Abstract
Objectives
Although clinical trials have demonstrated the benefit of adjuvant hormonal therapy for hormone receptor positive breast cancer, it is not known whether poor medication adherence might impact outcomes, particularly in the context of a low-income population traditionally under-represented in clinical trials. We explored the relationship between adherence to tamoxifen or selective aromatase inhibitors with cancer recurrence and death in a low-income, Medicaid-insured population.
Methods
Using a Medicaid claims-tumor registry and National Death Index data (NDI), we evaluated adherence to adjuvant hormonal therapy [defined by the Medication Possession Ratio (MPR)], cancer recurrence, and cancer-specific survival for female breast cancer diagnosed from 1998–2002, in North Carolina. Multivariate Cox Proportional Hazards models and logistic regression models were used to examine the role of adherence on cancer recurrence and survival.
Results
The sample consisted of 857 cases, mean age 67.7 years, 56.9% Caucasian, 60.9% local stage, with a mean follow-up of 4.4 years. Mean first year MPR was 77%. MPR adherence was not significantly associated with cancer-related death [adjusted HR =1.18 (95% CI 0.54 – 2.59)], or recurrence [adjusted OR= 1.49 (95% CI 0.78–2.84)]. There was also no significant interaction between adherence and use of concurrent CYP2D6 enzyme inhibitors.
Discussion
Hormonal therapy adherence was not associated with breast cancer outcomes in this low-income population with relatively poor adherence. Although suboptimal adherence is considered to be an important clinical problem, its effects on breast cancer outcomes may be masked by patient genetic profiles, tumor characteristics, and behavioral factors.
Keywords: adherence, adjuvant hormonal therapy, breast cancer, recurrence, survival
INTRODUCTION
Endocrine therapy is a crucial component of adjuvant treatment for women with hormone receptor positive breast cancer 1–10. However, oncology patient adherence to daily oral therapy is increasingly recognized as a challenge 11;12. For adjuvant hormonal therapy, reported adherence rates range from 50% to 75%11;13–16, with discontinuation rates particularly high during the first year 17–20. It has been estimated that half of breast cancer patients discontinue adjuvant endocrine treatment before the recommended five year treatment period21. At least two cohort studies have now linked poor hormonal therapy adherence with adverse outcomes for breast cancer patients including recurrence22 and mortality23.
One factor that has been hypothesized to modify the effect of hormonal therapies on breast cancer outcomes is concomitant use of medications that interfere with the activity of the cytochrome P450 2D6 (CYP2D6) enzyme that metabolizes tamoxifen24. It has been suggested that concurrent use of CYP2D6 inhibitor medications and tamoxifen may result in reductions in plasma tamoxifen metabolites25 and possibly reduced treatment efficacy26. Thus, concurrent use of CYP2D medications might be expected to moderate observed associations between adjuvant hormonal medication adherence and breast cancer outcomes. However, several recent studies failed to find an association between use of CYP2D6 inhibitor medications and poorer breast cancer outcomes in the context of adjuvant hormonal therapy22;27;28. Although Dezentje and colleagues22 did not find evidence of interactions between tamoxifen adherence and use of CYP2D6 inhibitor medications this possibility needs to be examined in a more diverse sample of women and for additional clinical endpoints.
We previously reported low adherence, with only 60% reporting with medication possession ratios (MPR) greater than 80%, to adjuvant hormonal therapy for early stage breast cancer29 among low income women identified from a linked database of North Carolina (NC) Medicaid and NC Central Cancer Registry (CCR)30;31. In this report, we describe the relationship of adherence to adjuvant hormonal therapy to breast cancer recurrence and death.
METHODS
This study was approved by the Institutional Review Boards at Wake Forest University School of Medicine and at Duke University Medical Center.
Database
Methods used to create the NC CCR-Medicaid linked dataset have been previously described32. In NC, Medicaid is almost entirely fee-for service with one small managed care program (<10,000 covered lives), thus exclusions for incomplete utilization data from HMO enrollees is minimal. Health care claims for persons enrolled in Medicaid with dual Medicare insurance (for those legally blind/disabled or 65+ years) are ‘crossed over’ to the Medicaid claims processing contractor, such that Medicaid pays the deductible and coinsurance for these individuals. As a result, our dataset includes detailed claims for both Medicaid and Medicare for the dually insured. For simplicity, we refer to all study claims as ‘Medicaid’ claims regardless of source of reimbursement.
