Abstract
Objectives
Inhibition of adenosine deaminase, with EHNA (erythro-9 (2-hydroxy-3-nonyl)-adenine), and the es-ENT1 transporter, with NBMPR (p-nitro- benzylthioinosine), entraps myocardial intracellular adenosine during on pump warm cross clamping (ACC) leading to a complete recovery of cardiac function and ATP during reperfusion. The differential role of entrapped intracellular and circulating adenosine in EHNA/NBMPR-mediated protection is unknown. Selective, DPCPX [8-cyclopentyl-1,3-dipropyl-xanthine], or non-selective, [8-(p-sulfophenyltheophyline], A1-receptor antagonists were used to block adenosine A1-receptor contribution in EHNA/NBMPR-mediated cardiac recovery.
Methods
Anesthetized dogs (n= 45), instrumented to measure heart performance using sonomicrometry, were subjected to 30 minutes of warm ACC and 60 minutes of reperfusion. Three boluses of the vehicle (Series A) or 100μM EHNA and 25μM NBMPR (Series B) were infused into the pump at baseline, before ischemia and before reperfusion. DPCPX (10 μM) or 8-SPT (100μM) was intra-aortically infused immediately after ACC distal to the clamp in Series (A) and Series (B). ATP pool and NAD+ was determined using HPLC.
Results
Ischemia depleted ATP in all groups by 50%. The ratios between adenosine to inosine were >10 fold higher in Series (B) than in Series (A) (p<0.001). ATP and function recovered in the EHNA/NBMPR-treated group (p<0.05 vs. control group). DPCPX or 8-SPT partially reduced cardiac function in Series (A) and (B) to the same degree but did not abolish EHNA/NBMPR-mediated protection in Series (B).
Conclusion
In addition to cardioprotection mediated by activation of adenosine receptors by extracellular adenosine, EHNA/NBMPR-entrapment of intracellular adenosine provides a significant component of myocardial protection despite of A1-R blockade.
Keywords: Cardiac surgery, Cardiopulmonary bypass, Ischemia and reperfusion, Myocardial protection, Nucleosides and Nucleotides, DPCPX, EHNA, NBMPR, 8-SPT
INTRODUCTION
In addition to pre-existing ischemic injury, reperfusion injury is implicated in poor cardiac recovery despite of excellent cardiac repair (1,2). Therefore, targeting post-ischemic reperfusion injury (3) is critical to improve intra- and post-operative out come. Adenosine has emerged as a promising adjuvant agent to augment cardioprotection in experimental models (4–6) and clinical trials (7–10) against ischemic and reperfusion injury. Blockade of A1-receptor abolishes cardioprotection mediated by ischemic preconditioning (4) while treatment with exogenous adenosine potentiates ischemic preconditioning effects (6). Unlike exogenous adenosine, endogenous adenosine generated during ischemia must be transported via the NBMPR-sensitive-es-ENT1 transporter 1 (es-ENT1) to extracellular domain (E-Fig-1A) in order to activate adenosine receptor subtypes (A1, A2A and B and A3) mediated signaling mechanisms of cardioprotection. Because of short half life of adenosine in human and animals, inhibition of adenosine deaminase by EHNA prolongs its half live and potentiates its pharmacologic efficacy. In addition, selective blockade of es-ENT1 transporter with NBMPR entraps myocardial adenosine and inosine at the site of production during ischemia and prevents its loss during reperfusion. Pre-ischemic treatment with EHNA/NBMPR entraps intracellular adenosine and results in a complete recovery of ATP and cardiac function (11–13) suggesting that the entrapped adenosine is intracellularly compartmentalized (E-Fig-1B). The specific binding sites of [3H]-NBMPR were identified in human and animals myocardial preparations (14). The relative contribution of entrapped intracellular adenosine and circulating extracellular adenosine in EHNA/NBMPR-mediated protection in relation to adenosine A1-receptor activation (E-Fig-1C) is not known. The present study was designed to determine the role of adenosine A1 receptor, in EHNA/NBMPR mediated post-ischemic recovery of function in a canine model of on pump warm aortic cross clamping and reperfusion. Selective blocking adenosine A1 receptor with DPCPX or non-selective blocking of subtypes of adenosine receptors with 8-SPT partially reduces but does not abolish post-ischemic recovery mediated by EHNA/NBMPR suggesting intracellular mechanisms of protection.
