Abstract
Objective
Our objective was to evaluate adherence among new users of zoledronate and IV ibandronate among U.S. Medicare enrollees.
Methods
We used data from the Medicare 5% random sample to evaluate new users of IV zoledronate and IV ibandronate with continuous Part A+B fee-for-service coverage. The outcome was adherence as quantified by the proportion of days covered (PDC), measured continuously and dichotomously (>= 80%). Follow-up time extended from 18–27 months for all individuals. Factors associated with low adherence with zoledronate were evaluated with logistic regression.
Results
We identified 775 new users of zoledronate and 846 new users of IV ibandronate. For both drugs, 30–48% of first infusions were given in an outpatient infusion center, not in a physician office. The mean PDC for zoledronate users was 82%, which was greater than the mean PDC for the IV Ibandronate users (58–62%, depending on time period, p < 0.0001). Approximately 30% of zoledronate users did not receive a second infusion. Factors associated with low adherence to zolendronate included older age and receipt of the first infusion in an outpatient infusion center rather than a physician’s office.
Conclusions
Less frequently dosed IV bisphosphonates have not resolved the problem of suboptimal adherence with prescription osteoporosis medications. Interventions continue to be warranted to improve long term adherence with osteoporosis treatments.
Keywords: osteoporosis, adherence, persistence, zoledronate, ibandronate, bisphosphonates
Introduction
Numerous studies have demonstrated poor adherence with oral bisphosphonates (1–3), and within the first year after starting therapy, up to half or more patients will discontinue therapy. As might be expected, poor adherence has been shown to compromise the anti-fracture benefit expected with osteoporosis medications (4–6). Besides bisphosphonates, low adherence has also been reported with teriparatide (7), a once daily injectable medication, despite parenteral administration, which should circumvent gastrointestinal side effects associated with oral bisphosphonate therapy(8). This finding is especially notable given that patients treated with teriparatide are likely to have more severe osteoporosis than patients using oral bisphosphonates and thus might be more motivated to be adherent.
The relevance of poor adherence with osteoporosis medications is underscored by the high prevalence, morbidity and mortality associated with osteoporosis and resulting fractures. In the U.S., approximately 44 million women and men age 50 and older are estimated to have osteoporosis or low bone mass, accounting for approximately 55% of people age 50 and older(9). Among individuals age 65 and older, estimates suggest that approximately 30% of individuals have osteoporosis and/or prior fracture(10). Using data from 2001 – 2008, a U.S. study suggested that treatment with oral bisphosphonates prevented more than 144,000 fractures among all U.S. women age 45 and older (11). This figure was based upon the medication-related fracture risk reduction observed for the 43% of patients with high adherence (>= 80%), an attenuated fracture benefit for the additional 20% of patients with lower adherence (50 – 79%), and no benefit for patients that were < 50% adherent. Based upon these results, the total number of fractures prevented represented only slightly more than half the number of fractures that could have been prevented if patients had been more adherent to their prescribed oral bisphosphonates. Improving the problem of non-adherence to osteoporosis therapies would therefore yield a demonstrable public health impact.
However, several strategies and interventions to improve adherence to osteoporosis medications have demonstrated only modest improvements, at best (12). Thus, the need for finding new solutions to improve adherence remains. A potential solution to poor adherence is the use of less frequently dosed medications. For example, use of weekly oral bisphosphonates has been sometimes shown to be associated with somewhat better (albeit still poor) adherence compared to daily oral bisphosphonates (13–16). For osteoporosis, intravenous bisphosphonates can be dosed once every 3 months (IV ibandronate) or every 12 months (zoledronate). At least in theory, adherence might be better with these therapies since they are given by a provider who can detect non-adherence when the patient fails to come back for retreatment. Additionally, their parenteral administration may minimize some side effects (e.g. gastrointestinal upset) and avoid the complex instructions required for taking oral bisphosphonates properly.
