Abstract
Background
Despite advances in the development of medications to treat alcohol dependence, few medications have been approved by the U.S. Food and Drug Administration. The use of certain anticonvulsant medications has demonstrated potential efficacy in treating alcohol dependence. Previous research suggests that the anticonvulsant levetiracetam may be beneficial in an alcohol-dependent population of very heavy drinkers.
Methods
In this double-blind, randomized, placebo-controlled clinical trial, 130 alcohol-dependent patients who reported very heavy drinking were recruited across 5 clinical sites. Patients received either levetiracetam extended-release (XR) or placebo and a Brief Behavioral Compliance Enhancement Treatment intervention. Levetiracetam XR was titrated during the first 4 weeks to 2000 mg/day. This target dose was maintained during Weeks 5 through 14 and was tapered during Weeks 15 and 16.
Results
No significant differences were detected between the levetiracetam XR and placebo groups in either the primary outcomes (percent heavy drinking days and percent subjects with no heavy drinking days) or in other secondary drinking outcomes. Treatment groups did not differ on a number of nondrinking outcomes, including depression, anxiety, mood, and quality of life. The only difference observed was in alcohol-related consequences. The levetiracetam XR treatment group showed significantly fewer consequences than did the placebo group during the maintenance period (p=0.02). Levetiracetam XR was well-tolerated, with fatigue being the only significantly elevated adverse event, compared with placebo (53%vs. 24%, respectively; p=0.001).
Conclusions
This multisite clinical trial showed no efficacy for levetiracetam XR compared with placebo in reducing alcohol consumption in heavy drinking alcohol-dependent patients.
Keywords: Alcohol Dependence, Levetiracetam, Keppra®, Medications Development, Alcohol Use Disorder
Introduction
Alcohol use disorders (AUDs) (alcohol dependence and abuse) are heterogeneous disorders that affect 18 million Americans (Grant et al., 2004), causing a wide range of medical, psychological, social, personal, and economic problems. Up to 40 percent of patients who are hospitalized have AUDs (de Wit et al., 2010). The total economic cost of alcohol use in the United States is estimated to be $235 billion annually (Rehm et al., 2009). Encouragingly, advances have been made in treatment approaches, especially in the development of medications specifically targeting alcohol drinking.
Currently, three medications (four formulations) are approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol dependence: disulfiram (Antabuse® or Antabus®), oral naltrexone (Revia® or Depade®), acamprosate (Campral®), and injectable naltrexone (Vivitrol®) (Johnson, 2008; Litten et al., 2005). While these medications have demonstrated small to moderate effect sizes in clinical trials, the search for new molecular targets and more efficacious drug compounds are under way in a number of clinical trials (ClinicalTrials.gov).
Several randomized, placebo-controlled trials (RCTs) suggest that anticonvulsant medications may reduce drinking among people with alcohol dependence (De Sousa, 2010; Book and Myrick, 2005). The most encouraging results, so far, have been with topiramate. Johnson et al. (2007) demonstrated, in a multi-site trial, efficacy for topiramate over placebo in reducing drinking in alcohol dependent patients—an effect greater than that observed with other alcohol medications in multi-site trials (Johnson, 2008; Litten et al., 2005). Gabapentin and zonisamide also have been tested and demonstrated some efficacy. Anton et al. (2009) reported that alcohol dependent patients who experienced pre-treatment withdrawal symptoms had fewer drinking days when treated with gabapentin in combination with flumazenil compared with placebo-treated patients. In addition, Anton et al. (2011) demonstrated that adding gabapentin to naltrexone for the treatment of patients with alcohol dependence resulted in fewer heavy drinking days than treatment with naltrexone alone. In a preliminary study, Arias et al. (2010) found that alcohol dependent patients treated with zonisamide had fewer heavy drinking days and consumed less drinks per week, compared with placebo.
Levetiracetam is another anticonvulsant medication that has shown promise for the treatment of alcohol dependence. Its mechanism of action is somewhat different from other antiepileptic medications. Levetiracetam activates the γ-aminobutyric acid (GABA) and glycine systems, interacts with the glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and partially depresses the Ncalcium current(Abou-Khalil, 2008; Carunchio et al., 2007; De Smedt et al., 2007a; Sanchis-Segura et al., 2006; Rigo et al., 2002)—all targets that have been associated with alcohol-seeking and drinking behavior (Litten et al., 2005). In addition, levetiracetam modulates the synaptic vesicle protein 2A (SV2A), an action that appears to correlate with seizure protection (Abou-Khalil, 2008; Kaminski et al., 2008; De Smedt et al., 2007a). The metabolism of levetiracetam is minimal; its elimination occurs almost completely through the urinary system (De Smedt et al., 2007b). Moreover, levetiracetam is well-tolerated, with fewer side-effects than observed with other anticonvulsants (Abou-Khalil, 2008; De Smedt et al., 2007b). Although supratherapeutic doses of levetiracetam(4000 mg) have met criteria for demonstrating abuse potential in the laboratory, levetiracetam has not been associated with reports of actual abuse in the marketplace (Feltner & Haig, 2011).
Levetiracetam appears promising as a medication for reducing or preventing harmful drinking. In animal models, levetiracetam reduced voluntary alcohol intake in alcohol preferring rats (Zalewska-Kaszubska et al., 2011). Although levetiracetam has been shown to be effective in treating alcohol withdrawal symptoms in several open label trials (Muller et al., 2010; Krebs et al., 2006), a recent multi-site trial failed to show an advantage over placebo, and diazepam was required as a rescue medication (Richter et al., 2010). Nonetheless, two recent open label studies have indicated the clinical efficacy of levetiracetam. Sarid-Segal et al. (2008) reported a significant decline in drinks per day, alcohol craving, and alcohol severity over 10 weeks of treatment with up to 2000 mg levetiracetam in 20 alcohol dependent patients. Mariani and Levin (2008) reported reduced alcohol consumption (i.e., drinking days/week, percent heavy drinking days, and percent days abstinent) and anxiety symptoms after 8 weeks of treatment with levetiracetam (up to 1500 mg twice daily) in a case series of three patients with comorbid alcohol dependence and an anxiety disorder. In addition, levetiracetam was well-tolerated in alcohol dependent patients in both studies.
