Abstract
BACKGROUND
Cellular therapy studies are often conducted at multiple clinical sites in order to accrue larger patient numbers. In many cases this necessitates use of localized Good Manufacturing Practices (GMP) facilities to supply the cells. To assure consistent quality, oversight by a quality assurance group is advisable. In this study we report the findings of such a group established as part of the Cardiovascular Cell Therapy Research Network (CCTRN) studies involving use of autologous bone marrow mononuclear cells (ABMMC) to treat myocardial infarction and heart failure.
STUDY DESIGN
Factors affecting cell manufacturing time were studied in 269 patients enrolled on 3 CCTRN protocols using Sepax-separated ABMMC. The cells were prepared at 5 GMP cell processing facilities and delivered to local treatment sites or more distant satellite Centers.
RESULTS
Although the Sepax procedure takes only 90 minutes, the total time for processing was approximately seven hours. Contributing to this were incoming testing and device preparation, release testing, patient randomization and product delivery. The average out-of body-time (OBT), which was to be <12 hours, averaged 9 hours. A detailed analysis of practices at each Center revealed a variety of factors that contributed to this OBT.
CONCLUSION
We conclude that rapid cell enrichment procedures may give a false impression of the time actually required to prepare a cellular therapy product for release and administration. Institutional procedures also differ and can contribute to delays; however, in aggregate it is possible to achieve an overall manufacturing and testing time that is similar at multiple facilities.
Keywords: Cellular therapy, Regenerative medicine, Cardiovascular cell therapy, Turnaround time
INTRODUCTION
Multicenter cellular therapy clinical protocols may use either centralized manufacturing of the product followed by shipment to the site of administration, or preparation of the cellular product at each clinical Center. The choice may be predetermined by the nature of the cellular product. Some cannot be cryopreserved for transportation, and time constraints may make it impossible to ship fresh cells. These restrictions often mandate processing of the cells at facilities associated with, or in close proximity to the clinical sites. When multiple processing facilities are involved simultaneously as in a network trial, this requires stringent quality assurance and control to minimize potential differences between the manufacturing sites, in contrast to the higher degree of reproducibility that can be achieved with centralized manufacturing. The National Heart, Lung and Blood Institute’s Cardiovascular Cell Therapy Research Network is conducting three multicenter clinical trials on the use of autologous bone marrow mononuclear cells for the treatment of myocardial infarction and heart failure or angina (1). In these studies the out-of-body time for the cells was set at a maximum of 12 hours, making centralized processing impossible. Each of the clinical sites, therefore, uses one of five cell processing facilities close to each of the five main clinical centers. Quality assurance (QA) was established under the auspices of an independent group, which is responsible for training, standard operating procedures, site visits and review of all documentation. As a part of the review process QA has tracked the times taken for the various components of marrow harvesting, transportation, processing, release testing, randomization, placebo preparation and return to the clinical center for administration. To our knowledge this is the first analysis of factors affecting turnaround time for manufacturing a cellular therapy product at multiple sites. The results reveal a variety of practices that can impact product preparation times.
MATERIALS AND METHODS
Clinical Protocols
The Transplantation in Myocardial Infarction Evaluation protocol (TIME) is a randomized, Phase II, double-blind, placebo-controlled trial to assess the effect on global and regional left ventricular function of the administration of 1.5×108 ABMMC infused via a 3.5 Fr infusion catheter in the left coronary artery at 3 or 7 days following acute myocardial infarction (MI) (2). The study involves 120 subjects with no prior history of coronary artery bypass grafting or who present with moderate to large MI and with an initial ejection fraction (EF) of ≤ 45%. The primary endpoints are changes in global and regional LV function. In the Late-TIME protocol (3), involving 87 subjects, the cells are administered 2–3 weeks post MI. This study has been completed and all products are part of this analysis.
The FOCUS protocol (4) is a blinded, placebo-controlled study of 87 subjects to assess the effect of 1×108 ABMMC delivered transendocardially via NOGA XP catheter and Myostar mapping to subjects with ischemic cardiomyopathy (EF <45%), left ventricular dysfunction and limiting heart failure and/or angina. The primary endpoints are changes in myocardial oxygen consumption, left ventricular end systolic volume and reduction in perfusion defects. This study has been completed after 92 subjects were enrolled and all products were included in this analysis.
