Abstract
Objective
Diets high in fat are implicated in the development and maintenance of obesity, and obese individuals display greater preferences for high-fat foods than do their lean counterparts. Weight-reduction bariatric surgery is associated with changes in food choice. In particular, after Roux-en-Y Gastric Bypass (RYGB), humans and rodents select or prefer foods which are lower in fat content. We asked whether a bariatric surgical procedure limited to the stomach, Vertical Sleeve Gastrectomy (VSG), causes a similar reduction of fat intake/preference.
Research Design and Methods
Rats received VSG or Sham surgery or remained surgically naïve, and were assessed for food preference using three diet-choice paradigms. Using progressive-ratio and conditioned taste aversion paradigms, we further asked whether surgically-induced changes in food choice are secondary to changes in the reward value of food and/or to the formation of a food aversion. Finally, food choice was compared between VSG and RYGB-operated rats.
Results
VSG rats decreased their intake of dietary fat, and shifted their preference toward lower caloric-density foods. This change in food choice was not associated with changes in motivated responding on a progressive-ratio schedule for either a fat or a carbohydrate food reinforcer. When VSG and RYGB were compared directly, both procedures caused comparable changes in food choice. The conditioned taste aversion paradigm revealed that VSG rats form an aversion to an intra-gastric oil administration whereas RYGB rats do not.
Conclusions
VSG and RYGB, two anatomically-distinct bariatric procedures, produce similar changes in food choice.
Keywords: Bariatric Surgery, Roux-en-Y Gastric Bypass, Macronutrient, Food Reward
INTRODUCTION
Diets high in fat are implicated in the development and maintenance of obesity1–3 in humans and animal models, and increasing concentrations of dietary fat cause dose-dependent body- weight gain in some strains of mice4. Furthermore, obese individuals display greater preferences for high-fat foods than their lean counterparts5, 6.
Weight-reduction bariatric surgery is the most effective long-term treatment for obesity. Despite its broad success, we understand remarkably little about how these procedures produce their potent effects. What is clear is that patients who undergo certain bariatric procedures, including Roux-en-Y gastric bypass (RYGB), frequently change their eating behavior after surgery. Several studies have demonstrated that humans7–10 and rodents11 who receive RYGB select or prefer different foods, particularly foods which are lower in fat. However, the effect of alternative bariatric procedures on food choice is less clear, and in particular, food choice following Vertical Sleeve Gastrectomy (VSG) has not been reported.
Bariatric surgeries have been classified as either restrictive, malabsorptive, or both. RYGB, which includes both gastric and intestinal modifications by shrinking the effective size of the stomach and rerouting the flow of nutrients, is classified as both restrictive and malabsorptive. In contrast, VSG is a comparatively simple procedure in which only the stomach is modified, and is therefore classified as a purely restrictive procedure. VSG involves removing approximately 80% of the stomach along the greater curvature, creating a gastric “sleeve” connecting the esophagus directly to the pylorus. Like RYGB, this procedure induces loss of weight and fat mass, and improves glucose tolerance in humans and in rodent models12–14.
We used a rat model of VSG to investigate whether rats undergoing VSG change their food choice after surgery. We hypothesized that, due to the decreased stomach volume, VSG rats would prefer the most calorically-dense food available in order to maximize caloric intake into their reduced stomach volume and would therefore increase fat intake relative to other macronutrients.
To investigate the mechanism underlying altered food choice, we also asked whether food reward, specifically fat-related food reward, is correspondingly altered. The reward value of food has been reported to increase in obesity15–17, and is predictive of weight gain in children18. It is therefore important to understand if and how food reward is modulated by bariatric surgery. The flip side may also be true. Many RYGB patients experience “dumping syndrome”, a cluster of aversive symptoms that includes gastrointestinal and vasomotor consequences, and which occurs when nutrients reach the small intestine too quickly19, 20. We hypothesized that a learned association of fat intake with these aversive consequences drives patients to eat less fat, and that a similar mechanism may operate in VSG as well. Therefore, we investigated whether RYGB and/or VSG would induce a conditioned aversion to food stimuli.
METHODS
Animals
Rats were housed at the Metabolic Diseases Institute of the University of Cincinnati under standard controlled conditions with free access to food and water except where noted. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. These studies used 3 cohorts. Male Long-Evans rats (Harlan Laboratories, Indianapolis, IN; 250–300g) were maintained on a high-fat diet (HFD; Research Diets, New Brunswick, NJ, D12451; 45% fat; 4.73kcal/g) for 6–8 weeks prior to receiving VSG, RYGB, or sham surgery, or remaining surgically naïve. Rats were matched for body weight and fat mass before being divided into the appropriate surgical groups. Rats were maintained on HFD before and after surgery except during diet selection testing, and all surgeries were conducted by the same surgeons. Cohort A included sham (n=13), VSG (n=14) and surgically naïve (n=7) rats. Cohort B included sham (n=20) and VSG (n=17) rats. Cohort C included sham (n=17), VSG (n=14) and RYGB (n=9) rats. The sham group was counterbalanced to include rats which received either a sham-VSG or sham-RYGB procedure as described below. No differences in any of the parameters measured were detected between the 2 sham procedures, and their data are therefore presented as a single group.
