Abstract
Background & Aims
Hepatocellular carcinoma (HCC) is a heterogeneous cancer in which sorafenib is the only approved systemic therapy. Histone deacetylases (HDAC) are commonly dysregulated in cancer and therefore represent promising targets for therapies, however their role in HCC pathogenesis is still unknown. We analyzed the expression of 11 HDACs in human HCCs and assessed the efficacy of the pan-HDAC inhibitor panobinostat alone and in combination with sorafenib in preclinical models of liver cancer.
Methods
Gene expression and copy number changes were analyzed in a cohort of 334 human HCCs, while the effects of panobinostat and sorafenib were evaluated in 3 liver cancer cell lines and a murine xenograft model.
Results
Aberrant HDAC expression was identified and validated in 91 and 243 HCCs, respectively. Upregulation of HDAC3 and 5 mRNAs were significantly correlated with DNA copy number gains. Inhibiting HDACs with panobinostat led to strong anti-tumoral effects in vitro and vivo, enhanced by the addition of sorafenib. Cell viability and proliferation declined, while apoptosis and autophagy increased. Panobinostat increased Histone H3 and HSP90 acetylation, downregulated BIRC5 (survivin) and upregulated CDH1. Combination therapy with panobinostat and sorafenib significantly decreased vessel density, and most significantly decreased tumor volume and increased survival in HCC xenografts.
Conclusions
Aberrant expression of several HDACs and copy number gains of HDAC3 and HDAC5 occur in HCC. Treatment with panobinostat combined with sorafenib demonstrated the highest preclinical efficacy in HCC models, providing the rationale for clinical studies with this novel combination.
Keywords: histone modification, signaling pathways, molecular therapies
Hepatocellular carcinoma (HCC) remains a major health problem worldwide as the third cause of cancer-related mortality and the primary cause of death among cirrhotic patients [1]. Hepatitis B and C, alcohol and aflatoxin have been identified as major risk factors leading to the development of HCC [2, 3]. Resection and transplantation are the only curative treatments available but are greatly hampered by high recurrence rates [3]. Currently, the multi-kinase inhibitor sorafenib is the only FDA-approved treatment for patients with advanced disease, necessitating the development of novel compounds that are effective against this devastating disease [4, 5]. A new class of histone deacetylase (HDAC) inhibitors is currently considered to be among the most promising anticancer agents in drug development, most likely due to their potent anti-tumoral effects demonstrated in preclinical studies of hematological malignancies and solid tumors and their promising therapeutic potential in early-phase clinical trials [6]. Vorinostat and Romidepsin are HDAC inhibitors (HDACi) that recently received FDA approval for the treatment of cutaneous T cell lymphoma [7].
HDAC inhibitors execute their anti-tumoral activities through hyperacetylation of histone and non-histone proteins, such as the molecular chaperone heat shock protein 90 (HSP90). Histone acetylation leads to decreased affinity for DNA and increased access of transcription factors, while HSP90 acetylation blocks its chaperone function and causes destabilization of client proteins implicated in several important signaling pathways [8]. In addition, HDACi can repress transcriptional activity directly or through destabilization and accelerated decay of mature transcripts. These different mechanisms of action lead to various anti-tumoral effects including the induction of apoptosis, autophagy and differentiation, cell cycle arrest and inhibition of tumor vascularization [9]. Although several key factors in cancer-related signaling pathways have been identified to be regulated by the HDAC family, no exclusive targets have been reported so far. HDACs are known to regulate the expression of some of the pivotal players of hepatocarcinogenesis including the apoptosis inhibitor BIRC5 (survivin), the tumor suppressor gene and Wnt-pathway regulator CDH1 (E-Cadherin), and the cyclin-dependent kinase inhibitor CDKN1A (p21) known to be implicated in growth arrest, senescence and apoptosis [10–14].
Panobinostat (LBH589) is a novel pan-HDAC-inhibitor with high efficacy in several preclinical models of cancer [15] and synergistic anti-tumoral activity when administered in combination with chemotherapy or molecular targeted therapies [14, 16]. Early clinical trials recently reported promising results with feasible tolerance and side effects, even indicating anti-tumoral activity in patients with Lymphoma [17].
Further evidence of the importance of the HDAC machinery in cancer is their observed dysregulation in tumoral tissues and their close correlation with aggressive tumoral behaviour. In cancer, aberrant expression of several of the 11 known classical HDAC family members (HDAC1–11, class I, II, IV) often correlates with disease progression and patient’s outcomes [18]. Overexpression of HDAC1 and 2 has been described in gastric and colorectal cancer [19, 20], and HDAC3 has recently been proposed as a potential biomarker for recurrence following liver transplantation in hepatits B (HBV)-associated HCC [21].