The primary clinical treatment sites were the Texas Heart Institute (THI), Houston; The Cleveland Clinic, Cleveland; The Minneapolis Heart Institute (MHI), Minneapolis; Vanderbilt University, Nashville and the University of Florida (UF), Gainesville. During the course of the study satellite treatment centers were established for the TIME and Late-TIME protocols. The UF satellite was Pepin Heart Hospital in Tampa; MHI satellites were at St. Paul Heart Clinic, St. Paul; Metropolitan Cardiology Consultants, Coon Rapids; the University of Minnesota, Minneapolis and the Mayo Clinic, Rochester (not active at the time of this report). The Cleveland satellite was at University Hospitals Case Medical Center (UHCMC); for THI the satellite was DeBakey Veteran’s Administration (not active at the time of this report).
Cell Processing Facilities
The Good Manufacturing Practices cell processing centers were located at Center for Cell and Gene Therapy, Baylor College of Medicine, Houston (Center #1) for THI; The Molecular & Cellular Therapeutics Facility, University of Minnesota, St. Paul (Center #), for MHI and satellites; Shands Hospital Stem Cell Laboratory, Gainesville (Center #3), for UF and satellite; Vanderbilt University Medical Center, Nashville (Center #5); Cell Therapy Service and UHCMC Ireland Cancer Center, Cleveland (Center #2), for the Cleveland Clinic and satellite. Cell processing facilities in Houston and Minneapolis are members of the Production Assistance for Cellular Therapy (PACT) contract from the National Heart, Lung and Blood Institute, under which cellular therapy products are provided free-of-charge to applicant investigators. Processing was performed in clean room facilities at Centers 1 and 4, requiring gowning of the staff. In the remaining facilities processing was performed in unclassified space. QA was performed by QA staff at the Baylor College of Medicine Center for Cell and Gene Therapy under contract to the CCTRN Data Coordinating Center. QA staff did not participate in any manufacturing activities.
Cell Processing
Details of the cell processing have been published previously (5). During the course of the study additional staff were trained at some of the centers. This resulted in the following final numbers available for processing at each center (Center #1 = 5, Center #2 = 3, Center #3 = 5, Center #4 = 6, Center #5 = 2. Routinely two technologists participated in hands-on manufacturing. Standardized methods were used for product transportation, processing, in-house release testing (cell counts, viability and endotoxin testing), randomization, placebo preparation and product release and distribution. Each center was responsible for working with the clinical staff and CCTRN Data Coordinating Center on scheduling processing and integrating it with other laboratory responsibilities.
Briefly the product used in all three protocols was a mononuclear fraction of autologous bone marrow prepared on a Sepax processor (Biosafe SA, Geneva, Switzerland).
The cells were adjusted to the required concentration and volume in saline containing 5% human serum albumin (HSA). Products were prepared for all patients and randomization to receive product or placebo was performed at the completion of release testing. Placebos consisted of saline/HSA plus or minus a small volume of autologous blood to provide color to assist in blinding. For the TIME and Late-Time protocols the cells were delivered in a 150ml transfer pack; for the FOCUS protocol delivery was in three 1ml syringes. At the time of this report 269 products have been prepared (90 TIME, 87 Late-TIME and 92 FOCUS) and are the basis for this analysis.
Release Testing
Release testing consisted of Gram stain [negative]; Endotoxin by Endosafe (Charles River, Wilmington, MA.) (6) [<5.0 EU/kg]; cell count (as per protocol) and viability by Trypan blue exclusion [>70% viable]. Non-release testing consisted of flow cytometry for CD34+ cells following the ISHAGE protocol (7) [report results – this was in addition to centralized more comprehensive flow cytometric analysis performed by the central study biorepository in Minneapolis, MN], and CFU-Hill and ECFC growth in Endocult and EGM-2 media (StemCell Technologies, Vancouver, BC) [report growth or no growth - this was in addition to centralized more comprehensive colony analysis performed by the central study biorepository in Gainesville, FL (8)].
