cue words were repeated during the test trials, and recall accuracy was evaluated only in a subsequent test phase conducted outside of the scanner. In the two remaining studies, cues eliciting successful rather than unsuccessful recall were associated with enhanced activity in regions that included the MTL, lateral parietal cortex and the posterior cingulate. These findings suggest that successful recall engages at least some of the same regions identified as recollection-sensitive in studies of recognition memory (see above), but they are subject to a caveat. This arises because the test procedure confounded memory for the retrieval cue with memory for its associate (recall would have been more likely when the retrieval cue was recognized than when it was not, but memory for the cue was not independently assessed). Therefore fMRI effects attributed to successful recall of the cue’s associate could have been driven, at least partially, by recognition of the cue itself.
An alternative to the paired associate procedure is to employ retrieval cues that correspond only partially to a studied item. This approach was adopted in two fMRI studies (Okada, Vilberg, and Rugg, in press; Schott et al., 2005) in which the cues were the first three letters (stems) of studied words (see Buckner et al. 1995; Rugg et al., 1998; Schacter et al., 1996; Squire et al., 1992 for earlier positron emission tomography (PET) studies of cued recall). In both fMRI studies it was reported that successful recall was associated with enhanced activity in regions – such as the MTL and the lateral parietal cortex – identified as recollection-sensitive in recognition memory experiments, adding further credence to the notion that recall and recollection-driven recognition depend upon the same core network.
The aim of the present study was to extend the prior findings for cued recall by directly assessing, within-subjects and using a single retrieval test, the amount of overlap between the regions engaged by recall of a study item and regions engaged by retrieval of its study context. Retrieval of contextual information is held to be a hallmark of successful recollection and, when contrasted with test trials on which contextual retrieval was unsuccessful, is the principal means by which recollection has been operationalized in fMRI studies of recognition memory (see above). If item recall does indeed depend upon the same processes that support recollection of episodic information more generally, the neural correlates of recall and successful contextual retrieval should overlap.
We addressed this issue with a test procedure first used in an event-related potential (ERP) study by Allan and Rugg (1998). Subjects attempted to recall study words in response to the presentation of three-letter stems. If recall was successful, a judgment was then required as to which of two encoding contexts the word had appeared in at study. Thus, it was possible to identify retrieval effects sensitive to successful recall and, additionally, effects sensitive to the successful retrieval of the study context of a recalled item. Allan and Rugg (1998) reported that the two classes of retrieval effect, as expressed in ERPs, were additive: the effects elicited by stems that gave rise to successful recall (relative to stems corresponding to new words) were enhanced when source memory was also successful, but maintained the same scalp topography. These findings suggest that at least some neural populations respond both to the successful recall of study items and, additionally, to the retrieval of their associated contexts. The present fMRI study allowed these populations to be identified. It was predicted that they will include regions previously implicated as members of the core recollection circuit described above.
Methods
Subjects
Subjects were right-handed, native English speakers aged between 18 and 30 yrs. A total of 23 individuals (11 female) took part in the experiment. Volunteers were right-handed, with normal or corrected-to-normal vision, no known history of neurological disease, and no other contraindications for MRI. Informed consent was obtained from each participant prior to participation in accordance with UCI Institutional Review Board guidelines. Five subjects were excluded from all analyses, one due to excessive motion artifact (greater than 3mm) and four due to poor behavior (either due to the contribution of fewer than 10 trials upon which both cued recall and source retrieval were successful or due to chance performance on the source judgment).
Stimuli
Critical stimuli were drawn from a pool of 560 words (based on the words originally employed by Rugg et al., 1998) in which the first three letters of each word were unique to the pool but were shared with at least four other words that were not included in the pool (words were five to nine letters long with a mean written frequency between 1 and 536 counts per million according to Kucera and Francis, 1967). Allocation of words to experimental conditions was randomized on a subject-specific basis. For each subject, two study lists were created containing 60 words each and cued recall test lists were comprised of 90 unique, three-letter word stems created from the 60 studied words and 30 unstudied words. An additional 24 words were selected from the stimulus pool to be used in a practice session, and another 12 were used as buffers in the study and test blocks. All stimuli were presented individually in white 40-point Helvetica font on a black background. Two buffer trials were added to the beginning and end of each study list, and two buffer trials were added to the beginning of each test list. Data from these buffer trials were excluded from all analyses in order to reduce the contribution of primacy and recency effects to the data.
Procedure
Subjects completed a short practice session outside of the scanner prior to beginning the first study session. The practice session consisted of two study-test blocks of 9 and 12 trials each, respectively. Subjects received instructions on how to perform both study and test tasks prior to beginning the first study practice. After practice, participants were informed that they would undergo two study-test cycles just as in the practice session. Participants were then positioned in the scanner and remained there for the duration of the two study-test cycles.
