Abstract
Objective
To investigate insulin sensitivity and secretion indices and determinants of glycemic control in youth with recent-onset type 2 diabetes at randomization in the TODAY study, the largest study of youth with type 2 diabetes to date.
Research Design and Methods
We examined estimates of insulin sensitivity [1/fasting insulin (1/IF), fasting glucose/insulin (GF/IF), 1/fasting C-peptide (1/CF), GF/CF], β-cell function [insulinogenic index (ΔI30/ΔG30), and ΔC30/ΔG30], and disposition index (DI) in the TODAY cohort of 704 youth (14.0±2.0 yr; diabetes duration 7.8±5.8 mo; 64.9% female; 41.1% Hispanic, 31.5% Black, 19.6% White, 6.1% American Indian, and 1.7% Asian) according to HbA1c quartiles at study randomization. The randomization visit followed a run-in period (median 71 days) during which glycemic control (HbA1c≤ 8% for at least 2 months) was achieved with metformin alone. These measures were also examined in relation to screening HbA1c levels prior to run-in.
Results
Insulin secretion indices declined with increasing HbA1c quartiles, at randomization and screening, (at randomization: ΔC30/ΔG30: 0.11±0.09, 0.10±0.19, 0.07±0.06, and 0.03±0.03 ng/ml per mg/dl, p<0.0001; DI: 0.03±0.03, 0.03±0.05, 0.02±0.02, and 0.01±0.01 mg/dl−1, p<0.0001) with no significant difference in insulin sensitivity. There were no significant differences in estimates of insulin sensitivity or secretion between genders or across the different racial groups. At randomization and screening, HbA1C correlated with DI (r=−0.3, p<0.001), with ΔC30/ΔG30, but not with insulin sensitivity estimates.
Conclusions
In youth with recent-onset type 2 diabetes treated with metformin, glycemic control, as measured by HbA1c, appears to be associated with residual β-cell function, and not insulin sensitivity.
Keywords: Insulin secretion, Insulin sensitivity, Disposition Index, Glycemic control, youth type 2 diabetes
Introduction
The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study is the first large multi-center trial examining treatment for youth with type 2 diabetes (1). Between May 2004 and August 2008, 1,211 young people between 10–17 years of age with type 2 diabetes of less than 2 years duration were screened for enrollment. Of those screened, 704 were randomized into a three-arm controlled clinical trial. The three arms consisted of treatment with metformin alone, metformin plus rosiglitazone or metformin plus an intensive lifestyle intervention program (1). This cohort represents the largest and most ethnically and geographically diverse group of pediatric patients with type 2 diabetes ever studied (2).
Using sensitive in vivo techniques to measure insulin sensitivity and secretion, youth with type 2 diabetes are reported to have impairments in both insulin sensitivity and secretion (3–6). In a previous investigation using the hyperinsulinemic-euglycemic and the hyperglycemic clamp, HbA1C correlated inversely with disposition index (DI) and first phase insulin, but not insulin sensitivity (7). In the present investigation, we aimed to examine if the latter observations hold true for a large population of youth with type 2 diabetes, uniformly treated with only metformin at randomization, using surrogate estimates of insulin sensitivity and secretion.
Research Methods and Design
Eligible individuals were 10–17 years of age, diagnosed with type 2 diabetes for <2 years at time of randomization in TODAY, had a body mass index (BMI) ≥ 85th percentile at the time of diagnosis or screening, and did not have laboratory evidence of β-cell autoimmunity. After screening, potentially eligible subjects entered a run-in period of 2–6 months with the goal to discontinue all diabetes medications except metformin, tolerate metformin at a dose of between 500 to 1000 mg twice daily, and maintain HbA1c ≤ 8% for at least 2 months on this regimen. Enrollment ended in February, 2009. Of the 1,211 subjects screened, 1091 (90%) did not have β-cell autoimmunity, 927 (76%) entered the run-in phase, and 704 (58%) successfully completed run-in and were randomized (2).