In light of a limited understanding regarding the adherence of patients initiating IV bisphosphonates, we examined patterns of use and the frequency of re-treatment with these agents for U.S. Medicare enrollees initiating these therapies. We hypothesized that adherence would be better with IV zoledronate compared to IV ibandronate, perhaps based upon the need for less frequent dosing. We also identified factors associated with low vs. high adherence with IV zoledronate to specifically evaluate whether the setting in which the initial infusion was given (i.e. physician office or a hospital infusion center) was associated with adherence.
Methods
Data sources and patient eligibility
After Institutional Review Board approval, the nationally-representative 5% random sample of Medicare data from the Center for Medicare and Medicare Services from 2005–2009 was used to identify individuals receiving the intravenous bisphosphonates ibandronate or zoledronate. Patients were required to be enrolled in fee-for-service Medicare with Part A and Part B coverage during a 12 months ‘baseline’ period prior to the first initiation of the IV bisphosphonate, defined as the ‘index date’, and had to retain this coverage throughout followup, which extended for at least 18 months after the index date. The baseline period was used to assess demographics and covariates of interest and to confirm that the patient had not used any IV bisphosphonate prior to the index date to fulfill a new user requirement (17). Covariates included age, gender, geographic region, comorbidities of high interest, location of the first infusion (i.e. hospital infusion center vs. physician office), prior DXA, prior osteoporosis drug use, and oral glucocorticoids. Patients with a diagnosis of Paget’s disease of bone (ICD-9 731.0) during the baseline period were excluded. Patients were required to be covered by the Medicare Part D outpatient drug benefit during at least part of the baseline period, depending on when they started IV bisphosphonate therapy (see below).
Identifying IV Bisphosphonate Users
IV ibandronate and zoledronate were first commercially available for osteoporosis in May 2006 and August 2007, respectively. Use of these agents in the CMS data was identified based upon their permanent Healthcare common Procedure Coding System (HCPCS) codes (J1740 and J3488, respectively). The permanent HCPCS code for zoledronate used for osteoporosis is distinct from the HCPCS code used for zoledronate when given for the treatment of skeletal related events associated with malignancy (J3487). Because parenteral medications are not assigned their permanent HCPCS codes until after the product is approved, early use of the IV bisphosphonates was identified based upon use of the “non-specific” HCPCS codes (J3490) coupled with osteoporosis diagnosis codes (e.g. 733.01), units dispensed (5 units for zoledronate, 3 units for ibandronate) and the billed costs for the infusion.
Patients were assigned to one of three mutually exclusive cohorts based upon the first IV BP that they started and the calendar month in which they initiated therapy. The ibandronate cohorts’ patient accrual periods were matched to the same duration (1 year) accrual period for the zoledronate users as follows: zoledronate users who initiated therapy from September 13, 2007 to September 12, 2008; comparison group 1: ibandronate users who initiated therapy in the corresponding period of calendar time, September 13, 2007 to September 12, 2008; and comparison group 2: ibandronate users who initiated therapy from May 1, 2006 to April 30, 2007, the first year that ibandronate was available. For the zoledronate cohort and comparison group 1, Part D coverage was required for the entire 12 month baseline period. For comparison group 2, patients were required to have Part D medication coverage in the last 4 months in the baseline period (January 2006 to April 2006). Since the Medicare Part D pharmacy benefit first began in the U.S. in January 2006, no additional outpatient medication data was available for Medicare beneficiaries prior to January 2006.
Adherence Assessment
On the date of each IV ibandronate infusion, patients were assigned 3 months of exposure; for zoledronate, patients were assigned 12 months of exposure. The primary outcome of interest of the analysis was adherence, defined as the proportion of days covered (PDC), which is similar to a medication possession ratio(18). The PDC is expressed as a proportion, computed by summing the number of days the patient is exposed to the medication, beginning with the first infusion and extending to the end of follow-up, and dividing by the amount of follow-up time. For patients who initiated zoledronate in September 2007, up to 27 months of follow-up time was available in the dataset. For comparability, follow-up for both ibandronate comparison cohorts was therefore truncated at a maximum of 27 months.