Study Population
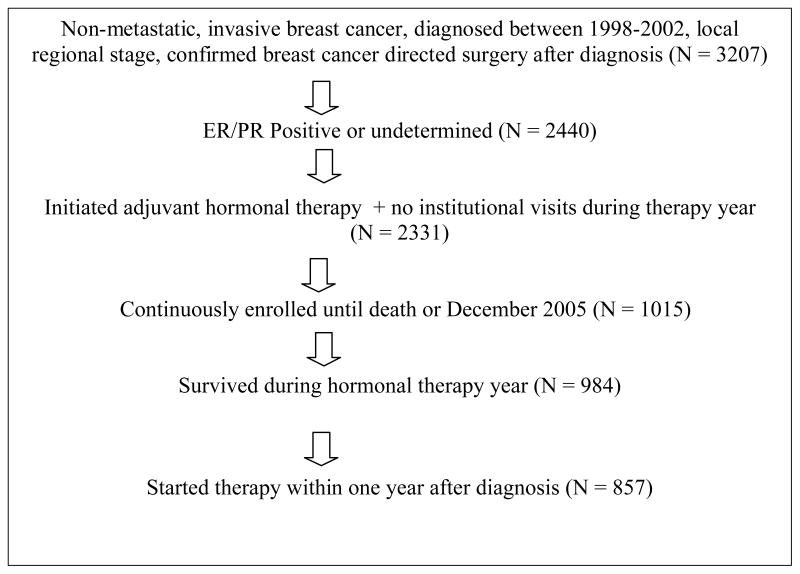
We used the NC CCR-Medicaid administrative database to identify 3207 women diagnosed with nonmetastatic, invasive breast cancer between 1998 and 2002 who had local or regional staging, and a confirmed breast conserving surgery or mastectomy after diagnosis. The sample was further limited to women whose tumors were hormone receptor positive, defined as estrogen receptor (ER) and/or progesterone receptor (PR) positive, or unknown, and who filled at least one prescription for tamoxifen or an aromatase inhibitor within a year of first cancer diagnosis, along with additional criteria summarized in Table 1. The final analytic sample was n=857.
Table 1.
Eligibility Criteria
|
Definition of Variables
Medication possession ratio (MPR)
Adherence is defined as the extent to which a medication is taken as prescribed33. One commonly used index for measuring medication adherence, the medication possession ratio (MPR), is defined as the ratio of the total days covered by the medication (using total day supply) divided by the days needing the medication34;35. MPR can be expressed as following: MPR = (p/d) × 100. Where p = total day supply minus surplus day supply, and d = total number of days (365) minus the number of days the patient spent in the hospital. As in a previous study of hormonal therapy adherence22, we focused on adherence during the first year of treatment to allow adherence to be treated as a time invariant predictor.
Medication persistence
Medication persistence was defined as continuous medication use during the year after start of adjuvant hormonal therapy. For our purpose, discontinuity was indicated if a gap of more than 3 months was found between medication refill date/end of therapy year and previous medication refill date plus day supply.
Medications
For the purposes of this study, adjuvant hormonal therapy included the following medications: tamoxifen, anastrozole (ArimidexTM), letrozole (FemaraTM), and exemestane (AromasinTM). In order to calculate adherence and persistence, these medications were treated as indistinguishable from each other if a patient switched to or concurrently took any of the medications. Additionally, a variable for the number of unique prescriptions for all conditions was calculated and defined as the unique number of medications (as defined by the first 9 digits of the National Drug Code) during the year after study medication start date, not limited to the study medications.
Sociodemographic and disease variables
Other independent variables, including breast cancer stage, hormone receptor status, tumor grade, urban/rural residence, and patient race/ethnicity, were obtained from the cancer registry, through which information was abstracted from medical charts by hospital registrars following North American Association of Central Cancer Registries (NAACCR) guidelines36. Staging was calculated by categories from Surveillance Epidemiology and End Results (SEER) summary stages37. SEER stages 1 and 2 defined local stage, and SEER stage 3, 4, or 5 comprised regional stage. ER and PR status were obtained from the registry. Race was defined as white or non-white. Medicare/Medicaid claims data consistent to the National Cancer Institute’s International Classification of Diseases 9th revision grouping methods for comorbidity38 were used to construct the Charlson Comorbidity Index, a weighted score of comorbidity. This index was calculated over the first two years after cancer diagnosis to better identify underlying conditions and distinguish cancer treatment related complications.
CYP2D6 inhibitor medications
To explore the possible effect of other medications that might decrease the efficacy of tamoxifen, which was used in 88.8% of these women, we identified concomitant use of drugs that were CYP2D6 inhibitors. We focused on medications used in a prior study27, including fluoxetine (Prozac), paroxetine (Paxil, Seroxat) cimetidine (Tagamet), and sertraline (Zoloft, Lustral), celecoxib, citalopram, escitalopram, levomepromazine, metoclopramide, levomepromazine, mirtazapine, amitriptyline, timolol, propranolol, venlafaxine, and zuclopenthixol.