This report utilizes data obtained at randomization and screening to compare surrogate estimates of insulin sensitivity and secretion, glycemic control, and body composition from the 704 randomized participants. Measures obtained at the randomization visit included anthropometrics (height, weight, and waist circumference), total and percent body fat (by DEXA), fasting lipid profile, and a 2-hour oral glucose tolerance test (OGTT) with fasting and stimulated glucose, insulin, and C-peptide levels. Blood testing, including the OGTT, was performed after an overnight fast and before 10 AM. If an OGTT was not performed (n=10), fasting glucose, insulin, and C-peptide levels were utilized for the respective estimates of insulin sensitivity and secretion. DEXA scans were obtained on all subjects except those whose weight was above 300 pounds (n=40). Glucose, insulin and C-peptide levels from fasting samples and the 2-hour OGTT were used to calculate estimates of insulin sensitivity (7–9), including 1/fasting insulin (1/IF), fasting glucose to insulin ratio (GF/IF) (9), 1/fasting C-peptide (1/CF), fasting glucose to C-peptide ratio (GF/CF) and estimates of insulin secretion: insulinogenic index (ΔI30/ΔG30), C-peptide index (ΔC30/ΔG30), and OGTT area under the curve (AUC) index: AUC C-peptide/AUC glucose (7–9). Disposition index (DI), an expression of β-cell function relative to insulin sensitivity, was calculated as 1/IF × ΔI30/ΔG30 (9) and 1/CF × ΔC30/ΔG30. These estimates of insulin sensitivity derived from fasting measures have been previously validated against hyperinsulinemic-euglycemic clamps in individuals with impaired glucose regulation (10–11) and type 2 diabetes (11, 12). Estimates of insulin secretion (insulinogenic index) and disposition index derived from the OGTT have proven to be good surrogate measures of β-cell function even in the setting of impaired glucose regulation and type 2 diabetes (9). They are also useful in predicting the risk of future type 2 diabetes and are particularly suitable for large epidemiologic studies (9). We preferentially utilized C-peptide derived measures of insulin sensitivity and secretion because some participants had received insulin before their enrollment in the TODAY study (screening visit) and because of differences in insulin clearance in different racial groups (13,14).
Randomization and screening HbA1c levels were divided into quartiles with the 1st quartile the lowest 25% of the HbA1C levels, and the 4th quartile- the highest (>75th percentile) of the total study population. Randomization HbA1c quartiles were: 1st quartile ≤5.5% (mean±SD: 5.2±0.2, n=176); 2nd quartile 5.5 to 5.9% (5.7±0.1, n=193); 3rd quartile 5.9–6.5% (6.2±0.2, n=188); and 4th quartile >6.5% (7.2±0.4, n=147). Screening HbA1c quartiles were: 1st quartile ≤5.9% (5.5±0.3, n=176); 2nd quartile 5.9 to 6.8% (6.4±0.3, n=181); 3rd quartile 6.9 to 8.5% (7.6±0.5, n=172); and 4th quartile >8.5% (10.5±1.4, n=173). Anthropometric and insulin sensitivity and secretion estimates were examined according to HbA1c quartiles at randomization and at the screening visit.
The protocol was approved by an External Evaluation Committee convened by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) and by the Institutional Review Boards of each participating institution. All participants provided informed consent and minor children confirmed assent according to local guidelines. A Data and Safety Monitoring Board convened by NIDDK reviews progress and safety regularly throughout the study.
Laboratory Methods
HbA1C levels were determined by an automated high-performance liquid chromatography system (G7, Tosoh Bioscience, San Francisco, CA). C-peptide was measured by a two-site immunoenzymatic assay (Tosoh, Bioscience, San Francisco, CA). The assay sensitivity is 0.05 ng/mL. Insulin was measured by a double-antibody radioimmunoassay developed by the Immunoassay Core Laboratory of the Diabetes Endocrinology Research Center, University of Washington, Seattle. All laboratory tests were performed at the Northwest Lipid Research Laboratory, University of Washington (Seattle, WA).
Statistical Methods
Data reported in this manuscript include descriptive statistics of the participants at the time of randomization. Group comparisons were made by ANOVA in the case of normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the chi-square test for categorical variables, with adjustments made as indicated in each table. When continuous outcome variables were significantly different by group, post-hoc tests were performed using the Tukey adjustment for multiple comparisons. Simple linear regression analysis was performed to evaluate the determinants of HbA1c as a dependent variable. Data are presented as mean±SD.
