Abstract
Objective
Obesity promotes hypertension, but it is unclear if sex differences exist in obesity-related hypertension. Angiotensin converting enzyme 2 (ACE2) converts angiotensin II (AngII) to angiotensin-(1–7) (Ang-[1–7]), controlling peptide balance. We hypothesized that tissue-specific regulation of ACE2 by high-fat (HF) feeding and sex hormones contributes to sex differences in obesity-hypertension.
Methods and Results
HF-fed females gained more body weight and fat mass than males. HF-fed males exhibiting reduced kidney ACE2 activity had increased plasma angiotensin II levels and decreased plasma Ang-(1–7) levels. In contrast, HF-fed females exhibiting elevated adipose ACE2 activity had increased plasma Ang-(1–7) levels. HF-fed males had elevated systolic and diastolic blood pressure that were abolished by losartan. In contrast, HF-fed females did not exhibit increased systolic blood pressure until females were administered the Ang-(1–7) receptor antagonist, D-Ala-Ang-(1–7). Deficiency of ACE2 increased systolic blood pressure in HF-fed males and females, which was abolished by losartan. Ovariectomy of HF-fed female mice reduced adipose ACE2 activity and plasma Ang-(1–7) levels, and promoted obesity-hypertension. Finally, estrogen, but not other sex hormones, increased adipocyte ACE2 mRNA abundance.
Conclusions
These results demonstrate that tissue-specific regulation of ACE2 by diet and sex hormones contributes to sex differences in obesity-hypertension.
Keywords: angiotensin-(1–7), blood pressure, losartan, ovariectomy
Obesity is a primary risk factor for the development of hypertension in the United States. Based on cross-sectional data, men demonstrate a steeper increase in blood pressure with age compared with women, which is reversed after menopause when the prevalence of obesity increases in females.1–3 It is unclear if obesity exerts similar mechanisms to promote hypertension in both men and women. The renin-angiotensin system (RAS) is activated in humans and experimental obesity-associated hypertension.4–7 Angiotensin converting enzyme 2 (ACE2) balances the activity of the RAS through catabolism of the vasoconstrictor peptide, angiotensin II (AngII), to form the vasodilator peptide, angiotensin-(1–7) (Ang-[1–7]). Expression of ACE2 by various cell types regulates actions of these peptides locally, as well as contributes to changes in the systemic balance of AngII/Ang-(1–7). Reductions in ACE2 expression and function, as has been demonstrated in adipose tissue of male obese mice,5 shifts the balance toward increased levels of AngII, favoring the development of hypertension.1
A variety of tissues express ACE2, including kidney, heart, lung, and adipose tissue.5,8 Adipose tissue possesses a local RAS capable of synthesizing AngII and forming Ang-(1–7) from ACE2.3 Previous studies in our laboratory demonstrated that ACE2 is regulated in adipose tissue of male mice fed a high-fat (HF) diet.5 Initial stimulation of ACE2 in adipose tissue of HF-fed males was lost when male mice exhibited robust obesity associated with increased plasma levels of AngII and obesity-induced hypertension. These results suggest that HF feeding in male mice shifts the ACE2-regulated AngII/Ang-(1–7) balance to favor high AngII in the development of obesity-associated hypertension. Because both adipose tissue3 and kidney2,8–10 express ACE2, obesity-induced regulation of ACE2 may differ between tissues and according to sex. Moreover, although several studies support sex differences in the RAS, which contribute to differences in blood pressure control,9–19 it is unclear whether sex differences extend to the development of obesity-related hypertension.
Recent results demonstrated that female spontaneously hypertensive rats exhibited a lower level of hypertension in response to AngII infusion than males, which was reversed by administration of an Ang-(1–7) receptor antagonist.19 These results suggest greater conversion of AngII to Ang-(1–7), presumably resulting from ACE2, in kidneys of female compared with male rats. Previous studies demonstrated that estradiol positively regulated renal ACE2 in a rat model of hypertensive renal disease.20 Taken together, these results suggest that differences in blood pressure responses to AngII between males and females may result from estrogen-mediated increases in ACE2 and increased production of the vasodilator, Ang-(1–7). It is unclear whether estrogen exerts tissue-specific regulation of ACE2 expression, and the role of estrogen regulation of ACE2 in the development of obesity-hypertension.
In the present study, we hypothesized that male and female mice exhibit sex differences in the development of obesity-induced hypertension because of differential tissue-specific regulation of ACE2 and a shift in the AngII/Ang-(1–7) balance. We focused on regulation of kidney versus adipose ACE2 activity in relation to plasma levels of AngII versus Ang-(1–7) in male and female mice made obese from consumption of a HF diet. Moreover, we defined the relative role of AngII versus Ang-(1–7) as mechanisms to initiate males or protect females, respectively, against obesity-associated hypertension. To define the mechanistic role of ACE2, we examined the effects of ACE2 deficiency in the development of obesity-associated hypertension in males versus females. Finally, we quantified effects of estrogen on ACE2 mRNA expression in adipocytes, and then defined effects of removal of female sex hormones on adipose ACE2 and the development of obesity-associated hypertension.