Stimuli at study were presented on either the left or right side of fixation whereas stimuli at test were presented in central vision. Study trials consisted of the presentation of a white fixation cross for 500 ms, followed by a red fixation cross for 200 ms, followed by a word for 2000 ms, and finally another white fixation cross for 1000 ms. Participants were instructed to evaluate whether each word was concrete or abstract and respond according to a four point scale where 1 = very concrete, 2 = somewhat concrete, 3 = somewhat abstract, and 4 = very abstract. Abstract judgments were made with the middle and index fingers of one hand and concrete judgments were made with the middle and index fingers of the other hand. Hand assignment was randomized across subjects.
In each test block, old and new word stems were pseudo-randomly interspersed such that no more than four trials of a given type (old or new) occurred in succession. All test trials began with a white fixation cross for 800 ms followed by a red fixation cross for 200 ms, followed by a word stem for 1000 ms. Then, a variable duration (1800–5800 ms) white fixation cross was presented, followed by a speech prompt (the word “speak!”) for 1000 ms, followed by another variable duration (3200–7200 ms) white fixation cross. These variable duration fixations were used to ensure that vocalization only occurred during the ‘silent’ period of a volume acquisition (see fMRI Data Acquisition), and were set such that the interval between the onset of successive test items was always 12 seconds. The interval between the onset of the test item and the speech prompt varied across trials between 2.8 and 6.8 seconds.
Subjects were instructed to attempt to complete each word stem with a studied word, or if this was not possible, to complete the stem with the first word that came to mind. When completing a stem with an unstudied word, instructions were to say the completion, and then ‘new’. When completing a stem with a studied word, instructions were to say the completion, and then ‘left’ or ‘right’ if the side of the screen on which the item was presented at study was remembered, or ‘don’t know’ if the location of the vocalized word at study could not be remembered. Participants were instructed to withhold vocalization until the prompt “speak!” appeared on the screen. Verbal responses were recorded via a scanner-compatible microphone.
fMRI Data Acquisition
High-resolution T1-weighted anatomical images (240 × 240 matrix, 1 mm3 voxels) and blood oxygenation level-dependent (BOLD), T2*-weighted echoplanar functional images (SENSE factor of 2, flip angle 70°, 80 × 80 matrix, FOV = 24 cm, TR = 3500 ms, TE = 30 ms) were acquired using a 3T Philips Achieva MRI scanner equipped with an 8 channel receiver head coil (Philips Medical Systems, Andover, MA). Three-hundred and twenty functional volumes were acquired during each test session. Each volume comprised 30 slices oriented parallel to the AC-PC line (thickness 3mm, 1mm inter-slice gap, 3mm × 3mm in-plane resolution) acquired in an ascending sequence. The first 2 volumes of each session (comprising the first 7 seconds of acquisition) were discarded to allow equilibration of tissue magnetization. Volumes were acquired with an acquisition time (TA) of 1479 ms and a repetition time (TR) of 3500 ms. The timing of successive trials was structured so as to ensure that each speech prompt onset immediately after data acquisition, providing a 2 sec window for a vocal response prior to acquisition of the succeeding volume. Thus, the fMRI data were not compromised by motion artifact associated with vocalization (see Henson et al., 2002).
fMRI Data Analysis
Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK), run under Matlab R2010a (The Mathworks Inc., USA) was used for fMRI data analysis. Functional imaging timeseries were subjected to realignment (to the mean image), slice-time correction, reorientation, spatial normalization to a standard EPI template (based on the Montreal Neurological Institute (MNI) reference brain; Cocosco et al., 1997), resampling into 3 mm isotropic voxels using nonlinear basis functions (Ashburner and Friston, 1999), and smoothing with an 8mm FWHM Gaussian kernel. Each subject’s anatomical volume was normalized to the MNI T1 template prior to averaging to create an across-subjects (N = 18) mean image. Analysis was performed using a General Linear Model (GLM) in which a 1 second box car was used to model neural activity at stimulus onset. To model the BOLD response, this function was convolved with a canonical hemodynamic response function (HRF; Friston et al., 1998). Six event-types were modeled: stems associated with correct recall that attracted correct source judgments (source hits), stems associated with correct recall and incorrect or ‘don’t know’ source judgments (source misses), studied stems completed with new words and endorsed as new (misses), stems corresponding to unstudied words completed with novel words and endorsed as new (correct rejections), speech onset cues, and events of no interest. For each test block, the model also included as covariates the across-scan mean and six regressors representing motion-related variance (three for rigid-body translation and three for rotation). Transient head movements greater than 1mm in any direction were eliminated via inclusion of the affected volumes as covariates in the first-level model. Such motion covariates were required for 9 subjects, with an average of 5.11 volumes excluded (range = 1–14). For each voxel, the image time-series was high-pass filtered to 1/128 Hz. An AR(1) model was used to estimate and correct for non-sphericity of the error covariance (Friston et al., 2002). The GLM was used to obtain parameter estimates representing the activity elicited by the events of interest.