The PDC was capped at 100% and was assessed beginning on the date of the first IV zoledronate or first IV ibandronate infusion given, which defined the ‘index date’. Patients were allowed to switch between IV bisphosphonates and still be considered adherent to IV bisphosphonate treatment. Since each calendar day was either treated as exposed or not exposed to IV bisphosphonate, overlapping days of exposure due to an early infusion of the same medication or switching to the other IV bisphosphonate did not affect the PDC. Switching from an IV bisphosphonate to an oral bisphosphonate or another osteoporosis medication did not satisfy the definition of adherence or affect the PDC calculation but was examined descriptively.
Statistical analysis
The PDC was plotted as a probability density function. Chi-square tests were used to compare high adherence (PDC >= 80%) both at 18 months and using all follow-up data between the zoledronate users and IV ibandronate users. Given that PDC was non-normally distributed, Wilcoxon rank sum tests were used to compare the PDC as a continuous measure between groups. Multivariable logistic regression was used to identify factors associated with low adherence to zoledronate (defined as < 80%, following prior conventions in the literature (4, 5, 11)). The main focus of this analysis was whether patients who received their first zoledronate infusion in a physician’s office (rather than an outpatient hospital-based infusion center) had better adherence. Other factors of interest were determined a-priori and included age, race, prior osteoporosis therapy (categorized mutually-exclusively as recent use [any prescription for an oral BP, calcitonin, raloxifene, or teriparatide within the last 4 months]; remote use [any of these prescriptions 5–12 month before the index date]; or neither); the specialty of the physician administering the infusion (relevant only for infusions given in a physician’s office); and oral glucocorticoid use. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
We identified 775 new users of IV zoledronate between September 13, 2007 and September 12, 2008; 275 new users of IV ibandronate who initiated treatment in the same calendar period as the zoledronate users; and 571 new users of IV ibandronate who initiated therapy within the first twelve months after the medication was commercially available. An additional 2–7% of individuals initiated treatment but died within the 18 months after the infusion and were therefore excluded from the analysis. Use of the non-specific HCPCS code was trivial (1%) for zoledronate but comprised approximately 15% of all infusions for the IV ibandronate comparison 2 cohort. An additional 12% of IV ibandronate infusions for both of the ibandronate cohorts were billed as outpatient (Part D) medications and taken to a healthcare provider rather than being billed directly by the provider as Part B medications.
Characteristics of the patients initiating IV zoledronate and ibandronate with at least 18 months of follow-up are shown in Table 1. Users of both IV bisphosphonates were predominantly white women. Between 23 and 40% of the individuals across the three cohorts had prior use of a different osteoporosis medication in the 4 months prior to the first infusion. Between one-third and one-half of the infusions were first given at an outpatient infusion center rather than a physician office. When they were given in a physician office setting, they were more often given by a rheumatologist or an endocrinologist.
Table 1.