In summary, preliminary results suggest that levetiracetam may be beneficial as a treatment for alcohol dependence. The purpose of this study was to assess both the efficacy and safety of levetiracetam XR (extended-release) in very heavy drinking alcohol dependent patients. A 4-month, randomized, multi-site, placebo-controlled, double-blind clinical trial was conducted assessing levetiracetam XR’s effect on drinking outcomes, alcohol-related consequences, quality of life, mood, anxiety, depression, and safety. The trial was registered in ClinicalTrials.gov (Identifier: NCT00970814).
Methods
Study Population
Randomized patients (n=130) included 99 men and 31 women who were diagnosed with alcohol dependence (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM–IV]; American Psychiatric Association, 1994). Interested candidates responded by telephone to advertisements at 5 academic sites in the United States between November 2009 and May 2010. During the initial telephone call screen, a brief set of standardized questions about drinking were asked to preliminarily determine whether study drinking criteria could be met and to ascertain the caller’s interest in study participation. Individuals who reported levels of drinking consistent with the study entry criteria were scheduled for an in-person screening visit. Interested patients who were able to provide a breath alcohol concentration (BAC) equal to 0.00 at a consent meeting then received information on the study and signed an informed consent form (approved by each clinical site’s Institutional Review Board1) before beginning any study assessments. Additionally, patients provided detailed baseline drinking histories and were given health screens and psychosocial assessments.
Key inclusion criteria included: 1) alcohol dependence, determined by DSM–IV criteria; 2) age 18 or older; and 3) drinking very heavily (10 or more drinks/drinking day for men; 8 or more drinks/drinking day for women) at least 40% of the days during any consecutive 60-day interval during the 90-day period before the clinic screening visit, with at least 1 heavy drinking day (5 or more drinks/drinking day for men; 4 or more drinks/day for women) occurring within the 14 days before randomization (a standard drink was 0.5 oz of absolute alcohol, equivalent to 10 oz of beer, 4 oz of wine, or 1 oz of 100-proof liquor) (Miller et al., 1991).
Key exclusion criteria included: 1) past-year DSM–IV dependence on any psychoactive substances other than alcohol and nicotine; 2) other psychiatric illnesses, including a lifetime DSM–IV diagnosis of panic disorder with or without agoraphobia, schizophrenia, bipolar disorder, or other psychosis; past-year diagnosis of major depression, or past-3-month diagnosis of an eating disorder; 3) inability to be safely withdrawn from alcohol on an outpatient basis (e.g., CIWA–AR score ≥10) or having undergone medical detoxification during the screening phase; 4) pharmacotherapy for alcohol dependence within 1 month before randomization; 5) current psychotherapy for alcohol problems; 6) abnormal calculated creatinine clearance defined as < 80 mL/min; 7) non-stable use of a Selective Serotonin Reuptake Inhibitor (SSRI) (defined as taking an SSRI for less than 3 months prior to informed consent); 8) current use of a dual uptake inhibitor, serotonin-norepinephrine reuptake inhibitor (SNRI), tricyclic antidepressant, or monoamine oxidase inhibitor (MAOIs) antidepressant; and 9) use of anticonvulsants, hypnotics, antipsychotics, psychomotor stimulants, or anti-anxiety agents in the 14 days prior to randomization.
Assessments
In-clinic assessments were carried out at screening, baseline, and at the beginning of Weeks 2, 3, 4, 6, 8, 10, 12, 14, 15, and 17, whereas brief telephone assessments were conducted at the beginning of Weeks 5, 7, 9, 11, 13, and 16 (Table 1). A follow-up telephone interview to assess safety and changes in drinking was performed at Week 19, approximately 2 weeks after the last in-clinic study visit. Patients were not allowed to complete in-clinic assessments unless they had a BAC ≤0.02%.
Table 1.
Assessment Schedule
| Assessment & Source | Schedule (weeks)a |
|---|---|
| Adverse events | Each Contact |
| Breath alcohol concentration (BAC) | SCR, BL, 2, 3, 4, 6, 8, 10, 12, 14, 15, 17 |
| Brief Drinking Assessment | SCR, 19 |
| Blood chemistriesb | SCR, 4, 15 |
| Clinical Institute Withdrawal Assessment for Alcohol - Revised (CIWR–AR) (Sullivan et al., 1989) | Each Contact |
| Concomitant medications | Each Contact |
| Demographics | SCR |
| Drinker Inventory of Consequences (DrInC) (Miller, 1995) | BL, 4, 8, 12, 15, 17 |
| Electrocardiogram (ECG) | SCR, 17 |
| Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991) | BL, 8, 15 |
| Hamilton Anxiety Scale (HAM-A) (Guy, 1976) | BL, 4, 8, 12, 15, 17 |
| Hematology | SCR, 4, 15 |
| Locator form | SCR |
| Medical history | SCR |
| MINI Screen/MINI | SCR |
| Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) | SCR, BL, 4, 8, 12, 15, 17 |
| Motivation to Reduce Drinking | SCR |
| Physical exam | SCR |
| Profile of Moods Questionnaire (POMS) | BL, 2, 3, 4, 12, 17 |
| Prior medications (past 30 days) | SCR |
| Quality of Life Short Form 12 (SF-12) (Szabo, 1996) | BL, 15 |
| Services utilization | BL, 8, 17 |
| Suicidality monitoring | Each Contact |
| Time-Line Follow-Back (Sobell and Sobell, 1992)c | SCR, BL, 2, 3, 4, 6, 8, 10, 12, 14, 15, 17 |
| Treatment compliance | 2, 3, 4, 6, 8, 10, 12, 14, 15, 17 |
| Urine drug screen | SCR, BL, 2, 3, 4, 6, 8, 10, 12, 14, 15, 17 |
| Urine pregnancy test | SCR, BL, 4, 8, 12, 17 |
| Vital signs (blood pressure, heart rate, and respiration rate) and weight | SCR, BL, 2, 3, 4, 6, 8, 10, 12, 14, 15, 17 |
SCR=screening; BL=baseline
All assessments were conducted in the clinic, except for weeks 5, 7, 9, 11, 13, 16, 19 which were conducted via telephone.
Blood chemistries were assessed after ≥2 hours of fasting; repeated after ≥8 hours if blood glucose or triglycerides were elevated.
TLFB methodology in conjunction with a modified Form 90 (Miller, 1996) was used to assess daily drinking for the past 90 days at baseline and since the last assignment during treatment.