A voxel-wise statistical threshold of p < .001, combined with a cluster extent threshold of 26 contiguous voxels, was employed for the principal unidirectional contrasts. As estimated with Monte-Carlo simulations implemented using the 3d ClustSim function in AFNI, this cluster extent threshold gives a corrected cluster-wise significance level of p < .05. Coordinates of significant effects are reported in MNI space. Effects of interest are displayed on sections of the included subjects’ mean normalized structural image or mapped onto a standard inflated (PALS-B12) fiducial brain represented in SPM5 space (Van Essen et al., 2001; 2005).
Results
Behavioral data
Behavioral performance is summarized in Table 1. As can be seen in the table, subjects recalled on average almost 50% of the study words while endorsing just over 80% of completions to unstudied stems as ‘new.’ Fewer than 10% of stems failed to elicit a response. Among the correctly recalled items, 60% were assigned to their correct encoding context. For stems eliciting correct recall, the mean probability of successful source recollection, estimated according to a single high-threshold model that takes the proportion of ‘don’t know’ responses into account (e.g., Park, Uncapher, and Rugg, 2008) was 0.35 (SD = 0.14). This value is significantly greater than the chance level of zero (t(17)=10.60, p < .001, one-tailed).
Table 1.
Mean performance indices for cued recall and source memory.
| Stem completed with | old word | new word | ||
|---|---|---|---|---|
| Response | “old” | “new” | “old” | “new” |
| New Stem | --- | --- | .111 (.027) | .838 (.028) |
| Old stem | .462 (.016) | .020 (.003) | .077 (.016) | .408 (.015) |
| Correct source | .597 (.034) | --- | --- | --- |
| Incorrect source | .166 (.026) | --- | --- | --- |
| Source don’t know | .237 (.046) | --- | --- | --- |
Note: Column headings correspond to subject responses. Standard error is given in parentheses. Source memory scores are proportionalized on all studied stems eliciting successful recall.
fMRI data
Analysis of the fMRI data took place in two principal stages. First, we identified where cue-elicited activity varied as a function of the accuracy of recall, contrasting cues corresponding to studied items according to whether or not they were associated with successful (hit) or unsuccessful (miss) recall.1 Subsequently, we asked whether activity in any of the regions demonstrating recall success effects (greater activity for successful vs. unsuccessful recall) was additionally sensitive to whether recall was accompanied by accurate versus inaccurate source memory.
Regions where cue-elicited activity was greater for successful than unsuccessful recall are illustrated in Figure 1, with peak co-ordinates and cluster sizes detailed in Table 2. Regions demonstrating enhanced activity for successful recall included posterior cingulate/retrosplenial/medial parietal cortex, bilateral lateral parietal cortex, right superior frontal gyrus, right anterior and medial prefrontal cortex and left parahippocampal/fusiform cortex. To ascertain whether these generic recall effects were driven by both classes of recall trial (source hits and source misses) we also performed separate source hit > miss and source miss > miss contrasts, each again thresholded at p < .001 (note the outcomes of these two contrasts do not inform the question of whether recall effects differ in their magnitude according to source accuracy. This question is addressed in a later section). As is evident from Figure 2 and Table 2, the two contrasts revealed patterns of recall effects that each resembled the generic effects illustrated in Figure 1. The one exception to this correspondence was the left parahippocampal/fusiform cortex, where recall effects exceeded the p < 0.001 threshold for source correct trials only.
Figure 1.
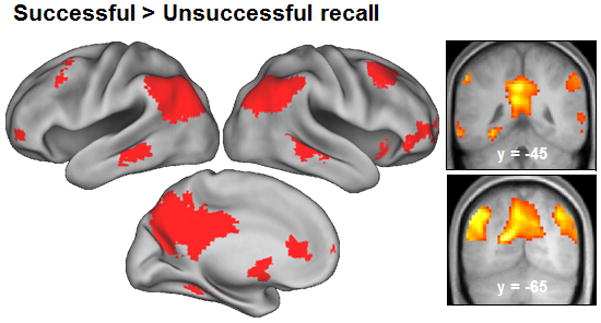
Regions showing greater activity for successful than unsuccessful cued recall (collapsed over source accuracy) are displayed on an inflated fiducial brain and coronal and sagittal across-subject mean structural images.