MATERIALS AND METHODS
Materials
Biochemical reagents, EHNA and NBMPR were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). DPCPX and 8 –SPT were purchased from Research Biochemical, Inc. (Natick, MA).
Animal Model
The following studies conform to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institute of Health (Publication No: 5377-3 (http://www.nap.edu/catalog/5140.html
Forty-five microfilaria-free adult mongrel dogs, of either sex, weighing 17–25 Kg were used in this study. Dogs were initially anesthetized with sodium pentobarbital, 35 mg/kg, i.v. (Nembutal, Abbott Laboratories, Chicago, Ill.). The surgical procedures and instrumentations were performed exactly as previously described (11). Cardiopulmonary bypass was established by subclavian artery and atrial venous cannulation. A membrane oxygenator (Medtronic, Minneapolis, MN) was used and primed with non-cross-matched homologous blood. Mean arterial reperfusion pressure was maintained at 60–65 mmHg while on bypass. Arterial blood gases, pH and hematocrit were routinely determined and maintained at the following levels: pO2=100–140 torr, pCO2=30–40 torr, pH=7.32–7.48, and hematocrit at about 30%.
Assessment of Left Ventricular Performance
The left ventricular performance was assessed, off bypass pump, from the relationship between stroke work and end-diastolic dimension using a sensitive and load-independent index of contractility (11) using pulse transit sonomicrometry (Triton Technology, San Diego, CA), and Millar pressure transducers. One pair of LTZ-piezoelectric hemispheric crystal was sutured to the anterior and posterior of the epicardial surface of the left ventricle wall in the minor axis (40–60mm). Analog data were digitized at 200 Hz and stored a personal computer hard disc and analyzed by interactive Crunch software developed in our laboratory. Data acquisition was performed at varying preloads via venous drainage creating diminishing work loops. The slope was obtained from the relationship between LV pressures (a surrogate of volume) against the end-diastolic lengths and is considered as a sensitive load-independent index of contractility.
Assessment of Adenine Nucleotide Pool Metabolism
Transmural Serial Tru-Cut needle biopsy specimens (5–10 mg) were obtained and immediately frozen in liquid nitrogen (−196°C), extracted, analyzed and quantified using HPLC and presented as nmoles/mg protein (11).
Experimental Protocol
After 30 minutes of stabilization period on-pump, dogs were assigned to two Series A and B. Three boluses (500 ml each) of the vehicle solution (saline containing 0.05% dimethylsulfoxide, DMSO) without (Series A) or with 25 μM NBMPR and 100 μM EHNA (Series B), were infused into the pump oxygenator prior to ischemia, before ACC just prior to releasing the ACC. Animals in Series A received a single intra-aortic infusion of 50 ml of saline (Control group, n=10), 100μM 8-SPT group (n=6) or 10μM DPCPX (n=7) distal to the clamp immediately following ACC. In Series B, dogs were treated with a single intra-aortic infusion of 50 ml of vehicle (EHNA/NBMPR-group, n=8), 100 μM 8-SPT (n= 7) or 10 μM DPCPX (n=7).
Statistical Analysis
Data are presented as mean ± SEM. Sequential measurements were compared using repeated measures analysis of variance (ANOVA) and Tukey’s post hoc tests using SAS (Statistical Analysis System Institute, Cary, NC). Differences were considered significant if the probability value for comparison of least squares means was less than 0.05 and the F ratios are presented in the figure legends.
RESULTS
Results of the control group and EHNA/NBMPR-treated group, have been previously published elsewhere (9,10) and are included in this study for comparison. This model represents a scenario of reversible global myocardial stunning without necrosis and all animals survived the length of the experiment in all groups.
Myocardial ATP
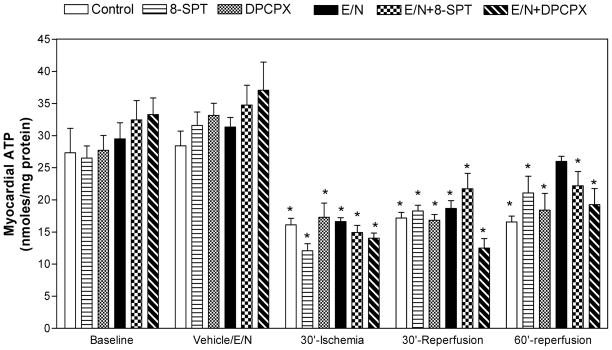
On-pump normothermic aortic cross-clamping for 30 minutes reduced myocardial ATP levels by about 50% in all groups (Fig 1). No recovery of ATP was observed in Series A groups during reperfusion. In Series B, ATP recovered at the end of reperfusion in the EHNA/NBMPR-group (25.98±0.8 nmol/mg protein, p=NS vs. baseline 28.41±2.3 nmol/mg protein). ATP levels increased in SPT–treated group in the presence of EHN/NBMPR to 22.2±2.2 nmol/mg protein. ATP remained depressed in the DPCPX groups in both Series A and B, 18.4±2.6 and 17.30±2.2 nmol/mg protein, respectively (p<0.05 vs. baseline).