Characteristics* of individuals who were new users of IV zoledronate or ibandronate
| Zoledronate cohort: New users during the period 9/13/07 – 9/12/08 (N = 775) % | Comparison Group 1: New Ibandronate users during the period 9/13/07 – 9/12/08 (N = 275) % | Comparison Group 2: New Ibandronate users during the period 5/01/06 – 4/30/07 (N = 571)% | |
|---|---|---|---|
| Female Gender | 761 (98.2) | 260 (94.5) | 538 (94.2) |
| Age at the index date | |||
| 65–69 | 64 (8.3) | 22 (8.0) | 64 (11.2) |
| 70–74 | 152 (19.6) | 63 (22.9) | 129 (22.7) |
| 75–79 | 246 (31.7) | 62 (22.5) | 168 (29.3) |
| 80–84 | 179 (23.1) | 78 (28.4) | 126 (22.2) |
| 85+ | 134 (17.3) | 50 (18.2) | 84 (14.6) |
| Geographic region | |||
| Northeast | 113 (14.6) | 53 (19.3) | 107 (18.7) |
| Midwest | 228 (29.3) | 57 (20.7) | 150 (26.5) |
| West | 110 (14.2) | 55 (20.0) | 97 (16.9) |
| South | 324 (41.9) | 110 (40.0) | 217 (37.9) |
| Comorbidities | |||
| Diabetes mellitus | 106 (13.7) | 39 (14.2) | 55 (9.6) |
| Fall-related conditions | 162 (20.9) | 53 (19.3) | 134 (23.5) |
| COPD | 126 (16.3) | 51 (18.5) | 91 (15.9) |
| Prior fracture | 79 (10.2) | 25 (9.1) | 44 (7.7) |
| Location/Specialty of Provider Giving 1st Infusion | |||
| Hospital-Based Outpatient Infusion Center (no provider specialty) | 327 (42.2) | 131 (47.6) | 176 (30.8) |
| Physician office: Internal medicine | 76 (9.8) | 35 (12.7) | 79 (13.8) |
| Physician office: Medical oncology | 112 (14.5) | 14 (5.1) | 28 (4.9) |
| Physician office: Rheumatology | 204 (26.3) | 73 (26.5) | 250(43.8) |
| Physician office: Other specialty | 56 (7.2) | 22 (8.0) | 38 (6.6) |
| Prior Osteoporosis drug use | |||
| Recent OP drug use (<4 months) | 308 (39.8) | 81 (29.5) | 133 (23.2) |
| Remote OP drug use (4–12 months) | 136 (17.6) | 66 (24.0) | N/A |
| Neither | 331 (42.7) | 128 (46.5) | N/A |
| Any Oral Glucocorticoid Use | 484 (62.5) | 199 (72.4) | 264 (46.1) |
COPD = chronic obstructive pulmonary disease; OP = osteoporosis; N/A = not applicable given the lack of part D pharmacy data available in the relevant time period for these individuals
a 12 month ‘baseline’ period prior to the first infusion was used for covariate assessment
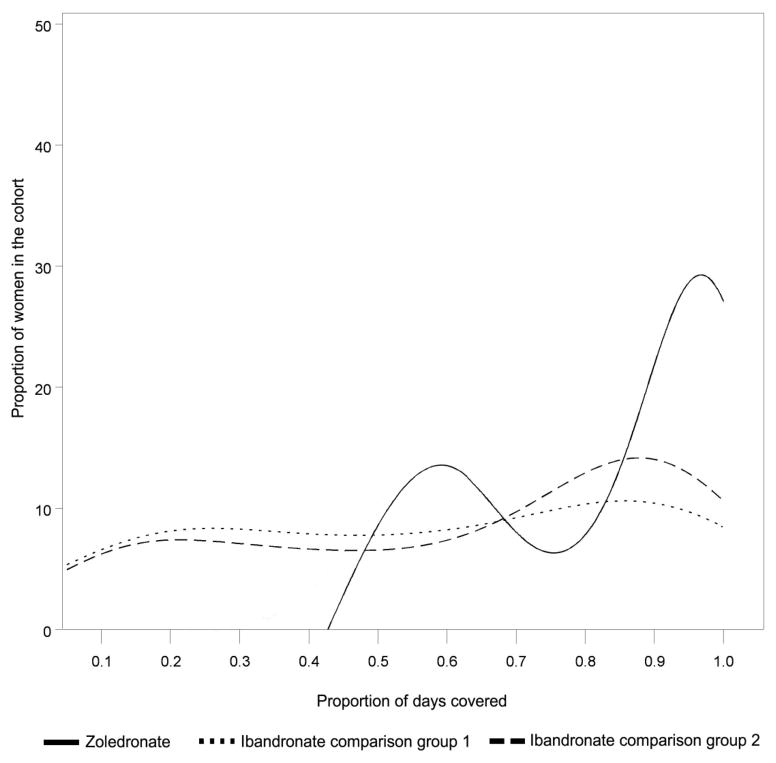