Table 2.
Regions Sensitive to Successful Recall
| Region | Hem | Location | Peak Z (k) | Zc | Zi |
|---|---|---|---|---|---|
| Successful > unsuccessful recall | |||||
| Lateral anterior prefrontal | L | −39, 51, −3 | 3.58 (43) | 3.73 | 3.17 |
| Anterior cingulate/medial frontal | LR | 3, 36, 12 | 5.80 (563) | 4.63 | 4.39 |
| Middle frontal gyrus | R | 33, 15, 39 | 5.66 (293) | 4.74 | 4.06 |
| L | −33, 18, 57 | 3.98 (45) | 3.32 | 3.76 | |
| Insula | R | 33, 18, −12 | 4.15 (29) | 3.88 | 3.13 |
| Thalamus | R | 15, 3, −15 | 4.59 (48) | 3.32 | 3.43 |
| LR | −6, 0, −3 | 4.62 (70) | 3.74 | 3.31 | |
| Middle temporal gyrus | L | −63, −33, −15 | 5.56 (143) | 4.85 | 4.81 |
| Fusiform/parahipp. cortex | L | −30, −45, −12 | 4.72 (74) | 4.97 | 2.76 |
| Posterior parietal cortex | R | 54, −54, 33 | 5.51 (844) | 4.97 | 4.54 |
| Retrosplenial/post. cingulate | L | −3, −72, 42 | 6.05 (2996)* | 5.61 | 5.24 |
| Supramarginal gyrus | L | −54, −57, 39 | 5.84 | 5.61 | 4.82 |
| Angular gyrus | L | −45, −72, 33 | 5.71 | 5.61 | 4.39 |
| Unsuccessful > successful recall | |||||
| Supplemental motor area | L | −6, 12, 60 | 4.94 (66) | 4.79 | 3.24 |
| Inferior frontal gyrus | L | −48, 12, 15 | 4.93 (342) | 4.27 | 5.27 |
| Post-central gyrus/anterior IPS | R | 51, −21, 51 | 4.73 (76) | 3.65 | 4.62 |
| L | −60, −27, 42 | 4.49 (144) | 3.97 | 4.54 | |
| Medial occipital | L | −12, −90, 21 | 3.80 (29) | 2.88 | 3.88 |
Notes: Hem = hemisphere; LR = left/right; IPS = intraparietal sulcus. K is the number of voxels in the cluster identified by the generic recall contrast collapsed across source accuracy. Zc refers to the Z-statistics for the source correct > miss and miss > source correct contrasts at the peak voxels identified in the generic contrast. Zi refers to the analogous contrasts between source incorrect and miss trials. Zc and Zi statistics are accurate to approximately +−.02. Each of these subsidiary contrasts is reliable at p < .001 with the exception of the fusiform/parahippocampal cortex for source incorrect > miss.
This cluster extends into both midline and left lateral parietal cortex. The loci of the two principal lateral peaks are given.
Figure 2.
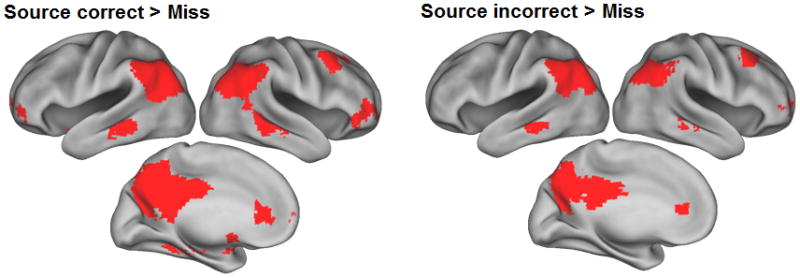
Recall success effects for source correct > miss (left) and source incorrect > miss (right) trials. Effects are rendered onto an inflated fiducial brain.
For the sake of comparability with our prior study (Okada, Vilberg and Rugg, in press) we performed the reverse contrast to identify regions where unsuccessful recall was associated with greater activity (miss > hit). As is shown in Figure 3 and Table 2, this contrast identified an extensive cluster in the left posterior inferior frontal gyrus (IFG), along with smaller clusters in bilateral post-central gyrus. In light of a prior finding of a reliable miss > hit effect in the anterior IPS (Okada, Vilberg, and Rugg, in press), we also performed the miss > hit contrast using a small volume correction within a 5 mm radius sphere centered on the peak co-ordinate of the prior effect (x = −52, y = −34, z = 48). The contrast was significant (peak at −54, −30, 48; Z = 2.74, corrected p < .025).