Shipping and Transportation
The cell processing facility and clinical center were within 15 minutes walking time in the case of THI, UF and Vanderbilt University. Transportation times were within 30 minutes by car for MHI and its satellites (with the exception of the Mayo Clinic) and for the Cleveland Clinic and its satellite. The only long distance transportation in the present study was between the Gainesville processing Laboratory and the Tampa satellite (128 miles one way). Cells were transported to and from the processing facilities in validated coolers at ambient temperature. In hot weather, cool (4°C) packs were placed in the coolers. Transportation was by laboratory staff or commercial couriers.
RESULTS
As reported previously (5), and confirmed in this larger analysis, the manufacturing process resulted in a uniform product from the Sepax at all of the processing facilities (Table 1A). In this report the CD34 measurements were performed by the flow cytometry facilities associated with each of the cell processing facilities and should be considered as preliminary values. Formal phenotypic analysis is ongoing at the CCTRN Minneapolis Biorepository (8). Details of the products prepared for administration are shown in Table 1B. It should be noted that products were prepared for all potential recipients whether or not they were subsequently randomized to the active or placebo arms of the studies. All products met release testing criteria.
Table 1.
Tables 1A and 1B
| 1A: Analysis of Sepax Clinical Separations
| ||||||||
|---|---|---|---|---|---|---|---|---|
| TNC | TRC | %TNC Rec | %TRC Depl | Total ISHAGE CD34+ | % Lymph | % Mono | % Gran | |
|
|
||||||||
| Mean | 5.51×108 | 1.85×109 | 23.6 | 100.0 | 3.33×106 | 42.4 | 9.2 | 38.0 |
| Std Dev | 3.21×108 | 2.65×109 | 5.9 | 0.0 | 1.65×106 | 17.2 | 9.3 | 15.6 |
| Median | 4.83×108 | 1.34×109 | 23.7 | 100.0 | 3.06×106 | 42.5 | 8.0 | 39.0 |
| Range | 0.094*–18.3×108 | 0.032*–4.0×1010 | 0.7*–39.7 | 99.9*–100.0 | 0.021*–10×106 | 0†–95.0 | 0†–112.7 | 0†–78.0 |
|
| ||||||||
|
1B: Analysis of Final Product Doses - 1/3 patients received placebo in place of cells‡
| ||||||||
| TIME/Late-TIME Products | FOCUS Products | |||||||
| TNC Dose | Total RBC Dose | TNC Dose | Total RBC Dose | |||||
|
|
||||||||
| N = | 177 | 177 | 92 | 92 | ||||
| Mean | 1.47×108 | 1.92×109 | 9.94×107 | 3.72×108 | ||||
| Std Dev | 1.55×107 | 1.75×1010 | 4.55×106 | 2.21×108 | ||||
| Median | 1.50×108 | 4.58×108 | 1.00×108 | 3.09×108 | ||||
| Range | 0.036 – 1.50×108 | 0.017 – 23.3×1010 | 0.61 – 1.01×108 | 0.13 – 1.58×109 | ||||
Lower value represents cells recovered after a Sepax device failure. Cells were recovered manually from the Sepax.
Lower values represent out of range results obtained from automated counters
Products were prepared for all recipients. Randomization to active or placebo arm occurred following product preparation. Results shown are for all products prepared.