Herein, we show that several HDAC-family members are aberrantly expressed in HCC with significant correlation of HDAC3 and 5 upregulation to DNA copy number gains. In addition, we provide evidence that panobinostat has consistent anti-tumoral efficacy in preclinical models of HCC, which is further enhanced when combined with sorafenib. Significantly decreased tumor volume and increased survival in vivo in response to this combination establish a rationale for clinical studies with this novel combination.
Material and Methods
Human samples, mRNA expression array and SNP analysis
A total of 230 human samples were obtained from patients with HCC treated with resection or liver transplantation within the HCC Genomic Consortium (Mount Sinai School of Medicine, New York; Hospital Clinic, Barcelona; Istituto Nazionale dei Tumori in Milan) as previously reported [22–25]. After patient’s informed consent and Institutional Review Board approvals were obtained, samples were collected and gene expression and SNP array studies were performed as described elsewhere [22]. The training set included 91 fresh frozen HCCs, along with 10 normal, 13 cirrhotic and 18 dysplastic liver samples for gene expression analysis (GSE9843). For SNP array, 101 HCC samples and 101 matching cirrhotic tissues (GSE9829) wre analyzed. The validation set included a publicly available dataset of 243 HCCs and paired non-tumoral liver tissue, and 2 pooled, normal liver samples (GSE14520). These patients had predominantly chronic HBV infection [26], while patients within the training set had HCV–related HCC.
Cell lines and in vitro drug treatments
Huh7 (Riken Bioresource Center), Hep3B and HepG2 (ATCC, Manassa, VA) cells were cultured as previously described. [22–24] Panobinostat was provided by Novartis Pharma AG (Basel, Switzerland) following a MTA agreement; sorafenib was purchased from LC Laboratories, Woburn, MA. Both compounds were diluted in DMSO and added 1, 2 and 3 days before cells were processed for further analyses. Final DMSO in all experiments was <0.05%.
Cell viability, proliferation assays, cell cycle analyses, western blot and real time PCR
MTT-, 3H-Thymidine Incorporation-, and fluorescent-activated cell sorting (FACS) assays were performed as previously reported [24]. Detailed information on western blot and real time PCR is given in the Supplementary Material.
HCC xenograft model and immunohistochemistry
All animal experiments were done following Mount Sinai School of Medicine Institutional Animal Care and Use Committee (IACUC) approval of protocols. Generation of the experimental model followed previously reported protocols [24]. A detailed description of the xenograft model, the treatment arms and the immunohistochemistry procedures can be found in the Supplementary Material.
Statistical Analysis
Bars represent the mean + standard error or standard deviation as explained in each figure’s legend. Two-tailed t-test or U-test for continuous variables were used for comparisons between groups and correlations were calculated with the non-parametric Spearman’s Coefficient (SC). SNP-array data and gene expression microarray analyses were performed as previously described [22]. Calculations were done by the SPSS package (SPSS 15.0, Chicago, IL).
Results
HDAC alterations in human hepatocellular carcinoma
Aberrant HDAC expression
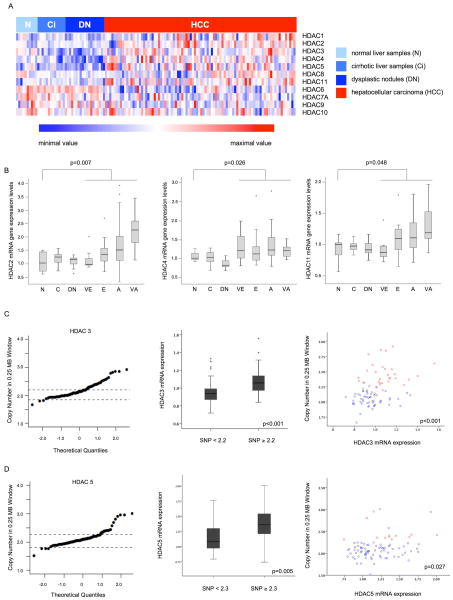
Gene expression levels of all 11 classic HDACs were analyzed by microarray in a training set of 91 HCC samples, 18 dysplastic nodules, 10 normal and 13 cirrhotic liver samples (Fig. 1A). A subset of HDAC mRNAs (HDAC1, 2, 4, 5, and 11) was significantly upregulated compared to normal liver, cirrhosis and dysplastic nodules, with the highest expression levels for HDAC2, 4 and 11 (Fig. 1B). HDAC3 was significantly upregulated in HCC compared to cirrhosis. In the contrast, HDAC6 and HDAC7 were significantly downregulated in HCC compared to normal liver (Suppl. Table 1). To validate these results in an independent set of HCC samples, we analyzed expression levels by independent microarray analysis in 243 paired HCC-cirrhotic samples and 2 pooled normal liver samples. Significant overexpression of 4 HDACs (1, 2, 4 and 5) and downregulation of HDAC6 and HDAC7 were confirmed (Suppl. Figure 1, Suppl. Table 2). Data for HDAC8 and HDAC10 was not available.