Recurrence algorithm
A study specific algorithm was developed to detect cancer recurrence. Recurrence was assumed if a patient had a cancer restaging procedure, followed in time by the presence of codes related to breast cancer directed surgery, radiation, or chemotherapy. A complete list of Current Procedural Terminology (CPT)/Healthcare Common Procedure Coding System (HCPCS), National Drug Code (NDC), International Classification of Diseases (ICD)-9, and Diagnosis Related Groups (DRG) codes used to identify treatment is available in Supplemental Digital Content 1. In addition, patients who were identified from the Master Death File as having died of cancer related causes after surgery were assumed to have had a recurrence.
Death Data
We linked these data to the U.S. Social Security Master Death File to record the event of death from all causes through the period of December 31, 2005. The Master Death File has been shown to be highly accurate, and inclusive of 93 percent to 96 percent of deaths occurring to members of the Social Security retirement benefits program when compared to data from the National Center for Health Statistics’ National Death Index, the most authoritative source of death information for the U.S. population39. Upon locating a match by Social Security Number, we verified the match based on first and last name contained in the registry. Only those matches with exact Social Security Numbers and names were classified as having the outcome of death. After we identified death from the Master Death File, cause of death was determined by the North Carolina Department of Health Statistics vital records database. Cancer-related deaths were defined as those deaths that had cancer listed as the underlying cause coded from the death certificate.
Data Analysis
The SAS system v9.2 was used for all statistical analyses. We first conducted bivariate and multivariate analyses to examine the relationship between adherence (measured by MPR and persistence) and both survival and recurrence. The relationship between adjuvant hormonal therapy adherence and cancer-related death starting one year after initiation of therapy was examined by fitting bivariate and multivariate Cox Proportional Hazard models to the data with cancer- related death treated as the outcome and adherence (MPR and persistence, in different models) as the predictor. The following variables were included as covariates in the multivariate analysis: index medication (Tamoxifen only, AI only, concurrent), age group (0–<45,45–<55,55–<65,65–<75,75+), race (white vs non-white), Charlson comorbidity (continuous), number of unique prescriptions (continuous), concurrent use of medication that decreases CYP2D6 activity (yes vs no), stage (local versus regional), hormone receptor status (positive or unknown), positive lymph nodes (0, 1–3, 4–9,10+), tumor grade using the Facility Oncology Registry Data Standards (FORDS) coding system (I, II, III, IV, and undetermined), type of surgery [breast conserving surgery or mastectomy], use of chemotherapy after diagnosis [yes vs no], use of radiation after diagnosis, urban residence, and year of therapy start (continuous 1998–2003).
Several model assumptions were checked. The proportional hazards assumption for both adherence models was checked by testing for the interactions with the log of follow-up time. The assumption of a linear relationship between the log hazard with MPR was examined using a likelihood ratio test which compared a model with additive splines for MPR to the original linear model.
The relationship between recurrence after one year of therapy initiation and adherence was then examined by use of a logistic regression where the dependent variable was patient recurrence one year after start of therapy and the independent variables were adherence plus the covariates described above. Non-linearity of MPR adherence was assessed as before.
Finally, a subgroup analysis was conducted by testing the interaction of CYP2D6 enzyme inhibitor medication use with MPR adherence. The interaction effect was entered into the multivariate model separately and tested using Type 3 Wald Chi square test.
RESULTS
Sociodemographic and Disease-related Characteristics
Characteristics of the 857 eligible women with nonmetastatic, hormone receptor positive or unknown, invasive breast cancer who had a filled prescription for adjuvant hormonal therapy within one year of diagnosis are shown in Table 2. Hormone receptor status was positive in 75.9% of the sample, and tumor grade was intermediate (grade two) or high (grade 3/4) in 40.5% and 24.2% of cases, respectively. The tumor was local stage in 60.9% of cases. With regard to other treatments, 67.0% had mastectomy, 43.1% radiation, and 33.3% chemotherapy. Mean age was 67.7 years and 56.9% of the sample was white, with 54.1% living in urban areas.
Table 2.