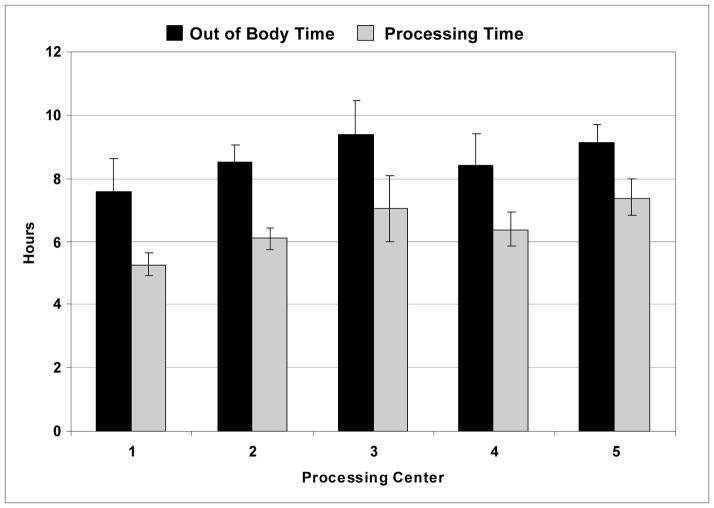
QA performed an analysis of the total OBT and processing time at each of the centers (Figure 1). This analysis revealed differences of up to 90 minutes in OBT, and of more than two hours in processing time between centers. In order to determine the source of these time differences a more detailed analysis was performed in which the OBT was subdivided into a number of component activities. These included marrow harvest and transport time, time required to prepare the marrow for processing (this included time to filter the marrow, take quality control (QC) samples, prepare buffers, load the disposable onto the Sepax device, etc.), processing time on the Sepax, time to adjust the cell concentration, take QC samples, perform QC testing and to randomize the recipient to the treatment or placebo arms, final preparation of the product or placebo, preparation of samples for ancillary studies and for the biorepositories (in some cases this was done after product delivery) and product delivery time to the cardiac catheterization laboratory. The time between product receipt and administration to the patient was also recorded. The results are shown in Figure 2. The marrow harvest and transportation times were comparable between all five centers, ranging between approximately 28 and 63 minutes. The preparation time was similar at three of the centers (~1 hour 20 minutes), half an hour longer at Center 5, and only 30 minutes at Center 1. Further examination revealed that staff at Center 1 performed the majority of preparative work (buffer preparation, installation and priming of the Sepax set, etc.) before and during the marrow harvest and, therefore, were able to get the marrow onto the Sepax more rapidly.
Figure 1. Out of Body and Processing Times.
The total out of body time for the bone marrow is shown in hours for each of the cell processing centers together with the time taken in the laboratory to perform cell processing. Bars indicate standard deviations.
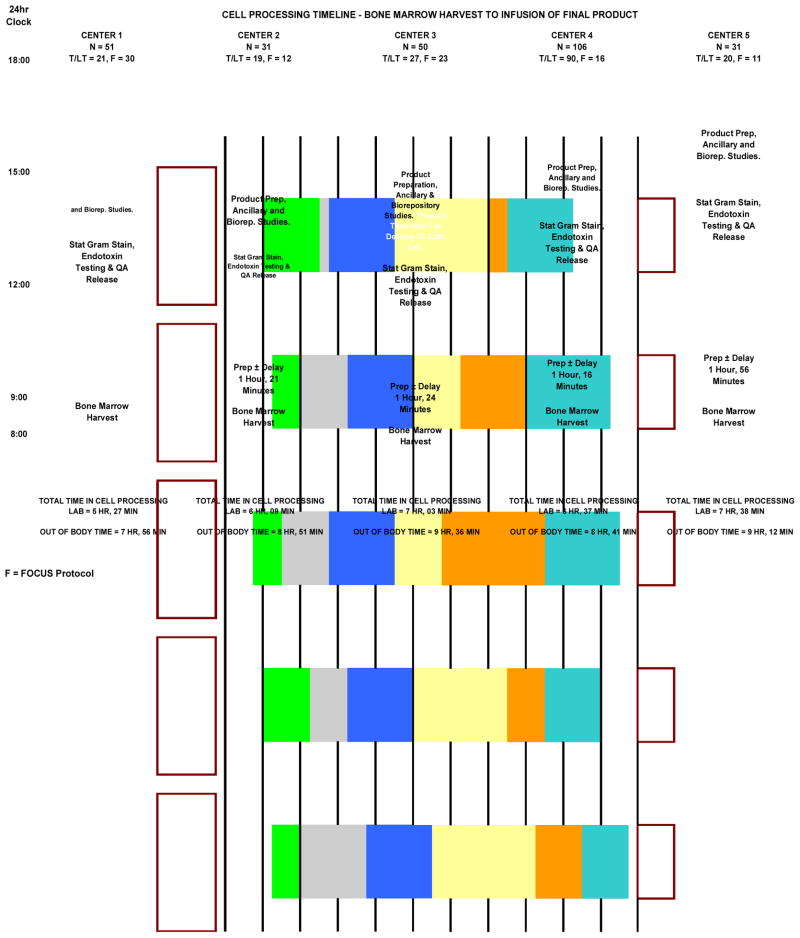
Figure 2.