Fig. 1. Aberrant HDAC mRNA expression and copy number changes in human HCC.
(A) Heatmap representing expression levels of all HDACs (HDAC1–11) in the training set. (B) Boxplots displaying gene expression levels of HDAC2, 4 and 11 in the hepatocarcinogenic process (N: normal liver, Ci: cirrhosis, DN: dysplastic nodule, and for HCC VE: very early, E: early, A: advanced and VA: very advanced). (C) HDAC3 and (D) HDAC5 DNA copy number levels, expression analyses and their correlation. QQplots show mean SNP array value for the gene’s locus for each tumoral sample and dashed lines represent highest/lowest copy number values in paired cirrhotic samples; Boxplots show mean expression values in high and low copy number samples. Dotplots display expression and copy number values per sample, red dots=copy number ≥2.2 and 2.3, respectively; blue dots=copy number <2.2 and 2.3, respectively). Stars in boxplots represent outlier samples.
Frequent copy number gains of HDAC3 and HDAC5 are associated to high gene expression levels
We used SNP array technology to evaluate DNA copy number alterations of all 11 HDAC-family members. Although no high-level amplifications were detected (cut-off: copy number value 3.4), HDAC3 and HDAC5 showed significant DNA gains (Fig. 1C, D). As expected, samples with increased copy numbers in HDAC3 and HDAC5 had significantly higher expression of HDAC3 and HDAC5 mRNA levels (p<0.001 and p=0.005, respectively) (Fig. 1C, D) and showed a significant correlation of amplification and gene expression (p<0.001 and p=0.0027, respectively) (Fig. 1C, D).
Expression of CDH1 and BIRC5 mRNA correlates with gene expression of multiple HDACs in human HCC
We next analyzed the mRNA expression of 2 previously described HDAC target-genes, CDH1 and BIRC5, in our cohort of samples. CDH1 was significantly downregulated in human HCC samples of our training set when compared to normal liver (p=0.001), cirrhotic liver (p<0.001) and to dysplastic nodules (p<0.001) (Suppl. Fig. 2A). BIRC5 mRNA was significantly upregulated in human HCC samples compared to normal liver, cirrhotic and dysplastic nodule samples (p<0.001) (Suppl. Fig. 3A). MRNA levels of both genes were correlated to gene expression of multiple HDACs, most significantly CDH1 to HDAC4 and 5 (Suppl. Fig. 2B) and BIRC5 to HDAC1, 2, and 11 (Suppl. Fig. 3B).
Effect of panobinostat alone or in combination with sorafenib in liver cancer cell lines
Panobinostat affects cell viability and proliferation alone and in combination with sorafenib in culture
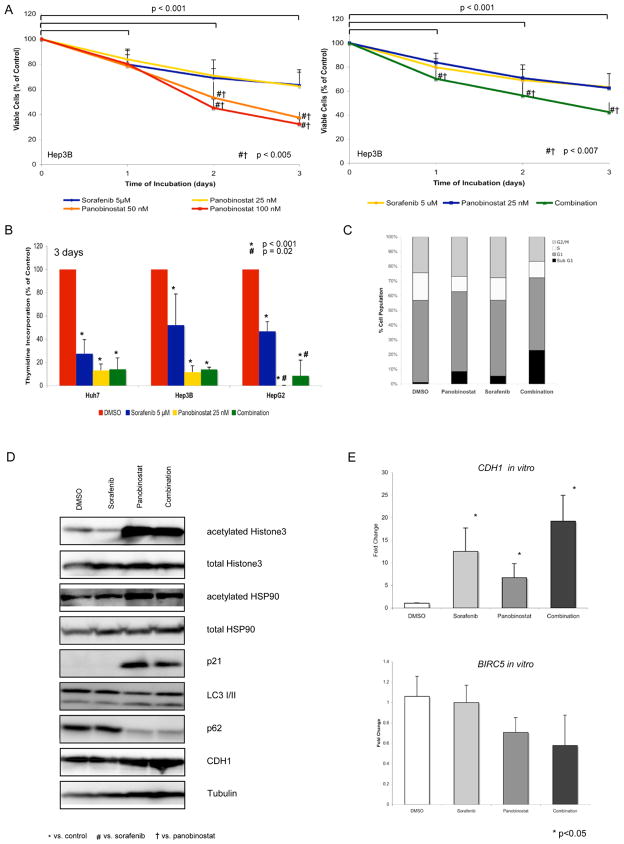
On the basis of the data obtained in human HCC analyses, we investigated the effects of panobinostat (Novartis Pharma AG, Basel, Switzerland) in experimental models of HCC. Panobinostat significantly decreased cell viability compared to control in a dose and time dependent manner at 25, 50 and 100 nM concentrations in Hep3B and HepG2 cells at 1, 2 and 3 days after treatment (12.1–77.7% decline, p<0.05) (Fig. 2A and Suppl. Fig. 4A, respectively). In Huh7 cells, cell viability significantly declined by 47.7% and 23%, respectively, 2 and 3 days after the administration of 50 and 100 nM concentrations compared to control (p<0.001) (Suppl. Fig. 4B). Interestingly, panobinostat 50 and 100 nM significantly decreased cell viability by 15.7–41.8% compared to sorafenib alone (p<0.004) 3 days after treatment in all 3 cell lines (Fig. 2A, Suppl. Fig. 4A, B). Combination therapy enhanced this effect on cell viability compared to single treatment in Hep3B and HepG2 cells at all time-points analyzed (Fig. 2A, Suppl. Fig 4C).
