Abstract
Objective
To evaluate sleep quality in women with hip pain due to daily activities involving the lower extremity joints.
Methods
We evaluated the association of the the Western Ontario MacMaster Osteoarthritis Index (WOMAC) hip pain severity score, with objective sleep measures, obtained by wrist actigraphy, in 2225 Caucasian women ≥ 65 years from the Study of Osteoporotic fractures study.
Results
Women had an increased odds of spending ≥1.5 hours awake after sleep onset (WASO) (OR: 1.28, 95% CI: 1.11–1.50) for every 5 units increase in hip pain score after adjustment for all covariates. Resting hip pain was the strongest predictor of sleep fragmentation (OR: 2.0, 95%CI 1.47–2.73), however standing pain was associated with higher WASO independent of pain while in bed (OR: 1.41, 95% CI: 1.07–2.01). Sleep disturbances increased significantly after the first two hours of sleep in women with severe hip pain compared to those without hip pain (1.4 ± 0.47 minutes per hour sleep p=<0.003). Similar associations were observed for long wake episodes greater than 5 minutes. There were no associations with daytime napping, sleep latency, sleep efficiency and total sleep minutes and WOMAC hip pain.
Conclusion
Fragmented sleep was greater in women with hip pain compared to those without hip pain. However, fragmented sleep in women with severe hip pain compared to those without hip pain was unchanged until after the first two hours of sleep. Further investigation, such as pain medications wearing off over time, or prolonged periods of inactivity decreasing the pain threshold are warranted.
Introduction
Hip pain from Osteoarthritis (OA), a major cause of chronic hip and knee pain in the elderly, is often triggered by important daily activities that involve the lower extremities such as walking, standing, and climbing stairs when compared to other diseases [1]. In addition, OA of the hip causes pain, stiffness and limited joint movement which can also be exacerbated by activities that reduce the range of hip movements such as sitting and lying down.
There are several health implications of chronic pain, including the effect of chronic pain on sleep quality in the elderly [2]. Chronic sleep disturbance is also associated with impaired daytime function, daytime sleepiness and fatigue, reduced quality of life, and increased health care utilization [3, 4]. Hence it is very important to study the effects of pain in the elderly to determine appropriate treatment options for patients with chronic pain and poor sleep.
A few studies have investigated the effect of chronic pain in patients with radiographic OA (RHOA) [5–7]. However these studies had a number of limitations including limited sample sizes, subjective sleep assessment, generic pain measures that may lead to mistakenly assessing elderly individuals with OA as pain free [1, 8], and the lack of information regarding the reliability between subjective sleep measures and objective sleep measures obtained from both actigraphy and the gold-standard measurement of sleep from polysomnography [9, 10].
Our unique dataset provides us with validated pain measurements assessed for specific activities involving the lower extremity joints and both subjective and objective sleep qualities in a large cohort of elderly women with data gathered on numerous potential confounders for adjustment. The aims of this study was 1) to determine the association of subjective and objective sleep disturbances with the WOMAC hip pain score and for hip pain while performing each activity; and 2) to determine if sleep disturbance varies during the night after sleep onset among those with severe, moderate and mild or no hip pain.
Methods
Participants
The Study of Osteoporotic Fractures (SOF) enrolled 9704 community-dwelling Caucasian women 65 years or older at baseline (1986–1988) from four regions in the United States. Women with bilateral hip replacement and unable to walk without assistance were excluded. [11]. Follow-up visits took place approximately every two years. The institutional review boards at each clinic site approved the study, and written informed consent was obtained from all participants.
Participants completed a self-administered mailed questionnaire approximately 2 weeks before actigraphy data collection. 3219 participants had wrist actigraphy data collected at the visit 8 exam (2002–2004) Of those 2,878 who wore an actigraph: 24(<1%) had an actigraph malfunction, 21 (<1%) had a software or initialization problem, and 34(1.2%) removed and did not replace the actigraph, leaving 2799 (97%) women with technically adequate actigraphy data for this analysis. 380 women with a total hip replacement or a confirmed hip fracture between baseline and visit 8 and 194 women with missing data for actigraphy, WOMAC measures and/or covariates for the multivariate models were excluded from the analysis. The final analysis sample included 2225 women unselected for hip pain.
Objective Sleep Parameters
Parameters of sleep-wake patterns were measured using an actigraph [12]. Actigraphy has been shown to provide an objective and reliable estimate of sleep/wake patterns [13]. Participants were instructed to wear the actigraph continuously for ≥3 consecutive 24hr periods, except while bathing. The actigraph is similar in size and weight to a standard wristwatch, and movement is detected via a piezoelectric bimorph-ceramic cantilever beam that generates a voltage each time the actigraph is moved. These voltages are gathered continuously and summarized over 1-min intervals. Data were collected in 3 modes but are reported here based on digital integration mode [14]. ActionW-2 software [12] was used to analyze the actigraphy data. Details of the actigraphy scoring algorithms utilized in the study have been published elsewhere [15, 16]. Participants were asked to keep a sleep log that was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”), and to delete time when the actigraph was removed. Inter-scorer reliability for editing the actigraphy data files has been previously found to be high in our group (intra-class coefficient for total sleep time (TST) = 0.95), and actigraphy has been shown to have good concordance with TST from polysomnography [15, 17].