Surgical Procedures
VSG surgery was conducted as previously described12. Briefly, the lateral 80% of the stomach was excised leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly. The VSG-sham procedure involved analogous isolation of the stomach followed by manually applying pressure with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. RYGB surgery was conducted as described previously21. Briefly, a small pouch was created at the proximal stomach, and physically separated from the remainder of the stomach. The jejunum was then transected, and the open end of the distal jejunum was anastomosed to the new pouch, creating the alimentary limb. The open end of the remaining proximal intestine (biliopancreatic limb) was anaostomed to the jejunum at a point 10-cm distal to the initial transection, creating the classic “Y.” For the RYGB-sham procedure, the jejunum was transected and re-anastomosed. Rats consumed liquid diet (Osmolite OneCal) for the first 3 post-operative days, and were transitioned back to solid diet by Day 5.
Body Weight, Food Intake, and Body Composition
Body weight and food intake (Cohort A) were recorded from the day of surgery until food restriction began on Day 27. Magnetic resonance imaging was performed 6-wk after surgery (Cohort A) to determine body composition using a whole-body composition analyzer (EchoMedical Systems, Houston, TX).
Diet Selection Testing
Three kinds of diet selection testing were employed. In the first paradigm (Cohorts A and C), three pure macronutrient diets (Harlan Teklad; TD.02521[carbohydrate], TD.02522[fat], and TD02523[protein]) were presented in separate containers simultaneously for 4d. In the second paradigm (Cohort B), rats were given a choice between two novel, nutritionally-complete pelleted diets, a high-fat diet which included sucrose (Research Diets, New Brunswick, NJ, D12331, 58% fat) and a low-fat diet (D12450B, 10% fat). To assess a possible preference for caloric density, rats in Cohort A were offered 2 liquid diets simultaneously: regular Ensure Plus™ (1.41kcal/g; 29% Fat, Abbott Nutrition, OH) and Ensure Plus that had been diluted by 50% with water, and intake of both diets was recorded over 48h.
Progressive-Ratio Paradigm
We employed a progressive-ratio lever-pressing paradigm to assess motivated responding for food cues (Cohort A). Prior to surgery, rats were trained to lever press for sucrose (Test Diet, Richmond, IN) and peanut oil (Planters brand/Nabisco, East Hanover, NJ) reinforcers in separate environments distinguished by light color, lever location, and presence or absence of a fan. This paradigm was chosen based on its ability to distinguish the reinforcing values of sucrose and peanut oil22. Training consisted of alternating 45min sessions in the sucrose and peanut-oil chambers, beginning with autoshaping and fixed-ratio sessions. Subsequently, rats were tested in a 1h Progressive-Ratio (PR) schedule. The response requirements of the PR schedule increase progressively, minimizing the effects of satiation22. Beginning 10d post-surgery, rats were retested on the PR or fixed-ratio schedules in an ad libitum or food-restricted condition.
Conditioned Taste Aversion
Three months after surgery (Cohort C), food aversion was assessed using a conditioned taste aversion paradigm in which a novel flavor (0.15% sodium saccharin) was paired with an intragastric (ig) infusion of 1ml peanut oil, 1ml water, or 1ml of the malaise-inducing agent, lithium chloride (0.15M LiCl) as a positive control. Rats were trained for 15d during which access to water or saccharin was limited to 2 brief exposures per day. The first 3 days consisted of acclimatization, in which rats were given 30min water access in the morning, followed immediately by ig infusion of 1ml water, and 45min water access in the afternoon. On Days 4–15, rats received 0.15% saccharin or water for 30min in the morning, and water for 45min in the afternoon. Each rat received 3 “test pairings” (30min saccharin access followed by ig infusion of the appropriate stimulus), and 3 “control pairings” (30min water access followed by ig water), for a total of 6 pairing sessions alternating with recovery days in which rats drank only water and received no ig infusion. Saccharin intake was recorded for all pairings, and rats which drank less than 2ml during the 30min exposure period were orally flushed with 1ml saccharin prior to administering the ig stimulus to ensure adequate flavor-stimulus pairing. On Day 17, following 16h water deprivation, a saccharin intake test was administered in which rats were given only saccharin to drink for 4h and their intake recorded.
Statistics
Data were analyzed using the appropriate ANOVA or student’s t-test. Where appropriate, Tukey’s post-hoc comparisons were used to determine pair-wise differences between groups. P<0.05 was considered significant for each of these analyses. All data were analyzed using GraphPad (Prism, San Diego, CA).