Patient Characteristics
| Study Population ER/PR Positive or Unknown N = 857 |
|
|---|---|
|
| |
| Index Medications | |
| Tamoxifen only | 702 (81.9%) |
| Tamoxifen concurrent with AI | 59 (6.9%) |
| AI only | 96 (11.2%) |
|
| |
| Age (years) | |
| Mean (std) [min,max] | 67.7 (13.2)[32,100] |
| <45 | 60 (7.0%) |
| 45 – <55 | 90 (10.5%) |
| 55 – <65 | 180 (21.0%) |
| 65 – <75 | 227 (26.5%) |
| 75 + | 300 (35.0%) |
|
| |
| Race | |
| Caucasian | 488 (56.9%) |
| Other | 369 (43.1%) |
|
| |
| Comorbidity (Charlson) | |
| Mean(std)[min,max] | 2.46 (2.25)[0,11] |
| 0 | 207 (24.2%) |
| 1 | 125 (14.6%) |
| 2 | 155 (18.1%) |
| 3 | 141(16.4%) |
| 4+ | 229 (26.7%) |
|
| |
| Number of unique prescription medications during study period | |
| Mean (Std)[min,max] | 15.1 (9.2)[1,69] |
| None – 5 | 52 (6.1%) |
| 5 – 10 | 205 (23.9%) |
| 10 – 20 | 402 (46.9%) |
| 20+ | 198 (23.1%) |
|
| |
| Number of Positive Lymph nodes | |
| Negative | 410 (47.8%) |
| 1–3 | 209 (24.4%) |
| 4–9 | 86 (10.0%) |
| 10+ | 39 (4.6%) |
| Not Examined | 113 (13.2%) |
|
| |
| Concurrent use of medication that decreases CYP2D6 activity | |
| Any | 403 (47.02%) |
| None | 454 (53.0%) |
|
| |
| Stage | |
| Local | 522 (60.9.%) |
| Regional | 335 (39.1%) |
|
| |
| Hormone receptor status | |
| Positive | 650 (75.9%) |
| Not determined | 207 (24.2%) |
|
| |
| Type of surgery | |
| BCS (Breast Conserving Surgery) | 283 (33.0%) |
| Mastectomy | 574 (67.0%) |
|
| |
| Chemotherapy1 | |
| No | 572 (66.7%) |
| Yes | 285 (33.3%) |
|
| |
| Radiation1 | |
| No | 488 (57.0%) |
| Yes | 369 (43.1%) |
|
| |
| Urban residence | |
| No | 393 (45.9%) |
| Yes | 464 (54.1%) |
|
| |
| Tumor Grade | |
| Low (grade 1) | 136 (15.9%) |
| Intermediate (grade 2) | 347 (40.5%) |
| High (grade 3/4) | 208 (24.2%) |
| Undetermined | 166 (19.4%) |
|
| |
| Year Initiation of Therapy | |
| 1998 | 85 (9.9%) |
| 1999 | 163 (19.0%) |
| 2000 | 164 (19.1%) |
| 2001 | 204 (23.8%) |
| 2002 | 185 (21.6%) |
| 2003 | 56 (6.5%) |
|
| |
| Survival in days 2 Mean (std) [min, max] | 1617.11 (565.4) [401,2860] |
| Cancer Related Deaths | 113 (13.2%) |
|
| |
| Patient recurrence | |
| No | 576 (67.2%) |
| Yes | 281 (32.8%) |
|
| |
| Mean MPR Adherence (std); % with MPR Adherence > 80% | |
| Year 1 (N = 857) | 77% (27); 63% |
| Year 2 (N = 812) | 71% (32); 62% |
| Year 3 (N = 705) | 70% (34); 60% |
| Year 4 (N = 489) | 65% (37); 55% |
| Year 5 (N = 290) | 58% (38); 46% |
|
| |
| Persistence during 1st year (std) | 82% (39) |
Chemotherapy and Radiation treatment as identified by codes in Table 2, with date of service within 6 months (chemotherapy) and 1 year (radiation) of diagnosis date.
Survival until cancer related death/censoring event. Patients who died within a year after start of therapy were excluded
Patients who recurred within a year after start of therapy were excluded.
Cancer Survival and Recurrence
During the study period, cancer-related death occurred in 113 (13.2%) of patients and 281 (32.8%) had tumor recurrence. follow-up in the sample starting from initiation of therapy was 1617 days (4.4 years), ranging from 401 days (1.1 years) to 2860 days (7,8 years) after initiation of hormonal therapy.
Hormonal Therapy Adherence
Mean MPR (ranging from 0 to 100) was 77% during the year after initiation, 71% at 2 years, 70% at 3 years, 65% at 4 years, and 58% at 5 years, restricted to patients with continuous enrollment during each year. The proportion of patients who achieved MPR at 80% from year 1 to 5 was 63%, 62%, 60%, 55% and 46% respectively. During the first year of treatment, 82% of the patients were found to be persistent.