The out of body time is broken down by component activity for each processing facility. The number of procedures performed by each facility is shown at the top of the appropriate column. At the base of the column is shown the mean processing time and total out of body time for the center. The activity descriptions are shown within the body of the columns.
The processing time on the Sepax was essentially identical at all centers (90 minutes) since the procedure is automated. Some of the greatest variability between centers occurred at the next stage during which the ABMMC are removed from the Sepax, re-filtered (if necessary) to remove any aggregates, brought to the correct concentration and release testing performed. This took from 90 minutes at Center 2 to 175 minutes at Center 5. Viability and endotoxin testing were performed within the processing laboratories and would, therefore, be expected to take similar times. Preparation time and running of samples on the Endosafe takes about 20 minutes. The test is somewhat sensitive and these times may be considerably prolonged if the sample has to be rerun after dilution in magnesium chloride and/or heat inactivated. The other variable is the time taken for the stat Gram stain. This was usually performed by the clinical laboratory associated with the processing center and took anywhere from 45 to 90 minutes.
Upon completion of release testing the centers enter the results into the CCTRN database which then assigns the recipient to receive the cells or placebo. Again this procedure took comparable times at each facility. It could be delayed if the nurse coordinators had not previously completed their required data entry steps. Subsequent tasks were handled differently by the centers resulting in turnaround times ranging from 37 minutes to more than two hours. The tasks that had to be accomplished were preparation of the placebo (if required) and packaging and labeling of the product to be delivered to the cardiac catheterization laboratory. In the case of FOCUS products the cells had to be drawn into syringes in a sterile field and the syringes placed into sterile bags for delivery. This took longer than TIME and Late-TIME products which were delivered in transfer packs. Some centers used this time to perform ancillary tests on residual cells and to prepare and package the samples to be sent to the CCTRN biorepositories, while others did this after delivery of the clinical product. At four centers the cells were delivered to the cardiac catheterization laboratories immediately after preparation. Depending upon the status of patient testing and preparation, the cells/placebo may then be administered immediately, or held temporarily until these procedures were completed. At Center 3 the cells were held in the processing laboratory until the patient was ready.
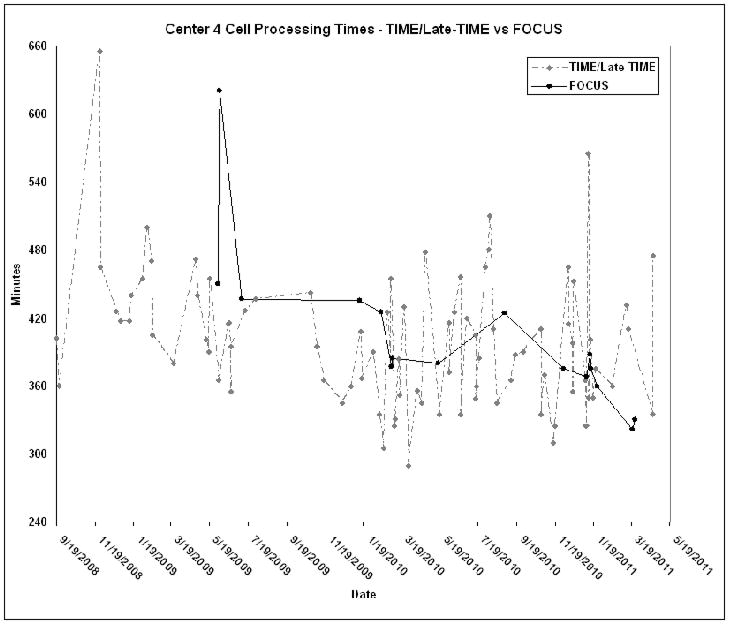
Transportation times to the site of administration, particularly those at satellite facilities, would be expected to influence the turnaround times. Center 1 did not deliver to satellites and was in close proximity to their clinical center. This is reflected in the fast turnaround time. In contrast, Center 3, whose satellite was 128 miles from the processing facility, achieved similar turnaround times for products that were administered locally or where driven to the satellite (Figure 3). This, however, also reflects the practice of holding the products for local delivery in the processing facility (rather than the catheterization laboratory) until the patient was prepared for the administration. The times between product delivery and initiation of administration ranged between an hour and 2 hours. This was primarily impacted by the method of administration. For FOCUS products delivery was transendocardially using a NOGA catheter and electro-mechanical mapping, whereas in TIME and Late-TIME simple intra-arterial infusion by stop-flow technique was used. There was no significant decrease in overall processing times over the course of the studies, although a slight trend was discernable, particularly with the FOCUS protocol (Figure 4). Since experience did not seem to decrease processing times it was felt that this reflected the efficacy of the training procedure.. As expected, there were occasional delays for specific products due to technical issues e.g. aggregate formation, separation problems, delays in testing etc (shown as sharp spikes in Figure 4).