Procedures
This study was conducted in accordance with good clinical practices (International Conference on Harmonisation, 1996). Patients who met eligibility criteria at the end of the screening visit were randomly assigned, within 14 days and in an approximate 1:1 ratio, to receive either levetiracetam XR or placebo using a permuted stratified block randomization procedure with clinical site (subsites were considered separate clinical sites) and SSRI use as stratification variables. SSRI use was defined as taking a stable regimen of SSRIs for the 3-month period prior to signing the informed consent with plans to continue the same SSRI regimen during the study. SSRI users were limited to no more than 30% of the target enrollment at each site. Stable SSRI use was acceptable for the study because many alcohol dependent women take SSRIs; and, thus, disallowing SSRI use could have negatively impacted the ability of the study to enroll female patients. Consequently, SSRI use was then treated as a stratification variable to control for its potentially confounding effect on study outcomes. Randomization was implemented via a voice-or Web-based randomization system.
Double-blind study medication was dispensed to patients for 16 weeks. Levetiracetam XR (Keppra XR®) was supplied in 500 mg over-encapsulated (OE) tablets with identical matching placebos. During the first 4 weeks after randomization, the dose was titrated from a starting dose of 500 mg/day up to a target dose of 2000 mg/day. This target dose was maintained during Weeks 5 through 14. A dose taper occurred during Weeks 15 and 16 during which the dose was reduced by half each week. Patients assigned to the placebo group received matched placebo and followed the same dosing schedule as the levetiracetam XR group. Patients unable to tolerate the 2000 mg/day target dose were allowed to continue in the study at a lower dose. Patients who discontinued medication during the study were allowed to remain in the study and participate in study assessments. Medication compliance was determined by corroborating the patient’s self-reported daily dose taken with the number of pills removed from the weekly blister pack, which was returned by the patient at each clinic visit.
All patients received Brief Behavioral Compliance Enhancement Treatment (BBCET) (Johnson et al., 2007). BBCET is a brief (15 to 30 minutes per session) standardized treatment platform used in conjunction with a pharmacological intervention for the treatment of alcohol dependence. The purpose of BBCET is to enhance compliance with the medication and with other aspects of the treatment regimen. BBCET sessions address patient issues relating to personal barriers of compliance, focusing on how medication can assist the patient in achieving his or her own goals related to the control of drinking, and, if necessary, addressing the management of adverse events. The first session was delivered at the randomization visit, with subsequent sessions occurring at each in-person clinic visit thereafter for a total of 11 sessions. BBCET administrators were certified and monitored for compliance with the BBCET guidelines throughout the study.
Analytic Plan and Statistical Methods
The primary efficacy outcomes were weekly percent days heavy drinking (HDDs) and percent of subjects with no heavy drinking days (PSNHDDs) during study Weeks 5–14. A grace period of the first 4 weeks was permitted to titrate levetiracetam XR to the selected therapeutic dose, whereas Weeks 15 and 16 were allowed for dose tapering. Secondary outcome measures included other drinking measures (drinks per day, drinks per drinking day, percent days abstinent, percent very heavy drinking days [10+/8+], and percent subjects abstinent) that also were assessed during Weeks 5–14, as well as alcohol-related consequences, depression, anxiety, mood, and quality of life. Skewed variables were transformed as follows: log transformations (percent very heavy drinking days, Montgomery-Asberg Depression Scale [MADRS], and Hamilton Anxiety Rating Scale [HAM-A])and square root transformations (drinks per day, drinks per drinking day, Drinker Inventory of Consequences [DrInC], and Profile of Mood States [POMS]).
All outcome measures were analyzed for an intention-to-treat (ITT) population that included all randomized patients (n=130). Continuous out comes were analyzed using a repeated-measures mixed effects model with patients treated as the random effect. A Toeplitz covariance matrix best fit the data and was used to model the correlations between repeated measures among patients. For descriptive purposes, least-square means (LSMEANs), standard errors (SEs), and 95% confidence intervals (CIs) are presented for each treatment group and were derived from unadjusted models with untransformed outcome variables and two predictors: week and treatment group. Corresponding Cohen’s d and p-values were derived from adjusted models with appropriately transformed outcome variables and included the covariates age, clinical site, SSRI use, and baseline value of the outcome. For continuous outcomes assessed at a single time (e.g., SF-12 and POMS), unadjusted means are presented for untransformed variables. Cohen’s d and p-values were derived from general linear models (ANCOVAs) with appropriately transformed outcome variables and included the same covariate scheme as the repeated-measures models. Single dichotomous drinking outcomes (i.e., abstinence and no heavy drinking) were computed to reflect drinking across the entire maintenance period (Weeks 5–14). Prevalence rates are presented for untransformed variables. Odds ratios (ORs) and p-values were derived from logistic regression models, again with the same covariate scheme. However, because there was no variability in the baseline equivalents of these outcome variables, percent days abstinent was used as the covariate for the abstinence outcome, and percent heavy drinking days was used as the covariate for the no heavy drinking outcome. All baseline drinking measures were computed during a 60-day period (Days 31–90 before the first screening visit). Outcomes for laboratory tests, safety measures, and vital signs were analyzed via paired t-tests that compared treatment group differences on change scores from baseline to Week 15 and by examining the frequencies of patients that shifted from a normal status at baseline to a clinically significant abnormal status at the end of treatment. To determine the effect of missing drinking data during treatment, models for the primary outcomes were rerun with missing drinking data imputed as heavy drinking days.
All analyses were re-run on an evaluable subsample in order to determine whether the treatment effect was improved among high compliance patients. The evaluable subsample was defined as those patients who took at least 500 mg (the minimal study dose) on at least 80% of study days during the maintenance period of the trial (i.e., on at least 56 of the 70 days during Study Weeks 5–14). Of the 130 randomized subjects, 95 (73.1%) were included in the evaluable subsample.
A series of additional exploratory subgroup analyses were conducted to determine if a differential treatment effect existed as a function of subgroup status for the primary outcome, percent heavy drinking days, during the maintenance period. Subgroups included gender, age of onset of regular drinking (early onset ≤ age 18; later onset > age 18), years of exposure to drinking (i.e., age – age of onset of regular drinking; dichotomized at the median = 27 years), and baseline MADRS (normal <7; depressed ≥7), and marijuana use based on a positive urine drug screen. For all subgroups except marijuana use, LSMEANS, 95% CIs, and p-values for treatment by subgroup interactions and simple effects were tested for significance via mixed effects models that included week, treatment group, subgroup, and treatment by subgroup interaction(adjusted for age, clinical site, SSRI use, and baseline percent heavy drinking days). Due to the small number of patients testing positive for marijuana use, a fully adjusted subsample analysis was run for the primary outcome variable on marijuana-negative patients only.