RESULTS
Body Weight, Body Composition, and Food Intake
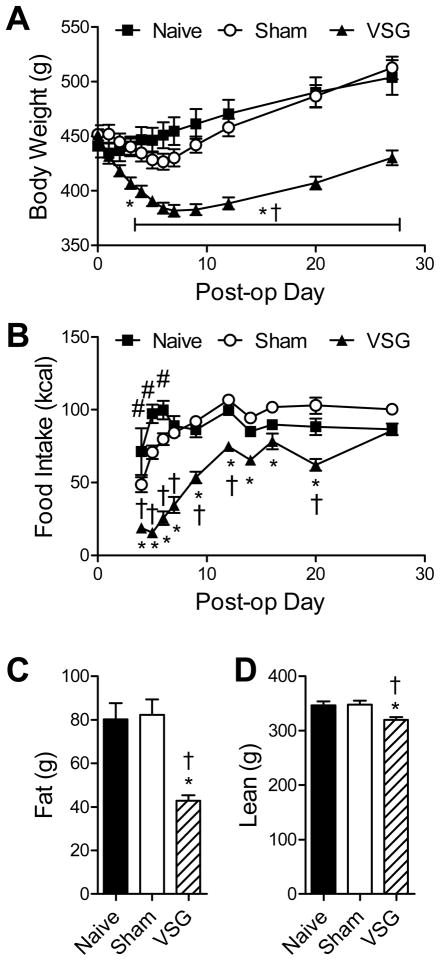
The body weight of Naïve (non-operated) and Sham rats did not differ significantly at any time-points, although Sham rats exhibited a trend toward weight loss in the first post-operative week that was subsequently recovered. VSG rats lost more body weight than both control groups (Naïve vs. VSG, p<0.05 starting at 4d, Sham vs. VSG p<0.05 starting at 3d) (Fig 1A). The decreased body weight of VSG rats compared to controls was sustained until sacrifice at 126d (Sham vs. VSG, p<0.001, data not depicted). Food intake was measured starting 4d post-operatively, when solid food (HFD) was re-introduced. Rats consumed HFD except during Days 16–20, when they were assessed for macronutrient selection, as described below. Naïve rats ate more than Shams from Days 4–6 (p<0.05), after which there were no longer significant differences. VSG induced a transient reduction in food intake compared to both control groups (Naïve vs. VSG, p<0.05 Days 4–20, Sham vs. VSG p<0.05 Days 4–12, and Day 20), an effect which was no longer apparent by Day 27 (Fig 1B). These results are in agreement with previous reports from our group 12, 23, and demonstrate a lack of rebound hyperphagia which occurs following food restriction and discontinuation of many other weight loss agents24–26. Body composition was measured 6 wk after surgery, and revealed that VSG rats had approximately 50% as much fat mass as controls (p<0.05 vs. both Naïve and Sham) (Fig 1C), and also had a modest decrease in lean mass (p<0.05 vs. Naïve and Sham) (Fig 1D).
Figure 1.
Rats which received Sham or VSG surgery, or remained surgically naïve, were monitored for body weight (A) and food intake (B), and body composition was assessed 6 wk post-operatively (C, D). * p<0.05 for Sham vs. VSG, † p<0.05 for Naïve vs. VSG, and # p<0.05 for Naïve vs. Sham.
Food Selection
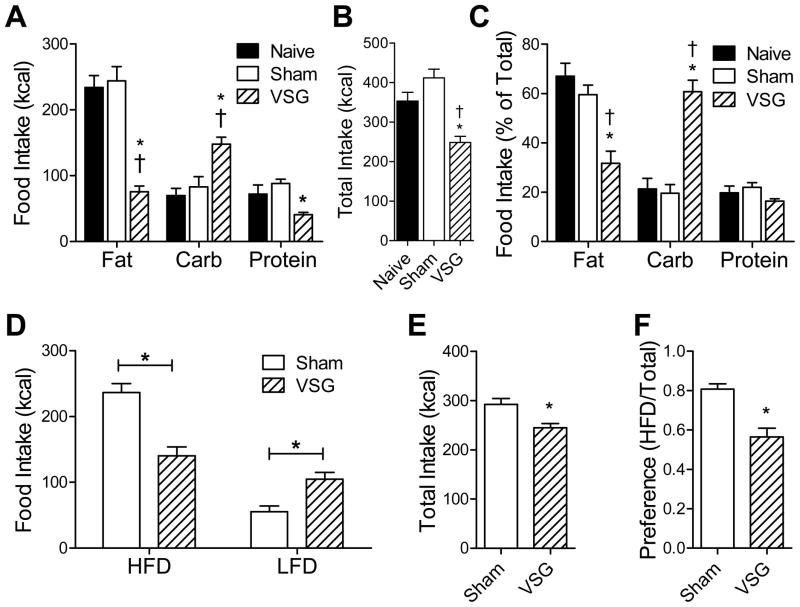
Prior to surgery, rats were tested for macronutrient selection, at which time there were no differences among groups (data not shown). Beginning at post-operative Day 16, rats were re-tested over 4d. VSG rats consumed less fat, more carbohydrate, and less protein than control rats (fat and carbohydrate: p<0.05 for Naive vs. VSG and Sham vs. VSG; protein: p<0.05 for Sham vs. VSG) (Fig 2A). Total caloric intake of VSG rats was significantly lower compared to Naïve and Sham (p<0.05) (Fig 2B). When the intake of the individual macronutrient diets was normalized for total caloric intake, fat intake and carbohydrate intake differed significantly between VSG and control groups (p<0.05 Naïve vs. VSG and Sham vs. VSG), whereas protein intake did not (Fig 2C). This effect was seen at later time-points relative to surgery (8wk, Cohort B, Supplemental Fig 1A) and was also observed in female VSG rats (Supplemental Fig 1B).
