Abstract
Objective
The Acute Respiratory Distress Syndrome (ARDS), the most severe form of Acute Lung Injury (ALI), is a highly-fatal, diffuse non-cardiogenic edematous lung disorder. The pathogenesis of ARDS is unknown but lung inflammation and lung oxidative stress are likely contributing factors. Since no specific pharmacologic intervention exists for ARDS, our objective was to determine the effect of treatment with ergothioneine---a safe agent with multiple anti-inflammatory and antioxidant properties on the development of lung injury and inflammation in rats insufflated with cytokines found in lung lavages of ARDS patients.
Method
Sprague-Dawley rats (3-10/group) were given 15 mg/kg or 150 mg/kg L-ergothioneine intravenously 1 hour before or 18 hours after cytokine (IL-1 and IFNγ) insufflation. Lung injury (lavage LDH levels) and lung inflammation (lavage neutrophil numbers) were measured 24 hours after cytokine insufflation.
Results
Ergothioneine pre- and post- treatment generally decreased lung injury and lung inflammation in cytokine insufflated rats.
Conclusion
Ergothioneine should be considered for additional testing as a potential therapy for treating and preventing ARDS.
Introduction
The Acute Respiratory Distress Syndrome (ARDS), the most severe form of Acute Lung Injury (ALI), kills approximately 40% of its victims and approximately 70,000 Americans yearly (Rubenfeld 2005). ARDS is characterized by the rapid development of a bilateral non-cardiogenic lung edema that produces severe hypoxemia requiring mechanical ventilation with high concentrations of oxygen (Levitt and Matthay 2006). The pathogenesis of ARDS is unknown but lung inflammation characterized by increased pro-inflammatory cytokines, the recruitment and activation of many neutrophils, and the development of oxidative stress in the lung are prominent features and likely contributors (Fudala 2008; Moine 2000; Repine 1992; Sittipunt 2001; Suter 1992). Insufflating cytokines into rats produces many of the lung abnormalities seen in ARDS patients and, accordingly, cytokine insufflation is often used to investigate the causes and identify potential treatments for ARDS (Wright 2003). The inflammatory and oxidative stress nature of ARDS has prompted the premise that anti-inflammatory and antioxidant interventions could be effective ways to protect the lung following ARDS inciting insults (Repine 1992). No treatments have been effective in reducing the mortality of patients with established ARDS (Levitt 2006).
In the present investigation, we evaluated the effect of ergothioneine - a molecule with many anti-inflammatory and antioxidant properties (Akanmu 1991; Aruoma, 1991; Aruoma 1997; Aruoma, 2011; Dong 2007; Franzoni 2006; Paul and Snyder 2010; Rahman 2003) on lung injury and lung inflammation in cytokine insufflated rats. Unlike many other interventions that have been advanced for treating ARDS (Levitt and Matthay 2006), ergothioneine is a normal body constituent and the body has ergothioneine transporters that increase ergothioneine concentrations in specific cells including the lung (Grundermann 2005). We found that administering ergothioneine generally decreases lung injury and inflammation in rats insufflated with cytokines that are increased in lung lavages of ARDS patients.
Methods
Reagents
Most reagents, buffers and substrates were purchased from Sigma Chemical Company (St. Louis, MO). Recombinant rat IL-1a (IL-1; 500-Rl-005) and IFNγ (285-IF-100) were purchased from R & D Systems (Minneapolis, MN). Ergothioneine was synthesized by the method of Yadan et al (Yadan and Xu 1995) and obtained from Cambridge Major Laboratories (Germantown, WI).
Cytokine Insufflation
Healthy male Sprague-Dawley rats (300-400 g body weight; Sasco, Omaha, NE) were fed a regular diet ad libitum. IL-1 (50 ng) and IFNγ (50 ng) in 0.5 ml of normal saline were delivered intratracheally into anesthetized rats as previously described (Wright 2003). Control rats were untreated or insufflated with saline alone. In some experiments, rats were injected with 15 or 150 mg/kg ergothioneine intravenously 1 hour before or 18 hours after cytokine insufflation. Twenty-four hours after cytokine insufflation, rat lungs were lavaged by pumping 7.0 ml of normal saline into and out of the lung three times before collection. Use of rats was approved by the University of Colorado Institutional Review Board protocol number #21910(07)1E.
Lung injury assessment
Lactate dehydrogenase (LDH) was measured on lung lavage samples using the Promega Cytotox 96 ® kit purchased from Fisher Scientific (Houston, Tx).