Hormonal Therapy Adherence and Cancer Outcomes
Higher MPR adherence to adjuvant hormonal therapy during the first year was not significantly associated with cancer recurrence (unadjusted OR = 1.21, 95%CI 0.70–2.06; adjusted OR = 1.49, 95%CI 0.78–2.84; Table 3) or to cancer-related death (unadjusted HR= 1.37, 95% CI 0.67–2.82; adjusted HR = 1.18, 95% CI 0.54–2.59; Table 3). Persistence during the first year was also not significantly associated with recurrence (unadjusted OR = 1.04, 95% CI 0.72–1.51; adjusted OR = 1.18, 95% CI 0.76–1.82) or to cancer-related death (unadjusted HR 1.25, 95% CI 0.75–2.09; adjusted HR = 1.22, 95% CI 0.70–2.15). No violations of the proportionality assumption (for MPR and persistence) or assumption of linearity (for MPR) were found. There were no significant interactions between use of CYP2D6 enzyme inhibitors and either measure of medication adherence on breast cancer recurrence or death (all p-values >.40). Elimination of patients not taking tamoxifen did not change the interaction results.
Table 3.
Multivariate Analysis of Hormonal Therapy Adherence and Cancer-Related Death and Cancer Recurrence.
| Outcome | ||
|---|---|---|
|
| ||
| Time to Cancer-Related Death Hazard Ratio (95% CI) |
Cancer Recurrence Odds Ratio (95% CI) |
|
|
| ||
| MPR Adherence (0–100%) | 1.18 (0.54–2.59) | 1.49 (0.78–2.84) |
|
| ||
| Age (Years) | ||
| <45 | 0.84 (0.41– 1.72) | 2.89 (1.42–5.88) |
| 45–54 | 0.69 (0.32–1.53) | 2.26(1.26–4.06) |
| 55–64 | 0.80 (0.44–1.43) | 1.57 (0.98–2.52) |
| 65–74 | 1.13(0.69–1.87) | 1.17 (0.75–1.81) |
| 75+ | Reference | Reference |
|
| ||
| Race, other vs white | 1.35 (0.89–2.03) | 1.81 (1.28– 2.56) |
|
| ||
| Cancer Stage (Local vs Regional) | 1.17 (0.30–4.58) | 0.35 (0.08–1.56) |
|
| ||
| Adjuvant Hormonal Therapy Medications | ||
| Tamoxifen only | 0.38(0.20– 0.70) | 0.89(0.47–1.67) |
| AI only | 0.25 (0.09–0.69) | 0.64 (0.29–1.39) |
| Tamoxifen concurrent with AI | Reference | Reference |
|
| ||
| Surgery Type, Breast-conserving vs mastectomy | 0.88 (0.49–1.55) | 1.86 (1.17– 2.95) |
|
| ||
| Adjuvant Cancer Treatment (yes vs no) | ||
| Chemotherapy | 1.40 (0.87–2.24) | 1.27 (0.85–1.88) |
| Radiation | 0.95(0.59–1.53) | 1.56 (1.02–2.38) |
|
| ||
| Number of Positive Lymph nodes | ||
| Negative | Reference | Reference |
| 1–3 | 1.70 (0.41–7.10) | 0.48 (0.10– 2.22) |
| 4–9 | 2.78 (0.69–11.28) | 0.92 (0.20– 4.24) |
| 10+ | 6.54 (1.53–28.00) | 3.44(0.62–19.06) |
| Not Examined | 0.95 (0.44–2.08) | 0.72 (0.42–1.25) |
|
| ||
| Tumor Grade | ||
| Low | Reference | Reference |
| Intermediate | 1.34 (0.58–3.11) | 0.88 (0.53–1.43) |
| High | 4.39 (1.95–9.87) | 2.37 (1.40–4.00) |
| Undetermined | 1.80 (0.75–4.30) | 1.24 (0.71–2.17) |
|
| ||
| Hormone Receptor Status (positive vs undetermined) | 0.85 (0.54, 1.34) | 0.83 (0.57, 1.20) |
|
| ||
| Charlson Comorbidity Index | 1.07 (0.97–1.18) | 1.05 (0.97– 1.20) |
|
| ||
| Use of CYP2D6 Inhibitor Medications (yes vs no) | 0.83 (0.54–1.25) | 0.93 (0.66–1.30) |
|
| ||
| Number of Unique Prescription Medications | 1.00 (1.00– 1.01) | 1.00 (1.00–1.00) |
MPR= Medication Possession Ratio; CYP2D6= Cytochrome P450 2D6 enzyme; Cox proportional hazard models were used to calculate the hazard ratio for time to cancer-related death from hormonal therapy initiation date. Logistic regression models were used to calculate the odds ratio for cancer recurrence. Multivariate analyses also controlled for year of initiation of hormonal therapy and urban vs non-urban residence.
