Abstract
The development of engineered skeletal muscle would provide a viable tissue for replacement and repair of muscle damaged by disease or injury. Current tissue engineering methods result in three-dimensional (3-D) muscle constructs that generate tension, but do not advance phenotypically beyond neonatal characteristics (Larkin et al., 2006). To develop to an adult phenotype, innervation and vascularization of the construct must occur. In this study, 3-D muscle constructs were implanted into the hindlimb of a rat along the sciatic nerve with the sural nerve isolated, transected and sutured to the construct to encourage innervation. Aortic ring anchors were sutured to the tendons of the biceps femoris muscle so that the construct would move dynamically with the endogenous muscle. After 1 week in vivo, constructs were explanted, evaluated for force production, and stained for muscle, nerve, and collagen markers. Implanted muscle constructs showed a developing capillary system, an epimysium-like outer layer of connective tissue, and an increase in myofiber content. The beginning of alpha-bungarotoxin clustering suggests that neuromuscular junctions (NMJ) could form on the implanted muscle given more time in vivo. Additionally, the constructs increased maximum isometric force from 192±41μN to 549±103μN (245% increase) compared to in vitro controls that increased from 276±23μN to 329±27μN (25% increase). These findings suggest that engineered muscle tissue survives 1 week implantation and begins to develop the necessary interfaces needed to advance the phenotype toward adult muscle. However, in terms of force production, the muscle constructs need longer implantation times to fully develop an adult phenotype.
Keywords: tissue engineering, implantation, skeletal muscle, innervation, vascularization
1. Introduction
The development of engineered skeletal muscle would provide a viable option for replacement and repair of damaged skeletal muscle in humans as well as a viable model for studying the effects of stressors and chemicals on muscle (Khodabukus et al., 2007). The current method for skeletal muscle repair, the muscle flap, is hindered by limited tissue availability and donor site morbidity. Our laboratory has developed a reproducible method for development of scaffold-less engineered muscle constructs. (Larkin et al., 2006). The engineered muscle we fabricate consists of a 3D construct with a fibroblast-produced extracellular matrix and myotubes aligned along the axis of strain. This in vitro construct can generate force either spontaneously or in response to electrical field stimulation. Engineered skeletal muscle is limited in vitro in that it does not develop beyond a neonatal phenotype in terms of strength characteristics and tissue organization (Larkin et al., 2006; Huang et al., 2010).
Two interfaces that we aimed to establish in our implanted constructs are neural and vascular. Our laboratory has enhanced engineered muscle in vitro through the co-culture of muscle and fetal nerve cells (Larkin et al., 2005). Other work has been done to successfully enhance scaffolded engineered muscle function by mimicking neural stimulation through bioreactors that deliver cyclic electric pulses (Donelly et al., 2010). In order for engineered muscles to grow to physiological sizes, a vascular network must be established. Dennis and Kosnik showed that myoblasts are unable to differentiate and proliferate more than 150μm away from oxygen and nutrient supplies (Dennis and Kosnik, 2000). Other research groups have proven that the foreign body response observed during in vivo implantation supports the in-growth of a capillary network into the implanted constructs (Beier et al., 2006; Saxena et al., 2001).
In addition to the benefits of neural and vascular interfaces, we expected our constructs to demonstrate a positive response to the cyclic mechanical stretching provided by the movement of the host muscles. Mechanical stimulation in vitro has been performed using bioreactors on scaffolded muscle tissue (Gonen-Wadmany et al., 2004; Powell et al., 2002) to enhance skeletal muscle phenotype. Gonen-Wadmany et al. (2004) showed that mechanical stimulation improves cell-mediated collagen compaction, resulting in improved cellular alignment. Thus, we hypothesized that the implantation of our muscle construct in an anatomical location to provide mechanical stretch during ambulation of the animal would enhance the phenotype when compared with the maintained in vitro construct.
The purpose of this research was to investigate the effect of one week of in vivo implantation on scaffold-less engineered skeletal muscle constructs with respect to contractile properties, structure, vascularization and innervation. We hypothesized that the one week of implantation of muscle constructs would shift the phenotype from neonatal toward adult and that muscle constructs would exhibit an increase in force production.
2. Materials and Methods
2.1 Animal model and animal care
Tissue engineering studies were carried out using muscle tissue from female Fischer 344 rats obtained from the Charles River Laboratories, Inc. (Wilmington, MA). All animals were acclimated to our colony conditions, i.e., light cycle and temperature, for 1 week prior to any procedure. The animals were fed Purina Rodent Chow 5001 laboratory chow and water ad libitum. Surgical procedures were performed to remove both soleus muscles and aorta from a subset of the female Fisher rats. These rats were obtained as retired breeders with an estimated age of 8-9 months of age. These harvested tissues were then used as an allogenic cell source for muscle precursor cells and anchoring tissues (aortic rings) for the constructs fabricated and implanted into the host animals. All surgical procedures were performed in an aseptic environment with animals in a deep plane of anesthesia induced by i.p. injections of sodium pentobarbital (65 mg/kg). Supplemental doses of pentobarbital were administered as required to maintain an adequate depth of anesthesia. All animal care and animal surgery procedures were in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85-23); the experimental protocol was approved by the University Committee for the Use and Care of Animals.