For bivariate comparisons, treatment group differences were tested for significance by t-tests for independent samples (for normally distributed variables) or Wilcoxon rank-sum tests (for skewed variables); prevalence rate differences were tested for significance via chi-square or Fisher’s exact tests. For all statistical tests, p<0.05 (two-tailed) was considered statistically significant. For the primary outcome, percent heavy drinking days, it was estimated that a sample size of 130 patients was required to obtain 104 evaluable patients (52 per treatment group), yielding 80% power to detect a treatment effect (Cohen’s d=0.66) with a two-tailed t-test at a .05 significance level. For the other primary outcome, percent subjects with no heavy drinking days, 80% power would be achieved given a placebo response rate of 30% and a medication response rate of 62%. Data were analyzed with SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Study Sample
A total of 295 patients consented for the study, 130 of which were randomized and 165 were excluded for either not meeting eligibility criteria or choosing not to participate. The main reason for screen failures included not meeting drinking criteria (25.5%), positive urine toxicology drug screen (17.6%) and not completing screening (10.3%). Of the 130 randomized patients, 66 were randomized to placebo and 64 were randomized to levetiracetam XR. One patient randomized to the placebo group dropped out of the study prior to receiving study medication. Except for a difference in age (placebo 47.0 years, levetiracetam XR 41.7 years; p=0.011) and POMS score (placebo 63.5, levetiracetam 54.6; p<0.0001)2, patients in the levetiracetam XR and placebo groups had statistically similar values on all baseline characteristics (Table 2). Randomized patients were mostly male, white, employed, and middle aged. On average, they drank very heavily—meeting or exceeding a 10/8 drinks per drinking day threshold for men and women, respectively, on approximately 75% of days. This rate of very heavy drinking translated to average consumption of about 16 drinks per drinking day. Despite high levels of heavy drinking, most patients had no or very mild depressive symptoms at baseline (64.3% were non-depressed [MADRS <7]; 28.7% had mild depression [MADRS = 7–19]; 7.0% had moderate depression [MADRS = 20–34]) (Montgomery and Asberg, 1979). In addition, very few patients displayed significant anxiety (96.9% were normal [HAM-A < 14]; 3.1% had at least mild anxiety [HAM-A ≥ 14]) (Guy, 1976). Also, at baseline, subjects had slightly above-normal physical functioning (SF12 physical aggregate score = 52.1) and slightly below-normal mental functioning (SF12 mental aggregate score = 47.2).
Table 2.
Baseline Characteristics of Patients
| Placebo (n=66) | Levetiracetam XR (n=64) | p-valuea | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| n | Mean or % | SD | n | Mean or % | SD | ||
|
|
|||||||
| Demographics | |||||||
| Age | 66 | 47.0 | 11.5 | 64 | 41.7 | 11.7 | 0.011 |
| Male | 52 | 78.8% | 47 | 73.5% | 0.474 | ||
| Employed | 46 | 69.7% | 38 | 59.4% | 0.219 | ||
| Married | 29 | 43.9% | 19 | 29.7% | 0.092 | ||
| Education (> high school) | 40 | 60.6% | 39 | 60.9% | 0.969 | ||
| Race/Ethnicity | |||||||
| White | 47 | 71.2% | 37 | 57.8% | 0.110 | ||
| Black | 17 | 25.8% | 24 | 37.5% | 0.150 | ||
| Hispanic | 1 | 1.5% | 1 | 1.6% | 1.000 | ||
| Other | 1 | 1.5% | 2 | 3.1% | 1.000 | ||
| Self-Reported Alcohol Consumptionb | |||||||
| Drinks per day | 66 | 13.7 | 7.1 | 64 | 15.4 | 9.5 | 0.568 |
| Drinks per drinking day | 66 | 15.8 | 9.6 | 64 | 17.2 | 9.2 | 0.242 |
| Percent days abstinent | 66 | 10.1 | 15.5 | 64 | 10.9 | 18.4 | 0.740 |
| Percent heavy drinking days | 66 | 88.3 | 16.2 | 64 | 88.4 | 18.6 | 0.976 |
| Percent very heavy drinking days (8+/10+) | 66 | 74.7 | 24.5 | 64 | 76.7 | 24.3 | 0.388 |
| Other Substance-Related Indicators | |||||||
| Drinker Inventory of Consequences (DrInC) score | 66 | 44.8 | 20.4 | 64 | 40.0 | 22.4 | 0.100 |
| Age onset regular drinking | 66 | 18.6 | 4.3 | 64 | 19.3 | 5.5 | 0.416 |
| Current smoker | 29 | 45.3% | 32 | 50.0% | 0.595 | ||
| Marijuana usec | 5 | 7.6% | 12 | 19.0% | 0.071 | ||
| GGT | 66 | 72.5 | 75.2 | 63 | 65.2 | 85.5 | 0.472 |
| Psychiatric Characteristicsd | |||||||
| SSRI User | 6 | 9.1% | 4 | 6.3% | 0.744 | ||
| Montgomery-Asberg Depression Rating Scale (MADRS) score | 66 | 6.8 | 7.1 | 63 | 6.0 | 6.3 | 0.588 |
| Hamilton Anxiety (HAM-A) score | 66 | 4.0 | 5.0 | 63 | 3.3 | 3.2 | 0.964 |
| SF-12 Mental Aggregate score | 64 | 46.2 | 10.4 | 64 | 48.2 | 10.5 | 0.216 |
| SF-12 Physical Aggregate score | 64 | 51.2 | 7.4 | 64 | 53.0 | 7.6 | 0.056 |
| Profile of Mood States (POMS) score | 64 | 63.5 | 20.5 | 64 | 54.6 | 14.4 | <0.0001 |
| Clinical Institute Withdrawal Assessment of Alcohol (CIWA) score | 66 | 2.2 | 3.0 | 64 | 1.7 | 2.0 | 0.751 |
Group mean differences are tested for significance by t-tests for independent samples for normally-distributed variables or Wilcoxon rank-sum tests for skewed variables. Group prevalence rate differences are tested for significance via chi-square or Fisher’s exact tests.
Reflects mean values during the 60-day period (Days 31–90) before screening.
Marijuana use based on positive urine drug screen.