DISCUSSION
Our finding of no association between hormonal therapy adherence and breast cancer outcomes contrasts with recent studies reporting significant associations between adherence and breast cancer event-free time22 and all cause mortality23. Importantly, these studies included women from the Netherlands and Scotland with very different sociodemographic characteristics and generally better levels of adherence (means or medians of 93% compared to only 77% in our population). In addition, the reported hazards ratios in these positive studies were small (HR= .99 for continuous adherence and recurrence and HR= 1.10 for poor adherence and mortality). Explanations for the lack of improvement in recurrence and survival with higher adherence rates in our sample may include factors unique to this population of patients and/or breast cancers that develop in this population, methodological limitations of claims data, and inability to detect what may have been a small effect.
Our measure of medical adherence, prescription refill data, was also used in prior studies of hormonal therapy and breast cancer outcomes, but has several limitations. First, it is possible that patients did not take their medications, even if they filled their prescription. Second, prescription refill data are subject to error introduced by receiving free samples of medications and use of discount medications ordered from other sources. We believe the later was unlikely to occur in this population because women in the cohort generally received prescription medications for free or at a very low cost ($1–$6).
There may be characteristics of this patient group that explain the lack of association between adherence and breast cancer recurrence and survival. Indeed, low socioeconomic status is known to be a risk factor for poorer outcomes after breast cancer generally40. Breast cancer treatment disparities, including underuse of adjuvant radiation following breast conserving surgery41, have been previously documented in this sample. In addition, there are known lifestyle factors, such as smoking, obesity, and physical inactivity that impact outcomes after breast cancer that are more common among women of low socioeconomic status 42–46. These factors may mask the effect of adherence to adjuvant hormonal therapies.
Tumor characteristics unique to this population may explain the lack of association between adherence and breast cancer outcomes. Similar to other registry studies47, nearly a quarter of the women in the study did not have hormone receptor data recorded in the cancer registry. All were prescribed adjuvant hormone therapy, but if a significant number of these “unknown” patients were actually ER/PR negative then the actual effect of adjuvant endocrine therapy may have been masked in this population.
We defined hormone receptor positive as ER positive and/or PR positive. The importance of the PR to the tumor’s response to hormonal therapy has been debated in the literature. Tumors that are both ER and PR positive, termed luminal A, respond more often to hormonal therapy than tumors that are ER positive and PR negative, termed luminal B, or those that are ER negative and PR positive48;49. It is possible that this population of low income women has a higher prevalence of ER positive, PR negative tumors that are less responsive to hormonal therapy. It has been reported that the luminal A tumor type is less common among black women50, who comprised 40.8% of this sample, Differences in the prevalence of breast cancer subtypes by socioeconomic status have not been described. The tumor registry data from 1998–2002 did not contain information on human epidermal growth factor receptor-2 (HER-2) status which distinguishes between the luminal subtypes. HER-2 positivity may indicate resistance to hormonal therapy48;51, especially in ER+/PR− subtypes. Controlling for tumor grade in the analysis may have partially accounted for this effect, since most HER-2 positive tumors are high grade.
Patient characteristics that decrease efficacy of adjuvant hormonal therapy are another consideration. It has been reported that side effects are a major determinant of adherence to adjuvant hormonal therapy11;15;17, such that patients who have less side effects are more adherent to therapy. Lack of side effects might be related to increased tolerance for certain side effects or to drug pharmacokinetics/pharmacogenetics. For instance, tamoxifen is metabolized by the cytochrome P450 system and patients who have low CYP2D6 enzyme activity or who are taking medications that interfere with the activity of this enzyme are less prone to side effects from tamoxifen because there are less active metabolites24. The “poor metabolizer” phenotype occurs in less than 10% of people and varies by ethnic group 52;53. It is unlikely that the prevalence of low CYP2D6 metabolism is higher in this low socioeconomic population, but, if it is, it could explain why we observed no association between adherence and outcomes. There are also many medications that interact with CYP2D6 and thereby decrease metabolism of tamoxifen to its more active metabolites24;52. We examined this possibility in our dataset and found that use of CYP2D6 enzyme inhibitor medications was not independently associated with breast cancer outcomes and did not interact with hormonal therapy adherence.