2.2 Preparation of Media
Unless otherwise indicated, all solutions and media were prepared and stored at 4°C prior to isolation and culture of cells and warmed to 37°C in a heated water bath immediately prior to use. Muscle growth medium (MGM) contained 300 mL of F-12 Kaighn's Modification Nutrient Mixture (Gibco BRL Cat# 21127-022), 125 mL of Dulbecco's Modified Eagle Medium (DMEM, Gibco BRL Cat# 11995-065), 75 mL of FBS (Gibco BRL Cat# 10437-028), 2.4ng/mL basic fibroblast growth factor (bFGF, Peprotech) and 5 mL of antibiotic-antimycotic (ABAM, Gibco BRL Cat# 15240-062). Muscle differentiation medium (MDM) was composed of 330 mL M199 (Gibco BRL Cat# 11150-059), 140 mL DMEM, 33mL FBS, 500 μl insulin transferring selenite-X (ITS-X) (1/1000 dilution), 362 μl 50 μM ascorbic acid 2-phosphate (Sigma Aldrich), and 5 mL ABAM (Gibco BRL Cat# 15240-062). Transport Medium (TM) consisting of Dulbecco's Phosphate-Buffered Saline (DPBS) pH 7.2 (Gibco BRL Cat# 14190-144) supplemented with 2% ABAM was used to transfer freshly isolated tissues from the surgical suite to the tissue culture facilities for isolation.
2.3 Preparation of Construct Dishes
Muscle constructs were engineered in individual 60 mm plates as described earlier (Larkin et al., 2006). Each 60 mm plate was coated with 5 ml of Sylgard (Dow Chemical Corporation, Midland, MI, type 184 silicon elastomer) and allowed to cure for 3 weeks prior to use. One-to-seven days prior to use, Sylgard coated plates were coated with laminin at 0-.71μg/cm2 per plate (20 μg of Natural Mouse Laminin [Gibco BRL Cat# 23017-015] and 4 ml of Dulbecco's Phosphate-Buffered Saline (DPBS) pH 7.2 [Gibco BRL Cat# 14190-144] per plate) and left to dry for 48 h. Salt crystals were dissolved and removed by rinsing the plates with 4 ml DPBS. The plates were then filled with 3 ml of previously described MGM and decontaminated with UV light (wavelength 253.7 nm) for 60 minutes and placed in a 37°C 5% CO2 incubator for up to one week prior to plating muscle cells.
2.4 Preparation of acellular aorta ring anchors
The descending aortas of Fischer 344 rats were surgically removed under aseptic conditions. Cells were removed from the aorta leaving just the extracellular matrix by immersing the tissue sequentially in one of five detergent solutions over a period of 15 days as outlined in Haase et al. (2003). The compositions of the solutions were as follows: Solution 1 - 7.3g ethylenediaminetetraacetic acid (EDTA), 0.5g sodium azide (NaN3), 800 mL Glycerol, 200 mL 0.9% NaCl; Solution 2 - 25g sodium deoxycholate; 0.26g NaN3, 600 mL distilled, and deionized H2O; Solution 3 -10g sodium dodecylsulfate (SDS), 0.52g NaN3, and 1000 mL distilled, deionized H2O; Solution 4 - 0.5g NaN3, 1000 mL 0.9% saline; Solution 5 - 15 mL Triton X-100, 0.25g NaN3, and 485 mL distilled H2O.
2.5 Dissection of Muscle and Isolation of Muscle Cells
Both soleus muscles were surgically removed under aseptic conditions, weighed, sterilized in 70% ETOH. Prior to dissociation, muscles were incubated in TM (at a concentration of 5mL per muscle dissected) for 5 minutes. A 20 mL dissociation solution consisting of 32 units of Dispase (Sigma Cat# P-3417; 0.4 units/mg) and 2390 units of Type 4 Collagenase (Gibco BRL Cat# 17104-019); 239 units/mg) per mL Ham's F12 (Gibco BRL Cat# 11765-054) was utilized. Two soleus muscles were dissociated for each 20 mL solution for a minimum of a 1.5 hour time period. The disassociated cell solution was poured through a 100μm filter, centrifuged and aspirated prior to being resuspended in 15 mL MGM and pre-plated overnight in a 100mm tissue culture dish. The supernatant from the pre-plate was removed and brought to a density of 1.3∧105 cells/mL. Three mLs of the cell suspension were plated in each of the previously prepared laminin-coated culture dishes and placed in a 37°C 5% CO2 incubator. Culture plates were not disturbed for at least 72 hours to allow cell adherence to the plates.