Results
Description of the TODAY cohort
Screening and entry criteria have previously been described in detail (1). Median screening HbA1c was 6.8%. The cohort of 704 randomized participants consisted of 457 females (65%) and 247 males (Table 1). At the time of randomization, the age of participants was 14.0±2.0 years, time since diagnosis 7.8±5.8 months, and HbA1c 6.0±0.8%. Most participants were in Tanner stage 4 or 5 of puberty. Participants were significantly obese with weight 95.6±25.3 kg, BMI 34.9±7.6 kg/m2 and BMI Z-score +2.2±0.4. The ethnic composition of the cohort was 41.1% Hispanic, 31.5% Non-Hispanic Black (NH Blacks), 19.6% Non-Hispanic White (NH Whites), 6.1% American Indian (AI), and 1.7% Asian. There were no significant differences in percent body fat among ethnic groups. Because of the small sample size of the Asian group (n=11), this group was not included in subsequent analyses that compared variables of interest across the different racial groups.
Table 1.
Anthropometric characteristics and surrogate estimates of insulin sensitivity and secretion of TODAY participants at randomization: total cohort and by race/ethnicity. All data are presented as mean±SD.
| Characteristic | Total Cohort (n=704) | Hispanic (n=290) | Non-Hispanic Black (n=221) | Non-Hispanic White (n=138) | American Indian (n=43) | Unadjusted p-value | BMI and Sex Adjusted p-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age (years) | 14.0± 2.0 | 14.0± 2.0 | 13.8± 2.0 | 14.1± 2.1 | 14.1± 2.2 | 0.54 | - |
|
| |||||||
| Months since diagnosis | 7.8± 5.8 | 7.6± 5.7 | 8.2± 6.0 | 7.8± 6.0 | 6.3± 4.3 | 0.25 | - |
|
| |||||||
| Female (n, %) | 457 (64.91%) | 178 (61.59%) | 156 (70.27%) | 82 (59.42%) | 32 (74.42%) | 0.05 | - |
|
| |||||||
| BMI (kg/m2) | 34.9± 7.6 | 34.5± 7.5c | 36.4± 8.1b,c | 33.7± 6.9 b | 34.9± 7.7 | <0.05 | - |
|
| |||||||
| BMI Z-score | 2.2± 0.4 | 2.1± 0.4c | 2.3± 0.4 b,c | 2.1± 0.5 b | 2.1± 0.5 | .0001 | - |
|
| |||||||
| Percent body fat | 38.1± 6.2 | 39.0± 6.1 | 37.4± 6.2 | 37.6± 6.4 | 38.0± 6.8 | 0.09 | - |
|
| |||||||
| Waist circumference (cm) | 108.6± 16.7 | 108.7± 16.1 | 111.2± 17.6 a | 106.8± 16.2 | 102.4± 14.1a | <0.01 | - |
|
| |||||||
| HbA1c (%) | 6.0± 0.8 | 6.0± 0.7 c | 6.2± 0.7 b,c | 5.9± 0.7 b | 5.9± 0.8 | <0.001 | - |
|
| |||||||
| Fasting glucose (mg/dL) | 111.3± 25.7 | 110.8± 25.0 | 110.9± 26.9 | 113.1± 23.5 | 113.9± 32.0 | 0.71 | - |
|
| |||||||
| Insulin Sensitivity Estimates | |||||||
|
| |||||||
| 1/IF (mL/μU) | 0.05± 0.04 | 0.05± 0.05 | 0.04± 0.05 | 0.05± 0.05 | 0.05± 0.03 | 0.28 | 0.80 |
| GF/IF (mg/dL per μU/mL) | 5.5± 5.6 | 5.7± 5.0 | 5.0± 7.4 | 5.8± 3.8 | 5.6± 4.7 | 0.50 | 0.92 |
| 1/CF (ng/mL)−1 | 0.31±0.14 | 0.30±0.13 c | 0.32±0.14 c | 0.31±0.16 | 0.32±0.22 | 0.37 | <0.01 |
| GF/CF (mg/dL per ng/mL) | 34.2±18.0 | 33.1±16.0 c | 35.1±16.4 c | 35.0±18.6 | 36.8±32.6 | 0.44 | 0.03 |
|
| |||||||
| Insulin Secretion Estimates | |||||||
|
| |||||||
| ΔC30/ΔG30 (ng/mL per mg/dL) | 0.08± 0.12 | 0.09± 0.16 | 0.08± 0.07 | 0.06± 0.07 | 0.09± 0.12 | 0.34 | 0.40 |