Methods
Animals and Diets
The institutional Animal Care and Use Committee at the University of Kentucky approved all procedures using animals. Male and female C57BL/6 mice (aged 2 months; The Jackson Laboratories, Bar Harbor, ME) were fed a low-fat (LF, 10% kcal as fat; D12450B; Research Diets Inc, New Brunswick, NJ; n = 10) or HF diet (60% kcal as fat; D12492, Research Diets Inc; n = 10) for 16 weeks. During week 16 of HF feeding, the angiotensin type 1 receptor (AT1R) antagonist, losar-tan (10 mg/kg per day), was administered in the drinking water of male mice. In separate studies, female HF-fed mice were infused with vehicle or the Ang-(1–7) mas receptor antagonist, D-Ala-Ang-(1–7) (168 ng/kg per minute, Alzet Model 2004, SC) during week 16 of HF feeding (n = 5 mice/group). In separate studies, male and female Ace2+/+ or Ace2−/− mice (aged 2 months; backcrossed 10× onto a C57BL/6 background; n= 10–15 mice/group) were fed the LF or HF diet for 16 weeks. In separate studies, HF-fed female mice (n = 10–15 mice/group) underwent either sham surgery or ovariectomy (Ovx), and 2 weeks later were fed the HF-diet for 16 weeks. Diets were matched in protein content (20% kcal) and provided energy at 3.85 or 5.25 kcal/g (LF and HF, respectively). Diets were provided to mice ad libitum. Fat and lean mass were measured on conscious mice at study end point using DEXA-IR. At study end point, oftlinemice were anesthetized with ketamine/xylazine (100/10 mg/kg, IP) for exsanguination and tissue harvest.
Measurement of Plasma Parameters
Plasma concentrations of AngII were quantified as described previously in male and female mice fed a LF or HF diet.5 Ang-(1–7) concentrations were quantified in mouse plasma (100 μL) using a commercial kit (Peninsula laboratories, LLC Bachem group, CA). The anti-Ang-(1–7) antibody exhibits minimal cross-reactivity to AngII.
Measurement of Glucose Tolerance
Glucose tolerance was measured in fasted (6 hours) mice injected with glucose intraperitoneally at a dose of 2 g/kg body weight. Blood glucose concentrations were measured at times 0, 15, 30, 60, 90, and 120 minutes after the injection.
Measurement of Blood Pressure
Blood pressure was measured by radiotelemetry during week 16, according to the methods described previously.5 Briefly, anesthetized (isoflurane, to effect) mice were implanted with carotid artery catheters during week 15 of LF or HF feeding, allowed 1 week to recover, and then pressure was recorded continuously (5 minute sampling) for 3 days. For female mice administered D-Ala-Ang-(1–7), systolic blood pressure (SBP) was measured by tail cuff as described previously21 and confirmed at study end point in anesthetized mice by femoral artery catheter.
Tissue ACE2 Measurements
ACE2 activity was measured in homogenates of kidney and adipose tissue using a commercial substrate (Mca-YVADAPK-[Dnp]-OH, R & D Systems, Minneapolis, MN). Standard curves were - generated with the reaction product, Mca-Pro-Leu-OH (Bachem, Torrance, CA), and data are expressed in specific activity (relative fluorescence units per minute per microgram).
Statistical Analysis
Data are expressed as mean ± SEM. All data were analyzed using SigmaStat (Version 11, 2008, Systat Software, Inc., Chicago, IL). For 2 factor analysis, a 2-way ANOVA was used to analyze end-point measures followed by Tukey test for post hoc analysis. Significance was accepted at P<0.05.
See online-only Data Supplement for additional experimental procedures.
Results
The Development of Obesity in HF-Fed Male and Female Mice
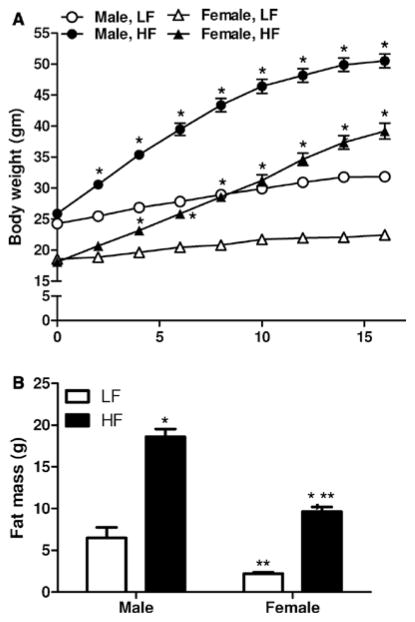
Male and female mice exhibited significant increases in body weight (Figure 1A, P<0.05) in response to the HF diet. Male HF-fed mice exhibited a 24.6 g increase in body weight, representing a 95% increase. By comparison, LF-fed males gained 7.5 g, representing a 31% increase. HF-fed female mice gained 21.1 g, representing an 117% increase. LF-fed females gained only 3.8 g, representing a 20% increase. After correcting for age-matched increases in body weights in LF-fed mice of each sex at week 16, female mice fed the HF diet gained more weight (97%) than males (64%). Body weight increased significantly in both sexes by week 4 of HF feeding. At study end point, body weights of HF-fed males were significantly increased compared with HF-fed females (Figure 1A).