To minimize night to night variability, the average of the sleep parameters over all the nights was used in this analysis. Three measures of sleep fragmentation were evaluated; wake after sleep onset (WASO, number of awake minutes in-bed scored from sleep onset to the end of the last sleep episode), number of long wake episodes >5 minutes duration (LWEP) and, sleep efficiency as the percentage of time the participant is sleeping while in-bed. Other sleep parameters include; TST as the mean minutes scored as nighttime sleep while in-bed, sleep latency as the number of minutes from the time the participant reported getting into bed to sleep onset that was scored on the actigraph; and napping behavior estimated as the mean minutes of periods of daytime inactivity.
We also scored the sleep actigraphy data at 2, 4, 6 and 8 hours after sleep onset. Wake minutes and sleep efficiency was scored at each of the time intervals for each night and then averaged over the 3–5 days that the actigraphy data was collected.
Subjective Measure of Sleep
Participants completed the Pittsburgh Sleep Quality Index (PSQI), a validated measure of subjective sleep quality and sleep disturbances over a one-month time period. Global PSQI scores range from 0–21, and a standard cutoff of ≥ 5 is indicative of poor sleep quality [18].
Hip Pain
The hip pain subscale from the Western Ontario MacMaster Osteoarthritis Index (WOMAC) was used to assess hip pain [19] via a self administered mailed questionnaire at visit 8. Five items such as hip pain while walking on a flat surface, standing upright, going up and down stairs, sitting or lying, and at night in bed were assessed. Women were asked to rate their pain intensity for each of the 5 pain items on a five point scale (0= none, 1=mild, 2=moderate, 3=severe, 4=extreme) for each hip and the score on the worst hip was used in the analysis.
We also dichotomized the WOMAC hip score as having moderate to extreme hip pain for ≥ 1 of the items verses having mild/no hip pain on all of the WOMAC items. The WOMAC pain items were also analyzed individually by classifying women with moderate to extreme hip pain during each activity as having hip pain for the activity, and women reporting mild or no hip on all activities as no hip pain.
Other Measurements
Participants who completed the mailed visit 8 questionnaire, answered questions about medical history, self-reported health, years of education, number of blocks walked for exercise, back pain, severity of back pain and recent worsening of arthritis pain. Functional status was measured with information on six instrumental activities of daily living (IADL) [20, 21]. Standard body mass index (BMI) was calculated using body weight and height measurements. Depressive symptoms and anxiety were assessed using the Geriatric Depression Scale (GDS, 0–15) [22] and The Goldberg Anxiety scale (0–9) [23] respectively. Fatigue was defined as those who reported lack of energy and feeling slowed down. Participants were asked to bring in all current medications used within the last 30 days, and a computerized medication coding dictionary was used to categorize the medications [24].
Statistical analysis
Characteristics of women including sleep parameters at visit 8 according to the category of WOMAC pain score were compared using analysis of variance for normally distributed continuous data, chi square tests for categorical data and Wilcoxon rank-sum non-parametric tests for skewed continuous covariates. To assess potential confounding we performed univariate tests of association between each of the sleep variables and potential confounders identified in table 1 (data not shown).
Table 1.
Characteristics of SOF women in this study by average WOMAC pain subscale
| WOMAC pain | |||
|---|---|---|---|
|
| |||
| Characteristics | Never or mild pain on all activities (N= 1708) | Moderate to extreme pain (>=1 activities) (N= 517) |
Whole cohort (N= 2225) |
| Demographics | |||
| Current age (years) | 83.82 +/− 3.29 | 83.72 +/− 3.27 | 83.8 +/− 3.28 |
| Body Mass Index (kg/m2) | 26.6 +/− 4.72 | 27.62 +/− 4.78*** | 26.83 +/− 4.75 |
| Education (years) | 701 (41.04) | 178 (34.43)** | 879 (39.51) |
| Health | |||
| Self rated health (Good vs. poor) | 1396 (81.73) | 344 (66.54)*** | 1740 (78.2) |
| Total Difficulty with IAL impairments (range 0–15) | 2 +/− 3.25 | 3.61 +/− 3.92*** | 2.37 +/− 3.48 |
| Geriatric depression score (0–15) | 2.1 +/− 2.39 | 2.97 +/− 2.78*** | 2.3 +/− 2.51 |
| Goldberg Anxiety scale (0–9) | 1.73 +/− 1.98 | 2.7 +/− 2.13*** | 1.95 +/− 2.05 |
| Fatigue | 1137 (66.57) | 447 (86.46)*** | 1584 (71.19) |
| Physical Activity | |||
| Number of blocks walked for exercise | 3.93 +/− 6.99 | 2.65 +/− 5.82*** | 3.63 +/− 6.76 |
| Non Hip pain | |||
| Recent Worsening of pain or Arthritis | 260 (15.22) | 142 (27.47)*** | 402 (18.07) |
| Back Pain | 1053 (61.65) | 453 (87.62)*** | 1506 (67.69) |
| Severity of back pain | |||