At 18 months, the proportion of zoledronate users that had a PDC >= 80% was 61%. The corresponding proportion for IV ibandronate comparison group 1 was 43%, and for comparison group 2 was 49% (p < 0.0001 and p < 0.0001 compared to IV zoledronate users). Using all available data (minimum 18 months, maximum 27 months), the proportion of patients with high adherence (PDC >= 80%) for the zoledronate and the two ibandronate cohorts was 62.8% versus 36.0%, and 33.3% (p < 0.0001 for both zoledronate-ibandronate comparisons). The distribution of the PDC (as a continuous variable) for new users of IV zoledronate and ibandronate is shown in Figure 1. The median and mean of the PDC is shown in Table 2, which demonstrated that the PDC for zoledronate users was significantly greater than for each IV ibandronate cohort. For the two ibandronate cohorts, 18.5% and 14.0% of patients received only a single dose, which was lower than the proportion of zoledronate patients who received only a single dose (32%). If the non-specific J codes for IV ibandronate were ignored, the mean PDC for the two ibandronate cohorts would have increased by 6% and 3%. Among the zoledronate cohort, 7% of patients used a non-IV bisphosphonate osteoporosis medication at any time during follow-up. Within each of the 2 ibandronate cohorts, 16% and 10% used a non-IV bisphosphonate at any time during follow-up.
Figure 1.
Distribution of Adherence (Proportion of Days Covered) for New Users of Zoledronate and IV Ibandronate*
* Proportion of days covered measured using all data, minimum 18 months, maximum 27 months, shown as a probability density function. The y axis represents the number of individuals at each value of PDC.
Table 2.
Adherence among New Users of IV Bisphosphonates, using All Follow-up Time*
| Proportion of Days Covered** | Number of Infusions | ||||
|---|---|---|---|---|---|
| Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | One Only, % | |
| IV Zoledronate | 92 (36) | 82 ± 18 | 2 (1) | 1.7 ± 0.5 | 31.9 |
| IV Ibandronate Comparison 1 | 61 (61) | 58 ±31 | 4 (5) | 4.4 ± 2.7 | 18.5 |
| IV Ibandronate Comparison 2 | 73 (56) | 62 ± 31 | 5 (5) | 5.3 ± 2.9 | 14.0 |
IQR = inter-quartile range; SD = standard deviation
range 18 – 27 months
PDC was compared between cohorts using non-parametric tests and was significantly greater for the IV zoledronate users compared to each IV ibandronate cohort, p < 0.0001 for each
Table 3 describes factors associated with low adherence to IV zoledronate. After multivariable adjustment, older patients and those with no use of osteoporosis medications in the preceding one year were more likely to be non-adherent. Compared to receiving the first dose of IV zoledronate in an outpatient hospital-based infusion center (i.e. not a physician’s office), patients receiving their first dose in an internists’, rheumatologists’ or endocrinologists’ office were more likely to be adherent.
Table 3.