Fig. 2. Panobinostat shows high efficacy in liver cancer cell lines, enhanced by sorafenib.
(A) Cell viability studies after treatment with panobinostat, sorafenib and their combination for 1, 2, and 3 days in Hep3B cells. (B) Proliferation studies after 3 days of treatment in Huh7, Hep3B and HepG2 cells. (C) Cell cycle analysis after 3 days of treatment in Huh7 cells. (D) Western blots of acetylated/total Histone H3, acetylated/total HSP90, p21, LC3, p62 and CDH1 in Huh7 cells. (E) Expression levels of CDH1 and BIRC5 in Huh7 cells after treatment for 1 day. Mean values + standard deviation are shown.
Twenty-five nM panobinostat also significantly decreased proliferation by up to 88.9% in Huh7 and Hep3B cells and completely blocked proliferation in HepG2 cells 2 and 3 days after administration (Fig. 2B, Suppl. Fig. 5). It is noteworthy that panobinostat led to greater decrease of proliferation than sorafenib treatment alone after 2 and 3 days of treatment (Fig. 2B, Suppl. Fig. 5). Nevertheless, combination treatment did not further enhance the effect on proliferation (Fig. 2B, Suppl. Fig. 5).
Panobinostat induces apoptosis, acetylation of Histone H3 and HSP90, and promotes autophagy in culture
We then analyzed the effects of panobinostat and the combination of panobinostat with sorafenib on the cell cycle by FACS analysis. Panobinostat alone increased the percentage of necrotic/apoptotic cells in sub-G1 to 8.5% compared to 1% in the control group. Combination with sorafenib significantly enhanced this effect to 23% in the combination group vs. sorafenib (p=0.002) and control (p=0.003), suggesting apoptosis as the main cause of cell death (Fig. 2C).
In order to confirm the previously described mechanism of action of panobinostat, we analyzed the acetylation status of the 2 known HDAC targets Histone H3 and the molecular chaperone HSP90. Panobinostat alone and in combination with sorafenib led to a significant hyperacetylation of Histone H3 and HSP90 in Huh7 cells 1 day after treatment (Fig. 2D).
We also observed a significant induction of the known HDAC target p21 at the protein level in the panobinostat treated cells 1 day after administration (Fig. 2D, Suppl. Fig. 6).
We finally aimed to assess autophagy activation in panobinostat and sorafenib treated Huh7 cells by analyzing the protein expression of LC3 and p62, two well-known autophagy markers [27]. Panobinostat administration, but not treatment with sorafenib, led to a significant decrease of p62 along with increased LC3 II levels, indicating that panobinostat might induce autophagy in Huh7 cells (Fig. 2D, Suppl. Fig. 6).
Panobinostat changes gene expression levels of CDH1 and BIRC5 in vitro
We further analyzed mRNA levels of CDH1 in Huh7 cells after treatment with sorafenib, panobinostat and their combination. Although both compounds increased CDH1 gene expression, only the combination therapy led to a statistically significant rise (p=0.02, Fig. 2E). Western Blot analysis showed up-regulation of CDH1 protein expression after panobinostat therapy for 1 day following treatment (Fig. 2E, Suppl. Fig. 6). The expression levels of BIRC5 mRNA were slightly decreased after treatment with panobinostat, but not with sorafenib, in Huh7 cells (Fig. 2E).