Figure 3.
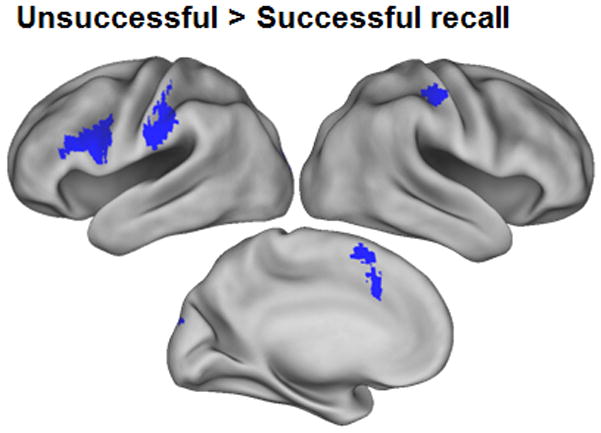
Regions where activity was greater for cues eliciting unsuccessful rather than successful recall. Effects are rendered onto an inflated fiducial brain.
To identify regions where recall success effects were enhanced when the associated source memory judgment was accurate we inclusively masked the hit > miss (i.e., recall success) contrast with the source correct > source incorrect contrast. For this analysis, we opted to maximize the sensitivity of the source accuracy contrast, since our principal goal was to identify recall-sensitive regions that are modulated by accuracy of source retrieval (cf. Allan and Rugg, 1998). Thus, we thresholded the hit > miss contrast at p < .001 (maintaining the 26 voxel extent threshold), and the source correct > source incorrect contrast at p < .05. The conjoint significance of these two orthogonal contrasts, as estimated by Fisher’s method, is p < 0.0005 (Lazar et al., 2002). As shown in Figure 4 (see also Table 3), several regions were identified where the effects of successful recall were modulated by source accuracy. These regions included the posterior cingulate/retrosplenial cortex, bilateral posterior parietal cortex, left parahippocampal/fusiform cortex, and medial prefrontal cortex. Figure 4 includes plots of peak parameter estimates from a sub-set of these regions. As is evident from the figure, activity in three of the highlighted regions (left and right lateral parietal cortex, and posterior cingulate) demonstrates a similar pattern across the different trial types: greatest for source hits, least for misses and correct rejections, and intermediate for source misses (the same pattern was also evident for the medial prefrontal cortex) . An exception to this pattern was found in the left parahippocampal/fusiform cortex, where activity elicited on source incorrect trials did not differ significantly from misses (p > .20, one-tailed).
Figure 4.
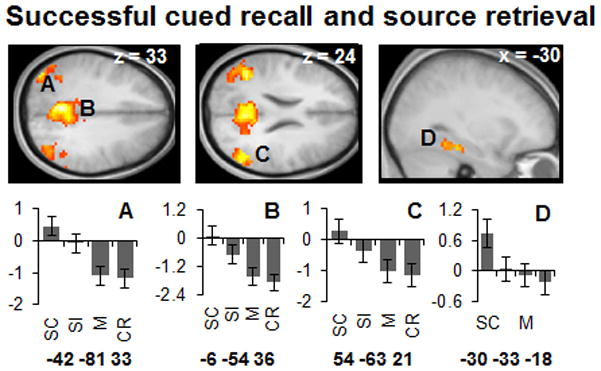
Upper: Regions demonstrating overlap between successful recall and source retrieval. Lower: Peak parameter estimates for the left angular gyrus (A), posterior cingulate (B), right temporoparietal junction (C), and parahippocampal /fusiform cortex (D). Coordinates are reported in MNI space. Note that parameter estimates for correct rejections are given for illustrative purposes; these trials were not employed in the contrasts identifying these regions.
Table 3.
Regions demonstrating overlap between recall and source retrieval success effects
| Region | Hem | Location | Peak Z (k) |
|---|---|---|---|
| Medial prefrontal cortex | LR | 12, 60, −9 | 2.69 (112) |
| Posterior cingulate | LR | −6, −24, 39 | 2.31 (32) |
| Fusiform/parahipp. cortex | L | −30, −33, −18 | 2.68 (47) |
| Middle temporal gyrus | R | 66, −36, −9 | 2.77 (98) |
| Retrosplenial/post. cingulate | L | −6, −54, 36 | 4.19 (807) |
| Posterior parietal cortex | L | −42, −57, 24 | 3.87 (297) |
| R | 54, −63, 21 | 3.90 (303) |
Notes: Hem = hemisphere; LR = left/right. Peak Z is for the outcome of the source hit > source miss contrast. K is the number of voxels in the identified cluster.