Figure 1.
The development of diet-induced obesity in male and female mice. A, Body weight in male and female low fat (LF)– and high fat (HF)–fed mice. B, Fat mass at the end of 16 weeks of LF or HF feeding in male and female mice. Data are mean± SEM from n = 10 to 15 mice/sex/diet. *P<0.05 versus LF within sex. **P<0.05 compared with males within diet.
In LF-fed mice, fat mass was significantly greater in male compared with female mice (Figure 1B). Fat mass increased significantly in both male and female mice fed the HF diet compared with LF-fed controls (Figure 1B). In addition, fat mass was significantly greater in HF-fed males compared with females (P<0.05). Compared with age-matched LF-fed controls, male HF-fed mice exhibited an 86% increase in fat mass (LF, 6.5±2.2; HF, 18.6±0.9 g, P<0.05). By comparison, female HF-fed mice exhibited a 236% increase in fat mass compared with age-matched LF-fed controls (LF, 2.2±0.2; HF, 9.6±0.6 g, P<0.05). Thus, female mice exhibited a relatively greater increase in body weight and fat mass in response to the HF diet compared with males.
Both male and female HF-fed mice exhibited impaired glucose tolerance (area under the curve for glucose tolerance tests; Figure I in the online-only Data Supplement; P<0.05) compared with LF controls. The magnitude of glucose intolerance was similar in HF-fed male (2.30-fold increase) and female (1.95-fold increase) mice compared with LF controls.
Effects of Obesity on Plasma AngII and Ang-(1–7) Concentrations and Tissue ACE2 Activity in Male and Female Mice
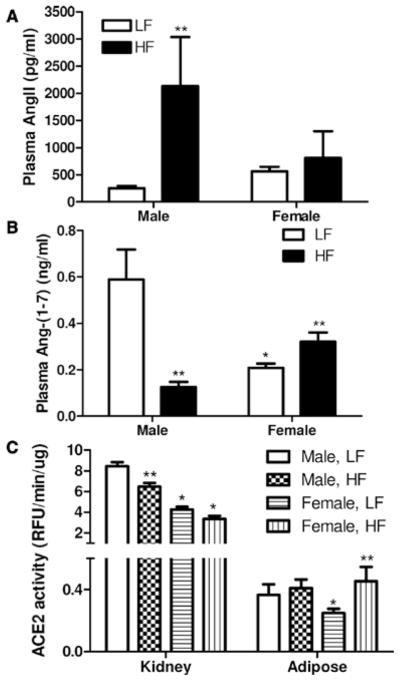
In LF-fed mice, plasma concentrations of AngII were similar between male and female mice (Figure 2A). With HF feeding, male, but not female mice, exhibited a signifi-cant increase in plasma concentrations of AngII (P<0.05). Plasma concentrations of Ang-(1–7) were significantly lower in age-matched LF-fed female compared with male mice (Figure 2B; P<0.05). In male mice fed a HF diet, plasma concentrations of Ang-(1–7) decreased significantly compared with LF-fed controls (Figure 2B; P<0.05). In contrast, HF-fed female mice exhibited a significant increase in plasma Ang-(1–7) concentrations compared with LF-fed controls (P<0.05).
Figure 2.
Effect of high-fat (HF) feeding on plasma concentrations of angiotensin II (AngII), angiotensin-(1–7) (Ang-[1–7]) and tissue angiotensin converting enzyme 2 (ACE2) activity in male and female mice. A, Plasma concentrations of AngII in low fat (LF) or HF-fed male and female mice after 16 weeks of HF feeding. B, Plasma concentrations of Ang-(1–7) in LF or HF-fed male and female mice after 16 weeks of diet feeding. C, Kidney or adipose ACE2 activity after 16 weeks of diet feeding. Data are mean ± SEM from n = 10 to 15 mice/sex/diet for plasma AngII or Ang-(1–7) levels, and n = 3 to 6 mice/sex/diet for tissue ACE2 activity. *P<0.05 compared with male within diet. **P<0.05 compared with LF within sex.