2.6 Preparation of Muscle Constructs
Muscle constructs were fabricated as previously described by Larkin et al. (2006) with the exception that aortic rings were used as anchors instead of adult rat tail tendon. Briefly, five days after initial plating, the cells were fed with MGM every 48 hours until the cells became confluent (approximately 7 days). Once the cells achieved confluence, they were fed with MDM every 48 hours until the myocytes fused to form multinucleated myotubes that began to contract spontaneously. At this time point, the aortic rings were pinned as anchors with minutien pins onto the muscle monolayer at a separation distance of 15mm. The aortic rings act as anchors and enable the muscle monolayer to roll up into a 3-D cylinder. Approximately 7 days later, a cylindrical 3-D muscle construct was formed in each dish. The cells were fed MDM every 48 hours during the co-culture of the muscle constructs. Five days post construct formation; the muscle constructs were tested for contractile function as described in section 2.8 below. At the conclusion of functional tests, each specimen was snap frozen in Tissue Freezing Media (Triangle Biological Sciences, Durham, NC) in isopentane chilled with dry ice, and stored at −80°C for subsequent histology.
2.7 Implantation of muscle constructs
F344 retired breeder rats were anaesthetized using isoflourane; the analgesic, Carprofen (5 mg/kg) was administered following induction of anesthesia and every 12 hours for 48 hours post-surgery. The left leg vastus lateralis and biceps femoris muscles were separated, exposing the sciatic nerve. Approximately 10mm of sural nerve was separated from the peroneal and tibial nerves and from surrounding connective tissue. A small surface blood vessel from the medial edge of the biceps was dissected exposing a 10 mm section, with the proximal end open. Muscle constructs were surgically placed in the hindlimb alongside the femur. The transected ends of sural nerve and blood vessel were placed next to the muscle belly and sutured to the construct to encourage innervation and vascularization of the construct. The aortic rings of the constructs were sutured with 9-0 suture silk to the proximal and distal tendons of the biceps femoris muscle so that they would experience mechanical stresses and move dynamically with the endogenous muscle. The vastus lateralis and biceps femoris muscles were sutured together using 7-0 suture (Ethicon) and the skin incision was closed using 9mm autoclips (Becton Dickinson). Animals were allowed to recover for 7 days. At the end of the 7 day implantation, the muscle constructs were dissected, placed in MDM, and contractile properties were measured.
2.8 Contractile Property Testing
Contractile properties were measured prior to (Day 0) and seven days after implantation of the muscle constructs. The protocol for measuring contractility of engineered muscle constructs was adapted from Dennis and colleagues (Dennis and Kosnik, 2000; Dennis et al., 2001; Kosnik et al., 2001) and Irintchev et al. (Irintchev et al., 1998). Briefly, the pin on one end of the construct was freed from the Sylgard and attached to a force transducer with canning wax. For field stimulation of the entire construct, platinum wire electrodes were positioned on either side of the construct. The temperature of the construct was maintained at 37±1°C using a heated aluminum platform. The diameter of the construct was determined and used to calculate cross-sectional area, assuming a circular cross section. Passive baseline force was measured as the average baseline passive force preceding the onset of stimulation. Twitches were elicited using a single 2.5 ms pulse at 10, 30, 60, and 90 mA, whereas maximum tetanic force was determined using a 1-s train of 2.5-ms pulses at 90 mA and 10, 20, 40, 60 and 80 Hz. Data files for each peak twitch force and peak tetanic force trace were recorded and subsequently analyzed using LabVIEW data acquisition software. Peak tetanic force was normalized for cross-sectional area to determine maximum specific force. Following the direct field stimulation of the entire construct, a micro-electrode was used to electrically stimulate the neural extensions projecting from the explanted constructs using the same stimulation parameters as described above for the field stimulation.