Figure 2.
Rats which received Sham or VSG surgery, or remained surgically naïve (Cohort A), were tested for food choice using a macronutrient-selection paradigm (A–C). Intake of each macronutrient (A), total combined food intake (B), and food intake of each macronutrient normalized to total food intake (C) are presented. Rats which received Sham or VSG surgery (Cohort B) were tested for food choice using a high-fat diet (HFD) versus low-fat diet (LFD) paradigm (D–F). Intake of each diet (D), total combined food intake (E), and HFD preference normalized to total food intake (F) are presented. * p<0.05 for Sham vs. VSG, † p<0.05 for Naïve vs. VSG, # p<0.05 for Naïve vs. Sham.
When rats selected between a novel high-fat diet (HFD) and a low-fat diet (LFD), VSG rats ate less HFD (p<0.05) and more LFD (p<0.05) than Sham rats (Fig 2D). When expressed as a preference (kcal of HFD / total kcal), VSG rats had a significant decrease in their preference for HFD (p<0.05) (Fig 2E). Total caloric intake of VSG rats was lower than that of Sham rats during the testing period (p<0.05) (Fig 2F).
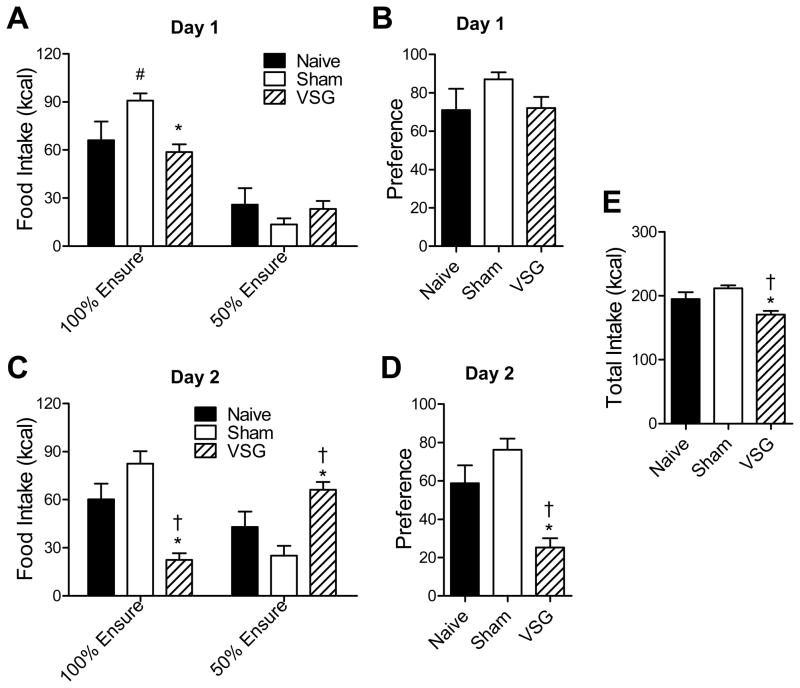
We hypothesized that VSG rats decreased their intake of fat due to the high caloric density of fat relative to the other macronutrients. To test this hypothesis, rats were given a choice between 2 liquid diets which differed in caloric density but not in relative nutrient composition (diluted[50%] or undiluted[100%] Ensure Plus™). During the first 24h, rats from all groups consumed the majority of their calories as the undiluted (100% Ensure) diet (Fig 3A), and there was no difference in dietary preference among groups (Fig 3B). However, during the second 24h, VSG rats decreased their intake of 100% Ensure (p<0.05 Naïve vs. VSG and Sham vs. VSG), and increased their intake of 50% Ensure (p<0.05 Sham vs. VSG) (Fig 3C), resulting in a decreased preference relative to Naïve and Sham rats (p<0.05) (Fig 3D). Total caloric intake was again lower in VSG rats compared to Naïve and Sham (p<0.05) (Fig 3E).
Figure 3.
Rats which received Sham surgery or VSG surgery, or remained surgically naïve, were tested for preference for caloric density using 2 liquid diets: undiluted Ensure (100%), or Ensure which had been diluted by 50% with water for 2 d. Data depicted are: intake of each diet for each day (A, C), preference (kcal of 100% Ensure/ total kcal) for each day (B, D), and total caloric intake over both days (E). * p<0.05 for Sham vs. VSG, † p<0.05 for Naïve vs. VSG, and # p<0.05 for Naïve vs. Sham.