Figure 3.
Breakdown of the out of body time is shown for all products prepared at a single center (far left column). The center column shows the data for products administered locally at the clinical site associated with that processing center. The right hand column shows data from products collected at a distant satellite, transported to the same processing center and then returned to the satellite for administration. The overall times are similar, reflecting the request to hold products for local delivery until requested by the cardiac catheterization laboratory. This compensated for the transportation time from and to the satellite clinical center.
Figure 4.
The cell processing times for Center 4 are shown from the receipt of the bone marrow to delivery of cells/placebo to the cardiac catheterization laboratory. This center was chosen since it had the median turnaround time and has processed the largest number of products There is a trend towards decreased overall processing times for the FOCUS protocol during the course of the study. Occasional delays, (shown as sharp spikes) in processing times shown are due to various technical issues.
DISCUSSION
Multicenter cell therapy protocols must determine whether centralized or local product manufacturing best fit the requirements of the protocol(s). In the present study the CCTRN established a maximum OBT of 12 hours for the cells. Given the geographical distribution of the participating centers this necessitated the establishment of processing facilities in close proximity to each treatment center. As clinical satellites were added to the network, in order to increase accrual, these were required to be within a distance that would allow delivery of the product within 12 hours of harvest. An automated cell enrichment procedure was selected in order to minimize processing differences between the laboratories and the results to date have supported this approach. Analysis of the manufacturing procedure has shown that the preparative steps before and after automated cell separation (e.g., quality control testing, concentration adjustments, release testing, packaging, labeling and delivery) contribute significantly to the turnaround time, such that the actual cell separation component represents only about one quarter of the total time taken. Clinical staff are often unfamiliar with the times required to manufacture and release a cellular therapy product, and this information needs to be emphasized during protocol development. In addition overall release time must particularly be taken into consideration when the trend is towards developing intraoperative methods for the preparation of cellular therapy products. An analysis of the procedure at each of the centers revealed a number of different practices that all fell within standard operating procedures. The first time discrepancy was associated with the preparation time before the marrow was processed on the Sepax. Most centers started preparation upon arrival of the marrow in the laboratory. This ensured that reagents, disposables etc, were not wasted in the event that the procedure was cancelled at short notice. In some laboratories other procedures were ongoing simultaneously and staff were not available for early set-up. In Center 1 the majority of preparation was performed early in the morning before the arrival of the bone marrow, such that the marrow went onto the Sepax within an average 30 minutes of arrival in the laboratory.
After Sepax processing was completed the cells had to be adjusted to the correct concentration. This was facilitated by providing worksheets that addressed all three possible scenarios (cells above, below or at the correct concentration after the Sepax). The time differences at this stage most probably reflect the time taken to perform stat Gram stains and the number of times that Endosafe endotoxin testing needed to be repeated. Repeat testing occurred most frequently at Center 1. Interestingly, this center had the shortest overall turnaround time. Further investigation revealed that at this center the Endosafe testing was performed by the Quality Control Laboratory, allowing the cell processing staff to continue their work while testing was ongoing. At the other centers the processing staff performed endotoxin testing. Cell counting and viability assessment was performed at all centers by cell processing staff, whereas Gram staining was carried out by the associated hospital clinical laboratories.