| None | 411 (24.06) | 75 (14.51)*** | 486 (21.84) |
| Mild | 536 (31.38) | 282 (54.55) | 818 (36.76) |
| Moderate | 106 (6.21) | 96 (18.57) | 202 (9.08) |
| Extreme | 260 (15.22) | 142 (27.47) | 402 (18.07) |
| Current Medications | |||
| Benzodiazepines | 105 (6.15) | 57 (11.03)** | 162 (7.28) |
| Analgesics/Opioid use | 66 (3.86) | 46 (8.9)*** | 112 (5.03) |
| Non steroidal Anti Inflammatory Drugs | 344 (20.14) | 162 (31.33)*** | 506 (22.74) |
| Actigraphy Measures | |||
| Total Sleep time in bed | 408.24 +/− 74.19 | 403.18 +/− 75.04 | 407.07 +/− 74.4 |
| Sleep Latency | 38.59 +/− 36.58 | 38.65 +/− 38.97 | 38.6 +/− 37.14 |
| Sleep Efficiency | 78.51 +/− 11.13 | 77.4 +/− 11.86 | 78.25 +/− 11.31 |
| Wake after sleep Onset | 72.56 +/− 45.33 | 79.8 +/− 49.24* | 74.25 +/− 46.36 |
| Napping | 73.39 +/− 61.73 | 77.72 +/− 64.3 | 74.39 +/− 62.35 |
| Long Wake periods after sleep onset | 6.45 +/− 3.04 | 6.88 +/− 3.32* | 6.55 +/− 3.11 |
p<0.01
p<0.001,
p <0.0001
Logistic regression models were used to determine the relationship between hip pain and sleep outcomes. The sleep parameters were expressed categorically based on clinically relevant cutpoints used in previous publications from the SOF study; [25–30] (total sleep time ≤5 h, sleep latency>=1 hr vs <1 hr; sleep efficiency <70% vs. ≥70%; WASO ≥90 min vs. <90 min; number of LWEP ≥8 vs. <8) or used standard cutpoints (PSQI >5 vs. ≤5). We also analyzed the sleep variables as continuous outcomes, and the conclusions from these analyses were consisted with our findings using the dichotomous variables.
Variables related (p<0.10) to ≥ 1 sleep outcome measure and WOMAC hip pain were included in multivariate analyses. To investigate mechanisms by which WOMAC hip pain might be associated with sleep disturbances, we constructed three multivariate regression models. The base model was adjusted for age, education years, BMI, back pain and recent worsening of arthritis. In a second model, we further adjusted by depression, fatigue and health status given the uncertainty of whether these covariates are in the causal pathway. The final multivariate models included all these covariates, plus further adjustment for anxiety, number of blocks walked for exercise and current use of benzodiazepines, opioids, or non-steroidal anti-inflammatory drugs (NSAIDs) medications. Finally, we performed regression models for each WOMAC hip pain item individually by adding both the WOMAC hip pain item and hip pain while in bed in a single multivariate model to determine if hip pain while performing other activities is associated with sleep disturbance, independent of hip pain in bed. The referent group for these analyses was women with mild or no hip pain for the specific activity.
To determine if the pattern in sleep disturbance between women with and without hip pain differed we performed random effect models with measures of total wake time at 2, 4, 6 and 8 hours after sleep onset. We categorized hip pain in 3 classes; severe/extreme pain on ≥1 WOMAC activity, moderate pain on any activity and mild/no hip pain on all activities. We included random effects term for the intercept and time to allow for individual time trends for each participant and tested interactions of time after sleep onset with; severe/extreme and moderate WOMAC hip pain compared to mild or no hip pain. Statistical analysis was performed using the statistical software program SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the study population
Among 2225 women (mean age 83.8 +/− 3.3 years), 517 women had moderate to extreme hip pain while performing ≥1 activities and 1708 reported mild or no hip pain for all of the activities (Table 1). The average WOMAC hip pain subscore was 2.0 ± 3.3.
Women with moderate to extreme hip pain were on average slightly overweight, had poorer self reported health, had greater difficulty with IADL impairments, more depressive symptoms, more fatigue and had higher anxiety scores compared to women with none or mild hip pain. These women were also less likely to exercise and the majority complained of moderate to extreme back pain compared to women without hip pain (Table 1).
Women with any moderate to extreme hip pain had higher rate of medication use for analgesics, opioids and NSAIDs when compared to women with no hip pain. Approximately 11% of women with moderate to extreme hip pain were taking benzodiazepines. 9% were on analgesics while a 31% were on NSAID medications and all were significantly higher when compared to women with no or mild hip pain (Table 1).
Inter-class correlation of WOMAC hip pain items
Standing pain and pain while climbing stairs were moderately correlated with walking pain (0.64, 0.61 respectively). Pain while sitting and while in bed were strongly correlated (0.755) (Table 2). In contrast, the correlation among those with resting hip pain with walking, standing and climbing stairs pain was lower (0.45, 0.50, 0.47 respectively).
Table 2.
