Abstract
We show that microRNA-21 is significantly elevated in peripheral B cells of HIV infected individuals who go on to develop AIDS-NHL (n=13, < 3 yrs prior to diagnosis) when compared to HIV negative (n = 18) or HIV positive controls (n = 21) (p < 0.01). Moreover, miR-21 is overex-pressed in activated B cells, and can be induced by IL4 alone, or with CD40 or IgM costimulation, and lipopolysaccharides (LPS), suggesting that miR-21 may help maintain B-cell hyperactivation, contributing to lymphomagenesis.
Most AIDS related non-Hodgkin lymphomas (AIDS-NHL) are of B cell origin, and include Burkitt's lymphoma (BL), diffuse large B cell lymphoma (DLBCL), primary central nervous system lymphoma (PCNSL), primary effusion lymphoma (PEL), and plasmablastic lymphomas [1]. The availability of specimens from the Multicenter AIDS Cohort Study (MACS), a large prospective longitudinal observational cohort study, allowed us to assess pre-diagnostic specimens for biomarkers that precede the development of such NHL. microRNAs (miRNA) have been found to be dysregulated in most cancers [2]. Tumor-specific miRNAs have been detected in body fluids such as serum/plasma, urine, saliva (reviewed in [3]) and cerebrospinal fluids [4], making them attractive candidates as bio-markers that might be perturbed early in the course of disease development.
miR-21 is the most commonly overexpressed miRNA in cancers. It is highly expressed in tumors arising from diverse tissue types, including brain, ovary, liver, breast, colon, lung, prostate, and stomach [5,6], as well as those of hematological origin, such as chronic lymphocytic leukemia [7], DLBCL [8], acute myeloid leukemia [9], and Hodgkin lymphoma [10]. A recent meta-analysis of 17 independent studies assessing the prognostic role for miR-21 in various cancer types concluded that high miR-21 expression in tumors correlated with poorer prognosis, especially in digestive system and head and neck squamous cell carcinomas [11]. In contrast, higher tumor miR-21 levels were associated with better prognosis in DLBCL [8]. miR-21 can also be a diagnostic marker for PCNSL [4] or a differential diagnostic maker for DLBCL subtypes [8]. In mouse models, over-expression of miR-21 in cells of the hematopoietic lineage led to pre-B-cell lymphoma [12], confirming the potential of miR-21 as an oncogene in B cell malignancies.
Viably frozen peripheral blood mononuclear cells (PBMC) from HIV infected men who went on to develop NHL (AIDS-NHL, n = 22), as well as from HIV uninfected (n = 18) and HIV infected (n = 21) men who did not develop NHL, were obtained from the MACS. AIDS-NHL subjects were separated into two groups based on whether samples were obtained < 3 years prior (n = 13) or > 4 years prior (n = 9) to NHL diagnosis. CD19+ B cells were isolated from PBMCs, followed by total RNA extraction and miR-21 quantification by qPCR, as described [13]. Because all clinical samples were anonymized, the UCLA medical IRB determined that the study was eligible for exemption from IRB review.
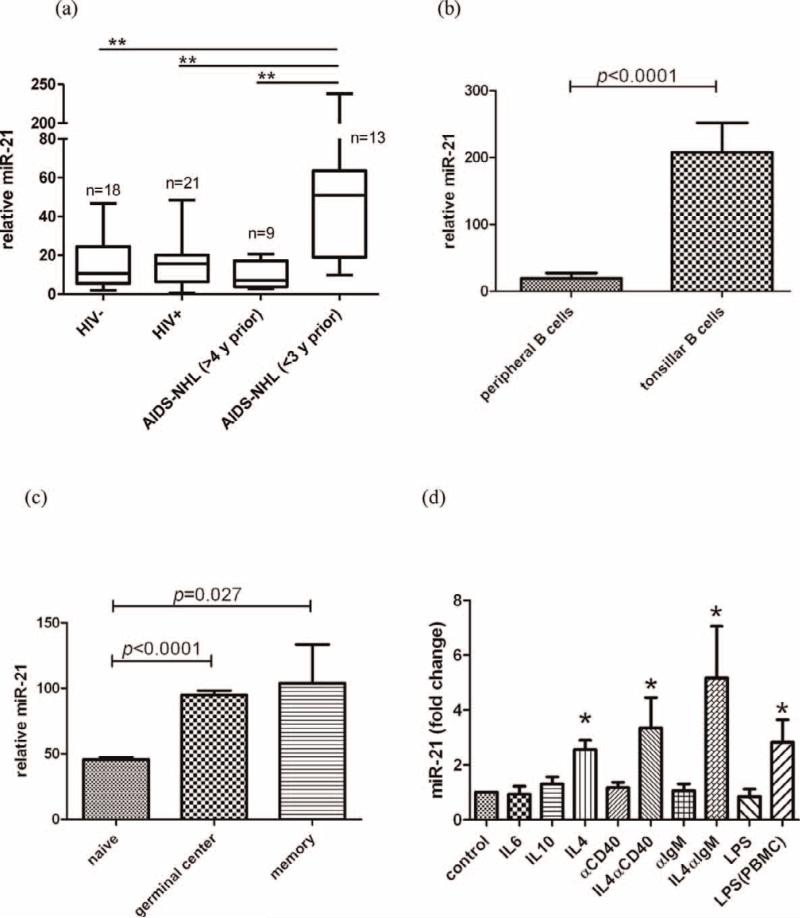
We found significant upregulation of miR-21 in CD19+ B cells from AIDS-NHL patients in the 3 years prior to NHL diagnosis, when compared to either HIV infected or uninfected groups (p < 0.01 for both comparisons), but not in AIDS-NHL patient samples obtained > 4 years prior to NHL diagnosis (Fig. 1a). The pre-diagnosis AIDS-NHL samples comprised of subtypes BL (n = 3), PCNSL (n = 4), and DLBCL (n = 6) in the < 3 years group, and BL (n = 1), PCNSL (n 2), and DLBCL (n = 6) in the > 4 years group. We observed no correlation between B cell Epstein-Barr virus (EBV) positivity and miR-21 overexpression in these samples (data not shown). These observations indicated that miR-21 is overexpressed in circulating B cells from HIV positive individuals who go on to develop NHL. Previously, miR-21 was also found to be elevated in serum and cerebrospinal fluid of DLBCL [14] and PCNSL [4] patients. Our observations indicate that the source of this elevated miR-21 may also include circulating B cells.