Following randomization the laboratories may be required to prepare the placebo and then must package and label the product for delivery. FOCUS products had to be packaged in syringes for use in a sterile field, which took longer than aliquoting into transfer packs for use in the other protocols. Several centers elected to use this time to prepare samples for shipment to the biorepositories and to set up cells for ancillary studies. Others performed these procedures following delivery of the cells to the clinical sites.
Patient preparation time also impacts overall OBT. In four of the Centers cells were delivered to the cardiac catheterization laboratory immediately after preparation and were held briefly in the catheterization laboratory until the patient was ready for product administration. During this period the patient may undergo required testing and, in the case of the FOCUS protocol, NOGA catheterization and electro-mechanical mapping. At Center 3 the cells were held in the processing facility rather than the catheterization laboratory. Other delays were due to transportation of the product to more distant clinical sites and satellites. The transportation conditions were all validated for greater than the maximum anticipated times and did not adversely affect cell viability of functionality as measured by colony formation. Additional delays occurring at the centers were generally minor and included labeling discrepancies and database problems.
In spite of the range of potential delays, patients who were generally harvested at 8am received the therapeutic cells or placebo by about 5pm. A review of the best turnaround times suggests that this could be reduced by approximately 1 hour if preparation and release testing times are optimized. The average OBT of 9 hours among all centers in these studies was approximately three hours longer than reported for the HEBE trial (9), where cells were prepared by manual density gradient centrifugation. In that trial the types of release testing are not described in detail, and we have shown in the CCTRN study that this can contribute significantly to the turnaround time.
This study reveals that proposed manufacturing turnaround times may be substantially underestimated if they are based only on the time taken to perform the cell separation procedure. In this report cell separation occupies only a quarter of the laboratory time. Potential delays can occur at a number of points, particularly during set-up, release testing and transportation. We hope that analyses of this type can be used to anticipate sources of delay and provide the information required to help optimize turnaround times in future cell therapy studies.
Acknowledgments
This study was supported by grant number U01-HL-087318 from the National Heart, Lung and Blood Institute (NHLBI). It was also supported in part by NHLBI contract numbers N01-HB-37164 and HHSN268201000008C to the Molecular and Cellular Therapeutics Facility, University of Minnesota) and N01-HB-37163 and HHSN268201000007C to the Cell Processing Facility, Baylor College of Medicine. We should like to thank the following for providing preliminary flow data: April Durett (Houston), Jose Iturraspe (Gainesville), Howard Meyerson (Cleveland), Kim Schaper (Minneapolis) and Bruce Greig (Nashville).
References
- 1.Simari RD, Moyé LA, Skarlatos SI, et al. Development of a network to test strategies in cardiovascular cell delivery: the NHLBI-sponsored Cardiovascular Cell Therapy Research Network (CCTRN) J Cardiovasc Transl Res. 2010;(1):30–6. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–63. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traverse JH, Henry TD, Vaughan DE, et al. Late TIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Willerson JT, Perin EC, Ellis SG, et al. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am Heart J. 2010;160:215–23. doi: 10.1016/j.ahj.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gee AP, Richman S, Durett A, et al. Multicenter cell processing for cardiovascular regenerative medicine applications: the Cardiovascular Cell Therapy Research Network (CCTRN) experience. Cytotherapy. 2010;12:684–91. doi: 10.3109/14653249.2010.487900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee AP, Sumstad D, Stanson J, et al. A multicenter comparison study between the Endosafe PTS rapid-release testing system and traditional methods for detecting endotoxin in cell-therapy products. Cytotherapy. 2008;10:427–35. doi: 10.1080/14653240802075476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland DR, Nayyar R, Acton E, et al. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009;11:595–605. doi: 10.1080/14653240902923161. [DOI] [PubMed] [Google Scholar]
- 8.Zierold C, Carlson MA, Obodo UC, et al. Developing Mechanistic Insights into Cardiovascular Cell Therapy - The Cardiovascular Cell Therapy Research Network (CCTRN) Biorepository Core Laboratory Rationale. American Heart Journal. 2011 doi: 10.1016/j.ahj.2011.05.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch A, Nijveldt R, van der Vleuten PA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2010 Dec 10; doi: 10.1093/eurheartj/ehq449. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]