Lung Inflammation measurement
Total, differential leukocyte and neutrophil counts were determined using H & E staining of lung lavage samples as previously described (Wright 2003).
Statistical Analyses
Data are expressed as the mean and standard error of the mean. Differences were assessed for significance using the Student’s t test. A p value of <0.05 was considered significant.
Results
Rats insufflated with cytokines (IL-1 and IFNγ) 24 hours before had increased lung lavage LDH concentrations compared to control rats (Figure 1). In contrast, rats treated intravenously with 15 mg/kg or 150 mg/kg ergothioneine 1 hour before cytokine insufflation, but not 150 mg/kg 18 hours after cytokine insufflation, had significantly decreased lung lavage LDH concentrations compared to untreated cytokine insufflated rats. Rats insufflated with cytokines 24 hours before had increased lung neutrophils compared to control rats (Figure 2). By comparison, rats treated intravenously with 150 mg/kg ergothioneine 1 hour before or 18 hours after cytokine insufflation, but not with 150 mg/kg ergothioneine 1 hour before cytokine insufflation, had significantly decreased lung neutrophils compared to untreated cytokine insufflated rats.
Figure 1. Effect of ergothioneine on lung injury (lavage LDH units/50 ul) in rats insufflated with cytokines (IL-1 and IFNγ).
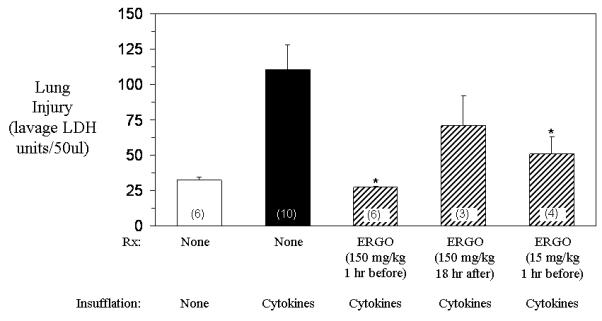
Lung injury was decreased 24 hours later in rats treated intravenously with 15 mg/kg or 150 mg/kg ergothioneine 1 hour before insufflation with cytokines, but not rats treated intravenously with 150 mg/kg ergothioneine 18 hours after cytokine insufflation, compared to untreated rats. Each value is the mean ± one standard error of the mean of the number of rats shown in the parentheses. * p value <0.05 compared to untreated cytokine insufflated rats.
Figure 2. Effect of ergothioneine on lung neutrophils (lavage neutrophils x 106) in rats insufflated with cytokines (IL-1 and IFNγ).
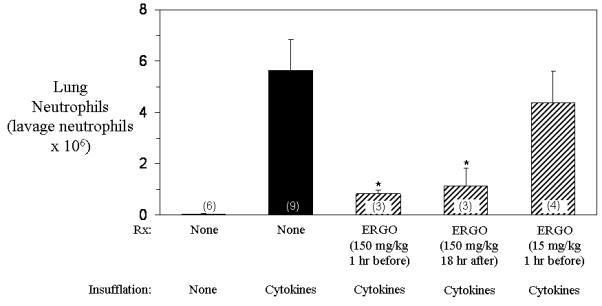
Lung neutrophils were decreased 24 hours later in rats treated intravenously with 150 mg/kg ergothioneine 1 hour before or 18 hours after insufflation with cytokines, but not rats treated intravenously with 15 mg/kg ergothioneine 1 hour before cytokine insufflation, compared to untreated rats. Each value is the mean ± one standard error of the mean of the number of rats shown in the parentheses. * p value <0.05 compared to untreated cytokine insufflated rats.
Discussion
We found that ergothioneine treatment decreases lung injury and inflammation in cytokine insufflated rats. Ergothioneine given intravascularly at doses of 15 or 150 mg/kg 1 hour before or 150 mg/kg 18 hours after cytokine insufflation was generally protective.