Scale range and interpretive values are as follows:
MADRS: (0–60) 0–6 normal; 7–19 mild depression; 20–34 moderate; 35–60 severe
HAMA: (0–56) <14 normal; 14–17 mild anxiety; 18–24 mild-moderate; 35–30 moderate-severe
SF-12: (T-score 0–100) 50 normal functioning
CIWA: (0–70) ≥10 indicative of alcohol withdrawal
Medication Compliance/Complete Drinking Data
Overall compliance with study medication, defined as the proportion of total prescribed medication taken during the maintenance phase of the study (Weeks 5–14), was 94.5% and was similar between the treatment groups (95.0% for the placebo group vs. 94.0% for the levetiracetam XR group; p=0.66). The average daily dose of medication taken was 1820 mg (or 3.64 of the 4 possible pills) in the placebo group and 1755 mg (or 3.51 of the 4 possible pills) in the levetiracetam XR group (p= 0.652). Research participation rate, defined as percent of patients with complete drinking data during the maintenance phase (Weeks 5–14), was 81.5% overall and was slightly higher in the placebo group than the levetiracetam XR group (84.8% vs. 78.1%, respectively), though this difference was not statistically significant (p=0.323). Overall, 37 patients (28.5%) discontinued the study drug prior to completing the study, with a larger proportion of patients discontinuing in the levetiracetam XR group compared with the placebo group (35.9% vs. 21.2%, respectively; p=0.063). Most discontinuation was the result of patients dropping out of the study (23 patients), but also included 14 patients who discontinued the study drug yet remained in the study for follow up.
Main Outcomes
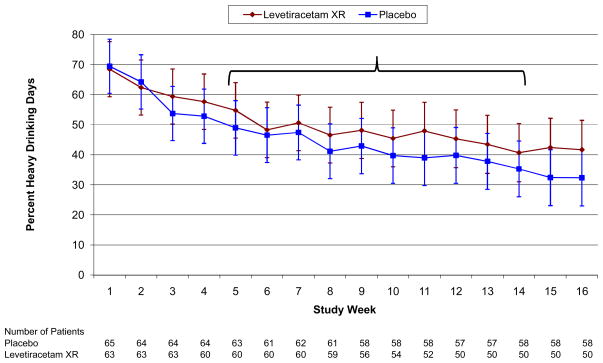
Two primary efficacy outcome measures, percent heavy drinking days (HDDs) and percent subjects with no heavy drinking days (PSNHDDs) were defined a priori. Regarding percent HDDs, the levetiracetam XR and placebo groups had statistically similar weekly unadjusted treatment group means during all weeks of the study (all p>0.14) and particularly during the study maintenance phase (Weeks 5–14) (all p>.19) (Figure 1). Fully adjusted models further failed to show significant differences between the treatment groups on this outcome (p=0.58), PSNHDDs (p=0.95), as well as five secondary drinking outcomes during Weeks 5–14, including percent days abstinent (p=0.92), drinks per day (p=0.84), drinks per drinking day (p=0.83), percent very heavy drinking days (p=0.80), and percent subjects abstinent (p=1.00) (Table 3). Similar results for the primary outcomes were obtained when missing drinking data were imputed as heavy drinking days (percent HDDs, p=0.93; PSNHDDs, p=0.95).
Figure 1.
Means are LSMEANS obtained from a mixed model that includes only study week and treatment group. Error bars are 95% confidence intervals. All weekly comparisons of levetiracetam XR and placebo are not significant (p>0.14); All p>0.19 during weeks 5–14. Note: treatment*week interaction (p=0.841).
Table 3.
Treatment Outcomes: Differences between Placebo and Levetiracetam XR
| Placebo | Levetiracetam XR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Drinking Outcomes | LSMEANa | SE | 95% CI | LSMEAN | SE | 95% CI | Cohen’s d | p-value | ||
|
| ||||||||||
| Drinks per day | 4.5 | 0.52 | 3.5 | 5.5 | 4.9 | 0.54 | 3.9 | 6.0 | −0.03 | 0.844 |
| Drinks per drinking day | 6.3 | 0.58 | 5.2 | 7.5 | 7.1 | 0.60 | 5.9 | 8.3 | −0.03 | 0.830 |
| Percent heavy drinking days | 41.2 | 4.39 | 32.5 | 49.9 | 45.8 | 4.97 | 36.9 | 54.7 | −0.09 | 0.575 |
| Percent very heavy drinking days (8+/10+) | 21.4 | 3.48 | 14.5 | 28.3 | 22.0 | 3.57 | 14.9 | 29.1 | 0.04 | 0.797 |
| Percent days abstinent | 44.0 | 4.38 | 35.3 | 52.6 | 43.2 | 4.49 | 34.3 | 52.1 | 0.02 | 0.917 |
|
|
||||||||||
| % | n | denom | % | n | denom | OR (95% CI)b | p-value | |||
|
|
||||||||||
| Percent subjects abstinent | 15.2 | 10 | 66 | 10.9 | 7 | 64 | 1.00 (0.32–3.10) | 0.998 | ||
| Percent subjects with no heavy drinking days | 18.2 | 12 | 66 | 14.1 | 9 | 64 | 0.97 (0.35–2.67) | 0.946 | ||
|
| ||||||||||
| Non-Drinking Outcomes | LSMEAN | SE | 95% CI | LSMEAN | SE | 95% CI | Cohen’s d | p-value | ||
|
| ||||||||||
| Drinker Inventory of Consequences (DrInC) score (Weeks 8, 12, 15) | 24.4 | 2.38 | 19.7 | 29.1 | 16.5 | 2.45 | 11.6 | 21.3 | 0.41 | 0.022 |
| Montgomery-Asberg Depression Rating Scale (MADRS) score (Weeks 8, 12, 15) | 3.8 | 0.60 | 2.6 | 5.0 | 3.2 | 0.64 | 1.9 | 4.5 | 0.09 | 0.588 |
| Hamilton Anxiety (HAM-A) score (Weeks 8, 12, 15) | 2.9 | 0.41 | 2.1 | 3.7 | 2.2 | 0.44 | 1.3 | 3.1 | 0.17 | 0.306 |
| Profile of Mood States (POMS) score (Week 12)c | 59.1 | 3.26 | 52.5 | 65.6 | 53.6 | 3.33 | 46.9 | 60.3 | −0.11 | 0.600 |
| SF-12 Physical Aggregate score (Week 15)c | 52.9 | 0.92 | 51.4 | 54.4 | 50.4 | 1.31 | 48.2 | 52.6 | 0.38 | 0.078 |
| SF-12 Mental Aggregate score (Week 15)c | 48.7 | 1.40 | 46.4 | 51.1 | 51.0 | 1.59 | 48.4 | 53.7 | −0.08 | 0.544 |
Abbreviations: LSMEAN = least square means; SE = standard error; 95% CI = 95% confidence interval; OR = odds ratio; n= numerator sample size; denom = denominator sample size.