Figure 1. Effect of 8-SPT and DPCPX on Myocardial ATP Levels.
ATP was determined in myocardial biopsies before and after saline (control) or drug administration and at the end of ischemia and during reperfusion. No significant differences between groups with respects to the time of sampling (ANOVA, F=0.04). * Represents significance <0.05 vs. baseline.
Myocardial Adenine Nucleosides
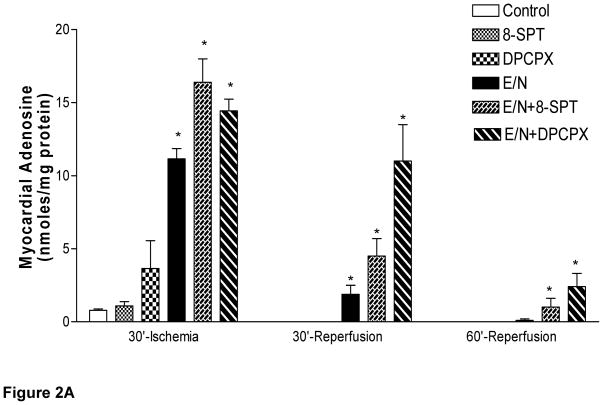
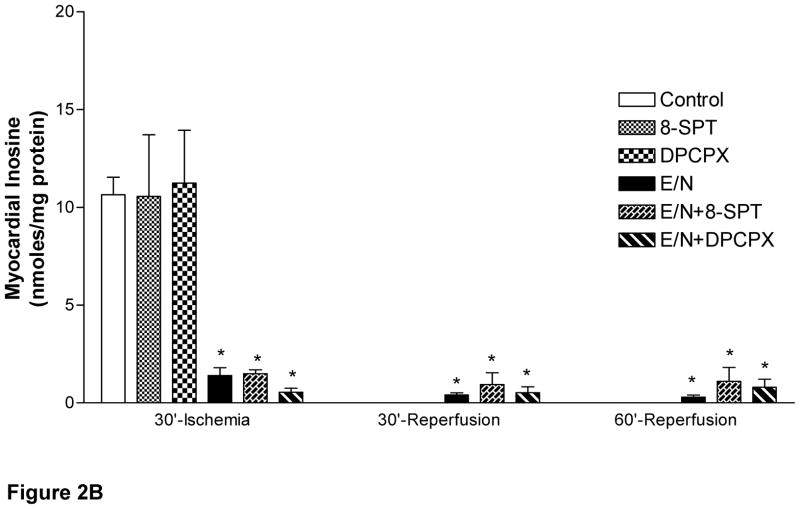
Both adenosine and inosine were not detected before or after EHNA/NBMPR treatment on pump. A small increase in myocardial adenosine occurred at the end of ischemia in the control group (0.8±0.1 nmol/mg protein). Inosine was the major (>90%) nucleoside present in the control, SPT, and DPCPX-treated groups of Series A at the end of ischemia (Fig 2B). Both adenosine and inosine generated during ischemia disappeared from the myocardium during reperfusion in all groups of Series A (Fig 2A). Myocardial adenosine increased in EHNA/ NBMPR-groups (Series B) during ischemia as follows: 11.15±0.7, 16.39±1.6 and 14.44±0.9 nmol/mg protein in the vehicle, SPT or DPCPX, respectively (p<0.05 vs. baseline and Series A groups). Inosine levels in Series (B) groups were also elevated; 1.40± 0.4, 1.49± 0.2, 0.55± 0.2 nmol/mg protein at the end of the ischemic period in the presence of vehicle, SPT or DPCPX, respectively (p<0.05 vs. baseline and Series A). During reperfusion, the ischemic myocardium lost all its adenosine and inosine in Series A groups but retained them in Series B groups (p>0.05 vs. baseline and Series A groups).