Ergothioneine appears worthy of additional study and further consideration as a therapy for ARDS. First, L-ergothioneine is a stable, water soluble, naturally occurring 2-thio-imidazole amino acid that exists predominantly in the thione form which does not autoxidize at physiologic pH in aqueous solution (Yadan and Xu 1995; Aruoma, 2011; Figure 3). Second, ergothioneine intersects a number of the putative mechanisms responsible for ARDS development (Repine 2011). The most notable include the ability of ergothioneine to scavenge free radicals (notably hydroxyl radical, peroxinitrite and hypochlorous acid), chelate divalent metals and increase levels of glutathione which decrease in ARDS patients (Akanmu 1991; Aruoma, 1997; Bernard 1997; Dong 2007). The latter properties make ergothioneine similar to N-acetylcysteine - another thiol compound that has had some success in the treating patients with ARDS (Bernard 1997). Ergothioneine also inhibits NF-kβ activation and limits the transcriptional activation of the gene for IL-8 – a chemotaxin which might account for neutrophil recruitment into lungs of patients with ARDS (Dong 2007; Fudala 2008) especially since macrophages recovered from ARDS patients have increased NF-kβ activities (Moine 2000). Third, a specific ergothioneine transporter exists which can increase the concentrations of ergothioneine intracellularly (Grundemann 2005). Cells expressing the ergothioneine transporter accumulate high levels of ergothioneine and monocytes express high concentrations of the transporter. Fourth, ergothioneine is normally in the body localized in certain tissues including the lung (Aruoma, 2011). Fifth, ergothioneine has an extensive safety record in humans (Schauss, 2010; Schauss, 2011).
Figure 3.
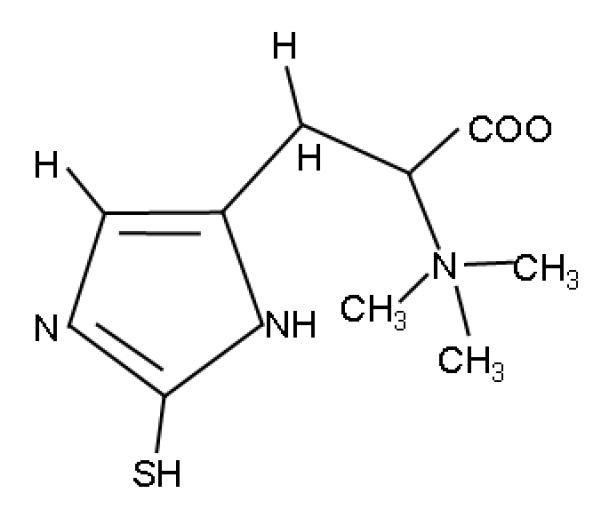
Structure of ergothioneine.
The potential beneficial effects of ergothioneine even when given after ARDS has been initiated by cytokine insufflation may be significant especially since the present treatment of ARDS begins only after ARDS is established. Unfortunately, at this time, many of these individuals are critically ill and in some instances may have already started to develop multiple organ failure---a common complication of ARDS (Deitch and Goodman 1999). The lack of success in treating established ARDS has prompted efforts to prevent ARDS. However, the development of ARDS in at-risk individuals is relatively uncommon, reportedly about 7%, when all predisposing conditions are analyzed in aggregate (Gajic 2010). Consequently, many individuals would need to be treated prospectively to prevent ARDS development in a relative few individuals. Although sophisticated clinical scoring systems are being developed and tested that might increase the predictability of an at-risk individual developing ARDS, predictability is now limited to approximately 18% and depends on the predisposing condition (Gajic 2010). Additional efforts using biomarkers are being evaluated that may further identify a sub-group of ARDS at-risk subjects who are more likely to develop ARDS. As these prediction strategies are improved and validated, safe therapies such as ergothioneine need to be tested to determine their potential in treating and/or preventing ARDS (Connelly and Repine 1997; Levitt and Matthay 2006; Repine 2011).
Conclusion
Ergothioneine, a safe natural compound with multiple anti-inflammatory and antioxidant properties, reduces lung inflammation and injury in rats insufflated with cytokines. Ergothioneine may be a candidate for treating or preventing ARDS but will need to be tested appropriately in clinical trials.
Highlights.
>ARDS is a highly-fatal, untreatable condition.
>Ergothioneine is an effective anti-inflammatory antioxidant.
>Ergothioneine decreases inflammation and injury in cytokine insufflated rats.
>Ergothioneine may be a candidate for treating ARDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: John E. Repine, MD owns stock, is a scientific advisory board member, and has a joint venture agreement with Oxis International for developing ergothioniene as an ARDS treatment for which a patent is pending.