Food Reward
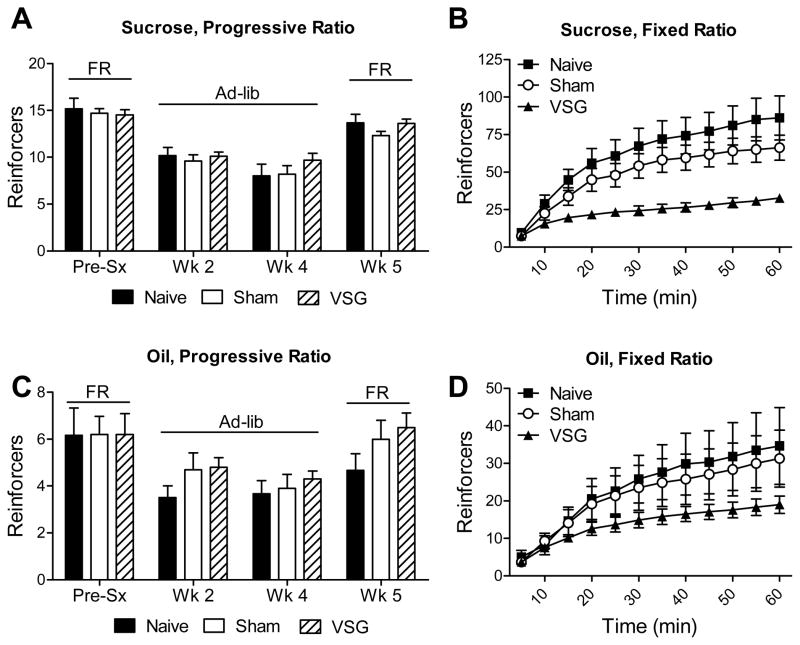
To test the hypothesis that decreased fat intake was due to decreased reward value of fatty foods, we used the progressive-ratio lever-pressing paradigm which assesses the motivation to work for sucrose or peanut oil food reinforcers. In this context, the continually increasing difficulty to obtain food rewards limits the total number of reinforcers earned, and therefore calories consumed. Rats were trained prior to surgery (Pre-sx) under food-restricted conditions (FR), at which time there were no differences among groups. Rats were re-tested at 2 and 4wk post-surgery under ad libitum-fed conditions (ad-lib), and again at 5wk post-surgery while food-restricted. No differences were found among groups at any of the time-points (Fig 4A, C). To validate that lever-pressing is a relevant paradigm for assessing food intake behaviors in VSG-operated rats, we used a fixed-ratio reinforcement schedule as a positive control. In these conditions, in which rats are easily able to obtain a large number of food rewards (presumably eating to the point of satiation), VSG rats earned significantly fewer sucrose and peanut-oil reinforcers than Naïve or Sham rats (P[interaction group x time]<0.0001 for sucrose, P=0.0297 for peanut oil) (Fig 4B, D). These data are in accordance with previous data that VSG rats eat smaller meals12.
Figure 4.
Rats which received Sham surgery or VSG surgery, or remained surgically naïve, were tested for motivated responding for food cues using a lever-pressing paradigm. No differences were found among groups for motivated responding (progressive-ratio) for sucrose (A) or oil (C) reinforcers under food-restricted (FR) or ad libitum-fed (Ad-lib) conditions. However, on a fixed-ratio schedule, VSG rats earned fewer food reinforcers compared to sham rats when pressing for either sucrose (B) or oil (D).
Food Selection in VSG and RYGB
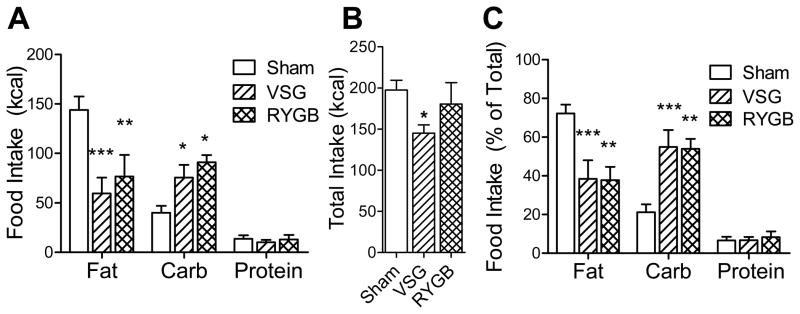
We used the macronutrient selection paradigm to directly compare food choice of VSG and RYGB-operated rats. We found that VSG and RYGB animals decreased their fat intake and increased their carbohydrate intake to a comparable degree relative to sham controls (p<0.05), and were not significantly different from each other (Fig 5A). This was the case both when the data are expressed as raw values, as well as when expressed as a percent of total food intake (Fig 5C). During the 2d testing period, total caloric intake was significantly lower for VSG rats (p<0.05), but not for RYGB rats, when compared to Sham controls (Fig 5B).
Figure 5.
Rats which received Sham surgery, VSG surgery, or RYGB surgery were tested for food choice using a macronutrient-selection paradigm. Intake of each macronutrient (A), total combined food intake (B), and food intake of each macronutrient normalized to total food intake (C) are presented. Symbols indicate significant differences when compared to Sham (* p<0.05, ** p<0.01, and *** p<0.001).