Unless otherwise noted, LSMEANS are based on the outcome variable (untransformed) and were obtained from a mixed model that includes only study week and treatment group. Corresponding Cohen’s d and p-values are based on the outcome variable (appropriately transformed) and were obtained from a mixed model that also includes the covariates clinical site, age, SSRI use, and the baseline value of the outcome. A positive value for Cohen’s d reflects a lower adjusted LSMEAN for the levetiracetam XR group than for the placebo group; a negative value for Cohen’s d reflects a higher adjusted LSMEAN for the levetiracetam XR group than for the placebo group.
Odds ratios and corresponding p-values are derived from a logistic regression model that includes the covariates clinical site, age, SSRI use, and baseline drinking. For the outcome percent subjects abstinent, percent days abstinent was used as the baseline drinking covariate. For the outcome percent subjects with no heavy drinking days, percent heavy drinking days was used as the baseline drinking covariate.
For the POMS and SF-12 outcomes, unadjusted means are presented on the untransformed variables. Corresponding Cohen’s d and p-values are based on the outcome variables (appropriately transformed) and were obtained from a general linear model that includes the covariates clinical site, age, SSRI use, and the baseline value of the outcome.
Treatment groups also did not differ significantly during the maintenance period on a number of nondrinking measures, including quality-of-life mental scale (SF-12) (p=0.54), anxiety (HAM-A) (p=0.31), depression (MADRS) (p=0.59) and mood (POMS) (p=0.60) (Table 3). Furthermore, smoking behaviors did not change during the study or between groups.
In contrast, the levetiracetam XR group had significantly lower alcohol-related consequences (DrInC total score) as compared with placebo during the treatment maintenance period (p=0.02). When the subscales of the DrInC were evaluated, the impulsive (p=0.03), physical (p=0.01), and intrapersonal (p=0.01) subscale scores were significantly lower in the levetiracetam XR group than the placebo group, whereas the interpersonal (p=0.13) and social (p=0.23) subscales scores did not differ significantly between the two groups (data not shown). Finally, the levetiracetam XR group displayed a trend (p=0.08) toward a lower quality of life on the SF-12 physical subscale score compared with the placebo group.
Subgroup Analyses
Across all outcomes, model results obtained with the high-compliance, evaluable subsample were similar to those results obtained with general ITT population. For example, treatment effects for both primary outcomes remained non-significant within the evaluable subsample (percent HDDs, p=0.37; PSNHDDs, p=0.45). A number of other analyses were performed to identify subgroups that might have responded to levetiracetam XR using the outcome percent heavy drinking days. Mixed model results revealed no significant differential treatment effects as a function of gender (interaction p=0.41), age of onset of regular drinking (p=0.80), years exposure to drinking (p=0.45), and depression (MADRS) score (p=0.78). There were also no differential treatment effects in the marijuana-negative drug screen subsample (p=0.72).
Safety and Adverse Events
Changes in mood, alcohol withdrawal (CIWA-AR≥10), vital signs, weight, electrocardiogram (ECG), and clinical laboratory results were unremarkable during the study and generally were similar between the treatment groups. Although GGT decreased in both groups, the decrease was greater in patients taking placebo than levetiracetam XR (−21 U/L vs. −14 U/L, respectively; p<.04); yet no patients from either group experienced a clinically significant shift from baseline in GGT values.
Of the 1029 adverse events reported (in 121 patients) during the treatment phase of the study, 56.9% of those events were mild, 38.3% were moderate, and 4.2% were severe, with statistically equal proportions within each category occurring among the levetiracetam XR and placebo groups. Treatment emergent adverse events occurring in at least 10% of patients included (from most to least frequent): fatigue, headache, insomnia, nausea, irritability, depressed mood, anxiety, arthralgia, somnolence, dizziness, paraesthesia, upper respiratory tract infection, vomiting, back pain, diarrhea, and pruritus (Table 4). Of these adverse events, fatigue was the only one that differed significantly by treatment group, with the prevalence rate in the levetiracetam XR group being more than two times that of the placebo group (53.1% vs. 24.2%, respectively; p=0.001).
Table 4.
Adverse Events Occurring in at Least 10% of Patients
| Number (%) of Patients with Adverse Eventsa | |||
|---|---|---|---|
| Adverse Event | Placebo (n=65) | Levetiracetam XR (n=64) | p-valueb |
| Fatigue | 16 (24.6) | 34 (53.1) | 0.001 |
| Headache | 22 (33.8) | 23 (35.9) | 0.803 |
| Insomnia | 15 (23.1) | 15 (23.4) | 0.961 |
| Nausea | 16 (24.6) | 14 (21.9) | 0.713 |
| Irritability | 13 (20.0) | 16 (25.0) | 0.496 |
| Depressed mood | 16 (24.6) | 12 (18.8) | 0.419 |
| Anxiety | 16 (24.6) | 9 (14.1) | 0.129 |
| Arthralgia | 8 (12.3) | 11 (17.2) | 0.434 |
| Somnolence | 7 (10.8) | 10 (15.6) | 0.415 |
| Dizziness | 7 (10.8) | 8 (12.5) | 0.759 |
| Paraesthesia | 10 (15.4) | 5 (7.8) | 0.272 |
| Upper respiratory tract infection | 5 (7.7) | 10 (15.6) | 0.181 |
| Vomiting | 8 (12.3) | 7 (10.9) | 0.808 |
| Back pain | 5(7.7) | 8 (12.5) | 0.397 |
| Diarrhea | 6 (9.2) | 7 (10.9) | 0.747 |
| Pruritus | 3 (4.6) | 8 (12.5) | 0.127 |
Computed among patients that received at least one dose of study medication; one patient in the placebo group did not receive study medication.
Group prevalence rates are tested for significance via chi-square or Fisher’s exact tests.