Alternatively, it is possible that current hormonal therapy dosing regimens are robust to occasional nonadherence, particularly if it is sporadic rather than sustained (e.g. missing one or two days a week, rather than entire weeks). Tamoxifen has a relatively long half life (approximately 7 days) and studies have found blood tamoxifen levels consistent with clinical response up to 21 days after drug discontinuation54. Anastrozole, letrozole, and exemestane have shorter half lives (1–3 days)55. Future studies should try to characterize the patterns of nonadherence.
One limitation of the current study is the possible absence of some important confounding variable that would explain the lack of significant associations between adherence and breast cancer outcomes, as is common among administrative or claims data. In a recent paper, Giordano and colleagues 56 illustrate the difficulties of replicating results from randomized clinical trials using administrative data. In an effort to reduce selection bias, we limited our analysis to cancer-related outcomes, rather than all-cause mortality. Relying solely on clinical trials data is not an option when researchers study groups that have traditionally under-represented in trials, such as the elderly and individuals of low socioeconomic status.
Finally, the sample size and/or length of follow-up may have limited our ability to detect differences in breast cancer outcomes, particularly for cancer-free survival. In clinical trials, survival differences with adjuvant hormonal therapy are typically seen at 5–10 years57, but cumulative reductions in mortality may be twice as big at 15 years1. It is more surprising that with a mean follow-up years, we also saw no association between adherence and breast cancer recurrence. The relatively poor adjuvant endocrine adherence observed in this population may limit our ability to detect difference in recurrence within the study time period. In addition, if the women in this study also had limited compliance with post-cancer treatment mammography surveillance, it is possible that detection of a breast cancer recurrence could have been delayed until after the follow-up period of this study.
In conclusion, in this database of low-income women with breast cancer who were enrolled in Medicaid, we did not observe a significant association between adherence to adjuvant hormonal therapies and breast cancer recurrence or death. Consistent with other recent studies 22;27, we also did not observe either an independent association between use of CYP2D6 enzyme inhibitor medications and breast cancer outcomes or an interaction with hormonal therapy adherence. Although suboptimal adherence is considered to be an important clinical problem, its effects on breast cancer outcomes may be masked by patient genetic profiles, tumor characteristics, and behavioral factors that may independently or interactively influence patient outcomes after breast cancer.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number R01-CA121317-3] and the Investigator-Sponsored Study Program of AstraZeneca.
Part of this study has been published in abstract form for the Annual meeting of the American Society of Clinical Oncology, 2009.
Footnotes
Authors’ Disclosure of Potential Conflicts of Interest
Drs. Weaver, Anderson, Hwang, and Kimmick received research funding from AstraZeneca to complete the study. During the time of this project, Dr. Kimmick was a speaker and consultant for Pfizer and Novartis.
Reference List
- 1.Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 6.Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 7.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 8.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. JNCI Journal of the National Cancer Institute. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 10.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 11.Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 13.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 14.Murthy V, Bharia G, Sarin R. Tamoxifen non-compliance: does it matter? Lancet Oncology. 2002;3:654. doi: 10.1016/s1470-2045(02)00895-1. [DOI] [PubMed] [Google Scholar]
- 15.Demissie S, Silliman RA, Lash TL. Adjuvant Tamoxifen: Predictors of Use, Side Effects, and Discontinuation in Older Women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 17.Lash T, Fox M, Westrup J, Fink A, Silliman R. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 18.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 19.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 20.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 21.van Herk-Sukel M, van de Poll-Franse L, Voogd A, Nieuwenhuijzen G, Coebergh J, Herings R. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5-years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–851. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 22.Dezentje VO, van Blijderveen NJC, Gelderblom H, et al. Effect of Concomitant CYP2D6 Inhibitor Use and Tamoxifen Adherence on Breast Cancer Recurrence in Early-Stage Breast Cancer. J Clin Oncol. 2010;28:2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 23.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz MP, Kamal A, Ames MM. Tamoxifen Pharmacogenomics: The Role of CYP2D6 as a Predictor of Drug Response. Clin Pharmacol Ther. 2007;83:160–166. doi: 10.1038/sj.clpt.6100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Desta Z, Stearns V, et al. CYP2D6 Genotype, Antidepressant Use, and Tamoxifen Metabolism During Adjuvant Breast Cancer Treatment. JNCI Journal of the National Cancer Institute. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. Br Med J. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahern TP, Pedersen L, Cronin-Fenton DP, Sorensen HT, Lash TL. No increase in breast cancer recurrence with concurrent use of tamoxifen and some CYP2D6-inhibiting medications. Cancer Epidemiol Biomarkers Prev. 2009;18:2562–2564. doi: 10.1158/1055-9965.EPI-09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson A, Johnson A, Quinlan P, et al. Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011;125:279–287. doi: 10.1007/s10549-010-1139-x. [DOI] [PubMed] [Google Scholar]