We performed a final analysis to determine whether source accuracy effects could be identified in regions outside of those demonstrating effects of successful recall. This was accomplished by exclusively masking the source correct > source incorrect contrast (thresholded at p < .001, 26 voxel cluster extent threshold) with the contrast for successful > unsuccessful cued recall (also p < .001, 26 voxel cluster extent threshold). No significant clusters were identified.
Discussion
The aim of this experiment was to determine whether regions sensitive to successful versus unsuccessful cued recall are also sensitive to the accuracy of a source memory judgment made on the recalled items (c.f. Allan and Rugg, 1998). In several regions, including bilateral posterior parietal cortex, posterior cingulate and parahippocampal cortex, recall effects were enhanced when subjects made an accurate rather than an inaccurate source judgment. Below, we discuss the implications of these findings.
Cued recall effects
The regions where activity was enhanced when recall was successful corresponded closely to those described in two previous studies of cued recall (Okada, Vilberg and Rugg, in press; Schott et al., 2005) Two aspects of the present findings are worth additional comment. First, as was also the case in Schott et al. (2005), recall success effects in lateral parietal cortex were bilateral (see Figure 1). This stands in contrast to the findings for recognition paradigms, when retrieval success effects for verbal (and non-verbal) test items are typically strongly left-lateralized (Vilberg and Rugg, 2008; but see Klostermann, Loui, and Shimamura, 2009). This test-based dissociation has an intriguing parallel in the ERP literature. A putative ERP correlate of successful recollection, the ‘parietal old/new effect’ (Rugg and Curran, 2007), parallels analogous fMRI effects in lateral parietal cortex in that it also is typically left-lateralized when elicited by recognition memory test items. The ERP correlates of successful cued recall, by contrast, are bilaterally distributed (see Allan and Rugg, 1997, for a direct contrast between the ERP effects elicited in each test). Why the lateralization of retrieval success effects in recall and recognition tests should differ is unclear (see Allan and Rugg (1997) for discussion). Nonetheless, the findings for cued recall indicate that left lateral parietal cortex does not invariably play a preeminent role in episodic retrieval relative to its right hemisphere counterpart (see Simons et al., 2010 for lesion evidence suggestive of a similar conclusion).
A second noteworthy aspect of the present findings concerns the recall effects localized to the IPS. Retrieval success effects in the mid-IPS are ubiquitous in studies of recognition memory and have variously been interpreted as evidence for a role in familiarity-driven recognition (Vilberg and Rugg, 2007), evidence accumulation (Donaldson, Wheeler and Petersen, 2010), and top-down attentional control (Cabeza et al., 2008). As was also the case in our previous study (Okada, Vilberg, and Rugg, in press), recall effects in the mid- and anterior IPS demonstrated a cross-over interaction: whereas the effects in the mid-IPS took the form of greater activity for hits than for misses, the anterior effects showed the reverse pattern. Together, the findings from these studies (see also Vilberg and Rugg, 2009c) clearly indicate that retrieval-related activity in the IPS is functionally heterogeneous (see Okada, Vilberg, and Rugg, in press for further discussion).
Source memory effects
The present test procedure allowed subjects to signal when source information about a recalled study word was unavailable (the ‘source don’t know’ response category of Table 1). One advantage of this procedure is that it reduces the diluting influence of ‘lucky guesses’ on the neural activity elicited by correct source judgments. Another advantage is that, in principle, it affords the opportunity to contrast activity elicited on trials where source information was unavailable with the activity on trials where an incorrect source judgment was made, raising the possibility of detecting effects associated with ‘false recollection’(cf. Wais, Squire, and Wixted, 2010). In the present study, however, limitations on trial numbers meant that it was not possible to separately model these two response categories, which were instead collapsed into a single source incorrect category (the response category employed in prior studies in which a ‘don’t know’ option was not included; see Introduction). Thus, it remains to be established to what extent effects associated with successful source retrieval would also be elicited by trials on which the retrieved source information was non-veridical.
Successful source retrieval was associated with enhanced activity in several of the regions where activity was enhanced when recall was successful, and no source effects were identified outside of these regions. The overlap between recall and source memory effects lends support to the proposal that the regions in which overlap was observed – and which have been implicated in episodic retrieval in numerous studies of recognition memory – constitute a ‘core’ recollection network.