Thirteen unique serious adverse events occurred during the maintenance phase of the trial, 3 in the levetiracetam XR group and 10 in the placebo group. Of the 3 in the levetiracetam XR group (inpatient alcohol detoxification, abortion of pregnancy, and thought of self-harm), none were considered related to the investigational product.
DISCUSSION
There are compelling reasons to conduct an early proof-of-concept clinical trial of levetiracetam for the treatment of alcohol-dependent patients. First, a number of other anticonvulsants, including topiramate, gabapentin, zonisamide, valproic acid, and carbamazepine, have shown promise in reducing drinking and acute alcohol withdrawal symptoms (Arias et al., 2010; De Sousa, 2010; Book and Myrick, 2005; Litten et al., 2005). Second, levetiracetam binds to several targets, including GABA, glycine, and N calcium channels, all of which have been shown to alter alcohol-seeking and drinking behavior (Perkins et al., 2010; Litten et al., 2005). Moreover, levetiracetam binds uniquely to SV2A, which recently has been correlated with the prevention of seizures and may be related to the reduction of alcohol consumption (Abou-Khalil, 2008; Kaminski et al, 2008). Third, two open label clinical trials have shown efficacy of levetiracetam in treating alcohol dependent patients (Sarid-Segal et al., 2008; Mariani and Levin, 2008). Finally, levetiracetam is well tolerated and has an attractive safety profile for an anticonvulsant. Levetiracetam has no cognitive side effects, an improvement over other anticonvulsants, including topiramate (Gomer et al., 2007).
In this multi-site controlled clinical trial, levetiracetam XR failed to demonstrate efficacy in reducing alcohol consumption in very heavy drinking alcohol dependent patients. There was no effect on the primary outcome measures of percent HDDs and PSNHDDs (Table 3). Moreover, there were no differences between levetiracetam XR and placebo groups in secondary drinking and non-drinking outcome measures (Table 3). In addition, there were no differences in non-drinking outcomes of depression, anxiety, and quality of life. Only the alcohol-related consequences, as measured using the DrInC questionnaire, showed a significant difference between the levetiracetam XR and placebo groups (with the former experiencing fewer consequences than the latter). However, this result is difficult to explain given the negative findings for alcohol consumption, especially because DrInC scores have been shown to correlate with drinking (Falk et al., 2010). Perhaps the greater fatigue associated with levetiracetam XR may mediate the relationship between treatment group and DrInC scores. For example, patients with greater fatigue may have less impulsive behavior associated with drinking. Alternatively, correction for multiple statistical tests was not used in this study; therefore, the significant DrInC result may be spurious.
A number of subgroup analyses further failed to demonstrate significant efficacy for levetiracetam XR above placebo. For instance, model results obtained from an evaluable subsample were similar to those obtained in the overall ITT population, suggesting that levetiracetam XR was no more efficacious than placebo even among patients with high compliance to the medication regimen. The treatment effect also remained non-significant in a subsample of patients who screened negative for marijuana at baseline. Likewise, the treatment effect did not significantly vary as a function of gender, age of onset of regular drinking, years of exposure to drinking, or baseline depression.
It is possible that the target dosage of levetiracetam XR in this study was not the therapeutic dose, explaining its lack of efficacy. Our target dose of 2000 mg daily is the dosage commonly used to treat seizures (De Smedt et al., 2007b; Abou-Khalil, 2008). Doses of 3000 mg and 4000 mg daily have also been used to treat seizures, although the number of adverse events, particularly irritability, appears to increase when doses of 3000 mg are used with alcohol dependent patients (D. Ciraulo, personal communication).
Medications for the treatment of AUDs are not uniformly and broadly effective; it appears that particular medications work for only particular patients or subgroups. We conducted a number of preliminary analyses for various subtypes to see if levetiracetam XR’s possible efficacy could be matched to a certain group of patients. We examined groups defined by gender, age of onset of regular drinking, years of exposure to drinking, DrInC scores (total and impulsivity subscales), anxiety, and depression. None of the subgroup analyses, however, revealed any differential treatment effect between the levetiracetam XR and placebo groups.
Levetiracetam XR was well tolerated by the alcohol dependent patients. Fatigue was the only side-effect that was significantly elevated in the levetiracetam XR group compared with placebo. In contrast, in a recent multi-site trial, the side-effect profile of topiramate yielded at least five side-effects that were significantly more frequent in alcoholic patients administered topiramate than in those who were taking placebo (Johnson et al., 2007). Perhaps, at least with anticonvulsants, the presence of side-effects is related to efficacy. Side effects may indicate the potency of the medication; or possibly, the side-effects are part of the mechanism that causes a person to reduce his or her drinking.
Because of the heterogeneity and complexity of AUDs, there is currently not one medication for the treatment of alcoholism that is effective and acceptable for all patients. Research is ongoing to develop more efficacious and safe medications to treat AUDs. It is important to make further progress in understanding the mechanisms underlying alcohol-seeking and drinking behavior, and it is hoped that this will lead to the discovery of new, more effective molecular targets for drug development. In this study, we recruited a subpopulation of very heavy drinkers who were functional, who did not require detoxification, and who were without signs of active and significant depression and anxiety. It is possible that other subpopulations might have positively responded to this compound. However, the present results do not support the use of levetiracetam XR at a dose of 2000 mg per day as a treatment for alcohol dependence.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Department of Health and Human Services. Fast Track Drugs and Biologics was the Coordinating Center.
National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinical Investigations Group, Study 2 (NCIG 002)
Boston University School of Medicine, Boston, MA: Ofra Sarid-Segal, M.D.; Maryam Afshar, M.D.; Chris Streeter, M.D.; Courtney Richambault, B.A.; Matthew Biondolillo, B.S.; Laurie Sickles-Colanari, R.N.
University of Pennsylvania Treatment Research Center, Philadelphia, PA: Elizabeth Mahoney, M.A.; Kelly Farraday, B.S.; Gail Kaempf, CRPN; Brenda Beitler, APRN; Cynthia Clark, CPRN; Margo Hendrickson, MSS, LCSW;DonnaGiles; William Dundon, Ph.D.
University of Virginia Center for Addiction Research and Education, Richmond, VA: Eva Jenkins-Mendoza, B.S., CCRP; Sean Sembrowich, R.N.; Ester Makanjuola, R.N.; Tricia Schirmer, B.S.