Conditioned Taste Aversion
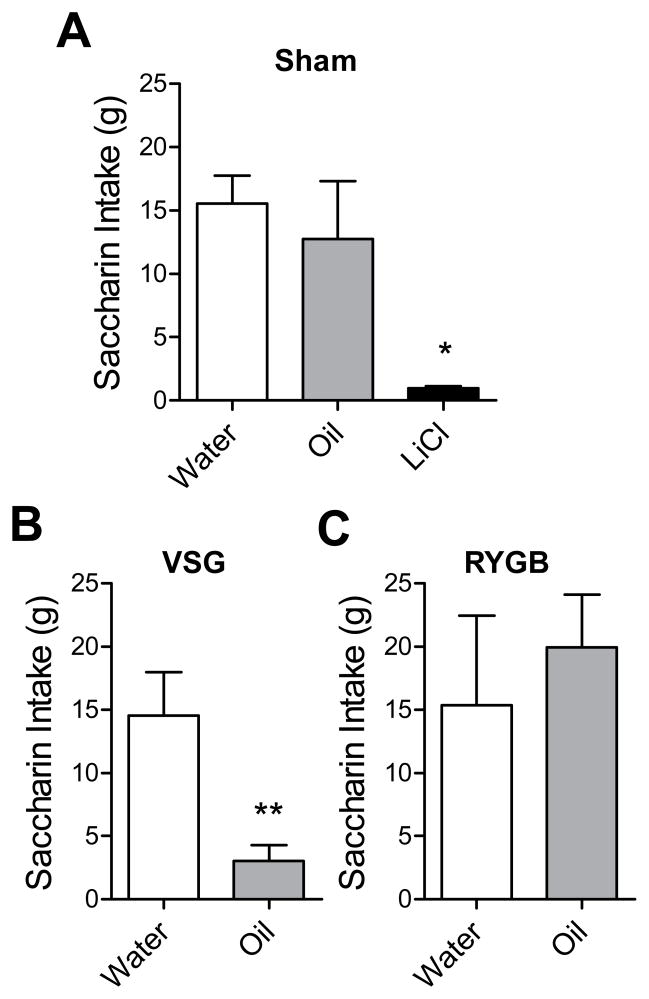
To determine whether the post-ingestive consequences of fat intake may be aversive to rats which have undergone VSG or RYGB, we trained rats to associate a novel flavor, saccharin, with an intra-gastric infusion of water, peanut oil, or the malaise-inducing agent LiCl as a positive control. Sham rats which received LiCl infusions formed a strong conditioned taste aversion, drinking far less saccharin than water-conditioned sham rats on the test day (water vs. LiCl p<0.05). Among rats that were conditioned with oil, Sham and RYGB rats formed no aversion, whereas VSG rats formed a strong aversion (p<0.05, Fig 6A–C). To ensure that RYGB surgery does not impair the ability to form associations between flavors and intra-gastric stimuli, we investigated whether RYGB rats would form a LiCl-induced taste aversion, and found that this was indeed the case (water-conditioned vs. LiCl-conditioned, p<0.01, Supplemental Fig 2).
Figure 6.
Rats which received Sham surgery, VSG surgery, or RYGB surgery were tested for conditioned taste aversion to an intra-gastric infusion of water, oil, or LiCl. * p<0.05 versus water, and ** p<0.01 versus water.
DISCUSSION
Increasing evidence suggests that RYGB and VSG are both metabolic surgeries, meaning that these surgeries influence metabolic outcomes in ways that are not fully explained by restriction and malabsorption alone. Our data support this hypothesis. If mechanical restriction of meal size was the sole reason animals reduce food intake, we would predict that rats would compensate for this by choosing the most calorically-dense food and thus maximize caloric intake. However, we observed exactly the opposite. In addition to decreasing intake of fat, the most calorically-dense macronutrient, VSG rats shifted their preference toward a less calorically-dense liquid diet, even when the relative proportions of macronutrient content were held constant. Furthermore, despite the anatomical differences between VSG and RYGB, both procedures resulted in the same changes in food selection. This suggests that a common underlying mechanism not involving direct manipulation of the intestine may be shared between VSG and RYGB.
Whether the changes in food choice may be secondary to changes in body weight remains unresolved. Although there are reports in humans that obese individuals have a greater preference for fat than lean individuals5, 6, it is not clear whether these taste preferences are a cause or consequence of obesity, and whether they may change with body weight gain or loss. In rodents, there are several reports that acute food restriction causes an increase in fat preference27–29, but they do not address the condition of chronic weight loss. Further studies will be needed to understand whether weight loss itself contributes to the potent effects of VSG and RYGB to alter food preferences.
Food intake is a complex behavior that, while necessary to obtain energy, is subject to numerous non-homeostatic influences, including the rewarding value of the food itself. We hypothesized that decreased fat intake following VSG is due to an impaired motivational drive for fat-related food stimuli. However, in the progressive-ratio lever-pressing paradigm, in which the amount of food acquired is severely limited by the increasing difficulty of the task, we found no deficits in motivational responding. This suggested that perhaps aversive consequences associated with ingestion of larger amounts of fat may instead be the culprit. While our conditioned taste aversion results indicate that this is a possibility following VSG, RYGB rats did not form an aversion to the intra-gastric fat stimulus. These results are in contrast to recent published data indicating that RYGB-operated rats form a taste aversion to an intra-gastric infusion of corn oil30. This difference may be due to disparate surgical RYGB procedures, as our procedure includes a much shorter alimentary limb (10 cm versus 50 cm) and a longer common channel (60 cm versus 30 cm), providing more distance during which biliopancreatic secretions mix with chyme, presumably favoring greater fat absorption in our model. Thus, although fat aversion may partially explain the decreased fat intake after VSG, it may not be the only mechanism, since RYGB rats decrease their fat intake even in the absence of an aversion.