In LF-fed mice, ACE2 protein (Figure II in the online-only Data Supplement) and enzymatic activity (Figure 2C; P<0.05) were significantly greater in kidneys of male compared with female mice. Similarly, ACE2 activity was significantly greater in adipose tissue from LF-fed male compared with female mice (Figure 2C). In HF-fed male mice, kidney ACE2 activity was significantly decreased compared with LF-fed controls (Figure 2C, P<0.05), and kidney ACE2 protein was modestly, but not significantly reduced (Figure IIIA in the online-only Data Supplement, P = 0.08). Interestingly, mas receptor protein increased significantly in kidneys from HF-fed male (Figure IV in the online-only Data Supplement), but not female mice (data not shown). There was no effect of HF feeding on kidney ACE2 protein (Figure IIIB in the online-only Data Supplement) or activity (Figure 2C; P >0.05) in females. Notably, whereas ACE2 activity in adipose tissue was not altered with HF feeding in male mice, it increased significantly in adipose tissue from HF-fed females compared with LF-fed controls (Figure 2C; P<0.05).
HF-Fed Male Mice Develop Obesity-Associated Hypertension That Is Abolished by Losartan, Whereas Administration Of D-Ala-Ang-(1–7) Promotes the Development of Obesity-Associated Hypertension in HF-Fed Females
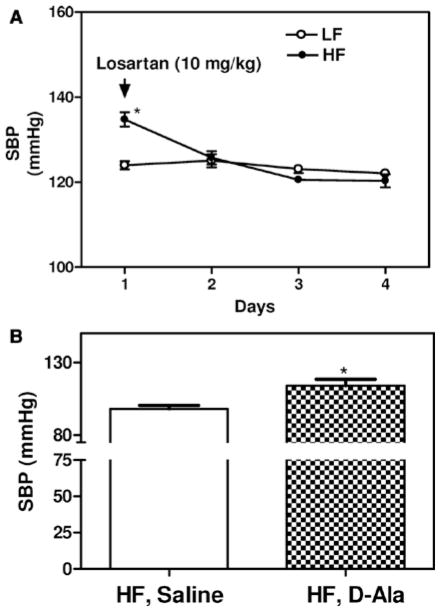
During the light cycle, SBP and diastolic blood pressure (DBP) were similar in LF-fed male and female mice (Figure 3A and 3C). However, during the night cycle, SBP, but not DBP, was significantly lower in LF-fed female compared with male mice (Figure 3B and 3D; P<0.05). As previously reported,5 HF-feeding male mice exhibited a significant increase in SBP during both the day and night compared with LF-fed controls (Figure 3A and 3B; P<0.05). During the night, DBP was also significantly increased in HF-fed male mice compared with LF-fed controls (Figure 3D; P<0.05). Male HF-fed mice exhibited significantly increased mean arterial pressure compared with LF-fed controls, with no differences in heart rate, pulse pressure, or activity (Table I in the online-only Data Supplement). In contrast, despite the robust development of obesity (Figure 1), female mice fed the HF diet did not exhibit a significant change in SBP or DBP during the day or at night (Figure 3A–3D). Although there was no effect of HF feeding on mean arterial pressure, pulse pressures, or activity, heart rate was significantly increased in HF-fed females compared with LF-fed controls (Table I in the online-only Data Supplement). Administration of the AT1R antagonist losartan had no significant effect on SBP in LF-fed male mice, but it significantly reduced SBP in HF-fed male mice to levels observed in LF-fed males (Figure 4A; P<0.05). Conversely, infusion of the mas receptor antagonist D-Ala-Ang-(1–7) to female HF-fed mice resulted in a significant increase in SBP compared with vehicle (Figure 4B; P<0.05).
Figure 3.
Effect of high-fat (HF) feeding on systolic blood pressure (SBP) and diastolic blood pressure (DBP) during the day/night cycle in male and female mice. SBP and DBP were measured by radiotelemetry from carotid artery catheters implanted during week 15 of diet feeding. Measurements are representative of 3 days of successive recordings. A and C, SBP and DBP, respectively, during the day. B and D, SBP and DBP, respectively, during the night. Data are mean± SEM from n = 10 to 15 mice/sex/diet. *P<0.05 compared with male within diet. **P<0.05 compared with low fat (LF) within sex.
Figure 4.
Losartan eliminates obesity-hypertension in male high fat (HF)–fed mice, whereas D-Ala-Ang-(1–7) promotes obesity-hypertension in female HF-fed mice. A, Administration of losartan (10 mg/kg per day) in the drinking water during week 16 resulted in a significant reduction in systolic blood pressure (SBP) (24 hours) in HF, but not low fat (LF)-fed mice. Data are mean± SEM from n = 10 mice/group. *P<0.05 compared with LF. **P<0.05 compared to days 1 to 3. B, Infusion of D-Ala-Ang-(1–7) (168 ng/kg per minute) during week 16 of HF feeding resulted in a significant increase in SBP in HF-fed female mice. Data are mean ± SEM from n = 5 mice/group. *P<0.05 compared with vehicle.