| AUC C-peptide (ng/mL/min) | 2890± 2081 | 3027± 2480 | 2813± 1826 | 2686± 1692 | 3045± 1647 | 0.40 | 0.20 |
|
| |||||||
| Disposition Index (DI) Estimates | |||||||
|
| |||||||
| 1/IF × ΔI30/ΔG30 (mg/dl)−1 | 0.06± 0.08 | 0.05± 0.08 | 0.06± 0.08 | 0.04± 0.05 | 0.06± 0.11 | 0.16 | 0.18 |
| 1/CF × ΔC30/ΔG30 (mg/dl) −1 | 0.02±0.03 | 0.02±0.04 | 0.03±0.02 | 0.02±0.02 | 0.03±0.05 | 0.22 | 0.18 |
Superscript pairs reflect statistically significantly different groups in Tukey post hoc comparison
p<0.05 for post hoc comparison of American Indian vs Black Non Hispanic
p<0.05 for post hoc comparison of Black Non Hispanic vs White Non Hispanic
p<0.05 for post hoc comparison of Black Non Hispanic vs Hispanics
Surrogate Estimates of insulin sensitivity and secretion by racial/ethnic groups (Table 1)
Racial/ethnic groups did not differ with respect to fasting glucose levels. After adjustment for sex and BMI, there were no significant differences in the fasting or OGTT-derived surrogate estimates of insulin sensitivity, secretion or DI across racial/ethnic groups except that 1/CF and GF/CF as measures for insulin sensitivity were higher in NH Blacks compared with the Hispanic group (1/CF= 0.34±0.01 vs. 0.30±0.01 ng/ml−1, post-hoc p=0.015 and GF/CF= 37.4± 1.1 vs. 32.8±1.0 mg/dl per ng/ml, p=0.014 in NH Blacks vs Hispanics, respectively).
Anthropometric characteristics and surrogate estimates of insulin sensitivity and secretion by gender
There were no gender differences in diabetes duration, BMI z-score, or HbA1c. Males had a larger waist circumference (112±18 cm vs. 107±16 cm, p<0.01), but lower percent body fat (33.9± 6.7% vs. 40.0±4.9%, p<0.01)than females. Among the 40 subjects too heavy to obtain DEXA scans, 65% were males. Females were approximately one year younger than males (13.7±2.1 years vs. 14.5±1.9 years, p<0.01) at the time of randomization despite similar diabetes duration. After adjusting for BMI as a reasonable measure of adiposity in this population (since not all participants had DEXA scans), females had lower insulin sensitivity than males estimated by GF/IF (5.1±4.1 vs. 6.1±7.6 mg/dl per μu/ml, p=0.012) and 1/IF (0.047±0.038 vs. 0.053±0.053 μu/ml−1, p=0.03), but not by 1/CF (0.3±0.1 vs. 0.3±0.2 ng/ml−1, p=0.2). There were no gender differences in measures of β-cell function ΔI30/ΔG30 (1.5±2.0 vs 1.4±2.3 μu/ml per mg/dl, p=0.3) or ΔC30/ΔG30 (0.56±0.07 vs 0.08±0.14 ng/ml per mg/dl, p=0.4). There was no difference between females and males in DI calculated as 1/IF × ΔI30/ΔG30 (5.8 ±10.2 vs. 5.4±6.1 mg/dl−1, p=0.5) or as 1/CF × ΔC30/ΔG30 (0.02±0.04 vs. 0.02±0.02 mg/dl−1, p=0.3).
Relationship of surrogate estimates of insulin sensitivity and secretion to HbA1c levels
Estimates of insulin sensitivity and secretion were assessed across HbA1c quartiles at randomization and at screening to evaluate the association between glycemic control and insulin sensitivity and β-cell function estimates in the TODAY participants.