Effect of panobinostat alone or in combination with sorafenib in HCC xenografts
Panobinostat and sorafenib additively reduce tumor volume and increase survival in vivo
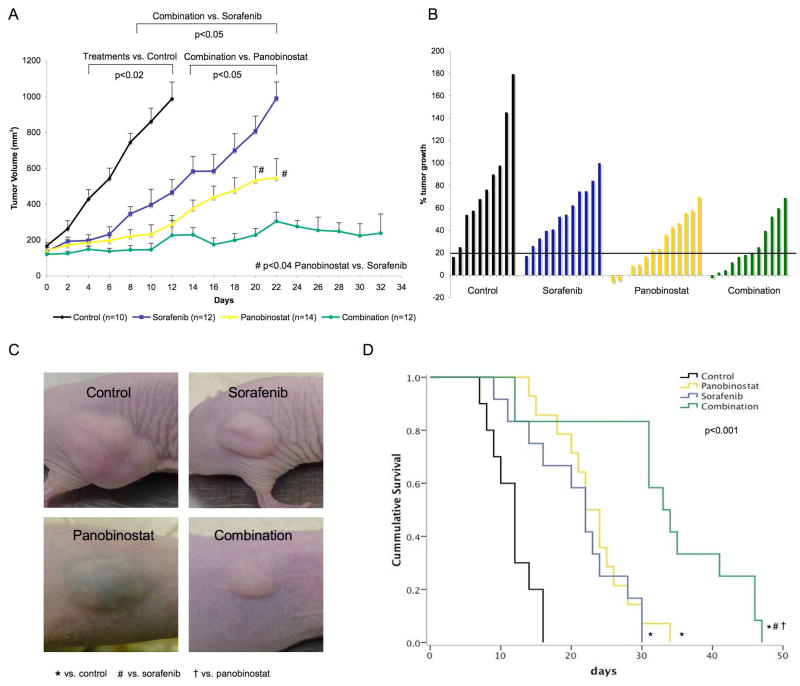
Treatment with panobinostat as single agent, and as expected with sorafenib, led to a significant delay in tumor growth compared to control mice (p<0.02, day 4–12) (Fig. 3A). After 12 days of treatment the median tumor volume was 987.3 mm3 for control, 288.2 mm3 for panobinostat and 464.9 mm3 for sorafenib treated mice. Tumor volume was further reduced to 225.7 mm3 in the group that received combination therapy (p<0.05, Fig. 3A). After 20 days of treatment this additive effect was still evident with a median tumor volume of 227 mm3 in the combination group, 531 mm3 in the panobinostat group and 806 mm3 in mice receiving sorafenib (Fig. 3A, p=0.04 for panobinostat vs. sorafenib, p=0.0002 for combination vs. sorafenib, and p=0.005 for combination vs. panobinostat). To assess the response rate for each group, we analyzed the tumor growth rate less than 20% at day 12 (Fig. 3B). This effective delay in tumor growth was evident for 7/12 (58.3%) mice of the combination group, compared with 6/14 (42.9%) in the panobinostat, 1/10 (10%) in the sorafenib and 1/12 (8.3%) in the control group. This low growth rate was maintained in 6/12 (50%) panobinostat treated mice until the study endpoints were reached (data not shown). As an example, Fig. 3C shows xenograft tumors 5 minutes before euthanization: while in the panobinostat group 2 intratumoral hematomas occurred, not interfering with continuation of the therapy, no adverse events could be observed in the other treatment arms. Nevertheless, panobinostat treatment at 15 mg/kg per day induced weight loss of up to 20% of the body weight in 5/14 (35.7%) animals, and this toxicity led us to use a dose of 7.5 mg/kg in the combination arm. Overall survival increased in the single treatment groups (median 22 days for sorafenib, 23 days for panobinostat) compared to control (median survival 12 days, p<0.001), while combination treatment further enhanced the median survival to 34 days in comparison to control and single treatments (p<0.001, Fig. 3D).
Fig. 3. Combination of panobinostat and sorafenib mostly decreases tumor volume and improves survival in HCC xenografts.
(A) Tumor volume of HCC xenografts treated with panobinostat, sorafenib and the combination of both. Plotted values represent mean tumor volume until half of the mice for each treatment arm have been sacrificed. Mean values + standard error are shown. (B) Waterfall plot showing % tumor growth from randomization to day 12 of treatment. Bars below 20% show stable disease or response. (C) Representative pictures of HCC xenografts of each group at the day of euthanization. (D) Kaplan-Meyer survival analyses of mice in different treatment arms.