2.9 Histochemical and Immunohistochemical Analysis of 3D Muscle Constructs
Unfixed muscle constructs were placed into TBS medium (Triangle Biological Sciences, Durham, NC), frozen in cold isopentane and stored at −80°C until needed. Samples were sliced in the muscle portion of the construct to obtain cross sections with a cryostat at a thickness of approximately 12 μm, adhered to Superfrost Plus microscopy slides and used for staining. Sections were stained for general morphology observations with hematoxylin and eosin (H&E) as previously described (Luna, 1968). For immunohistochemical analysis frozen sections were fixed with ice cold methanol for 10 min and rinsed with Phosphate Buffered Saline (PBS). Sections were blocked for 30 min with PBS-0.05%Tween20 (PBST) containing 20% calf serum (PBST-S) at room temperature. Sections were incubated overnight at 4°C with the primary antibodies diluted in PBST-S. Immunofluorescent staining with specific antibodies was performed to detect the presence of myosin heavy chains (MCH; MF-20 mouse monoclonal antibody 1:5 dilution, obtained from the Developmental Studies Hybridoma Bank, Iowa City, IA), alpha-actinin (mouse monoclonal antibody;, 1:200 dilution, Sigma, St. Louis, MO), nebulin (mouse monoclonal antibody, 1:100 dilution, Abcam, Cambridge, MA), PAX7 (rabbit polyclonal antibody, 1:100 dilution, Abcam, Cambridge, MA), collagen type 1 (rabbit polyclonal antibody, 5 μg/mL, Chemicon International, Temecula, CA), blood vessels (rabbit polyclonal anti-CD31 antibody; 10 μg/ml; Abbiotec, San Diego, CA), and nerve fibers (rabbit polyclonal anti-S-100 antibody, 1:100 dilution, Abcam, Cambridge, MA, and mouse monoclonal anti-pan-axonal neurofilament marker SMI312 antibody, 1:500, Covance, Emeryville, CA). Following three washes in PBST a one hour room temperature incubation with Cy3-conjugated anti-mouse or anti-rabbit antibody (Jackson ImmunoResearch Lab., West Grove, PA) was used for visualization. Following three washes in PBST nuclei were stained by 5 min incubation with DAPI solution (Sigma, St. Louis, MO) in PBST. As per previously described methods of Kostrominova (2011), Staining of sections with fluorescein labeled wheat germ agglutinin (WGA; 5 μg/ml for 5 min; Molecular Probes, Eugene, OR) was used for visualization of connective tissue. For visualization of developing neuromuscular junctions, muscle sections were incubated for 10 min at room temperature in the solution of alpha-bungarotoxin (alpha-BTX-rhodamine; Sigma, St. Louis, MO; 1 μg/mL). The sections were examined and photographed with a Leica microscope and cross-sections of the constructs were analyzed with a software package, Image J.
2.10 Statistics