What is responsible for the additive effects of successful recall and source memory on retrieval-related activity? One possibility is that activity in these regions co-varies with the amount of information recollected. By this argument (cf. Vilberg and Rugg, 2008) the regions support processes that contribute to the maintenance or representation of retrieved information but which are indifferent to the form of that information. On the assumption that more retrieved information needs to be retrieved to identify both a studied word and its context than is needed to identify just the word itself, activity sensitive to amount of retrieved information should be modulated by source accuracy. This is not to say however that the regions where recall and source retrieval effects overlapped are functionally homogenous. For example, in some regions item and context information might be represented as separately retrieved features of the study episode (cf. Diana, Yonelinas, and Ranganath, 2007), whereas other regions might support the integration of these features into a unified episodic representation. We have previously proposed that lateral parietal cortex in the vicinity of the angular gyrus (BA 39) might play such an integrative role (Vilberg and Rugg, 2008; see Shimamura, 2011 for a related proposal).
Unlike other regions where recall and source memory effects overlapped, retrieval success effects in the vicinity of posterior left parahippocampal/fusiform cortex were evident only when recall was accompanied by an accurate source judgment. This finding is consistent with proposals that parahippocampal cortex plays a more important role in the processing of spatial (and, perhaps, non-spatial) context than it does in the processing of item information (Bar, 2004; Diana, Yonelinas, and Ranganath, 2007). However, the present data do not preclude the alternative possibility that retrieval-related activity in this region is detectable only when a relatively large amount of information is retrieved, regardless of whether the information is context- or item-related. Resolution of this issue will require studies in which the amounts of retrieved item and contextual information are independently varied.
Overlapping effects of successful recall and source memory were found in only a subset of the regions demonstrating effects for successful recall (cf. Figures 1 and 2). Notably, source memory effects were absent in the lateral prefrontal regions that demonstrated recall effects. While null findings should be treated cautiously, the lack of source memory effects in these regions is inconsistent with the proposal that successful source retrieval is associated with engagement of left lateral prefrontal cortex (Lundstrom et al., 2003, 2005). The findings are, however, consistent with the idea that retrieval success effects in lateral prefrontal cortex do not reflect processes supporting the representation or maintenance of retrieved information but rather strategic or control processes that operate downstream of retrieval (Herron, Henson and Rugg, 2004; Ranganath and Knight, 2002).
In addition to the regions where activity was enhanced when either recall or source memory retrieval was successful, other regions were identified where activity was greater for cues associated with unsuccessful recall (‘reversed’ recall effects). One of these regions – in the vicinity of the anterior IPS – was discussed above. The left dorsal inferior frontal gyrus also demonstrated reversed effects, replicating prior findings (Rugg, et al., 1998; Okada, Vilberg, and Rugg, in press). As has been proposed previously (Rugg et al., 1998), this finding likely reflects the role of this region in supporting the selection of additional candidate completions of word stems that failed to elicit successful recall.
The present pattern of reversed recall effects differed from those reported previously (Okada, Vilberg, and Rugg, in press) in that effects were not detected in right dorsolateral prefrontal cortex. The prior findings were accounted for by appealing to the idea that this region supports the evaluation and monitoring of the outcome of a retrieval attempt (Fletcher and Henson, 2001; Henson et al., 2000; but see Han, Huettel, and Dobbins, 2009), on the assumption that monitoring is more heavily taxed when a retrieval cue is employed iteratively (that is, when recall fails), than when the cue elicits successful recall. Unlike in prior studies, though, here successful recall was associated with the requirement to attempt to retrieve source information. We conjecture that the failure to find reversed recall effects in right dorsolateral prefrontal cortex arose because the monitoring demands imposed by the source memory judgment on recalled items roughly matched the demands associated with searching memory when recall was unsuccessful.
Concluding comments
The present findings strengthen the proposal that retrieval of qualitative information about a prior study episode – recollection – is associated with the engagement of a set of primarily posterior cortical regions that constitute a content- and task-independent recollection network. Elucidation of the functional significance of recollection-related activity in each of these different regions is an important future challenge.
Acknowledgments
NIMH grant R01-MH072966
Footnotes
We elected to use miss rather than correct rejection trials as the ‘baseline’ for assessing the effects of successful recall to avoid a confound between successful and unsuccessful recall and the familiarity of the associated retrieval cues. Analyses employing correct rejections instead of misses gave rise to findings very similar to those reported here.