Panobinostat induces apoptosis, decreases proliferation and reduces vessel density in HCC xenografts
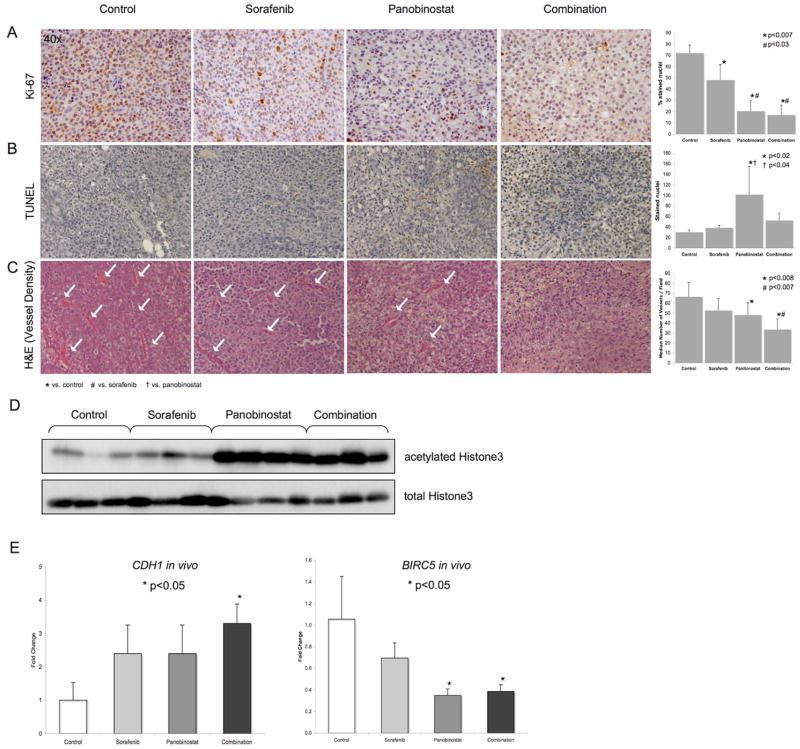
To further assess proliferation and apoptosis in vivo, Ki-67 and TUNEL immunohistochemistry was performed. We observed a significant decrease in the proliferation index (stained nuclei per field) from 72.1±7% in control animals to 48.1±13.8% in sorafenib treated mice, 20.5±9.3% in the panobinostat and 17±8.9% in the combination group (p<0.007) (Fig. 4A). Panobinostat and combination therapy led to a significantly lower proliferation index than sorafenib treatment alone (p<0.03) (Fig. 4A), but no synergism could be detected. TUNEL staining revealed a significant increase of apoptotic cells in the panobinostat treated mice compared to the control and sorafenib group (p<0.04). Although combination treatment increased apoptosis compared to sorafenib, the difference was not statistically significant (Fig. 4B). Finally, we analyzed neoangiogenesis in vivo by assessing the vessel density in each tumor. Panobinostat treatment alone led to a significant decrease of vessel density compared to control animals, which could be enhanced by combination with sorafenib leading to a significantly lower vessel density compared to control and sorafenib treated mice (p<0.007) (Fig. 4C). We also confirmed the activity of panobinostat in HCC xenografts with a significant increase of acetylated Histone H3 in all analyzed tumors (Fig. 4D).
Fig. 4. Drug-induced effects on proliferation, apoptosis, angiogenesis and target-gene expression in HCC xenografts.
(A) Ki-67 immunostaining for proliferation analysis, (B) TUNEL immunostaining for apoptosis analysis and (C) H&E staining for vessel density assessment in HCC xenografts. Original magnification for all pictures is 40x. (D) Western blot results for acetylated and total Histone H3 in HCC xenografts. (E) Expression levels of CDH1 and BIRC5 in vivo. Mean values + standard deviation are shown.
Panobinostat suppresses BIRC5 and induces CDH1 gene expression in combination with sorafenib in vivo
Finally, we analyzed the gene expression of CDH1 and BIRC5 in HCC xenografts collected from mice treated with panobinostat, sorafenib and their combination. Although sorafenib and panobinostat alone increased CDH1 expression levels, only the combination led to a statistically significant increase of CDH1 mRNA in vivo (p=0.02) (Fig. 4E). BIRC5 gene expression levels significantly declined after treatment with panobinostat alone (p=0.01) and after treatment with combination (p=0.04). Although sorafenib led to a mild decrease of BIRC5 levels, no synergistic effect could be observed (Fig. 4E).
Discussion
After sorafenib approval in advanced HCC, the scientific community aimed to further enhance the survival benefit with combination therapies and/or novel drugs [28, 29]. Combination therapies are currently tested in one phase III study (sorafenib with erlotinib) and in several phase II studies [5]. Nonetheless, none of them is testing the efficacy of an HDAC inhibitor in this difficult-to-treat cancer, a family of drugs already approved in other malignancies [6].
This study provides compelling data supporting the role of HDACs as novel targets for the treatment of HCC. We report aberrant expression of most HDACs and identify HDAC3 and HDAC5 overexpression to be associated with copy number gains in human HCC. In addition, we show that treatment with the HDAC inhibitor panobinostat is highly efficient in preclinical models of HCC, boosted by the combination with sorafenib.
Recently, dysregulation of several HDACs has been reported for gastric, prostate, breast and liver cancer [19, 30]. These aberrant expression profiles are believed to contribute to carcinogenesis by disturbing the balance of histone acetylation and non-acetylation needed for normal cell growth and proliferation. In accordance with this data, we detected and confirmed several significantly dysregulated HDACs in 2 large datasets of human HCC.