Values are presented as means ± SE. Statistical analysis was performed by using Staveiw (SAS Institute Inc., Cary, NC). A one way analysis of variance (ANOVA) was conducted to compare the differences between muscle constructs implanted in vivo for one week and in vitro constructs. Differences were considered significant at P < 0.05.
3. Results
3.1 Morphology of muscle constructs
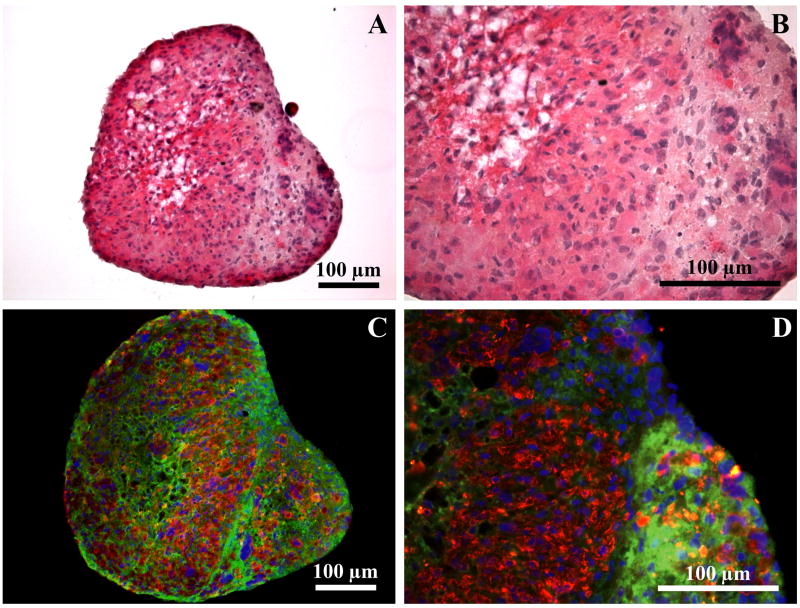
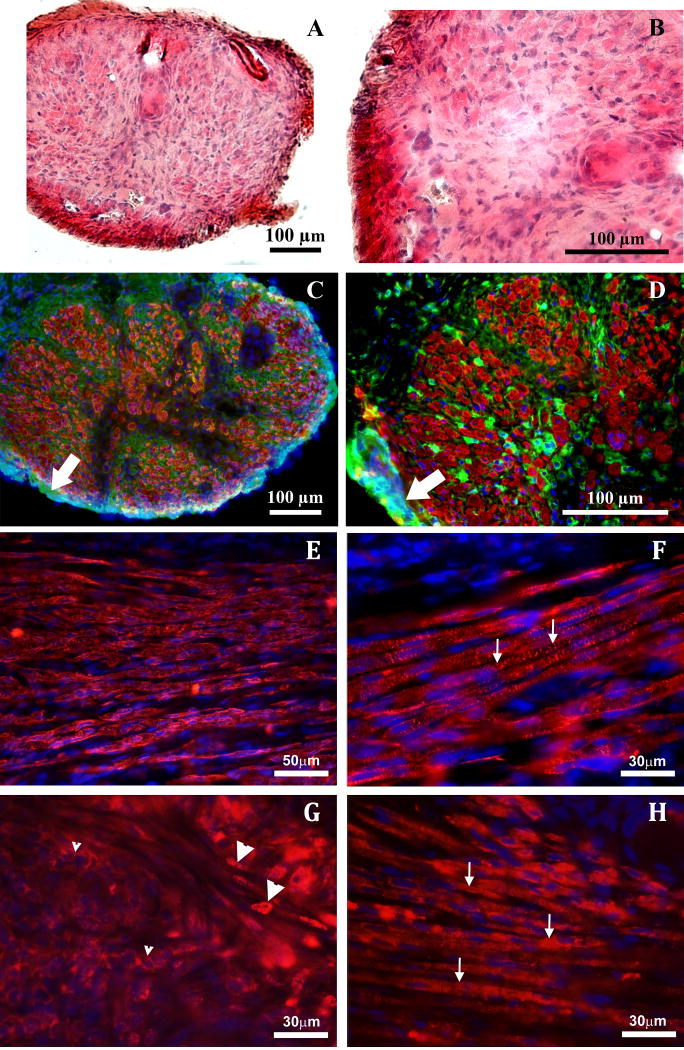
H&E staining of the engineered muscle constructs was performed in order to compare general morphology of in vitro constructs (Figures 1A and 1B) with one week implanted muscle constructs (Figures 2A and 2B). Immunostaining was used to visualize sarcomeric myosin heavy chains (red) and collagen type I (green) in the in vitro (Figures 1C and 1D) compared to implanted (Figures 2C and 2D) constructs. Cell nuclei were visualized with DAPI (blue). Collagen 1 immunostaining showed a distinct epimysium-like outer layer of collagen formed at the periphery of explanted constructs (arrows in Figures 2C and 2D). Additional muscle marker staining for alpha-actinin and nebulin show striations due to developed sarcomeres (Figures 2F and 2H) and staining for PAX7 showed differentiating multinucleated myosin (Figure 2G). Sarcomeric myosin was present at the higher level in the one week implanted compared to in vitro control constructs. Analysis of cross-sections of the constructs with a software package, Image J, showed 20.2% by area more myofiber staining in implanted constructs than in in vitro controls (66.5±2.1% of the total area versus 45.3±4.9%, respectively).
Figure 1.
Cross-section of an in vitro muscle construct stained with Hematoxylin and Eosin (A and B) and for myosin heavy chains (red), collagen I (green), and nuclei (DAPI, blue) (C and D).
Figure 2.
Cross-section of an explanted muscle construct stained with Hematoxylin and Eosin (A and B) and for myosin heavy chains (red), collagen I (green), and nuclei (DAPI, blue) (C and D). Immunostaining of the longitudinal sections of muscle construct after implantation in vivo with antibodies against MCH (E), alpha-actinin (F), PAX7 (G) and nebulin (H). Nuclei were stained with DAPI (blue in E-H). Small arrows in F and H indicate striations due to the presence of the developed sarcomeres. Small arrowheads in G indicate perinuclear staining of the non-differentiated myoblasts. Large arrowheads in G indicate nuclear staining of the myoblasts in the process of differentiation and fusion.
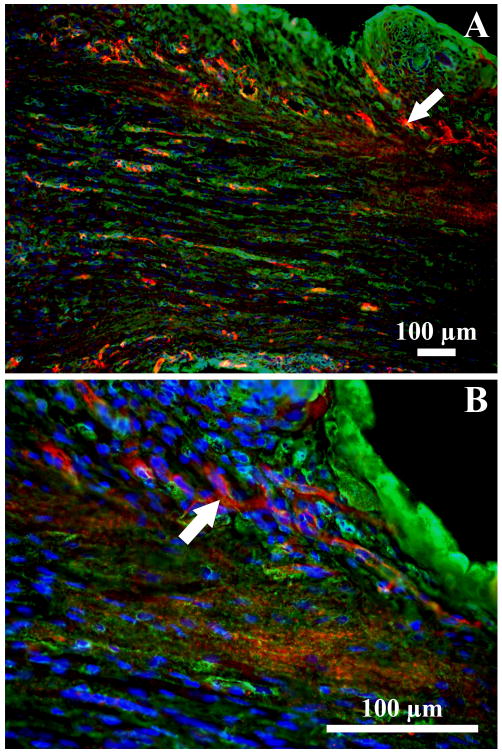
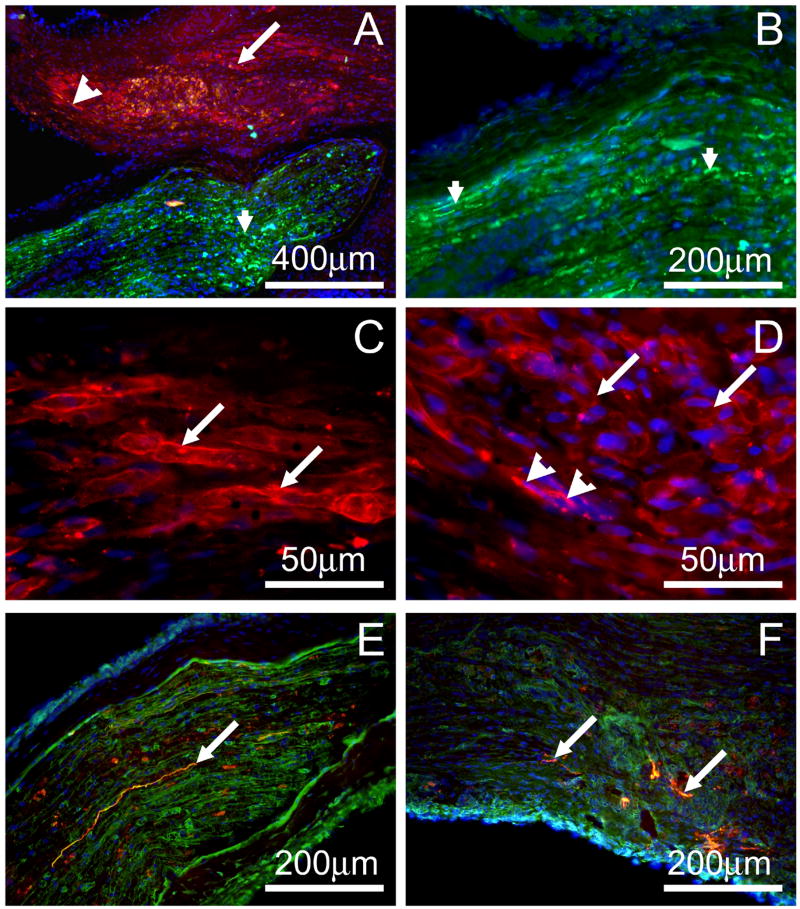