Inter-Class Correlation Coefficients for WOMAC hip pain Item
| Pain while | Walking | Standing | Climbing Stairs | Sitting/Lying | In bed at night |
|---|---|---|---|---|---|
| Walking | ---- | 0.64 | 0.61 | 0.49 | 0.45 |
| Standing | 0.64 | ---- | 0.58 | 0.54 | 0.50 |
| Climbing Stairs | 0.61 | 0.58 | ---- | 0.47 | 0.47 |
| Sitting/Lying | 0.49 | 0.54 | 0.47 | ---- | 0.76 |
| In bed at night | 0.45 | 0.5 | 0.47 | 0.76 | ---- |
Subjective Sleep measures and WOMAC hip pain
About two thirds of women with moderate to extreme hip pain had poor self-reported sleep quality on the PSQI and 28.4% of women reported trouble sleeping due to pain ≥3 times a week compared to 6.6% of women with no or mild hip pain (Table 3). We compared women with actigraphy and subjective sleep measures and found that among women with PSQI >5, 31% had ≥ 90 minutes WASO, 9% had TST ≤5 hours and 20% napped ≥ 2,1/2 hours. Similarly among women with trouble sleeping ≥3 times a week; 41% had ≥ 90 minutes WASO, 13% had TST ≤5 hours and 24% napped ≥ 2,1/2 hours. After adjustment for covariates, moderate to extreme WOMAC hip pain for ≥ 1 activity was not significantly associated with poor sleep quality on the PSQI scale (1.22; 0.97–1.54) compared to women without hip pain (Table 4). However, WOMAC items for resting hip pain were significantly associated with PSQI (Table 4) in multivariate models. All WOMAC hip pain items were strongly correlated with trouble sleeping due to pain ≥3 times a week after adjustment for all covariates (Table 4).
Table 3.
Categorical Subjective and objective sleep measures for SOF women by WOMAC hip pain subscale
| WOMAC pain | |||
|---|---|---|---|
|
| |||
| Characteristics | Never or mild pain on all activities (N= 1708) | Moderate to extreme pain (>=1 activity) (N= 517) |
Whole cohort (N= 2225) |
| †Self reported sleep quality | |||
| Poor sleep based on PSQI >5 | 814 (47.66) | 323 (62.48)*** | 1137 (51.1) |
| Trouble sleeping due to pain | |||
| Less than once a week | 128 (7.49) | 79 (15.28)*** | 207 (9.3) |
| Once or twice a week | 139 (8.14) | 92 (17.79) | 231 (10.38) |
| Three or more times a week | 113 (6.62) | 147 (28.43) | 260 (11.69) |
| †Sleep quality from Actigraphy | |||
| Total Sleep time in bed ≤ 5hrs vs. >5hrs | 1070 (62.65) | 320 (61.9) | 1390 (62.47) |
| Sleep Latency ≥ 1hr vs. < 1hr | 290 (16.99) | 82 (15.86) | 372 (16.73) |
| Sleep Efficiency <70% vs. ≥ 70% | 295 (17.27) | 104 (20.12) | 399 (17.93) |
| Wake after sleep Onset ≥ 90mins vs. <90mins | 447 (26.19) | 173 (33.46)* | 620 (27.88) |
| Napping ≥ 2 hrs vs. <2 hrs | 311 (18.44) | 101 (19.8) | 412 (18.75) |
| Average number of Long Wake periods after sleep onset >5 minutes ≥ 8 vs. <8 | 435 (25.47) | 166 (32.11)* | 601 (27.01) |
Unadjusted models.
p<0.05,
p <0.005
Table 4.
OR ratio and 95% confidence interval for objective sleep measures with WOMAC hip pain
| Current Type of Pain | † N | Base Model | Base model +health + depression+ fatigue | MV model |
|---|---|---|---|---|
| OR (95% Confidence Interval) | OR (95% Confidence Interval) | OR (95% Confidence Interval) | ||
| Wake after sleep onset ≥ 90 minutes compared to <90 minutes | ||||
| WOMAC pain subscale (0–20) per 5 points change | 1.39 (1.19, 1.60) | 1.29 (1.1, 1.50) | 1.28 (1.11, 1.50) | |
| Moderate to extreme pain in 1 or more activity | 517 | 1.44 (1.15–1.81) | 1.33 (1.05–1.68) | 1.33 (1.05–1.68) |
| Moderate to extreme while … | ||||
| walking on a flat surface | 279 | 1.76 (1.32, 2.33) | 1.54 (1.16, 2.06) | 1.52 (1.13, 2.03) |
| standing upright | 282 | 1.85 (1.42, 2.46) | 1.63 (1.22, 2.17) | 1.62 (1.21, 2.17) |
| going up or down stairs | 309 | 1.50 (1.14, 2.00) | 1.38 (1.04, 1.82) | 1.36 (1.02, 1.80) |
| in bed at night or | 257 | 1.88 (1.41, 2.52) | 1.69 (1.26, 2.28) | 1.68 (1.22, 2.24) |