Up to now only very limited data about transcriptional regulation of HDACs has been available. Mutation, methylation and copy number analyses of different HDAC isoforms need to be performed in order to confirm our observed gains in HDAC3 and 5 and to detect additional mechanisms of regulation. HDACs themselves act as transcriptional regulators, either through inhibiting transcription by histone deacetylation or through activating expression by functioning as transcriptional coactivators [31]. It has previously been shown that CDH1 is silenced by a repressor complex containing HDAC1 and 2 in pancreatic cancer cells, and that it can be reexpressed after treatment with the HDACi Trichostatin A or HDAC2 specific siRNA knockdown [11]. In accordance with this report from in vitro data, we observed highly significant correlations of several HDACs with CDH1 gene expression in human HCC. The other reported HDAC target BIRC5 is frequently downregulated by HDAC inhibition in many cancer models including HCC [10, 32]. Nonetheless, no human data had shown correlation between HDACs and BIRC5 gene expression. Even though a vast number of putative HDAC target genes have been reported, we chose CDH1 and BIRC5 as 2 pivotal players with recently reported contributions to the development of HCC as part of a molecular signature discriminating dysplastic nodules from early HCC [25, 33].
HDACis are currently among the most promising anti-tumoral drugs in both preclinical and clinical development. Preliminary results suggest high anti-tumoral efficacy also in solid tumors, particularly when administered in combination with chemotherapy, radiotherapy or molecular targeted agents [18]. The pan-HDACi panobinostat demonstrated high anti-tumoral activity in preclinical models of solid tumors and seemed to be well tolerated after oral administration in clinical studies [34]. We observed that combining panobinostat with sorafenib strongly potentiated individual treatment efficacy in vitro and in vivo. Similar effects have been reported with lapatinib, a dual EGFR/HER2 inhibitor, in colon cancer cells [14], irradiation in non-small lung cancer cells [35] and doxorubicin in multiple myeloma cells [16].
Although combination treatment led to several significant additive effects in vitro (e.g. cell viability, apoptosis, CDH1 expression), the underlying mechanism could not be elucidated. Recent reports showed that sorafenib increases CDH1 levels through the Raf-MAPK-pathway and that HDACs directly suppress CDH1 expression and this might partially explain the observed additive effect on CDH1 expression in our study [36]. Assumed additive effects on cell viability still require further investigation, since our hypothesis of panobinostat further altering the RAS/MAPK- and PI3K/Akt/mTOR pathway along with sorafenib was not confirmed (data not shown).
The fact that panobinostat alone led to 85–100% decrease in proliferation depending on the cell line analyzed might be related to the status of p53 activity in different cell lines and also to the panobinostat-specific inhibition of BIRC5 [37]. This might result in the observed p21 up-regulation together with a possible direct p21 induction through inhibition of a HDAC cofactor. Other authors have thoroughly explored the mechanisms of induction of apoptosis by HDACi both by p53-dependent and p53-independent pathways [38]. Panobinostat, but not sorafenib, is also able to induce autophagy in liver cancer cells, a mechanism already observed in lymphoma cells [37]. Nonetheless, we were unable to confirm that this occurs through alteration of the Akt/mTOR pathway [39]. Autophagy activation might indeed be a separate mechanism by which panobinostat modulates cell viability in HCC, but this definitely requires further investigation.
Translated into our xenograft model, the combination of panobinostat with sorafenib led to the highest decrease of tumor volume and the most significantly improved survival rates in treated mice along with a significant decrease of vessel density in HCC xenografts. While sorafenib targets neo-angiogenesis through the VEGF pathway [4], it has been demonstrated that panobinostat reduces angiogenesis by inhibition of endothelial cell formation [40]. In this setting, the additive effect of both compounds reported in this study compellingly warrants further investigation.
Concerns over the toxicity of amplified drug toxicity in combination treatment trials have been the current bottleneck to translating positive preclinical experiments into clinical trials in HCC. This has been the case with some combinations of molecular targeted therapies in HCC and recent reports suggest that combining sorafenib and everolimus appears difficult to manage [41], Thus, the combination of sorafenib and panobinostat should be explored in the setting of phase I–II studies, particularly assessing a potential synergistic anti-angiogenic toxicity.
In conclusion, dysregulation of the HDAC-family might play a significant role in hepatocarcinogenesis. While copy number changes lead to aberrant HDAC gene expression in HCC, additional mechanisms need to be explored. Inhibition of all classical HDACs certainly leads to high anti-tumoral efficacy in preclinical models of HCC. Combining panobinostat with the standard of care sorafenib greatly enhanced its anti-tumoral activity, supporting the rationale for clinical studies with this combination.
Supplementary Material
Acknowledgments
Financial support:
Josep M Llovet is supported by grants from the US National Institutes of Diabetes and Digestive and Kidney Diseases (1R01DK076986-01), the European Commission’s Framework Programme 7 (HEPTROMIC, proposal no: 259744), the Samuel Waxman Cancer Research Foundation and the Spanish National Health Institute (SAF-2010-16055). The study was supported by the Landon Foundation-American Association for Cancer Research Innovator Award for International Collaboration in Cancer Research. Scott Friedman has grants from the National Institutes of Health (1RO1DK37340, 1RO1DK56621). Anja Lachenmayer was supported by a fellowship from the German Research Foundation (DFG) and Sara Toffanin received a fellowship from National Cancer Institute, Milan, Italy. Clara Alsinet is supported by a grant from the Instituto de Salud Carlos III.