ACE2 Deficiency Increases Blood Pressure in HF-Fed Male and Female Mice
Based on the above described results, we hypothesized that ACE2 deficiency would increase blood pressure in male HF-fed mice, primarily by increasing AngII/AT1R-mediated effects. In contrast, we hypothesized that female ACE2-deficient mice would develop obesity-hypertension because of an increase in the AngII/Ang-(1–7) balance (Figure 2B). ACE2-deficient male and female mice exhibited significant increases in body weight and fat mass with HF feeding (data not shown). After 16 weeks of HF feeding, body weights of HF-fed ACE2-deficient male and female mice were modestly, but significantly lower than HF-fed wild-type controls (Table II in the online-only Data Supplement, P<0.05).
Plasma concentrations of Ang-(1–7) in HF-fed Ace2−/y male mice (Ace2−/y: 0.15±0.03 ng/mL) were not significantly different from levels in HF-fed wild-type male mice (Ace2+/y: 0.13±0.02 ng/mL). However, plasma Ang-(1–7) concentrations in HF-fed Ace2−/y males were lower than those observed in wild-type male mice fed the LF diet (0.59±0.14 ng/mL; Figure 2B; P<0.05). In contrast to findings from wild-type HF-fed females (Figure 2B), HF feeding did not result in an increase in plasma concentrations of Ang-(1–7) in ACE2-deficient females (Ace2+/+: 0.31±0.04; Ace2−/−: 0.25±0.05 ng/mL).
As demonstrated (Figure 3), night cycle SBP was signifi-cantly greater in HF-fed male compared with female Ace2+/+ mice (Figure 5A; P<0.05). Similar findings were observed for day cycle SBP (data not shown). ACE2 deficiency significantly increased SBP and mean arterial pressure in HF-fed male and female mice compared with wild-type controls of each sex (Figure 5A; Table II in the online-only Data Supplement; P<0.05). However, male HF-fed ACE2-deficient mice had significantly greater SBP compared with ACE2-deficient females (Figure 5A; P<0.05). To determine if male and female ACE2-deficient mice fed the HF diet exhibited increased SBP from elevated systemic AngII effects at AT1R, we administered losartan to Ace2+/+ and Ace−/y mice. In HF-fed male mice, administration of losartan significantly lowered SBP (24 hours) in both genotypes (Figure 5B; P<0.05). However, losartan-mediated reductions in SBP were more pronounced in ACE2-deficient mice compared with wild-type male mice (P<0.05). In HF-fed female mice, losartan had no effect on SBP in Ace2+/+ females (data not shown), but significantly lowered blood pressure in Ace2−/− females (prelosartan: 137±2; postlosartan: 111±4 mm Hg; P<0.05).
Figure 5.
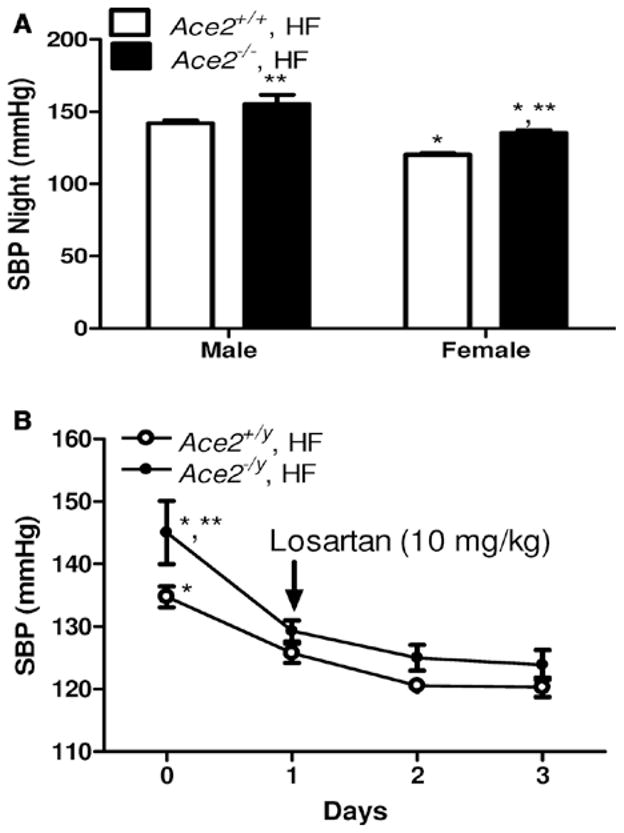
Effect of angiotensin converting enzyme 2 (ACE2) deficiency on systolic blood pressure (SBP) in male and female High fat (HF)–fed mice. A, SBP during the night cycle in HF-fed wild type (Ace2+/+) and ACE2-deficient mice (Ace2−/− female, Ace2−/y male). B, Effect of administration of losartan (10 mg/kg in the drinking water, indicated by arrow) for 3 days on SBP (24 hours) in HF-fed Ace2+/+ and Ace2−/y male mice. Data are mean± SEM from n = 10 to 15 mice/genotype (A) or n = 8 mice/genotype (B). A, *P<0.05 compared with male within diet. **P<0.05 compared with Ace2+/+ within sex. B, *P<0.05 compared to days 1 to 3 within genotype. **P<0.05 compared with Ace2+/+, HF.