| when sitting or lying | 216 | 2.00 (1.47, 2.73) | 1.79 (1.31, 2.46) | 1.78 (1.29, 2.45) |
| * Referent :No or mild hip pain | 1708 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8 or more wake episodes greater than 5 minutes vs. < 8 | ||||
| WOMAC pain subscale (0–20) per 5 points change | 1.25 (1.11, 1.45) | 1.24 (1.07, 1.45) | 1.24 (1.06, 1.44) | |
| Moderate to extreme pain in 1 or more activity | 517 | 1.34 (1.06–1.68) | 1.28 (1.01–1.62) | 1.28 (1.01–1.61) |
| Moderate to extreme hip pain while | ||||
| walking on a flat surface | 279 | 1.58 (1.19, 2.10) | 1.45 (1.08, 1.94) | 1.43 (1.06, 1.92) |
| standing upright | 282 | 1.66 (1.25, 2.19) | 1.52 (1.13, 2.03) | 1.51 (1.13, 2.03) |
| going up or down stairs | 309 | 1.39 (1.05, 1.83) | 1.31 (0.99, 1.74) | 1.28 (0.96, 1.70) |
| in bed at night | 257 | 1.85 (1.39, 2.48) | 1.73 (1.28, 2.32) | 1.69 (1.25, 2.28) |
| when sitting or lying | 216 | 1.99 (1.46, 2.71) | 1.86 (1.36, 2.55) | 1.85 (1.34, 2.55) |
| * Referent :No or mild hip pain | 1708 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Poor sleep based on PSQI >5 | ||||
| WOMAC pain subscale (0–20) per 5 points change | 1.41 (1.22–1.63) | 1.21 (1.04–1.41) | 1.2 (1.03–1.41) | |
| Moderate to extreme pain in 1 or more activity | 517 | 1.52 (1.23–1.88) | 1.24 (0.99–1.55) | 1.22 (0.97–1.54) |
| Moderate to extreme hip pain while | ||||
| walking on a flat surface | 279 | 1.51 (1.15–1.98) | 1.15 (0.86–1.54) | 1.11 (0.83–1.5) |
| standing upright | 282 | 1.49 (1.13–1.96) | 1.2 (0.89–1.6) | 1.17 (0.87–1.57) |
| going up or down stairs | 309 | 1.5 (1.15–1.95) | 1.17 (0.89–1.55) | 1.18 (0.89–1.56) |
| in bed at night | 257 | 1.95 (1.42–2.67) | 1.51 (1.08–2.11) | 1.49 (1.06–2.09) |
| when sitting or lying | 216 | 1.86 (1.39–2.48) | 1.47 (1.08–2) | 1.46 (1.07–2) |
| * Referent :No or mild hip pain | 1708 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Trouble sleeping due to pain 3 or more times a week | ||||
| WOMAC pain subscale (0–20) per 5 points change | 1.64 (1.35–2) | 1.57 (1.29–1.93) | 1.57 (1.28–1.93) | |
| Moderate to extreme pain in 1 or more activity | 318 | 1.95 (1.41–2.69) | 1.87 (1.35–2.6) | 1.9 (1.36–2.65) |
| Moderate to extreme hip pain while | ||||
| walking on a flat surface | 175 | 2.24 (1.52–3.3) | 2.01 (1.35–2.99) | 2.02 (1.35–3.03) |
| standing upright | 182 | 2.43 (1.65–3.56) | 2.21 (1.49–3.26) | 2.22 (1.49–3.31) |
| going up or down stairs | 201 | 2.25 (1.56–3.26) | 2.1 (1.44–3.07) | 2.07 (1.41–3.04) |
| in bed at night | 155 | 3.04 (2.03–4.55) | 2.87 (1.9–4.32) | 2.81 (1.86–4.25) |
| when sitting or lying | 194 | 2.25 (1.55–3.27) | 2.13 (1.45–3.11) | 2.14 (1.46–3.14) |
| * Referent :No or mild hip pain | 1708 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
Base model adjusted for: age, education years, BMI, back pain and recent worsening of arthritis.
MV models adjusted for age, education years, BMI, back pain and recent worsening of arthritis, depression, fatigue, health status, anxiety, number of blocks walked for exercise and current use of benzodiazepines, opioids, or NSAIDs medications
Number of women with hip pain and sleep disturbance
referent group with no hip pain for all activities in the WOMAC scale
Objective sleep measures and WOMAC hip pain
In univariate analyses, women with moderate to extreme hip pain for ≥ 1 activity had worse objectively measured sleep fragmentation compared to those with no hip pain. 33% had ≥90 minutes of WASO (p=0.0012) and 32% had ≥8 LWEP lasting ≥5 minutes (p=0.0029, Table 3). There were no significant univariate associations between hip pain and napping for ≥ 2 hours, having < 70% sleep efficiency, TST ≤5 hours and sleep latency ≥1hr. The absolute mean differences in continuous sleep parameter were small although statistically significant for WASO and LWEP (Table 1).