Bibliography
- Akanmu D, Cecchini R, Aruoma OI, Halliwell B. The antioxidant action of ergothioneine. Arch Biochem Biophys. 1991;288:10–16. doi: 10.1016/0003-9861(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Whiteman M, England TG, Halliwell B. Antioxidant action of ergothioneine: Assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Comm. 1997;231:389–391. doi: 10.1006/bbrc.1997.6109. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Spencer JP, Mahmood N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem Toxicol. 1999;37:1043–1053. doi: 10.1016/s0278-6915(99)00098-8. [DOI] [PubMed] [Google Scholar]
- Auroma OI, Coles S, Landes B, Repine JE. Characteristics and bioefficacy of ergothioneine---a unique natural dietary antioxidant. J Age Management Med. 2011 (in press) [Google Scholar]
- Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS: The Antioxidant ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- Connelly KG, Repine JE. Markers for predicting the development of acute respiratory distress syndrome. Annu Rev Med. 1997;48:429–445. doi: 10.1146/annurev.med.48.1.429. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Goodman ER. Prevention of multiple organ failure. Surg Clin North Am. 1999;79:1471–1488. doi: 10.1016/s0039-6109(05)70088-8. [DOI] [PubMed] [Google Scholar]
- Dong KK, Damaghi N, Kibitel J, Canning MT, Smiles KA, Yarosh DB. A comparison of the relative antioxidant potency of L-ergothioneine and idebenone. J Cosmet Dermatol. 2007;6:183–188. doi: 10.1111/j.1473-2165.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Franzoni F, Colognato R, Galetta F, Laurenza I, Barsotti M, Di Stefano R, Bocchetti R, Regoli F, Carpi A, et al. An in vitro study on the free radical scavenging capacity of ergothioneine: comparison with reduced glutathione, uric acid and trolox. Biomed Pharmacother. 2006;60:453–457. doi: 10.1016/j.biopha.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Fudala R, Krupa A, Stankowska D, Allen TC, Kurdowska AK. Anti-interleukin-8 autoantibody: interleukin-8 immune complexes in acute lung injury/acute respiratory distress syndrome. Clin Sci (Lond) 2008;114:403–412. doi: 10.1042/CS20070272. [DOI] [PubMed] [Google Scholar]
- Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, 3rd, Hoth JJ, Mikkelsen ME, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2010;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schomig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JE, Matthay MA. Treatment of Acute Lung Injury: Historical perspectives and potential future therapies. Semin Respir Crit Care Med. 2006;27:426–438. doi: 10.1055/s-2006-948296. [DOI] [PubMed] [Google Scholar]
- Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, Moore EE, Abraham E. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000;13:85–91. doi: 10.1097/00024382-200013020-00001. [DOI] [PubMed] [Google Scholar]
- Paul BD, Snyder SH. The usual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17:1134–1140. doi: 10.1038/cdd.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Gilmour PS, Jimenez LA, Biswas SK, Antonicelli F, Aruoma OI. Ergothioneine inhibits oxidative stress- and TNF-alpha-induced NF-kappa B activation and interleukin-8 release in alveolar epithelial cells. Biochem Biophys Res Commun. 2003;21:860–864. doi: 10.1016/s0006-291x(03)00224-9. [DOI] [PubMed] [Google Scholar]
- Repine JJD, Crader KM, Elkins ND, Wilson P, Repine JE. Preventing the Acute Respiratory Distress Syndrome. Prevent Med. 2011 doi: 10.1016/j.ypmed.2011.12.007. (In press) [DOI] [PubMed] [Google Scholar]
- Repine JE. Scientific perspectives on the adult respiratory distress syndrome. Lancet. 1992;339:466–469. doi: 10.1016/0140-6736(92)91067-i. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcome of acute lung injury. N Engl J Med. 2005;353:685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J. 2002;16:1713–1720. doi: 10.1096/fj.02-0331com. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Vertesi A, Endres JR, Hirka G, Clewell A, Qureshi I, Pasics I. Evaluation of the safety of the dietary antioxidant ergothioneine using the bacterial reverse mutation assay. Toxicology. 2010;278:39–45. doi: 10.1016/j.tox.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Beres E, Vertesi A, Frank Z, Pasics I, Endres J, Aruoma OI, Hirka G. The effect of ergothioneine on clastogenic potential and mutagenic activity: Genotoxicity evaluation. Int J Toxicol. 2011 doi: 10.1177/1091581811405856. in press. http://ijt.sagepub.com/content/early/2011/07/16/1091581811405856. [DOI] [PubMed] [Google Scholar]
- Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- Wright RM, Ginger LA, Kosila N, Elkins ND, Essary B, McManaman JL. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am J Respir Cell Mol Biol. 2003;30:479–490. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- Yadan JC, Xu J. Process for the preparation of ergothioneine. number 5, 438, 151 US patent. 1995