Ovx Reduces ACE2 Activity in Adipose Tissue and Promotes Obesity-Associated Hypertension in Female Mice
Estrogen has been reported to positively regulate ACE2 activity in female rat kidney.20 Therefore, we quantified effects of Ovx on tissue ACE2 activity and blood pressure in HF-fed females. Ovx increased body weights of HF-fed females compared with sham-operated controls (Sham, 41±3; Ovx, 50±2 g; P<0.05). In kidneys from HF-fed females, Ovx had no effect on ACE2 activity (Figure 6A). In contrast, ACE2 activity in adipose tissue from HF-fed females was significantly reduced by Ovx (Figure 6A; P<0.05). Plasma concentrations of Ang-(1–7) in HF-fed females were significantly decreased by Ovx (Figure 6B; P<0.05) to levels that were comparable with those in LF-fed female mice (Figure 2B). Notably, SBP (night cycle) was significantly increased by Ovx (HF, sham: 120±1; HF, Ovx: 141±3 mm Hg, Figure 6C; P<0.05) to levels similar to those observed in HF-fed males (Figure 3). Similar findings were observed in day cycle SBP (data not shown) and mean arterial pressure (Table III in the online-only Data Supplement). To determine if sex hormones regulate adipocyte ACE2 expression, we quantified concentration-dependent effects of estrogen, progesterone, or testosterone on ACE2 mRNA abundance in 3T3-L1 adipocytes. Incubation of differentiated 3T3-L1 adipo-cytes with estrogen, but not other sex hormones, resulted in a concentration-dependent increase in ACE2 mRNA abundance (Figure V in the online-only Data Supplement; P<0.05).
Figure 6.
Effect of ovariectomy (Ovx) on tissue angiotensin converting enzyme 2 (ACE2) activity, plasma levels of angio-tensin-(1–7) (Ang-[1–7]), and systolic blood pressure (SBP) in high fat (HF)–fed females. Females underwent sham surgery or Ovx for 2 weeks before HF feeding for a total of 16 weeks. Plasma Ang-(1–7) concentrations in HF-fed sham and Ovx. A, ACE2 activity in kidney or adipose tissue from HF-fed sham (HF) and Ovx females. B, females. C, SBP during the night cycle in HF-fed sham and Ovx females. Data are mean± SEM from n = 3 to 6 mice/group (A), or n = 10 to 15 mice/group (B and C). *P<0.05 compared with HF.
Discussion
The current study provides evidence that the AngII/Ang-(1–7) balance is regulated differently in male and female mice with diet-induced obesity, and contributes to diverging susceptibilities to obesity-hypertension. In males, HF feeding shifts the balance toward AngII/AT1R stimulation and the development of hypertension, and plasma Ang-(1–7) levels are suppressed. In contrast, females exhibit increased plasma Ang-(1–7) concentrations with obesity and are resistant to the development of obesity-hypertension. AT1R antagonism eliminated obesity-hypertension in HF-fed males, whereas mas receptor antagonism promoted obesity-hypertension in females. ACE2 was demonstrated as a mechanism for differences in obesity-hypertension between males and females, because ACE2 deficiency promoted obesity-hypertension in both sexes. Administration of losartan decreased effects of ACE2 defi-ciency to promote obesity-hypertension in males and females. Female sex hormones were demonstrated to contribute to tissue-specific regulation of ACE2 and the development of obesity-hypertension, since Ovx of females reduced adipose ACE2 activity and promoted obesity-hypertension. Notably, regulation of adipose ACE2 activity by HF feeding and Ovx in females paralleled blood pressure changes in females, whereas kidney ACE2 activity paralleled blood pressure changes in males. These results support the hypothesis that tissue spe-cific regulation of ACE2 by obesity and female sex hormones results in a shift in the AngII/Ang-(1–7) balance contributing to sex differences in the development of obesity-associated hypertension.
To our knowledge, this is the first study to examine sex differences in the development of obesity-associated hypertension between male and female mice. In the present study, mice fed HF diets exhibited several characteristics of the metabolic syndrome, including obesity, glucose intolerance, and hypertension, some of which exhibited sex differences. For example, in contrast to male mice, female mice were resistant to the development of obesity-hypertension despite more pronounced increases in fat mass and body weight. Although it is generally considered that females exhibit lower levels of cardiovascular diseases compared with males, the age-adjusted prevalence of uncontrolled blood pressure is higher in females compared with males.22 In addition, although blood pressure increases with age in both women and men,3,22–25 the age-related increase is more rapid in women after menopause than in men.3,26 Results from this study suggest that changes in the AngII/Ang-(1–7) balance, favoring an increase in systemic levels of Ang-(1–7) associated with HF-induced activation of ACE2 in adipose tissue, contribute resistance to the development of obesity-hypertension in female mice. Given that females exhibited greater elevations in fat mass with obesity than males, elevated ACE2 activity in the expansive mass of adipose tissue of HF-fed females would predictably influence the local and systemic RAS. As females gained more weight and fat mass compared to males fed the HF-diet, the lack of hypertension development in females cannot be attributed to diminished development of obesity. Moreover, because glucose intolerance was similar between male and female HF-fed mice, the differences in insulin sensitivity most likely did not contribute to sex differences in the development of hypertension.