References
- Allan K, Rugg MD. An event-related potential study of explicit memory on tests of cued recall and recognition. Neuropsychologia. 1997;35:387–97. doi: 10.1016/s0028-3932(96)00094-2. [DOI] [PubMed] [Google Scholar]
- Allan K, Rugg MD. Neural correlates of cued recall with and without retrieval of source memory. Neuroreport. 1998;9:3463–6. doi: 10.1097/00001756-199810260-00023. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nature Reviews Neuroscience. 2004;5:617–29. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. Journal of Neuroscience. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–56. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan RS, Evans A. Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S425. [Google Scholar]
- de Zubicaray G, McMahon K, Eastburn M, Pringle AJ, Lorenz L, Humphreys MS. Support for an auto-associative model of spoken cued recall: evidence from fMRI. Neuropsychologia. 2007;45:824–35. doi: 10.1016/j.neuropsychologia.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE. Remember the source: dissociating frontal and parietal contributions to episodic memory. Journal of Cognitive Neuroscience. 2010;22:377–91. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RNA, Graham KS. Stimulus content and the neural correlates of item and source memory. Brain Research. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. Journal of Neuroscience. 2005;25:3280–6. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher PC, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: Applications. NeuroImage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. NeuroImage. 2011;55:801–7. doi: 10.1016/j.neuroimage.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L. Neural correlates of availability and accessibility in memory. Cerebral Cortex. 2008;18:1720–6. doi: 10.1093/cercor/bhm201. [DOI] [PubMed] [Google Scholar]
- Han S, Huettel SA, Dobbins IG. Rule-dependent prefrontal cortex activity across episodic and perceptual decisions: an fMRI investigation of the critical classification account. Journal of Cognitive Neuroscience. 2009;21:922–37. doi: 10.1162/jocn.2009.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–23. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–72. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Josephs O, Dolan RJ. Functional magnetic resonance imaging of proactive interference during spoken cued recall. NeuroImage. 2002;17:543–58. [PubMed] [Google Scholar]
- Herron JE, Henson RNA, Rugg MD. Probability effects on the neural correlates of retrieval success: an fMRI study. NeuroImage. 2004;21:302–310. doi: 10.1016/j.neuroimage.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cerebral Cortex. 2007;17:2507–15. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal and ventral networks in episodic memory retrieval. NeuroImage. 2010;50:1648–57. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Klostermann EC, Loui P, Shimamura AP. Activation of right parietal cortex during memory retrieval of nonlinguistic auditory stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:242–8. doi: 10.3758/CABN.9.3.242. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. NeuroImage. 2002;16:538–50. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. NeuroImage. 2003;20:1934–43. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27:824–34. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Meltzer JA, Constable RT. Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. NeuroImage. 2005;24:384–97. doi: 10.1016/j.neuroimage.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Okada K, Vilberg KL, Rugg MD. Comparison of the neural correlates of retrieval success in tests of cued recall and recognition memory. Human Brain Mapping. doi: 10.1002/hbm.21229. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztekin I, Long NM, Badre D. Optimizing design efficiency of free recall events for fMRI. Journal of Cognitive Neuroscience. 2010;22:2238–50. doi: 10.1162/jocn.2009.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learning and Memory. 2008;15:417–25. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Knight RT. Prefrontal cortex and episodic memory: Integrating findings from neuropsychology and functional brain imaging. In: Parker A, Wilding EL, Bussey TJ, editors. The Cognitive Neuroscience of Memory: Encoding and Retrieval. Hove, UK: Psychology Press; 2002. pp. 83–99. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Allan K, Frith CD, Frackowiak RS, Dolan RJ. Neural correlates of memory retrieval during recognition memory and cued recall. NeuroImage. 1998;8:262–73. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. PNAS: Proceedings of the National Academy of the United States of America. 1996;93:321–5. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. [Google Scholar]
- Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, Duzel E. Redefining implicit and explicit memory: the functional neuroanatomy of priming, remembering, and control of retrieval. PNAS: Proceedings of the National Academy of the United States of America. 2005;102:1257–62. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cognitive, Affective, and Behavioral Neuroscience. 2011;11:277–91. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–91. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex. 2010;20:479–85. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner EI, Fernandez MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–79. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory. PNAS: Proceedings of the National Academy of the United States of America. 1992;89:1837–41. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An Integrated Software System for Surface-based Analyses of Cerebral Cortex. Journal of the American Medical Informatics Association. 2001;41:1359–78. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–99. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Functional significance of retrieval-related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Human Brain Mapping. 2009a;30:1490–501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Left parietal cortex is modulated by amount of recollected verbal information. Neuroreport. 2009b;20:1295–9. doi: 10.1097/WNR.0b013e3283306798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. NeuroImage. 2009c;45:562–71. doi: 10.1016/j.neuroimage.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. Journal of Cognitive Neuroscience. 2010;22:109–23. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–32. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]