Factors* Associated with Low Adherence (<80%) to IV Zoledronate
| Crude Odds Ratio | Adjusted Odds Ratio | |
|---|---|---|
| Gender | ||
| Female | 1.0 | 1.0 |
| Male | 3.11 (1.03 – 9.37) | 2.30 (0.73–7.24) |
| Age at the index date | ||
| 65–69 | 1.0 | 1.0 |
| 70–74 | 1.01 (0.53–1.93) | 1.97 (0.50–1.89) |
| 75–79 | 1.45 (0.79–2.65) | 1.35 (0.73–2.51) |
| 80–84 | 1.57 (0.84–2.92) | 1.58 (0.83–2.99) |
| 85+ | 2.80 (1.47–5.31) | 2.87 (1.49–5.55) |
| Location/Specialty of Provider Giving 1st Infusion | ||
| Hospital-Based Outpatient Infusion Center | 1.0 | 1.0 |
| Internal medicine | 0.35 (0.19–0.63) | 0.35 (0.19–0.64) |
| Rheumatology/Endo | 0.58 (0.40–0.84) | 0.59 (0.40–0.86) |
| Other specialty | 1.21 (0.69–2.14) | 1.22 (0.68–2.20) |
| Medical oncology | 0.72 (0.46–1.13) | 0.72 (0.45–1.15) |
| Prior Osteoporosis drug use | ||
| Recent | 1.0 | 1.0 |
| Remote | 1.37 (0.89–2.09) | 1.42 (0.91–2.20) |
| Neither | 1.73 (1.25–2.40) | 1.78 (1.27–2.50) |
| Recent DXA (within the last year) | 1.34 (0.99–1.81) | 1.34 (0.98–1.84) |
| Geographic region* | ||
| Northeast | 1.0 | 1.0 |
| Midwest | 1.00 (0.63–1.60) | 0.89 (0.55–1.45) |
| South | 0.87 (0.56–1.36) | 0.91 (0.57–1.44) |
| West | 1.13 (0.66–1.93) | 1.12 (0.64–1.96) |
DXA and geographic region was forced into the model based upon clinical interest. All other factors listed in Table 1 that are not represented here did not have a significant association with adherence.
Conclusions
The reasons for suboptimal adherence with osteoporosis medications are multifactorial. As we showed, approximately 30% of zoledronate patients did not receive a second dose during a follow-up period that ranged between 18 to 27 months from the first dose. The mean adherence with zoledronate was 82%, which was significantly greater than the mean adherence (approximately 60%) for users of IV ibandronate. Among the factors associated with low adherence to zoledronate were older age and receipt of the first infusion in an outpatient hospital infusion center, rather than a physicians’ office.
As a practical matter, a key element of promoting adherence on an infrequent dose IV therapy requires ensuring that the patient is scheduled to repeat the infusion and remembers to return. Verifying the reliability of the processes of care to schedule the next infusion and remind patients at the time it is needed is likely to be an important factor in ensuring high adherence with IV bisphosphonates. This is one potential explanation for why patients initiating IV bisphosphonate therapy in an outpatient infusion center were more likely to be non-adherent; namely, it may be more difficult to coordinate processes of care to ensure that the patient continues to receive therapy and be rescheduled for the next infusion if they are receiving the infusion outside of their physician’s office. Besides use of less frequently dosed osteoporosis medications, a variety of other factors have also been suggested to improve osteoporosis medication adherence (19–22). These include more frequent follow-up, monitoring such as with bone turnover markers, physician education from pharmacists, and patient education and counseling via mail and telephone. Unfortunately, to date, most of these have had very modest effect sizes, at best (12). Additional strategies such as those that reduce medication copayments, tailor messages to patients about their adherence, and involve use of health information technology, may hold additional promise (23–25) to help improve adherence.
Although we found that adherence with IV bisphosphonate therapy was not optimal, it may be better than the adherence observed in prior reports of oral bisphosphonate users. For example, a recent systematic literature review found that 50% of oral bisphosphonates discontinued therapy after only 6 months, and the mean medication possession ratio at 12 months was only 67% (1). For teriparatide, the parenterally administered drug given by injection once daily, only 57% of patients were persistent at 1 year (7). Adherence with zoledronate observed in this study was better than that observed in a Korean study of 259 patients, among whom only 36% returned for a second zoledronate infusion (26).