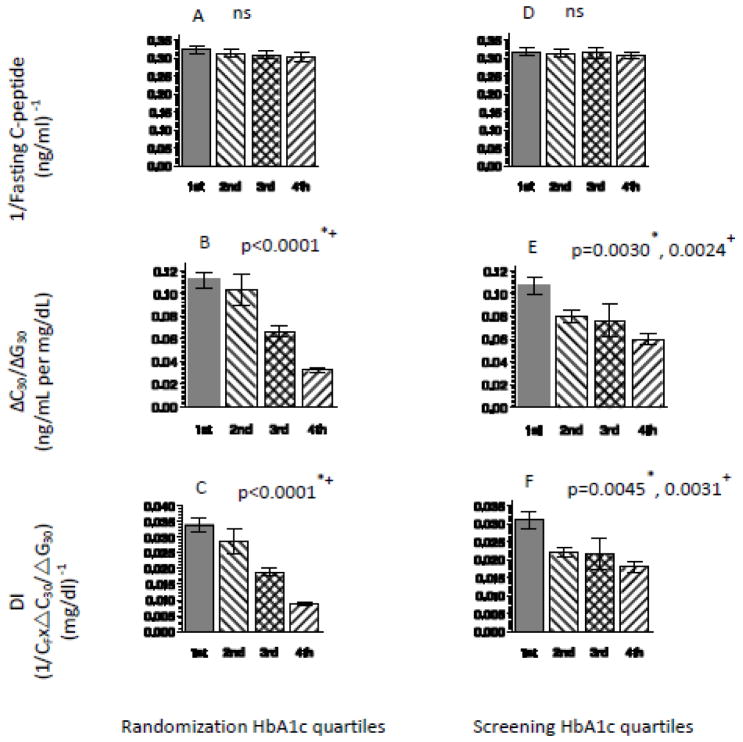
At randomization (Figure 1A–C) and at screening (Figure 1D–F), ΔC30/ΔG30 and DI decreased significantly with increasing quartiles of HbA1c with no difference in insulin sensitivity (1/CF).
Figure 1.
(A)- 1/Fasting C-peptide (CF), (B)- ΔC30/ΔG30, (C)- DI across randomization HbA1c quartiles (left panel); (D)- 1/Fasting C-peptide (CF), (E)- ΔC30/ΔG30, (F)- DI across screening HbA1c quartiles (right panel).
Unadjusted p-value, + adjusted for BMI, race and sex.
These trends persisted after adjusting for BMI, race, and sex. There were no significant differences in age and BMI or BMI Z-scores across the HbA1c quartiles.
Correlations between randomization HbA1c and surrogate estimates of insulin secretion and disposition index (DI)
At randomization, HbA1c andβ-cell function correlated inversely (albeit weakly), with the highest correlation with DI (r=−0.30, p<0.0001), followed by AUC C-peptide (r=−0.20, p<0.0001), and ΔC30/ΔG30 (r=−0.11, p=0.003); there was no correlation with measures of insulin sensitivity. Similarly, screening HbA1c correlated inversely with DI (r=−0.1, p=0.009). In a multiple regression analysis, with HbA1c as the dependent variable and DI, BMI, Tanner stage, sex, and race as independent variables (R2=0.1233, p<0.0001), all variables except Tanner stage contributed to the variance in HbA1c (p-values for individual covariates: DI p<0.0001; BMI p=0.05; sex p=0.02; and race p=0.0008), and DI explained 8 % of the variance in HbA1c.
Discussion
This study describes surrogate indices of insulin sensitivity and secretion in the largest multi-ethnic cohort of youth with type 2 diabetes studied to date. The main findings of this report are: 1) residual β-cell function is the major determinant of glycemic control in pediatric patients with recent-onset type 2 diabetes treated with metformin; and 2) glycemic control in youth with type 2 diabetes is related to β-cell function relative to insulin sensitivity.