Figure 2. Effect of SPT and DPCPX on Myocardial Adenine Nucleosides Levels.
Adenine nucleosides, adenosine (A) and inosine (B) were determined in myocardial biopsies before and after saline (control) or drug administration and at the end of ischemia and during reperfusion. Statistically significant differences were found between groups with respect to the time of sampling (p<0.001, ANOVA, F=11.8 and 8.8). * represents significance <0.05 vs. baseline
Left Ventricular Performance
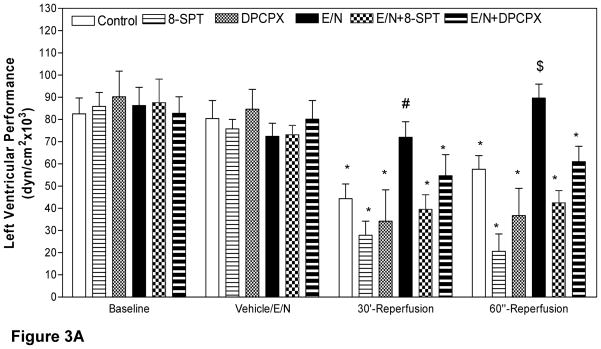
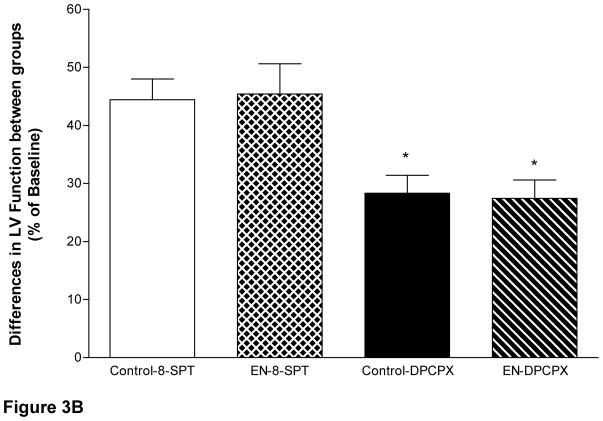
Administration of the vehicle solution or EHNA/NBMPR did not affect left ventricular contractility before ischemia (Fig 3A). In Series A, LV function in the control group recovered to only 55% and 71.6% of preischemic function (44.27±6.7 dyn/cm2 × 103 and 57.58±6.1 dyn/cm2 × 103) after 30 and 60 minutes of reperfusion, respectively (p<0.05 vs. baseline = 80.42±8.1 dyn/cm2 × 103). SPT significantly reduced cardiac function in Series A, below the control level by 36.7% and 27.2% at 30 and 60 minutes of reperfusion, respectively (p<0.05 vs. preischemia, 75.73± 4.3 dyn/cm2 × 103). In the presence of DPCPX, LV function also declined at 30 and 60 minutes of reperfusion to 40.37% and 43.21%, respectively, (p<0.05 vs. preischemia 84.65±8.9 dyn/cm2 × 103). In EHNA/NBMPR-group the left ventricular function returned to 99.42% and 119.34% of the pre ischemic values after 30 and 60 minutes of reperfusion respectively, (p=NS vs. preischemia 72.4±5.9 dyn/cm2 × 103). Intra-aortic infusion of the non-selective antagonist SPT in the presence of EHNA/NBMPR (Series B) resulted in a significant decline in post-ischemic recovery to 54.03% and 57.99% of preischemic function (39.5±6.6 and 42.4±6 dyn/cm2 ×103) after 30 and 60 minutes reperfusion, respectively (p<0.05 vs. baseline 73.1±4.2 dyn/cm2 × 103,). DPCPX administration in EHNA/NBMPR-treated groups resulted in a modest decline in LV functional recovery following global ischemia to 68.23% and 76.06% of preischemic function (54.7±9 and 60.9±7 dyn/cm2 × 103) at 30 and 60 minutes of reperfusion, respectively, (p<0.05 vs. baseline values of 80.1±8 dyn/cm2 × 103). When the differences between percents recovery for each corresponding antagonists were plotted for each antagonist in the presence or absence of EHNA/NBMPR (Fig 3B), it clear that the reduction of function was identical in Series A and B with respect to each antagonist.