These results are surprising considering clinical reports that RYGB patients, but not VSG patients, experience the aversive symptoms including nausea and abdominal pain known as “dumping syndrome.” One possibility is that the paradigm we employed did not capture the effects of the classical dumping syndrome, and that the conditioned aversion produced in the VSG rats is indicative of some other kind of gastro-intestinal distress which is not experienced by RYGB rats. Indeed, there is evidence that administering lipids directly into the proximal intestine of unoperated rats can cause an aversion31. This would not happen in RYGB rats since the administered oil enters more distally in the intestine than in the VSG procedure, which maintains a normal pyloric-duodenal juncture. Another consideration is that our rat RYGB procedure maintains a proportionally larger gastric pouch (30% of initial volume) than the standard human procedure (10% of initial volume), and we cannot rule out that this difference may explain the lack of the expected fat aversion. Alternatively, separate mechanisms (aversion in VSG vs. an unknown mechanism in RYGB) could operate in the two procedures causing the same food choice changes.
Although altered food choice has been reported following RYGB and some other bariatric procedures7–11, the physiology that underlies this phenomenon has not been identified. However, there are a number of possibilities. The first is altered hormone profiles, particularly those of glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and ghrelin. All three hormones have been reported to modulate intake of certain kinds of foods or the choice between foods, and each has been reported to be altered following VSG and RYGB. Post-prandial GLP-1 and PYY levels are greatly enhanced after both surgeries13, 14, 21. One study found that peripheral administration of the GLP-1 receptor agonist Exendin-4 causes a relative increase in carbohydrate intake32, and that it is more effective at reducing food intake following an intra-gastric preload of fat than of carbohydrate33. Similarly, PYY injected into the paraventricular nucleus of the hypothalamus was reported to stimulate carbohydrate intake to a greater degree than fat intake34. Ghrelin is another peptide hormone which several reports have linked to changes in food choice. One study found that centrally-administered ghrelin preferentially increases fat intake over carbohydrate intake35, and another study demonstrated that ghrelin stimulates the intake of fat-rich palatable foods, whereas genetic or pharmacological inhibition of ghrelin causes a decrease in the intake of palatable foods36. Regarding bariatric surgery, ghrelin has been reported to decrease following both VSG and RYGB surgery13, 37, although controversy remains whether these changes are consistently observed in RYGB38. While the literature on this topic is relatively sparse, each of these hormones has been linked to changes in food choice in normal rodents in a direction that may explain the effects of VSG and RYGB on food choice. However, variation in dose, route of administration (peripheral vs. central), and the specific types of foods used in the food choice studies makes it difficult to predict precisely if and how changes in GLP-1, PYY, and/or ghrelin may account for changes in food choice following bariatric surgery.
Changes in taste acuity and/or neuronal responses to food cues may influence food choice after bariatric surgery. Two studies have found that RYGB patients have enhanced taste acuity, especially for sweet tastants39, 40. Interestingly, when examined by functional magnetic resonance imaging, humans who underwent RYGB displayed reduced activation in mesolimbic reward areas, and this effect was more pronounced in response to high-calorie than for low-calorie foods41. Animal models will make possible a wider range of the experiments necessary to directly test this hypothesis.
Altered lipid handling by the gastrointestinal tract is another potential explanation for the changes in food choice following VSG and RYGB. Both surgeries decrease plasma triglycerides and increase plasma bile acids42–46. Plasma bile acids are an indicator of intraintestinal bile acid levels, which are involved in the processing of dietary fat. Recent work from our group indicates that in VSG this difference is likely due to decreased secretion of dietary lipid from the intestine. Several lines of evidence link lipid metabolism to food choice. First, inhibition of fatty acid oxidation by 2-mercaptoacetate (2-MA) selectively decreases fat intake relative to carbohydrate and protein47, and this effect is believed to be mediated via vagal afferents48 originating in the small intestine49. Second, enterostatin, which is co-secreted with pancreatic co-lipase, and whose regulation is thereby coupled to lipid metabolism50, also decreases fat intake relative to other macronutrients51, 52. Finally, genetic ablation of CD36, a fatty acid translocase expressed in both the intestine and lingual papillae, abolishes fat preference in a 2-bottle choice test53.
Taken together, these studies indicate that lipid processing in the intestine results in signaling events to the CNS that contribute to food selection. However, the fact that one agent that inhibits fat utilization (2-MA) and two others that are positively associated with fat absorption or breakdown (enterostatin and CD36) all inhibit dietary fat intake creates a challenge for understanding both the relationship of lipid metabolism and food choice, and also how these effects translate to VSG and RYGB. While there remains considerable uncertainty, altered lipid handling provides a compelling alternative to restriction and malabsorption as a common mediator of a variety of effects caused by VSG and RYGB.