Studies on the pathophysiology of type 2 diabetes in adults (15) demonstrate reduced insulin sensitivity as well as decreased β–cell function. Longitudinal studies in adults indicate that worsening metabolic control over time is associated with decreasing β-cell function irrespective of the mode of therapy (16). A limited number of cross-sectional pediatric studies (3–6) similarly revealed that the pathophysiology of type 2 diabetes involves both a decrease in insulin action (3,5) as well as a β-cell secretory defect (3–6). The TODAY cohort study confirms the findings from adult studies and the limited clinical studies in children of a defect in both insulin sensitivity and secretion in youth with type 2 diabetes. Moreover, our findings demonstrate that, in youth with type 2 diabetes treated with metformin, residual β-cell function, and not insulin sensitivity, appears to be the major determinant of glycemic control as measured by HbA1c. Indeed, when the TODAY participants are evaluated according to screening or randomization HbA1c quartiles, insulin sensitivity indices were not significantly different, but insulin secretion parameters were worse, with increasing HbA1c quartiles. The disposition index (DI), which reflects insulin secretion relative to insulin sensitivity (9,15), showed a significantly declining pattern with increasing HbA1c quartiles suggesting that the β-cell defect (loss of insulin secretion) relative to the severity of insulin resistance determines the level of glycemic control. This is consistent with findings from other pediatric studies (3,5). On the other hand, one might argue that the glycemic control could be modulating β-cell function through glucotoxicity. However, this seems unlikely since all participants in this report had HbA1c levels under 8% for at least 2 months prior to randomization.
A limitation of this report is that participants at the screening visit were on different treatment regimens, including insulin therapy or no pharmacotherapy. A higher HbA1c at screening may reflect exposure to less intensive therapy and/or a higher degree of glucotoxicity (17) resulting in lower insulin secretion. However, the finding of decreased insulin secretion indices across HbA1c quartiles was observed not only at screening, but also at the randomization visit, at which time all participants were treated uniformly with metformin only (1) and all had relatively good metabolic control (HbA1c ≤ 8%) for at least two consecutive months. During this early stage of type 2 diabetes, some recovery of β-cell function is expected (18) and glucotoxicity is unlikely to be the major determinant responsible for our findings. Females in our study were a year younger than males at randomization in the study despite similar diabetes duration. This suggests earlier age of onset of type 2 diabetes in females, likely related to earlier onset of puberty and its associated insulin resistance (19). To what degree this earlier onset of puberty-related insulin resistance contributes to earlier decompensation of β-cell function is unclear.
In conclusion, our data demonstrate that in youth with type 2 diabetes treated with metformin, residual β-cell function, and not insulin sensitivity, is the major determinant of glycemic control as measured by HbA1c. Future reports from the TODAY study will further contribute to our understanding of the natural history and efficacy of different treatment modalities in maintaining glycemic control and preserving β-cell function in youth with type 2 diabetes.
Appendix
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043–45 (Childrens Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi-aventis.
The following individuals and institutions constitute the TODAY Study Group (* indicates principal investigator or director):
CLINICAL CENTERS Baylor College of Medicine: S. McKay*, B. Anderson, C. Bush, S. Gunn, M. Haymond, H. Holden, K. Hwu, S. M. Jones, McGirk, B. Schreiner, S. Thamotharan, M. Zarate Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), A. Davis, A. Haider, S. Huestis, C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan, D. Rogers Childrens Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, J. Sanchez, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda Children’s Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, K. Gralewski, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi Children’s Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti Columbia University Medical Center: R. Goland*, R. Cain, I. Fennoy, D. Gallagher, P. Kringas, N. Leibel, R. Motaghedi, D. Ng, M. Ovalles, M. Pellizzari, R. Rapaport, K. Robbins, D. Seidman, L. Siegel-Czarkowski, P. Speiser Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, M. Malloy, K. Milaszewski, L. Orkin, A. Rodriguez-Ventura Massachusetts General Hospital: D. Nathan*, L. Bissett, K. Blumenthal, L. Delahanty, V. Goldman, A. Goseco, M. Larkin, L. Levitsky, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, V. Kociela, T. Whelan, B. Wolff State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, A. Hull, M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, J. Smith, S. Sternlof, R. Swisher University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters Washington University School of Medicine: N. White*, A. Arbeláez, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, M. Mignosa, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle
PROJECT OFFICE National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S. Marcovina*, J. Chmielewski, M. Ramirez, G. Strylewicz DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang Diet Assessment Center (University of South Carolina): E. Mayer-Davis*, Y. Liu, M. Nichols Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, D. Laughlin, G. Leibach, C. Massmann, M. Mills, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, A. Vannucci
OTHER Centers for Disease Control: P. Zhang Hospital for Sick Children, Toronto: M. Palmert State University of New York at Buffalo: L. Epstein University of Florida: J. Silverstein
WRITING GROUP: Bacha F, Pyle L, Nadeau K, Cuttler L, Goland R, Haymond M, Levitsky L, Lynch J, Weinstock R, White N, Caprio S, and Arslanian S.