In the base models, women had higher odds of spending ≥1.5 hours WASO (1.28, 1.10–1.50) for every 5 units increase in the WOMAC hip pain score after adjustment of all covariates. The strongest predictor of sleep disturbance was moderate to extreme hip pain while sitting or lying (base model; 2.00, 1.47–2.73) for WASO ≥90 minutes and 1.99(1.46, 2.71) for ≥8 wake episodes. In addition, hip pain in bed at night, while standing and walking were also significantly associated with sleep disturbances in the base models. These associations were attenuated by further adjustments but remained statistically significant in the final multivariate models (Table 4). There was no significant association between the WOMAC hip pain score and daytime napping, TST, sleep latency, and sleep efficiency in base models and in multivariate models.
We assessed the association of the WOMAC hip pain items with sleep fragmentation as measured by WASO, independent of hip pain while in bed at night. Hip pain while walking (1.28, 0.94–1.74) or while going up or down stairs (1.10, 0.81–1.49) was not associated with sleep disturbance independent of hip pain at night while in bed (Table 5). However, hip pain while standing was associated with ≥ 90 minutes WASO (1.41, 1.07–2.01) independent of hip pain while in bed at night (1.37, 0.98–1.90). Models with hip pain while in bed and while sitting were not performed due to the strong correlation between the two hip pain items (correlation coefficient=0.76).
Table 5.
Odds Ratio and 95% CI of Multivariate models for each WOMAC hip pain adjusted for moderate to extreme hip pain while in bed for wake minutes >=90 after sleep onset
| † Moderate to extreme WOMAC pain while | * Multivariate Model + pain while in Bed | ** Pain while in Bed |
|---|---|---|
| Walking | 1.28 (0.94–1.74) | 1.45 (1.06–2.00) |
| Standing upright | 1.41 (1.07–2.01) | 1.37 (0.98–1.90) |
| Going down stairs | 1.10 (0.81–1.49) | 1.54 (1.12–2.14) |
Models for pain while sitting or lying were not done due to high correlation with pain while in bed.
OR for each WOMAC activity adjusted for covariates and pain while in bed
OR for moderate to extreme pain in bed, when adjusted for covariates and each WOMAC hip pain activity.
Covariates: age, education years, BMI, back pain and recent worsening of arthritis, depression, fatigue, health status, anxiety, number of blocks walked for exercise and current use of benzodiazepines, opioids, or NSAIDs medications
Wake minutes after sleep onset at 2 hour intervals
Wake minutes after sleep onset increased steadily with hours asleep in both women with and without hip pain as defined by the WOMAC pain scale. During the first 2 hours of sleep, there was no difference in total wake minutes between women with and without hip pain. However, as sleep time progressed, the rate of change in wake minutes per hour asleep was higher in women with severe/extreme hip pain compared to women with mild/no hip pain (1.40 ± 0.47 more minutes per hour sleep, interaction p=<0.003) (Figure 1). Wake times after sleep onset per hour asleep in women with moderate hip pain was significantly different from those with mild/no hip pain (p=0.02), though the change in wake time per hour sleep was small (0.77 ± 0.34 per hour asleep). There were no significant interactions of antidepressant, NSAIDs or opioids medication use with WOMAC hip pain categories (data not shown).
Figure 1.
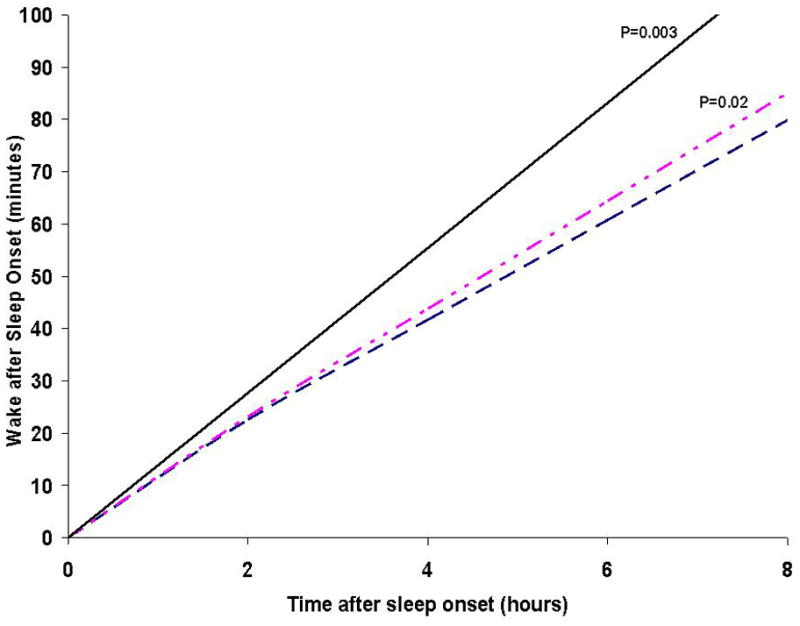
Rate of change in Wake Minutes for every 2 hour intervals after sleep onset in women with severe to extreme, moderate and mild or no WOMAC hip pain
_______ Severe to Extreme WOMAC hip pain on >=1 activity
__ _ _ __ Moderate WOMAC hip pain any activity
__ __ __ Mild or No WOMAC hip pain on all activities
Adjusted for age, education years, BMI, back pain and recent worsening of arthritis, depression, fatigue, health status, anxiety, number of blocks walked for exercise and current use of benzodiazepines, opioids, or NSAIDs medications.