Fig. 1. miR-21 levels in peripheral B cells from MACS subjects, in normal B cell subpopulations, and upon in vitro stimulation.
(a) miR-21 level is significantly elevated in circulating B cells preceding AIDS-NHL diagnosis. Relative miR-21 levels were normalized to RNU48 and calculated using the 2-dCt method. miR-21 levels were compared across HIV-, HIV+, and pre-AIDS-NHL subjects grouped into < 3 yrs or > 4 years prior to NHL diagnosis. The box represents the median, 25th and 75th percentile; and the whiskers, the range of values. P values were obtained by one way ANOVA with Bonferroni's multiple comparison test. **signifies p < 0.01 (b) Comparison of miR-21 expression in CD19+ B cells isolated from peripheral blood (n = 7) and tonsils (n = 4). Error bars represent the mean ± SD of the biological replicates. P value was calculated using two tailed t-test. (c) miR-21 expression in tonsillar CD19+ B cell subsets (n = 3). The subsets were separated by FACS based on the following immunophenotype: naïve (IgD+, CD38lo), germinal center (IgD-,CD38hi), and memory (IgD-,CD38lo) as described [13]. Bars represent mean ± SD. P values were calculated using two tailed t-test. (d) Effect on miR-21 levels by B-cell stimulatory molecules. B cells were incubated for 48 hrs with IL6 (200pg/ml), IL10 (10ng/ml), IL4 (10ng/ml), anti-CD40 (500ng/ml), anti-IgM (1ug/ml), or LPS (100ng/ml). All stimulants (except LPS(PBMC)) were directly added to CD19+ B cells isolated from PBMC. For LPS(PBMC), LPS was added to whole PBMC, and 48 hrs later CD19+ B cells were subsequently isolated for analysis. miR-21 expression values were normalized to control stimulation (set to 1). Error bars represent mean (± SD) of at least three independent experiments. P values were calculated by two tailed paired t-test in comparison to control stimulation. (*, p < 0.05)
One pathway that has been suggested to be critical in lymphomagenesis is B cell activation. In HIV+ individuals, several B-cell stimulatory factors such as IL6 [15,16], IL10 [16,17], B-lymphocyte stimulator (BLyS) [18], LPS [19] and the B cell chemokine CXCL13 [20] are elevated in the serum. Additionally, increased surface expression of TNFα on T lymphocytes [21], and the CD40 ligand on T lymphocytes [17] and HIV virions [22,23], also contribute to B cell activation. B cell activation also occurs in secondary lymphoid organs such as lymph nodes, spleen, or tonsils following B cell antigen encounter, and maturation via the germinal center (GC) reaction.
To explore whether miR-21 levels were differentially modulated in activated B cells, we measured miR-21 levels in tonsillar and peripheral B cell populations, obtained from tonsillectomies (n = 4) or healthy donors (n = 7), respectively. We observed a 10.8 fold higher signal for miR-21 in tonsillar B cells when compared to peripheral B cells (p < 0.0001) (Fig. 1b). Within tonsillar B cell subsets, we found significantly higher miR-21 expression in the GC (p < 0.0001) and memory (p = 0.027) subsets compared to naïve subsets (Fig. 1c). Thus, miR-21 is overexpressed in activated B cells, particularly in the GC and memory subsets.
To further explore possible pathways for miR-21 overexpression in B cells, we utilized several molecules to activate B cells in vitro. We found significant miR-21 upregulation by stimulation with IL4 alone (p = 0.002), or in combination with anti-CD40 ab (p = 0.024) or anti-IgM ab (p = 0.021), but not by IL6 (p = 0.720) or IL10 (p = 0.181) (Fig. 1d). Although anti-CD40 or anti-IgM ab augmented IL4 induced miR-21 expression, they were insufficient for miR-21 induction, suggesting that the helper T cell produced cytokine IL4 is important for miR-21 upregulation.