Figure 3. Effect of SPT and DPCPX on left Ventricular Performance.
The slope of the relationship between the stroke-work/end diastolic dimensions of the left ventricle at each time, except during ischemic arrest, is plotted in Figure 3A. There are statistically significant differences between groups with respect to the time of sampling (p<0.05, ANOVA, F=4.18). * Represents significance <0.05 vs. baseline. Figure 3B demonstrates the effect of SPT and DPCPX on the differences between recoveries of left Ventricular Performance. The differences between the percent of recovery are identical in both Series A and B suggesting that the contribution of extracellular adenosine is identical.
DISCUSSION
Results from previous studies (11,12), also included here, have demonstrated that selective modulation of adenosine deamination with EHNA and transport with NBMPR during ischemia entraps intracellular adenosine and improves post-ischemic ATP and functional recovery during reperfusion. Because myocardial adenosine was elevated during ischemia more than 10 folds higher in the EHNA/NBMPR treated groups compared to controls, it is has been postulated that adenosine receptors subtypes (A1, A2A, A2B, A3) are involved in EHNA/NBMPR-mediated post-ischemic recovery. Indeed, endogenous adenosine-triggered activation of adenosine receptors is involved in ischemic preconditioning and that DPCPX or 8-SPT abolishes infarct size limitation mediated by ischemic preconditioning (4–6).
In the present study, pre-ischemic treatment with EHNA/NBMPR elevated myocardial adenosine and improved functional and ATP recovery during reperfusion in the absence or presence of adenosine A1-receptor blockade. These results suggest that only a part of the recovery is mediated by extracellular adenosine and activation of membrane surface adenosine receptors. The remaining component of recovery could be mediated by intracellular adenosine and possibly involved intracellular signaling mechanisms of protection. Grube et al have recently shown evidence for an intracellular localization of A2B adenosine receptor in rat cardiomyocytes (15).
It has been long assumed that endogenous adenosine, produced during the onset of ischemia is solely derived from cardiac ATP depletion. This may be true in isolated perfused hearts or cardiomyocytes preparations. Myocardial ischemia triggers sympathetic stimulation and a surge of co-released neurotransmitters and ATP (16–18). Like cardiac adenosine, non-cardiac ATP is rapidly broken down in circulation to adenosine, inosine, hypoxanthine, and xanthine providing substrates for free radicals’ production catalyzed by xanthine oxidase (E-Fig 1). It is possible that extracellular adenosine is activating both the DPCPX-sensitive A1 receptor and 8-SPT-sensitive A1, A2A, A2B and A3 subtypes. Administration of DPCPX or 8-SPT abolished the contribution of only surface adenosine A1 receptor and other subtypes in the recovery of function without affecting intracellular adenosine mediated protection. It has been shown that adenosine receptors cross-talk and work together in concert to induce cardioprotection (19). Indeed, activation of A2A, A2B and A3 is required for A1 receptor activation (20,21). Therefore, we postulate that DPCPX or 8-SPT sensitive contribution in EHNA/NBMPR mediated recovery is taking place regardless of entrapment intracellular adenosine. The remaining component of functional recovery could be related to unique, yet unknown, intracellular mechanisms of protection.
Adenosine levels were greater in the presence of DPCPX or SPT in EHNA/NBMPR-treated groups than EHNA/NBMPR alone (Fig 2A). This could be explained, in part, by additive inhibitory effects between on EHNA/NBMPR and SPT or DPCPX on adenosine deamination and transport. Despite of a greater accumulation of myocardial adenosine in SPT and DPCPX groups of Series (B), a modest yet significant recovery cardiac function and ATP repletion were observed. Again, post-ischemic repletion of myocardial ATP confirms that entrapped adenosine is compartmentalized in ischemic cells during ischemia and reperfusion.