Footnotes
Author contributions: FB: first author, manuscript design and data collection and interpretation; LP: George Washington University Coordinating Center for TODAY, data management, statistical data analysis, and writing; KN: data collection, interpretation and writing; LC: PI of TODAY at Case Western Reserve University, Chair of Publication and Presentation Committee, study design, data collection and interpretation, and manuscript edits; RG: PI of TODAY at Columbia University Medical Center, data collection and interpretation, and manuscript edits; MH: PI of TODAY at Baylor College of Medicine, study and manuscript design, data collection and interpretation, and coordinator of the writing group; LL: study and manuscript design, data collection and interpretation, and editing of manuscript; JL: study and manuscript design, data collection and interpretation, and manuscript edits; RW: PI of TODAY at State University of New York Upstate Medical University, study and manuscript design, data collection and interpretation, and manuscript edits; NW: PI of TODAY at Washington University School of Medicine, study and manuscript design, data collection and interpretation, and writing and editing of manuscript; SC: PI of TODAY at Yale University, co-chair of the writing group, study and manuscript design, data collection and interpretation, and manuscript edits; and SA: PI of TODAY at Children’s Hospital of Pittsburgh, co-chair of the writing group, study and manuscript design, data collection and interpretation, and manuscript writing and critical editing.
Disclosures: None of the authors has any conflict of interest to declare.
References
- 1.Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, Kaufman FR, Linder B, Marcovina S, McGuigan P, Pyle L, Tamborlane W, Willi S TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–67. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes mellitus: Insulin resistance, beta-cell failure or both? Diabetes Care. 2005;28:638–644. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder DA, Woo JG, D’Alessio DA. Impaired β-cell sensitivity to glucose and maximal insulin secretory capacity in adolescents with type 2 diabetes. Pediatr Diabetes. 2010;11:314–21. doi: 10.1111/j.1399-5448.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacha F, Gungor N, Lee S, Arslanian S. In vivo insulin sensitivity and secretion in obese youth: What are the differences between NGT, IGT and type 2 diabetes? Diabetes Care. 2009;32:100–105. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54:1735–1743. doi: 10.2337/diabetes.54.6.1735. [DOI] [PubMed] [Google Scholar]
- 7.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care. 2010;33:632–8. doi: 10.2337/dc09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 11.Pontiroli AE, Pizzocri P, Caumo A, Perseghin G, Luzi L. Evaluation of insulin release and insulin sensitivity through oral glucose tolerance test: differences between NGT, IFG, IGT, and type 2 diabetes mellitus. A cross-sectional and follow-up study. Acta Diabetol. 2004;41:70–76. doi: 10.1007/s00592-004-0147-x. [DOI] [PubMed] [Google Scholar]
- 12.Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, Hori Y, Yano Y, Adachi Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Arslanian S, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American Children. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 14.Kwame O, Schuster D, Samuel K, Albert GBA. Race and ethnicity determine serum insulin and c-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African Ancestry and White Americans. Metabolism. 1997;46:53–58. doi: 10.1016/s0026-0495(97)90167-0. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SE. The relative contributions of insulin resistance and [beta]-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulfonylurea failure in non-insulin dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Jonas J, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal MA. Glucose regulation of islet stress responses and beta cell failure in type 2 diabetes. Diabetes, obesity and metabolism. 2009;11:65–81. doi: 10.1111/j.1463-1326.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med. 2001;18:10–6. doi: 10.1046/j.1464-5491.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 19.Hannon T, Janosky J, Arslanian S. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–63. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]