Longitudinal sections of the implanted muscle constructs stained with antibodies against CD31 indicated that after just one week, there was vascularization of the constructs (Figures 3A and 3B). Immunostaining with anti-hemoglobin antibody to demonstrate vessel functionality could not be performed due to the developing muscle being stained as well, probably due to the cross-reaction with myoglobin (data not shown). However, the CD31 staining provides good evidence of a developing vasculature in one week explants. Staining with antibodies against NCAM showed nerve fibers in the sural nerve located in the proximity to the muscle constructs (small arrowheads in Figures 4A and 4B). There was no NCAM-positive immunostaining in the muscle constructs. Staining with alpha-bungarotoxin for acetylcholine receptors showed intensive staining of the plasma membrane of the developing muscle fibers (arrows in Figures 4A, 4C and 4D). In a few areas clustering of the acetylcholine receptors was observed (large arrowheads in Figures 4A and 4D) suggesting an initiation of neuromuscular junction formation. Immunostaining with antibodies against pan-axonal neurofilament marker showed presence of nerve fibers in the sural nerve located in the proximity to the muscle construct (arrow in Figure 4E). We were able to detect nerve fiber sprouting in one section of one muscle construct (arrows in Figure 4F) suggesting the outgrowth of axonal processes from the sural nerve into the muscle construct. However, functional testing suggested that one week implanted muscle constructs did not have well formed neuromuscular junctions capable of transmitting action potentials to the muscle. Aortic rings proved an acceptable anchor for the constructs as the aorta-muscle interface stayed intact during all testing (data not shown).
Figure 3.
Longitudinal section of an explanted muscle construct stained for blood vessels (CD31, red), connective tissue (WGA, green), and nuclei (DAPI, blue) (A and B).
Figure 4.
Longitudinal section of an explanted muscle construct stained with antibodies against S100 to visualize nerve fibers (green in A and B), alpha-bungarotoxin to visualize acetylcholine receptors (red in A, C and D) and with antibodies against axonal neurofilament marker (red in E and F). Nuclei were stained with DAPI (blue in Figures A-F). Connective tissue in E and F was stained with WGA lectin (green). Small arrowheads in A and B indicate nerve fibers in the sural nerve placed in the proximity of the muscle construct. Arrows in A and C indicate developing muscle fibers with alpha-bungarotoxin stained plasma membrane. Large arrowheads in A and D indicate small clusters of acetylcholine receptors at the developing muscle fibers. Arrows in E and F indicate nerve fibers in sural nerve and muscle construct, respectively.