The mechanisms of EHNA/NBMPR-mediated augmentation of post-ischemic recovery could be related to: a) prolonging the half life of intracellular and extracellular adenosine; b) salvaging intracellular adenosine for ATP, c) interruption of the formation and the release of inosine as a precursor for purines-free radical substrates hypoxanthine and xanthine and (11) and d) intracellular adenosine activates unknown intracellular signaling mechanisms mediated by intracellular adenosine receptor subtypes. Xia et al (22) have reported that inhibition of adenosine deaminase with EHNA (without NBMPR) abolished the formation of free radical species after global ischemia. In isolated rabbit heart model, EHNA/NBMPR abolished ischemic preconditioning effects on infarct size (23). Infusion of hypoxanthine and xanthine, in animals pretreated with EHNA/NBMPR, diminished LV function recovery to the control group despite of entrapment of intracellular adenosine (11). The latter observations implicated the xanthine-oxidase mediated oxygen free radicals generation in post-ischemic vascular injury “stunning,” (24). A cohort study on myocardial protection with specific blocker of the es-ENT1 transport demonstrated that administration of EHNA/NBMPR after 20 minutes or warm aortic cross clamping but prior to 100 minutes of hypothermic intermittent retrograde coronary sinus cardioplegia abolished myocardial stunning regardless of cardioplegic arrest and reperfusion (unpublished results). Our results provide evidence that, besides adenosine/adenosine receptor protective mechanisms, entrapment of adenosine and inosine provides alternative (rather additive, if combined) cardioprotection that may contribute to functional recovery. Our findings may be considered in future clinical trials of adenosine and adenosine modulating agents to reduce the doses and augments pharmacologic efficacy. Our current results supported the concept that nucleoside entrapping is effective in attenuating post-ischemic reperfusion injury in a severe scenario of warm global ischemia. It is anticipated that this intervention would be more efficacious under intraoperative procedures of myocardial protection. This modality has important clinical potentials to improve clinical out come of off - or on-pump cardiac surgery especially in sicker and elder patients with low metabolic and contractile reserves.
Limitations
The present study has obvious experimental model limitations that must be considered by the reader such as species, age and model related differences. The issue of species-dependent presence of xanthine oxidase remains debatable. However, it is believed that porcine heart has resemblance to human anatomy and coronary distribution. Finally the does of EHNA and NBMPR were used according to our previous reports (3,4) which are greater than the Ki values (0.05 nM). The dose response relationship was not intended in this canine model aortic cross clamping. The timing of administration of vehicle and EHNA/NBMPR was considered to target specific modulation of adenosine metabolism and compartmentalization during global warm ischemia and reperfusion.
CONCLUSIONS
In addition to adenosine A1 receptor, and other subtypes, mediated protective mechanisms by extracellular adenosine, selective entrapment of intracellular adenosine significantly improved post-ischemic functional and ATP recovery after severe warm global ischemia. Modulation of adenosine metabolism and compartmentalization could serve as an important new modality to improve intraoperative recovery of stunned hearts and attenuate acute reperfusion injury in the settings of thrombolytic therapy, PCI, CABG and resuscitation from hemorrhagic or cardiogenic shock.
Supplementary Material
Acknowledgments
Supported in part by the National Institute of Health Grant # HL 5-1090 (AS Abd-Elfattah)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangano DT, Miao Y, Tudor IC, Dietzel C Investigators of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group; Ischemia Research and Education Foundation (IREF). . Post-reperfusion myocardial infarction: long-term survival improvement using adenosine regulation with acadesine. J Am Coll Cardiol. 2006;48:206–14. doi: 10.1016/j.jacc.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Abd-Elfattah A, Higgins R, Latifi R, Merrell R. Targeting Post-Ischemic Reperfusion Injury: Scientific Dream and Clinical Reality. New Surgery. 2001;1:41–51. [Google Scholar]
- 4.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1adenosine receptors in rabbit heart. Circulation. 1991;84:350–6. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 5.Schultz R, Rose J, Post H, Heusch G. Involvement of endogenous adenosine in ischemic preconditioning in swine. Pflugers Arch. 1995;430:273–282. doi: 10.1007/BF00374659. [DOI] [PubMed] [Google Scholar]