Previously, it was shown that B cells express the LPS receptor CD14, and are functionally responsive to LPS [24]. Direct stimulation of B cells by LPS did not change miR-21 levels (Fig. 1d, LPS, p = 0.340), however, when LPS was added to whole PBMC (Fig. 1d, LPS(PBMC)), it significantly induced miR-21 overexpression in B cells (p = 0.021), suggesting a role for activated monocytes and T cells in B cell miR-21 upregulation.
Overall, these data suggest that miR-21 overexpression is a consequence of B cell activation seen in HIV infection, with a shift to a GC phenotype among circulating B cells. Increased appearance of circulating B cells with GC or immature/transitional phenotype [25] is a feature of HIV disease progression. The phenotype of cells contributing to increased miR-21 expression in AIDS-NHL remains to be defined. In summary, elevated levels of the B-cell activation induced miR-21 in circulating B cells are seen in those persons who go on to develop AIDS-NHL, suggesting that miR-21 may be an important biomarker for assessing NHL risk in HIV infected individuals.
Acknowledgements
We would like to thank all the MACS participants and staff, Dr. Najib Aziz, Larry Magpantay, the UCLA AIDS Institute virology core, the UCLA Tissue Procurement Core Laboratory, and the UCLA JCCC/CFAR Flow Cytometry Core Facility.
Source of Funding
This work was supported by grants from the Margaret E. Early Trust, the National Institute of Health (NCI supplement to U01-AI35040, R01-CA57152, P30-AI28697), and fellowship support from California HIV/AIDS Research Program (D08-LA-311) to DRT. This work was carried out in the facilities of the UCLA AIDS Institute, which were supported, in part, by funds from the James B. Pendleton Charitable Trust and the McCarthy Family Foundation, and by NIH grant AI-028697: UCLA Center for AIDS Research (CFAR).
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Raphael M, Borisch B, Jaffe ES. In: Lymphomas associated with infection by the human immune deficiency virus (HIV). World Health Organization Classification of Tumors, Pathology and Genetics of Tumours of Haemotopoietic and Lymphoid Tissues. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. IARC Press; Lyon: 2001. pp. 260–263. [Google Scholar]
- 2.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- 5.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2008;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4981. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 8.Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121:1156–1161. doi: 10.1002/ijc.22800. [DOI] [PubMed] [Google Scholar]
- 9.Jongen-Lavrencic M, Sun SM, Dijkstra MK, Valk PJ, Lowenberg B. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111:5078–5085. doi: 10.1182/blood-2008-01-133355. [DOI] [PubMed] [Google Scholar]
- 10.Navarro A, Gaya A, Martinez A, Urbano-Ispizua A, Pons A, Balague O, et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008;111:2825–2832. doi: 10.1182/blood-2007-06-096784. [DOI] [PubMed] [Google Scholar]
- 11.Fu X, Han Y, Wu Y, Zhu X, Lu X, Mao F, et al. Prognostic role of microRNA-21 in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2011;41:1245–1253. doi: 10.1111/j.1365-2362.2011.02535.x. [DOI] [PubMed] [Google Scholar]
- 12.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 13.Thapa DR, Li X, Jamieson BD, Martinez-Maza O. Overexpression of microRNAs from the miR-17–92 paralog clusters in AIDS-related non-Hodgkin's lymphomas. PLoS ONE. 2011;6:e20781. doi: 10.1371/journal.pone.0020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 15.Breen EC, van der Meijden M, Cumberland W, Kishimoto T, Detels R, Martinez-Maza O. The development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–299. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 16.Crabb Breen E, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller F, Aukrust P, Nordoy I, Froland SS. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood. 1998;92:3721–3729. [PubMed] [Google Scholar]
- 18.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. Plasma levels of B-lymphocyte stimulator increase with HIV disease progresssion. AIDS. 2003;17:1983–2000. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 19.Benchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 20.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, et al. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005;25:702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 21.Macchia D, Almerigogna F, Parronchi P, Ravian A, Maggi E, Romagnani S. Membrane tumor necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 22.Epeldegui M, Thapa DR, De La Cruz J, Kitchen SG, Zack JA, Martinez-Maza O. CD40 Ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PloS ONE. 2010;5:e11448. doi: 10.1371/journal.pone.0011448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbeault M, Ouellet M, Giguere K, Bertin J, Belanger D, Martin G, Tremblay MJ. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J Virol. 2011;85:2189–2200. doi: 10.1128/JVI.01993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler-Heitbrock HW, Pechumer H, Petersmann I, Durieux J-J, Vita N, Labeta MO, Strobel M. CD14 is expressed and functional in human B cells. Eur J Immunol. 1994;24:1937–1940. doi: 10.1002/eji.1830240835. [DOI] [PubMed] [Google Scholar]
- 25.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O'Shea MA, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci USA. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]