3.2 Contractile Properties
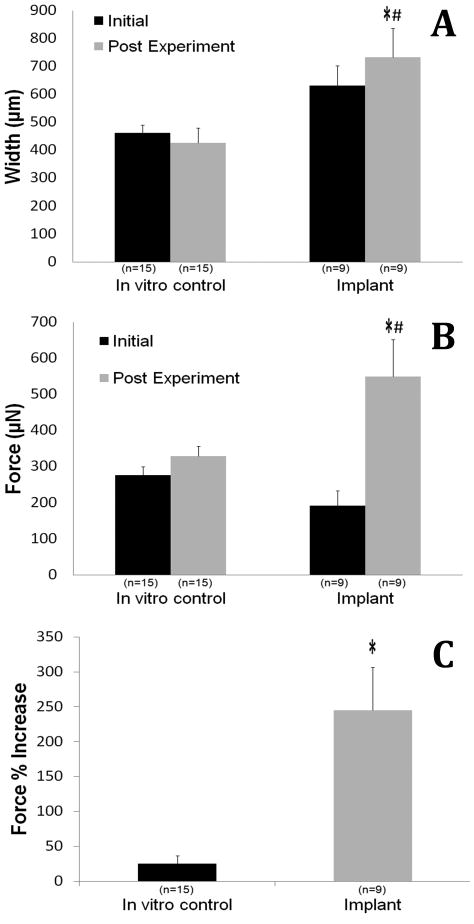
The muscle constructs exhibited spontaneous baseline activity and could elicit both a twitch and a tetanus response to field stimulation. Following one week of implantation, the explanted muscle constructs increased in size on average from 630±73μm to 732±105μm while the in vitro constructs shrank, but not significantly, changing on average from 462±29μm to 427±53μm (Figure 5A). The implanted constructs increased maximum isometric force from 192μN to 549μN (245% increase) compared to in vitro controls that increased from 276μN to 329μN (25% increase) over the same time period (Figure 5B and 5C). The change in isometric force generated between the two groups was significant (P > 0.05). Following the field stimulation of the muscle construct, microelectrodes were used to electrically stimulate the neural extensions radiating from the construct. In all 9 implanted constructs, there was no response to direct stimulation of the regenerating nerve extension, indicating a lack of functional innervation (data not shown).
Figure 5.
Contractile properties measured prior to (Day 0■, labeled Initial) and seven days after implantation (Day 7
 , labeled Post Experiment) of the muscle constructs. The (A) construct width, (B) maximum isometric force and (C) maximum specific force. Values are means ± SEM. A one way ANOVA was performed on all groups to detect significant differences between the in vitro controls vs the implants and the initial (Day 0) force production vs the Day 7 force production. * indicates a significant difference (P>0.05) in implants vs controls at the same experimental time point. # indicates a significant difference (P>0.05) in implants Day 0 vs Day 7 experiment.
, labeled Post Experiment) of the muscle constructs. The (A) construct width, (B) maximum isometric force and (C) maximum specific force. Values are means ± SEM. A one way ANOVA was performed on all groups to detect significant differences between the in vitro controls vs the implants and the initial (Day 0) force production vs the Day 7 force production. * indicates a significant difference (P>0.05) in implants vs controls at the same experimental time point. # indicates a significant difference (P>0.05) in implants Day 0 vs Day 7 experiment.
4. Discussion
The purpose of this research was to investigate the effect of a one week in vivo implantation on advancement of phenotype in scaffold-less engineered skeletal muscle constructs. We hypothesized that one week of implantation with mechanical stimulation and surgical supplementation of nerve and vasculature would shift the phenotype of the engineered muscle from neonatal toward adult and that muscle constructs would exhibit an increase in force production.
Following one week exposure to the in vivo environment, including mechanical stresses, the muscle constructs increased isometric force production. Previous studies from our lab showed that when muscle constructs were exposed to one week of innervation with the tendon ends of the construct sutured to the belly of a muscle it resulted in necrosis of the muscle tissue (data not shown). Although a blood vessel was not routed to the constructs in this pilot study, the constructs were quickly encapsulated in a fibrotic sheath and vascularized. This suggests that the introduction of the blood vessel and/or the mechanical stresses placed on the construct by anchoring it to the tendons of the biceps femoris drove the construct to develop a more adult phenotype. This increase in adult phenotype was concluded from the data measuring a 20.2% increase in the myosin staining in the muscle tissue. In addition to the increase in myosin staining, we observed that the cross-sections of the implanted construct were larger. Assessment of the construct in longitudinal sections indicated an increase in the longitudinal alignment of muscle fibers along the axis of the muscle from end to end.
The beneficial effect of neurotization on muscle tissue development has been studied extensively (Baltich et al., 2006; Das et al., 2010). The appearance of small acetylcholine receptor clusters in our explants in the proximity to the junction of endogenous nerve and engineered muscle tissue suggests that the muscle tissue responds to the regenerating nerve signaling by clustering of acetylcholine receptors. In a similar study performed on scaffolded muscle by Dhawan et al (2007), scaffolded constructs were implanted with or without nerve transection to promote neurotization of the constructs. In the Dhawan et al (2007) study, the explanted neurotized constructs generated a force of 649 μN while explanted non-neurotized constructs generated only 124 μN. The Dhawan et al (2007) findings support our hypothesis that neurotization will play a large role in increasing the maximum isometric force of our constructs as compared to in vitro controls given more time in vivo. As stated above, unpublished pilot studies from our lab in which nerve but not vasculature was supplied to the regenerating muscle in vivo suggest that immediate vascularization may initially play a bigger role in tissue survival than innervation. Two- and four-week implants have been proposed as a future research goal for our lab to improve the level of neurotization and vascularization of the constructs.