- 6.McCully JD, Uematsu M, Parker RA, Levitsky S. Adenosine-enhanced ischemic preconditioning provides enhanced postischemic recovery and limitation of infarct size in the rabbit heart. J Thorac Cardiovasc Surg. 1998;116:154–62. doi: 10.1016/S0022-5223(98)70254-5. [DOI] [PubMed] [Google Scholar]
- 7.Mentzer RM, Jr, Rahko PS, Molina-Viamonte V, Canver CC, Chopra PS, Love RB, Cook TD, Hegge JO, Lasley RD. Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery. Am J Cardiol. 1997;79(12A):38–43. doi: 10.1016/s0002-9149(97)00262-2. [DOI] [PubMed] [Google Scholar]
- 8.Mentzer RM, Jr, Birjiniuk V, Khuri S, Lowe JE, Rahko PS, Weisel RD, Wellons HA, Barker ML, Lasley RD. Adenosine myocardial protection: preliminary results of a phase II clinical trial. Ann Surg. 1999;229:643–9. doi: 10.1097/00000658-199905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J. 2006;27:2400–5. doi: 10.1093/eurheartj/ehl094. [DOI] [PubMed] [Google Scholar]
- 10.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW AMISTAD-II Investigators. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45(11):1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Abd-Elfattah AS, Jessen ME, Lekven J, Doherty NE, 3rd, Brunsting LA, Wechsler AS. Myocardial reperfusion injury. Role of myocardial hypoxanthine and xanthine in free radical-mediated reperfusion injury. Circulation. 1988;78(5 Pt 2):III224–35. [PubMed] [Google Scholar]
- 12.Abd-Elfattah AS, Jessen ME, Lekven J, Wechsler AS. Differential cardioprotection with selective inhibitors of adenosine metabolism and transport: role of purine release in ischemic and reperfusion injury. Mol Cell Biochem. 1998;180:179–91. [PubMed] [Google Scholar]
- 13.Abd-Elfattah AS, Ding M, Wechsler AS. Intermittent aortic cross clamping prevents cumulative adenosine triphosphate depletion, ventricular fibrillation, and dysfunction (stunning): Is it preconditioning? J Thorac Cardiovasc Surg. 1995;110:328–39. doi: 10.1016/S0022-5223(95)70228-8. [DOI] [PubMed] [Google Scholar]
- 14.Abd-Elfattah AS, Hoehner J, Wechsler AS. Identification of nucleoside transport binding sites in the human myocardium. Mol Cell Biochem. 1998;180:105–10. [PubMed] [Google Scholar]
- 15.Grube K, Rüdebusch J, Xu Z, Böckenholt T, Methner C, Müller T, Cuello F, Zimmermann K, Yang X, Felix SB, Cohen MV, Downey JM, Krieg T. Evidence for an intracellular localization of the adenosine A2B receptor in rat cardiomyocytes. Basic Res Cardiol. 2011;106(3):385–96. doi: 10.1007/s00395-011-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson JG, Jr, Schrader J. Role of extracellular and intracellular adenosine in the attenuation of catecholamine evoked responses in guinea pig heart. J Molec Cell Cardiol. 1984;16:813–822. doi: 10.1016/s0022-2828(84)80005-x. [DOI] [PubMed] [Google Scholar]
- 17.Poelchen W, Sieler D, Wirkner K, Illes P. Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience. 2001;102(3):593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Purine cotransmission. Exper Physiol. 200;94:20–24. doi: 10.1113/expphysiol.2008.043620. [DOI] [PubMed] [Google Scholar]
- 19.Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47(5):684–90. doi: 10.1016/j.yjmcc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urmaliya VB, Pouton CW, Ledent C, Short JL, White PJ. Cooperative cardioprotection through adenosine A1 and A2A receptor agonism in ischemia-reperfused isolated mouse heart. J Cardiovasc Pharmacol. 2010;56(4):379–88. doi: 10.1097/FJC.0b013e3181f03d05. [DOI] [PubMed] [Google Scholar]
- 21.Zhan E, McIntosh VJ, Lasley RD. Adenosine A2A and A2B receptors are both required for adenosine A1 receptor-mediated cardioprotection. Am J Physiol Heart Circ Physiol. 2011 Jul 8; doi: 10.1152/ajpheart.00264.2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia Y, Khatchikian G, Zweier JL. Adenosine deaminase inhibition prevents free radical mediated injury in the postischemic heart. J Biol Chem. 1996;271:10096–102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- 23.Walsh RS, Abd-Elfattah AS, Daly JJF, Wechsler AS, Downey J. Selective blockade of nucleoside transport channels prevents preconditioning of rabbit myocardium: Surg Forum. 1993;XLIV:234–236. [Google Scholar]
- 24.Zughaib ME, Abd-Elfattah AS, Jeroudi MO, Sun JZ, Sekili S, Tang XL, Bolli R. Augmentation of endogenous adenosine attenuates myocardial ‘stunning’ independently of coronary flow or hemodynamic effects. Circulation. 1993;88(5 Pt 1):2359–69. doi: 10.1161/01.cir.88.5.2359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.