In agreement with previous studies,4,5 plasma concentrations of AngII were increased in male mice exhibiting obesity-associated hypertension. To our knowledge, this is the first report that obesity results in sex-dependent differences in plasma concentrations of Ang-(1–7). Marked reductions in plasma Ang-(1–7) concentrations in male HF-fed mice favored an increase in the AngII/Ang-(1–7) balance, which was supported by data demonstrating pronounced reductions in obesity-hypertension in males administered losartan. As ACE2 converts AngII to Ang-(1–7), we hypothesized that tissue-specific regulation of ACE2 in HF-fed male mice contributed to a shift in the AngII/Ang-(1–7) balance. Indeed, HF-fed male mice, but not females, exhibited reductions in kidney ACE2 protein and activity that likely contributed to elevated plasma levels of AngII and reduced levels of Ang-(1–7). In addition, as plasma levels of Ang-(1–7) in HF-fed male mice were similar to those of ACE2-deficient male mice, the increased blood pressures in ACE2-deficient male mice fed the HF diet most likely resulted from augmented effects of AngII. This conclusion was supported in the present study by more pronounced losartan-mediated reductions in blood pressure in ACE2-deficient male mice compared with controls. Administration of losartan has been demonstrated previously to lower blood pressure to a greater extent in obese compared with lean male rats.27,28 These findings suggest that diet-induced obesity in male mice is associated with a shift in the AngII/Ang-(1–7) balance toward high AngII, potentially resulting from dysregulated kidney ACE2.
Whereas deficiency of ACE2 augmented obesity-hypertension in male mice, it induced obesity-hypertension in female mice. Mechanisms of ACE2 deficiency to induce the development of obesity-hypertension in HF-fed females included reductions in plasma Ang-(1–7) levels. Recent findings demonstrated increased conversion of AngII to Ang-(1–7) and lower blood pressure responses to AngII in female compared with male spontaneously hypertensive rats.19 Our results extend these findings by demonstrating that ACE2 deficiency in female mice eliminates HF diet–induced increases in plasma Ang-(1–7) and induces obesity-hypertension. In addition, because administration of losartan normalized blood pressures of ACE2-deficient HF-fed females, these results suggest that ACE2 deficiency augments obesity-hypertension in male and female mice by increasing the AngII/Ang-(1–7) balance. Recent results demonstrated that a polymorphism in the ACE2 gene is associated with a lower risk for fatal cardiovascular events in females29, potentially related to 2 alleles of the X-linked ACE2 gene in females compared with males. It is unclear whether gene dosage differences in ACE2 expression between male and female mice in this study contributed to resistance to obesity-hypertension in females. However, as plasma Ang-(1–7) levels, as well as kidney and adipose ACE2 levels, were lower in LF-fed females than males, these results do not support a role for gene dosage effects of ACE2 in females. Rather, male and female mice appear to respond differently to consumption of a HF diet in the tissue-specific regulation of ACE2 and AngII/Ang-(1–7) balance. Tissue-specific regulation of angiotensin peptide balance by deficiency of ACE2 has been reported recently to impact pregnancy and gestational weight gain in pregnant female mice.30
Our results indicate that female sex hormones also contribute to protection of females against obesity-hypertension, and that this protection may involve estrogen-mediated regulation of ACE2 in adipose tissue. Previous studies demonstrated that estrogen protects female rats against hypertension by amplifying the vasodilator contributions of Ang-(1–7) and reducing formation of AngII.31,32 Additional studies by this group33 demonstrated that administration of estradiol to ovariectomized apolipoprotein E-deficient female mice decreased kidney ACE2 mRNA abundance. In contrast, in a renal wrap model of hypertension, Ovx decreased kidney ACE2 activity, which was restored by estradiol.20 However, this same group recently demonstrated that Ovx of female mice increased renal ACE2 activity,34 prompting the authors to speculate that estrogen regulation of ACE2 in kidney is different between normal and disease conditions. Our findings do not support a role for female sex hormones as regulators of kidney ACE2 activity in the disease condition of obesity. Rather, adipose ACE2 activity and plasma Ang-(1–7) levels were reduced by Ovx of HF-fed females and blood pressure increased, supporting adipose ACE2 as a target of female sex hormones in protection against obesity-hypertension. Further, estrogen, but not other sex hormones, increased ACE2 mRNA abundance in an adipocyte cell culture system, suggesting that in vivo effects of Ovx to decrease adipose ACE2 activity resulted from a lack of estrogen. Previous investigators demonstrated that the ACE2 promoter has 2 putative estrogen response elements.32 Interestingly, obesity is associated with altered effects of estrogen in adipose tissue,35,36 suggesting that estrogen may exert tissue-specific effects on adipose tissue of obese female mice. Moreover, a loss of estrogen in postmenopausal women has been suggested as a contributor to an increased prevalence of obesity in this population.37,38 Indeed, in the present study, Ovx promoted the development of obesity, which may have contributed to increased blood pressure in ovariectomized HF-fed females.