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CI
confidence interval
- FACS
fluorescent-activated cell sorting
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- HSP90
heat shock protein 90
- IACUC
Institutional Animal Care and Use Committee
- IHC
immunohistochemistry
- PBS
phosphate buffered saline
- SC
spearman’s coefficient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 7.Boumber Y, Issa JP. Epigenetics in cancer: what’s the future? Oncology (Williston Park) 2011;25:220–226. 228. [PubMed] [Google Scholar]
- 8.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 9.Witt O, Lindemann R. HDAC inhibitors: magic bullets, dirty drugs or just another targeted therapy. Cancer Lett. 2009;280:123–124. doi: 10.1016/j.canlet.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Mahalingam D, Medina EC, Esquivel JA, 2nd, Espitia CM, Smith S, Oberheu K, et al. Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin Cancer Res. 2010;16:141–153. doi: 10.1158/1078-0432.CCR-09-1385. [DOI] [PubMed] [Google Scholar]
- 11.von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–371. e361–365. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labonte MJ, Wilson PM, Fazzone W, Russell J, Louie SG, El-Khoueiry A, et al. The Dual EGFR/HER2 Inhibitor Lapatinib Synergistically Enhances the Antitumor Activity of the Histone Deacetylase Inhibitor Panobinostat in Colorectal Cancer Models. Cancer Res. 2011;71:3635–3648. doi: 10.1158/0008-5472.CAN-10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009;5:601–612. doi: 10.2217/fon.09.36. [DOI] [PubMed] [Google Scholar]
- 16.Maiso P, Colado E, Ocio EM, Garayoa M, Martin J, Atadja P, et al. The synergy of panobinostat plus doxorubicin in acute myeloid leukemia suggests a role for HDAC inhibitors in the control of DNA repair. Leukemia. 2009;23:2265–2274. doi: 10.1038/leu.2009.182. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson M, Ritchie D, DeAngelo DJ, Spencer A, Ottmann OG, Fischer T, et al. Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin Lymphoma. Br J Haematol. 2009;147:97–101. doi: 10.1111/j.1365-2141.2009.07837.x. [DOI] [PubMed] [Google Scholar]
- 18.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 19.Weichert W, Roske A, Gekeler V, Beckers T, Ebert MP, Pross M, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 20.Weichert W, Roske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669–1677. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
- 21.Wu LM, Yang Z, Zhou L, Zhang F, Xie HY, Feng XW, et al. Identification of histone deacetylase 3 as a biomarker for tumor recurrence following liver transplantation in HBV-associated hepatocellular carcinoma. PLoS One. 2010;5:e14460. doi: 10.1371/journal.pone.0014460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toffanin S, Hoshida Y, Lachenmayer A, Villanueva A, Cabellos L, Minguez B, et al. MicroRNA-Based Classification of Hepatocellular Carcinoma and Oncogenic Role of miR-517a. Gastroenterology. 2011;140:1618–1628. e1616. doi: 10.1053/j.gastro.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. 1983 e1971–1911. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 26.Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 32.Lu YS, Kashida Y, Kulp SK, Wang YC, Wang D, Hung JH, et al. Efficacy of a novel histone deacetylase inhibitor in murine models of hepatocellular carcinoma. Hepatology. 2007;46:1119–1130. doi: 10.1002/hep.21804. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Geng L, Cuneo KC, Fu A, Tu T, Atadja PW, Hallahan DE. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res. 2006;66:11298–11304. doi: 10.1158/0008-5472.CAN-06-0049. [DOI] [PubMed] [Google Scholar]
- 36.Nagai T, Arao T, Furuta K, Sakai K, Kudo K, Kaneda H, et al. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10:169–177. doi: 10.1158/1535-7163.MCT-10-0544. [DOI] [PubMed] [Google Scholar]
- 37.Ellis L, Bots M, Lindemann RK, Bolden JE, Newbold A, Cluse LA, et al. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009;114:380–393. doi: 10.1182/blood-2008-10-182758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Fazio P, Schneider-Stock R, Neureiter D, Okamoto K, Wissniowski T, Gahr S, et al. The pan-deacetylase inhibitor panobinostat inhibits growth of hepatocellular carcinoma models by alternative pathways of apoptosis. Cell Oncol. 2010;32:285–300. doi: 10.3233/CLO-2010-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YL, Yang PM, Shun CT, Wu MS, Weng JR, Chen CC. Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy. 2010;6:1057–1065. doi: 10.4161/auto.6.8.13365. [DOI] [PubMed] [Google Scholar]
- 40.Qian DZ, Kato Y, Shabbeer S, Wei Y, Verheul HM, Salumbides B, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 41.Finn RS, Poon RTP, Yau T, Klumpen H, Chen L, Kang Y, et al. Phase I study of everolimus in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29(suppl; abstr 4074) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.