Johns Hopkins University School of Medicine: George E. Bigelow, Ph.D.; Jenna Cohen M.S.; Delphine Duschel, LPN; Joseph Harrison, B.S.; Kori Kindbom, M.A.; Jennifer Mucha, M.A.; Leticia Nanda, M.S., CRNP; Kimberly Nelson, LPN; Shirley Savage, LPN.
Dartmouth Medical School, Lebanon, NH: Audrey Kern, M.D.; Christopher O’Keefe, M.A.; Shannon Rondeau, R.N., Marjorie Weeks, M.P.A; Joseph Rancourt
FastTrack Drugs and Biologics, North Potomac, MD: Ngami Donovan, B.S.; Marian Mannion, B.S.; Katrina Kell, B.S., CCRA; Katarina Ujhazy, M.D., CCRP; Hermon Gebrehiwet, B.S., CCRP and Josh Berman, M.D., Ph.D.
Footnotes
Each clinical site also obtained a Certificate of Confidentiality issued by NIAAA.
Baseline POMS was not chosen as a covariate in mixed models because of its very low correlation with drinking and non-drinking (non-POMS) outcomes.
References
- Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatric Dis Treat. 2008;4:507–523. doi: 10.2147/ndt.s2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Publishing, Inc; Washington, DC: 1994. [DSM–IV] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168:709–717. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, Stewart SH, Waid R, Malcolm R. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29:334–342. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Feinn R, Oncken C, Covault J, Kranzler HR. Placebo-controlled trial of zonisamide for the treatment of alcohol dependence. J Clin Psychopharmacol. 2010;30:318–322. doi: 10.1097/JCP.0b013e3181db38bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book SW, Myrick H. Novel anticonvolusants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14:371–376. doi: 10.1517/13543784.14.4.371. [DOI] [PubMed] [Google Scholar]
- Carunchio I, Pieri M, Ciotti MT, Albo F, Zona C. Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia. 2007;48:654–662. doi: 10.1111/j.1528-1167.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P. Levetiracetam: The profile of a novel anticonvulsant drug-Part I: Preclinical data. CNS Drug Rev. 2007a;13:43–56. doi: 10.1111/j.1527-3458.2007.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P. Levetiracetam: Part II, the clinical profile of a novel anticonvulsant drug. CNS Drug Rev. 2007b;13:57–78. doi: 10.1111/j.1527-3458.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa A. The role of topiramate and other anticonvulsants in the treatment of alcohol dependence: a clinical review. CNS Neurol Disord Drug Targets. 2010;9(1):45–49. doi: 10.2174/187152710790966696. [DOI] [PubMed] [Google Scholar]
- De Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Felter EF, Haig G. Evaluation of the subjective and reinforcing effects of diphenhydramine, levetiracetam, and valproic acid. J Psychopharmacol. 2011;25(6):763–773. doi: 10.1177/0269881109359095. [DOI] [PubMed] [Google Scholar]
- Gomer B, Wagner K, Frings L, Saar J, Carius A, Harle M, Steinhoff BJ, Schulze-Bonhage A. The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486–494. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM–IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Guy W. EDCEU Assessment Manual. U.S. Department of Health and Human Services; Rockville, MD: 1976. HAMA Hamilton Anxiety Scale. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation. [accessed September 21, 2011];ICH E6: Good Clinical Practice: Consolidated guideline. 1996 http://ec.europa.eu/health/files/eudralex/vol-10/3cc1aen_en.pdf.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–75. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Matagne A, Leclercq K, Gillard M, Michel P, Kenda B, Talaga P, Klitgaard H. SV2A protein is a broad-spectrum anticonvulsant target: Functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54:715–720. doi: 10.1016/j.neuropharm.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Krebs M, Leopold K, Richter C, Kienast T, Hinzpeter A, Heinz A, Schaefer M. Levetiracetam for the treatment of alcohol withdrawal syndrome: An open-label pilot trial. J Clin Psychopharmacol. 2006;26:347–349. doi: 10.1097/01.jcp.0000219926.49799.89. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig J, Mattson ME, Egli M. Development of medications for alcohol use disorders: Recent advances and ongoing challenges. Expert Opin Emerging Drugs. 2005;10:323–343. doi: 10.1517/14728214.10.2.323. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Levetiracetam for the treatment of co-occurring alcohol dependence and anxiety: Case series and review. Am J Drug Alcohol Abuse. 2008;34:683–691. doi: 10.1080/00952990802308213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. the Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. In: Mattson M, Marshall LA, editors. NIAAA Project MATCH Monograph Series. Vol. 4. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. [Google Scholar]
- Miller W. Form 90: A structured assessment interview for drinking and related behaviors (Test Manual) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1996. [Google Scholar]
- Miller W, Heather N, Hall W. Calculating standard drink units: International comparisons. Br J Addict. 1991;86:43–47. doi: 10.1111/j.1360-0443.1991.tb02627.x. [DOI] [PubMed] [Google Scholar]
- Montgomerty S, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muller CA, Schafer M, Schneider S, Helimann HM, Hinzpeter A, Volkmar K, Forg A, Heinz A, Hein J. Efficacy and safety of levetiracetam for outpatient alcohol detoxification. Pharmacopsychiatry. 2010;43:184–189. doi: 10.1055/s-0030-1249098. [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Therap. 2010;127:53–65. doi: 10.1016/j.pharmthera.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Richter C, Hinzpeter A, Schmidt F, Kienast T, Preuss UW, Plenge T, Heinz A, Schaefer M. Levetiracetam for the treatment of alcohol withdrawal syndrome. J Clin Psychopharmacol. 2010;30:720–725. doi: 10.1097/jcp.0b013e3181faf53e. [DOI] [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, Leprince P, Moonen G, Selak I, Matagne A, Klitgaard H. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA-and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid-Segal O, Piechniczek-Buczek J, Knapp C, Afshar M, Devine E, Sickles L, Uwodukunda E, Richambault C, Koplow J, Ciraulo D. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: An open label study. Am J Drug Alcohol Abuse. 2008;34:441–447. doi: 10.1080/00952990802082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sullivan J, Sykora k, Schniederman J, Naranjo C, Sellers E. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-AR) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Szabo S. The World Health Organization Quality of Life (WHOQOL) In: Spiker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 355–362. [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Czarnecka E, Dyr W, Gorska D. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with levetiracetam: A preliminary study. Physiol Behav. 102:538–541. doi: 10.1016/j.physbeh.2010.12.021. [DOI] [PubMed] [Google Scholar]