The strengths of our study include use of national U.S. Medicare data, which yields results that can be generalized to the entire Medicare fee-for-service population. Also contained within the data were the setting in which the drugs were given (physician office or hospital) and the specialty of the administering prescriber. We evaluated use of the IV bisphosphonates during the period in which it was billed using a non-specific J code; not having used the non-specific J codes would have misclassified approximately 15% of ibandronate infusions and erroneously assigned the date of the first infusion. This would have the effect of including in the study prevalent IV bisphosphonate users (who had previously received the drug billed under a non-specific J code) and would have misclassified them as incident users. It also would have missed incident users (who received their infusion billed via the non-specific J code) who quickly discontinued and never went on to receive the medication billed as the specific J code. Both of these problems yielded biased estimates of adherence, resulting in better apparent adherence for IV ibandronate. The use of the non-specific J code was less problematic for zoledronate given that it was approved for Paget’s disease of bone before osteoporosis and most of its use after August 2007 was via its specific J code. Validation of the analytic procedure used to assign the non-specific J codes is not yet available but is anticipated from ongoing work.
Our results must be interpreted in lights of our study design. We required that patients have at least 18 months of observability after their index date, which excluded the small proportion of individuals who died during this follow-up period. This was done intentionally in order to remove individuals who might be judged to not warrant ongoing osteoporosis treatment in light of high expected near-term mortality. We also lacked information on certain covariates that might be associated with adherence such as results from bone mineral density testing; it is possible with patients with more severe osteoporosis might be more likely to persist and this might partially explain somewhat higher adherence compared with prior reports of adherence with oral bisphosphonates. We also recognize that zoledronate is approved for the prevention (as well as for treatment) of osteoporosis, and when used for a prevention indication it may be given every 2 years. However, Medicare does not pay for zoledronate when associated with an osteoporosis prevention diagnosis. Futhermore, zoledronate did not obtain its prevention indication until the summer of 2009, making it unlikely to have affected our results. There is no prevention indication or less frequent dosing interval available for IV ibandronate. We also lacked information on the reason for non-adherence; some may have been deliberate on the part of patients who elected to discontinue therapy, but some non-adherence may have been unintentional if both the patient and the infusion center forgot to have the patient return for re-treatment (i.e. an administrative reason). Finally, we recognize that patients may switch from IV bisphosphonates to a different non-IV osteoporosis therapy; however, this occurred in fewer than 20% of the IV bisphosphonate patients examined.
In conclusion, we found higher adherence with IV zoledronate compared to IV ibandronate, and absolute rates of adherence that were lower than optimal but higher than most published studies of adherence with oral bisphosphonates. Although the results of our analysis suggest that adherence may be improved with less frequent osteoporosis medication dosing, a significant fraction of patients will still benefit from additional interventions to improve medication adherence.
Significance & Innovation.
While substantial research has shown that adherence with oral bisphosphonates is poor, less frequently dosed parenteral osteoporosis medications might yield improved adherence due to more convenient dosing, avoidance of GI side effects, and more direct control of administration by healthcare providers. However, this possibility is largely unexplored.
Using a national random sample of Medicare beneficiaries, we studied adherence at 18 months and beyond to the IV bisphosphonates zoledronate and IV ibandronate. We found that the mean adherence to IV zoledronate was 82%, which was significantly higher than the mean adherence (approximately 60%) to IV ibandronate (p < 0.0001). Approximately 30% of IV zoledronate users received only a single infusion.
A key factor associated with low adherence was administration of the medication in an outpatient center rather than in a rheumatologist, endocrinologist, or internal medicine physicians’ office.
Despite somewhat better adherence with IV bisphosphonates compared to prior literature reporting adherence with oral bisphosphonates, less frequent, parenterally dosed agents have not solved problems of low adherence to osteoporosis therapies.
Acknowledgments
Dr. Curtis receives support from the NIH (AR 053351) and AHRQ (R01 HS018517). Dr. Saag receives salary support from the National Institutes of Health (AR052361), and the Agency for Healthcare Research and Quality (U18 HS016956).
This research was supported by a contract between UAB and Amgen, Inc. Only the authors from UAB had access to the Medicare data used. The analysis, presentation and interpretation of the results were solely the responsibility of the authors.