In conclusion, results from this study demonstrate sex differences in the development of obesity-associated hypertension related to ACE2-mediated regulation of the AngII/Ang-(1–7) balance. Male mice exhibited obesity-hypertension associated with enhanced AngII/AT1R effects, whereas protection of female mice against obesity-hypertension was abolished by Ang-(1–7) mas receptor antagonism. Although ACE2 deficiency promoted obesity-hypertension in males and females, tissue-specific regulation of ACE2 by diet and sex hormones may have contributed to sex differences in obesity-hypertension. Regardless of sex differences in obesity-hypertension, these results suggest that stimulation of ACE2 may be beneficial in the treatment of obesity-associated hypertension in both males and females.
Supplementary Material
Acknowledgments
The authors acknowledge Dr Thomas Coffman and Dr Susan Gurley (Duke University) for the ACE2-deficient mice, and Dr Vinayak Shenoy for consultation in ACE2 activity assay.
Sources of Funding
This work was supported by the National Institutes of Health (HL73085; P20RR021954 Dr Cassis), a predoctoral fellowship from the American Heart Association (Dr Gupte), and a postdoctoral fellowship from the National Institutes of Health (F32HL095281; Dr Thatcher).
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.248559/-/DC1.
Disclosures
None.
References
- 1.Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 2.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton PC, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 3.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8(10 Pt 1):978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 4.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 5.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 7.Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 8.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension. 1998;31:90–96. doi: 10.1161/01.hyp.31.1.90. [DOI] [PubMed] [Google Scholar]
- 10.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35(1 Pt 2):480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 11.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1-7) Peptides. 2001;22:927–933. doi: 10.1016/s0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 13.Zapater P, Novalbos J, Gallego-Sandín S, Hernández FT, Abad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. 2004;43:737–744. doi: 10.1097/00005344-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol. 2005;83:413–422. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 15.Xue B, Pamidimukkala J, Hay M. Sex differences in the of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 16.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 17.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, JF Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–R390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol. 2008;93:648–657. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 21.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation Brown reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 22.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 23.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 25.Taddei S. Blood pressure through aging and menopause. Climacteric. 2009;12 (Suppl 1):36–40. doi: 10.1080/13697130903004758. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-López FR, Chedraui P, Gilbert JJ, Pérez-Roncero G. Cardiovascular risk in menopausal women and prevalent related comorbid conditions: facing the post-Women’s Health Initiative era. Fertil Steril. 2009;92:1171–1186. doi: 10.1016/j.fertnstert.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Keen HL, Brands MW, Alonso-Galicia M, Hall JE. Chronic adrenergic receptor blockade does not prevent hyperinsulinemia-induced hypertension in rats. Am J Hypertens. 1996;9(12 Pt 1):1192–1199. doi: 10.1016/S0895-7061(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 28.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R181–R186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- 29.Vangjeli C, Dicker P, Tregouet DA, Shields DC, Evans A, Stanton AV MORGAM project. A polymorphism in ACE2 is associated with a lower risk for fatal cardiovascular events in females: the MORGAM project. J Renin Angiotensin Aldosterone Syst. 2011;12:504–509. doi: 10.1177/1470320311405557. [DOI] [PubMed] [Google Scholar]
- 30.Bharadwaj MS, Strawn WB, Groban L, Yamaleyeva LM, Chappell MC, Horta C, Atkins K, Firmes L, Gurley SB, Brosnihan KB. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension. 2011;58:852–858. doi: 10.1161/HYPERTENSIONAHA.111.179358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol. 1997;273(6 Pt 2):R1908–R1915. doi: 10.1152/ajpregu.1997.273.6.R1908. [DOI] [PubMed] [Google Scholar]
- 32.Haynes MP, Russell KS, Bender JR. Molecular mechanisms of estrogen actions on the vasculature. J Nucl Cardiol. 2000;7:500–508. doi: 10.1067/mnc.2000.109958. [DOI] [PubMed] [Google Scholar]
- 33.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol. 2008;93:658–664. doi: 10.1113/expphysiol.2007.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced CR, hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Gorres BK, Bomhoff GL, Gupte AA, Geiger PC. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol. 2011;110:1046–1053. doi: 10.1152/japplphysiol.00541.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebuffé-Scrive M, Eldh J, Hafström LO, Björntorp P. Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metab Clin Exp. 1986;35:792–797. doi: 10.1016/0026-0495(86)90217-9. [DOI] [PubMed] [Google Scholar]
- 38.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP., Jr Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37:1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.