Mechanical stimulation has been determined to play an important role in the development of skeletal muscle in vitro. In a 2010 study, Candiani et al. showed that ten days on a bioreactor resulted in an eight-fold increase in myosin accumulation in stretched constructs as compared to static controls. Comparatively, Figures 1C and 2C show an increase in staining for myosin heavy chain in implanted constructs as compared to in vitro controls. In support of this data was an increase in force production. Future studies from our lab will look at the contribution of mechanical stress on the development of a more advanced phenotype by placing a subset of engineered muscle constructs on bioreactors prior to implantation. The timing for muscle development must be weighed with the decreased diffusion of substrate into larger more developed muscle constructs in vitro and increased risk of cell death in the central area of the constructs due to the lack of nutrients and oxygen.
In conclusion, one week of an in vivo environment resulted in constructs with multinucleated muscle fibers and alpha-actinin stained sarcomeres, as well as greater force producing capabilities. This advancement of muscle phenotype seen after implantation brings us closer to our goal of using engineered muscle constructs for tissue repair. Future studies in our laboratory will continue to examine the development of the neural and vascular interfaces with our engineered muscle constructs and the viability of replacing whole muscles with engineered tissue.
Acknowledgments
This research was supported by a NIH, NIAMS, NIBIB funded grant R01 AR054778-02. We acknowledge Rose Lee and Aaron Adams for technical assistance.
References
- Baltich J, Hatch-Vallier L, Adams AM, Arruda EM, Larkin LM. Development of a scaffoldless three-dimensional engineered nerve using a nerve-fibroblast co-culture. In Vitro Cell Dev Biol Anim. 2010;46:438–44. doi: 10.1007/s11626-009-9260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JP, Stern-Straeter J, Foerster VT, Kneser U, Stark GB, Bach AD. Tissue engineering of injectable muscle: three-dimensional myoblast-fibrin injection in the syngeneic rat animal model. Plast Reconstr Surg. 2006;118:1113–24. doi: 10.1097/01.prs.0000221007.97115.1d. [DOI] [PubMed] [Google Scholar]
- Candiani G, Riboldi SA, Sadr N, Lorenzoni S, Neuenschwander P, Montevecchi FM, Mantero S. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J Appl Biomat Biomech. 2010;8:68–75. [PubMed] [Google Scholar]
- Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–4888. doi: 10.1016/j.biomaterials.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis RG, Kosnik PE. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In vitro Cellular & Developmental Biology. 2000;36:327–35. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dennis RG, Kosnik PE, 2nd, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol. 2001;280:288–95. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Lytle IF, Dow DE, Huang YC, Brown DL. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 2007;13:2813–21. doi: 10.1089/ten.2007.0003. [DOI] [PubMed] [Google Scholar]
- Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16:711–8. doi: 10.1089/ten.TEC.2009.0125. [DOI] [PubMed] [Google Scholar]
- Gonen-Wadmany M, Gepstein L, Seliktar D. Controlling the cellular organization of tissue-engineered cardiac constructs. Ann N Y Acad Sci. 2004;1015:299–311. doi: 10.1196/annals.1302.025. [DOI] [PubMed] [Google Scholar]
- Haase SC, Rovak JM, Dennis RG, Kuzon WM, Jr, Cederna PS. Recovery of muscle contractile function following nerve gap repair with chemically acellularized peripheral nerve grafts. J Reconstr Microsurg. 2003;19:241–8. doi: 10.1055/s-2003-40580. [DOI] [PubMed] [Google Scholar]
- Huang NF, Lee RJ, Li S. Engineering of aligned skeletal muscle by micropatterning. Am J Transl Res. 2010;2:43–55. [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Rosenblatt JD, Cullen MJ, Zweyer M, Wernig A. Ectopic skeletal muscles derived from myoblasts implanted under the skin. J Cell Sci. 1998;111:3287–3297. doi: 10.1242/jcs.111.22.3287. [DOI] [PubMed] [Google Scholar]
- Khodabukus A, Paxton JZ, Donnelly K, Baar K. Engineered muscle: a tool for studying muscle physiology and function. Exerc Sport Sci Rev. 2007;35:186–91. doi: 10.1097/jes.0b013e318156df01. [DOI] [PubMed] [Google Scholar]
- Kosnik PE, Faulkner JA, Dennis RG. Functional development of engineered skeletal muscle from adult and neonatal rats. Tissue Eng. 2001;7:573–84. doi: 10.1089/107632701753213192. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY. Application of WGA lectin staining for visualization of the connective tissue in skeletal muscle, bone, and ligament/tendon studies. Microsc Res Tech. 2011;74:18–22. doi: 10.1002/jemt.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149–58. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol. 2005;42:75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. McGraw-Hill; New York, NY, USA: 1968. pp. 94–95. [Google Scholar]
- Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557–65. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed Mater Eng. 2001;11:275–81. [PubMed] [Google Scholar]
- Smietana MJ, Syed-Picard FN, Ma J, Kostrominova T, Arruda EM, Larkin LM. The effect of implantation on scaffoldless three-dimensional engineered bone constructs. In Vitro Cell Dev Biol Anim. 2009;45:512–22. doi: 10.1007/s11626-009-9216-3. [DOI] [PubMed] [Google Scholar]