- 29.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimmick G, Camacho F, Foley KL, Levine EA, Balkrishnan R, Anderson R. Racial differences in patterns of care among medicaid-enrolled breast cancer patients. Journal of Oncology Practice. 2006;2:205–213. doi: 10.1200/jop.2006.2.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimmick GG, Camacho F, Balkrishnan R, Anderson R. Patterns of care among breast cancer patients with financial need: Information from a Medicaid-claims and tumor registry linked database. J Clin Oncol. 2005;23:537S. [Google Scholar]
- 32.Anderson RT, Kimmick GG, Camacho F, et al. Health system correlates of receipt of radiation therapy after breast-conserving surgery: a study of low-income Medicaid-enrolled women. Am J Manag Care. 2008;14:644–652. [PubMed] [Google Scholar]
- 33.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 36.Jean-Baptiste R, Gebhard IK, editors. Series IV: Cancer case ascertainment. Springfield, IL: North American Association of Central Cancer Registries; 2002. Procedure guidelines for cancer registries. [Google Scholar]
- 37.Johnson CH, Adamo M. SEER program coding and staging manual 2007. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 38.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 39.Hill ME, Rosenwaike I. The Social Security Administration’s Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001;64:45–51. [PubMed] [Google Scholar]
- 40.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health. 2009;18:883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 41.Foley K, Kimmick G, Camacho F, Levine E, Balkrishnan R, Anderson R. Survival disadvantage among Medicaid-insured breast cancer patients treated with breast conserving surgery without radiation therapy. Breast Cancer Res Treat. 2007;101:207–214. doi: 10.1007/s10549-006-9280-2. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski RT, Aiello E, McTiernan A. Weight Loss in Breast Cancer Patient Management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 43.Irwin ML, Smith AW, McTiernan A, et al. Influence of Pre- and Postdiagnosis Physical Activity on Mortality in Breast Cancer Survivors: The Health, Eating, Activity, and Lifestyle Study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogunleye A, Holmes M. Physical activity and breast cancer survival. Breast Cancer Research. 2009;11:106. doi: 10.1186/bcr2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship Between Potentially Modifiable Lifestyle Factors and Risk of Second Primary Contralateral Breast Cancer Among Women Diagnosed With Estrogen Receptor-Positive Invasive Breast Cancer. J Clin Oncol. 2009;27:5312–5318. doi: 10.1200/JCO.2009.23.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120:2672–2677. doi: 10.1002/ijc.22575. [DOI] [PubMed] [Google Scholar]
- 47.Dunnwald L, Rossing M, Li C. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Research. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arpino G, Weiss H, Lee AV, et al. Estrogen Receptor-Positive, Progesterone Receptor-Negative Breast Cancer: Association With Growth Factor Receptor Expression and Tamoxifen Resistance. JNCI Journal of the National Cancer Institute. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 49.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone Receptor Status Significantly Improves Outcome Prediction Over Estrogen Receptor Status Alone for Adjuvant Endocrine Therapy in Two Large Breast Cancer Databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 50.Kwan M, Kushi L, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Research. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowsett M, Harper-Wynne C, Boeddinghaus I, et al. HER-2 Amplification Impedes the Antiproliferative Effects of Hormone Therapy in Estrogen Receptor-positive Primary Breast Cancer. Cancer Res. 2001;61:8452–8458. [PubMed] [Google Scholar]
- 52.Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic Differences in Genetic Polymorphisms of CYP2D6 in the U.S. Population: Clinical Implications. Oncologist. 2006;11:126–135. doi: 10.1634/theoncologist.11-2-126. [DOI] [PubMed] [Google Scholar]
- 53.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nature Reviews Cancer. 2009;9:576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 54.Fabian C, Sternson L, El-serafi M, Cain L, Hearne E. Clinical pharmacology of tamoxifen in patients with breast cancer: Correlation with clinical data. Cancer. 1981;48:876–882. doi: 10.1002/1097-0142(19810815)48:4<876::aid-cncr2820480403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 55.Buzdar AU, Robertson JFR, Eiermann W, Nabholtz JM. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer. 2002;95:2006–2016. doi: 10.1002/cncr.10908. [DOI] [PubMed] [Google Scholar]
- 56.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