Footnotes
Conflicts of Interest:
JRC: Merck, Lilly, Amgen, Roche (consulting and research)
KGS: Lilly, Novartis, Amgen, Sanofi, Merck, Areda (consulting and research)
ED: Amgen (research)
Others: none
Bibliography
- 1.Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–51. doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 2.Netelenbos JC, Geusens PP, Ypma G, Buijs SJ. Adherence and profile of non-persistence in patients treated for osteoporosis--a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int. 2011;22(5):1537–46. doi: 10.1007/s00198-010-1372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold DT, Silverman S. Review of adherence to medications for the treatment of osteoporosis. Curr Osteoporos Rep. 2006;4(1):21–7. doi: 10.1007/s11914-006-0011-8. [DOI] [PubMed] [Google Scholar]
- 4.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. The Benefit of Adherence with Bisphosphonates Depends on Age and Fracture Type: Results From an Analysis of 101,038 New Bisphosphonate Users. J Bone Miner Res. 2008;23(9):1435–41. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoer A, Seidlitz C, Gothe H, Schiffhorst G, Olson M, Hadji P, et al. Influence on persistence and adherence with oral bisphosphonates on fracture rates in osteoporosis. Patient Prefer Adherence. 2009;3:25–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Foster SA, Foley KA, Meadows ES, Johnston JA, Wang SS, Pohl GM, et al. Adherence and persistence with teriparatide among patients with commercial, Medicare, and Medicaid insurance. Osteoporos Int. 2011;22(2):551–7. doi: 10.1007/s00198-010-1297-z. [DOI] [PubMed] [Google Scholar]
- 8.Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, et al. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115(3):209–16. doi: 10.1016/s0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 9.Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Dept of Health and Human Services; 2004. [Google Scholar]
- 10.Cheng H, Gary LC, Curtis JR, Saag KG, Kilgore ML, Morrisey MA, et al. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res. 2011;26(1):3–11. doi: 10.1002/jbmr.189. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson T, Iversen MD, Avorn J, Brookhart AM, Katz JN, Losina E, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheehy O, Kindundu CM, Barbeau M, LeLorier J. Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int. 2009;20(8):1369–76. doi: 10.1007/s00198-008-0795-8. [DOI] [PubMed] [Google Scholar]
- 14.Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19(6):811–8. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 15.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80(7):856–61. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 16.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–52. doi: 10.1007/s00198-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Shu AD-H, Stedman M, Polinski JM, Jan S, Patel M, Truppo C, et al. Adherence to Osteoporosis Medications After Patient and Physician Brief Education: Post Hoc Analysis of a Randomized Controlled Trial. Am J Manag Care. 2009;15(7):417–24. [PMC free article] [PubMed] [Google Scholar]
- 20.Clowes JA, Peel NF, RE The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 21.Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Gleeson T, Iversen M, Avorn J, Brookhart MA, Lii J, et al. A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int. 2010;21(1):137–44. doi: 10.1007/s00198-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollmer W, Williams A, Vupputuri S, Rand C, Smith D, Feldstein A, et al. C-B4-03: Promoting Adherence to Improve Effectiveness of Cardiovascular Disease Therapies (PATIENT): Implementing a Medication Adherence Intervention Using Health Information Technology. Clin Med Res. 2011;9(3–4):152. [Google Scholar]
- 24.Vollmer W, Feldstein A, Smith D, Dubanoski J, Waterbury A, Schneider J, et al. Use of Health Information Technology to Improve Medication Adherence. American Journal Managed Care. 2011;17(12 Spec No):SP79–SP87. [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhry NK, Fischer MA, Avorn J, Schneeweiss S, Solomon DH, Berman C, et al. At Pitney Bowes, value-based insurance design cut copayments and increased drug adherence. Health Aff (Millwood) 2010;29(11):1995–2001. doi: 10.1377/hlthaff.2010.0336. [DOI] [PubMed] [Google Scholar]
- 26.Lee YK, Nho JH, Ha YC, Koo KH. Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1881-x. [DOI] [PubMed] [Google Scholar]