P values are for interaction of Moderate WOMAC pain and Severe to Extreme hip pain with time after sleep onset compared to those with no WOMAC hip pain
Discussion
This cross–sectional analysis of 2225 elderly Caucasian women suggests an association between the presence of hip pain and objectively measured sleep fragmentation. This association was primarily driven by resting hip pain for both subjective and objective sleep measures. This was further supported by a strong correlation between moderate to extreme hip pain at night in bed and trouble sleeping due to pain ≥3 times a week. Differences in sleep fragmentation among those with severe to extreme pain and without hip pain were more pronounced as the night progressed. There were no associations observed between hip pain and sleep duration, sleep latency, napping or sleep efficiency.
To our knowledge this is the first study to examine the association of objectively and subjectively measured sleep disturbance with the WOMAC hip pain subscore for performing activities involving the lower extremity joints. Women with a higher WOMAC pain score had greater risk of spending ≥ 1.5 hours WASO and a greater number of wake episodes lasting ≥ 5 minutes. These results are similar to other studies that have shown a higher prevalence of trouble staying asleep as one of the most common sleep disturbances among the elderly with symptomatic OA [5, 7]. Poor sleep measured by PSQI was not associated with the overall WOMAC hip pain score in multivariate models, however resting hip pain items was significantly associated with poor sleep.
Disturbed sleep was most prevalent in those with hip pain at rest suggesting that the pain while lying or at night in bed, is most disruptive to sleep. This association was independent of hip pain from other activities such as walking or climbing stairs. Pain while sitting/lying or in bed at night may be indicative of pain due to inflammation of the joints, commonly caused by trochanteric bursitis [31] or caused by osteoarthritis (OA) where prolonged periods of inactivity can worsen inflammation at night in bed when pressure is placed on the hip joint and subsequently increase the pain intensity [32–34].
The correlation between pain while standing and resting hip pain was low. We showed that standing pain was significantly associated with increased WASO minutes, independent of pain while in bed at night. This association was not seen with pain while walking or climbing stairs and given that the correlation between standing pain and pain while walking or climbing stairs was moderate, this suggests that standing hip pain may be measuring a different type of pain that warrants further investigation. Pain while in bed at night and sitting pain were strongly correlated and hence it was difficult to determine which rest item attributed to sleep disturbance. It is likely that both pain items are describing the same type of pain in this cohort.
It is also well documented that depression, fatigue and poor health are strong correlates with poor sleep [5, 35]. Furthermore, the combined effects of depression and pain on sleep may be greater in patients with OA as studies have shown that depression increases the pain perception on these patients [36, 37]. Although these covariates attenuated the association, we found that disturbed sleep was significantly associated with increased hip pain independent of the effects of fatigue, poor health and depression suggesting that other mechanisms by which hip pain affects sleep are plausible.
Our study is unique in that, after the first two hours after sleep onset, disruptions to sleep were compounded by each additional hour of sleep, especially in those with severe to extreme hip pain compared to those without hip pain, although the differences were not clinically relevant. More importantly, we found that the wake minutes in the first two hours of sleep did not differ between those with and without hip pain. One possible explanation for this is that pain medications analgesic effects wear off during the night due to the time lapse between administering pain medications and bed time. As a result the pain medication may be more potent in the early night and wear out as the night progresses. Another plausible explanation is the longer one is at rest, the worse pain becomes and thus disrupts sleep. This is further supported by the finding that there was no difference among those with and without hip pain for sleep latency, suggesting that in the beginning of the night, the sleep experience is similar, but worsens as the night progresses among those with severe pain compared to those with mild or no hip pain.
There are several strengths in our study. The large population of older women was mostly community-dwelling, and was not selected for inclusion on the presence of hip pain, OA, or sleep problems. Sleep outcomes were gathered both objectively and subjectively and we used a validated pain measure for OA related hip pain. Adjustments for multiple potential confounding factors were made, suggesting these associations were not explained by other covariates including depression, fatigue, anxiety, medication use, education, or lifestyle.
There are also several limitations in our study that should be addressed. We were unable to determine if the women with hip pain had RHOA. However, the WOMAC pain scale for OA related pain in the knee and hip [19] has gained growing acceptance since its introduction in 1986 and has demonstrated much stronger associations with indicators of hip disease, such as restriction in movement. It is possible that the hip pain experienced may in part be due to trochanteric bursitis which may affect sleep quality, however we were unable to determine this as it was not assessed. The comparability of subjective and objective sleep measures was not investigated as it was not the scope of this analysis, however we showed that only 31% with poor sleep measured by PSQI had ≥90 minutes of WASO, suggesting that subjective sleep measures and actigraphy measures may be measuring different aspects of sleep.
Also all participants were elderly Caucasian women and given that there are a large number of factors that may influence pain and sleep quality in men and individuals from different ethnicities, more studies employing a more diverse sample are warranted.
More recently a complex bidirectional association of chronic pain with poor sleep [2, 38] has been suggested. However the cross-sectional design of this study prevented us from determining the direction of the relationship between pain and sleep.
In conclusion, hip pain while performing lower extremity activities is related to fragmented sleep. Sleep disturbances tend to occur later in the night suggesting that further investigation, such as pain medications wearing off over time, or prolonged periods of inactivity decreasing the pain threshold are warranted. In addition, given the complex relationship between chronic pain and sleep disturbance, appropriate interventions should be utilized in patients suffering from hip pain while performing daily activities and poor sleep.
Supplementary Material
Significance and Innovation.
This manuscript is of importance since it explores the relationship between disturbed sleep and pain in the lower extremities often experienced by patients with OA. Given that poor sleep and OA is prevalent among the elderly, it is important to study the effects of pain in the elderly to determine appropriate treatment options for patients with chronic pain and poor sleep. One unique aspect of this analysis was that we were able to look at two hour intervals after sleep onset and determine the differences in women with and without pain with increased wake minutes due to disturbed sleep. This is a novel approach that to our knowledge has not been done. We were able to show that the first two hours of sleep is unchanged in those with and without pain, and disturbed sleep increased incrementally with each additional hour after the first two hours after sleep onset.
Acknowledgments
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720, K24-AR04884
References
- 1.Keefe FJ, et al. Osteoarthritic knee pain: a behavioral analysis. Pain. 1987;28(3):309–21. doi: 10.1016/0304-3959(87)90066-2. [DOI] [PubMed] [Google Scholar]
- 2.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154(10):1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2003;(1):CD003161. doi: 10.1002/14651858.CD003161. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox S, et al. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48(10):1241–51. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 6.Leigh TJ, et al. Comparison of sleep in osteoarthritic patients and age and sex matched healthy controls. Ann Rheum Dis. 1988;47(1):40–2. doi: 10.1136/ard.47.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KD, et al. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35(6):1102–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai PF, Means KM. Osteoarthritic knee or hip pain: possible indicators in elderly adults with cognitive impairment. J Gerontol Nurs. 2005;31(8):39–45. doi: 10.3928/0098-9134-20050801-13. [DOI] [PubMed] [Google Scholar]
- 9.Perlis ML, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Gehrman P, et al. Towards an understanding of self-reports of sleep. J Sleep Res. 2002;11(3):229–36. doi: 10.1046/j.1365-2869.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 12.Action-W User’s Guide. Ambulatory Monitoring, Inc; Ardsley NY: [Google Scholar]
- 13.Morgenthaler T, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 14.Motionlogger User’s Guide: Act Millenium. Ambulatory Monitoring, Inc; [Google Scholar]
- 15.Blackwell T, et al. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28(12):1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 16.Jean-Louis G, et al. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105(2):185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell T, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31(2):283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim SA, et al. Older patients’ perceptions of quality of chronic knee or hip pain: differences by ethnicity and relationship to clinical variables. J Gerontol A Biol Sci Med Sci. 2003;58(5):M472–7. doi: 10.1093/gerona/58.5.m472. [DOI] [PubMed] [Google Scholar]
- 20.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1(21):1–115. [PubMed] [Google Scholar]
- 21.Pincus T, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh JI, Yesavage JA. A knowledge assessment test for geriatric psychiatry. Hosp Community Psychiatry. 1985;36(11):1160–6. doi: 10.1176/ps.36.11.1160. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg D, et al. Detecting anxiety and depression in general medical settings. BMJ. 1988;297(6653):897–9. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahor M, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 25.Tranah GJ, et al. Postmenopausal hormones and sleep quality in the elderly: a population based study. BMC Womens Health. 2010;10:15. doi: 10.1186/1472-6874-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe K, et al. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69(3):237–42. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell T, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 28.Ensrud KE, et al. Use of selective serotonin reuptake inhibitors and sleep disturbances in community-dwelling older women. J Am Geriatr Soc. 2006;54(10):1508–15. doi: 10.1111/j.1532-5415.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 29.Mehra R, et al. Interpreting wrist actigraphic indices of sleep in epidemiologic studies of the elderly: the Study of Osteoporotic Fractures. Sleep. 2008;31(11):1569–76. doi: 10.1093/sleep/31.11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spira AP, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136–43. doi: 10.1097/JGP.0b013e3181871345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal NA, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88(8):988–92. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor M, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 33.Poole AR, HDMR, Howell DS, Altman RD, Buckwalter JA, Goldberg VM, Saunders W. Osteoarthritis. 3. 2001. Etiopathogenesis of osteoarthritis; p. 40. [Google Scholar]
- 34.Moldofsky H, Lue FA, Saskin P. Sleep and morning pain in primary osteoarthritis. J Rheumatol. 1987;14(1):124–8. [PubMed] [Google Scholar]
- 35.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158(10):1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 36.Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J Rheumatol. 1999;26(8):1785–92. [PubMed] [Google Scholar]
- 37.Tsai PF, et al. Testing a theory of chronic pain. J Adv Nurs. 2003;43(2):158–69. doi: 10.1046/j.1365-2648.2003.02690.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith MT, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