In conclusion, in contrast to our original hypothesis, VSG decreases the preference for high-fat or calorically-dense foods, and neither restriction nor caloric malabsorption can account for these effects. Despite the drastically different surgical manipulations, VSG and RYGB result in such remarkably similar changes in food choice that it suggests a common underlying mechanism. It is important to note that these changes in food choice are not crucial to the weight-loss effects of these surgical procedures given that weight loss occurs even when rats are maintained with access to only the single high-fat diet that caused their obesity in the first place. Nevertheless, these results demonstrate a compelling effect of VSG to alter ingestive behavior beyond its anorexic effect, and that this altered food choice does not require post-surgical dietary advice and counseling typically provided to human bariatric surgical patients. This highlights the important changes in signaling to the CNS that occur during these bariatric surgical procedures that result in profound changes in behavior. While further experiments will be necessary to delineate the key underlying physiological changes that result in altered signals to the CNS, understanding these mechanisms could lead to development of less invasive targeted therapies to enable more successful strategies to reduce body weight.
Supplementary Material
A) Rats from Cohort B which received Sham surgery (n=17) or VSG surgery (n=16) were tested for food choice using a macronutrient selection paradigm 8 weeks after surgery (A). A separate cohort of male (sham n=5, VSG n=6) and female (sham n=5, VSG n=6) rats were tested for food choice 2 weeks following sham or VSG surgery (B). ** p<0.01.
Rats from Cohort C which received RYGB surgery were tested for formation of a conditioned taste aversion to a 1-ml intra-gastric load of water or LiCl, using unsweetened grape Kool-Aid as the novel flavor. ** p<0.01 compared to water-conditioned RYGB rats.
Acknowledgments
Grant Support: This work was supported by NIH grants DK54890 and DK82480 and Ethicon Endo-Surgery. HWP is supported by NIH training grant T32 HD07463 and a grant from the Ryan Foundation.
We thank Jose Berger, April Haller, Alfor Lewis, Kenneth Parks, Kathi Smith, and Mouhamadoul Toure for their surgical expertise in conducting the VSG, RYGB, and sham surgeries. Thanks also to Brad Chambers and Jon Davis for technical assistance with the Progressive Ratio experiments.
Footnotes
CONFLICT OF INTEREST
Randy J. Seeley- Johnson & Johnson (Ethicon Endo-Surgery), Zafgen, Merck, Pfizer, Mannkind, Roche Darleen A. Sandoval - Johnson & Johnson (Ethicon Endo-Surgery), Pfizer, Mannkind, Novonordisk Stephen C. Benoit - Johnson & Johnson (Ethicon Endo-Surgery)
Supplementary information is available at the International Journal of Obesity’s website.
References
- 1.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 (Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 2.Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr. 2000;130(2S Suppl):284S–288S. [PubMed] [Google Scholar]
- 3.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49(2):79–90. [PubMed] [Google Scholar]
- 4.de Wit NJ, Boekschoten MV, Bachmair EM, Hooiveld GJ, de Groot PJ, Rubio-Aliaga I, et al. Dose-Dependent Effects of Dietary Fat on Development of Obesity in Relation to Intestinal Differential Gene Expression in C57BL/6J Mice. PLoS One. 2011;6(4):e19145. doi: 10.1371/journal.pone.0019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite. 1992;18(3):207–21. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- 6.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav. 1985;35(4):617–22. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JR, Marcus E. High and low fat food selection with reported frequency intolerance following Roux-en-Y gastric bypass. Obes Surg. 2008;18(3):282–7. doi: 10.1007/s11695-007-9336-3. [DOI] [PubMed] [Google Scholar]
- 8.Thirlby RC, Bahiraei F, Randall J, Drewnoski A. Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J Gastrointest Surg. 2006;10(2):270–7. doi: 10.1016/j.gassur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Ernst B, Thurnheer M, Wilms B, Schultes B. Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg. 2009;19(3):274–80. doi: 10.1007/s11695-008-9769-3. [DOI] [PubMed] [Google Scholar]
- 10.Lindroos AK, Lissner L, Sjostrom L. Weight change in relation to intake of sugar and sweet foods before and after weight reducing gastric surgery. Int J Obes Relat Metab Disord. 1996;20(7):634–43. [PubMed] [Google Scholar]
- 11.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–36. 2436, e1–3. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234–41. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 14.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 15.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27(1):41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 16.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87(5):1121–7. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr. 2010;91(2):300–8. doi: 10.3945/ajcn.2009.28632. [DOI] [PubMed] [Google Scholar]
- 18.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7--10-y-old children. Am J Clin Nutr. 2009;90(2):276–81. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 19.Abell TL, Minocha A. Gastrointestinal complications of bariatric surgery: diagnosis and therapy. Am J Med Sci. 2006;331(4):214–8. doi: 10.1097/00000441-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6(10):583–90. doi: 10.1038/nrgastro.2009.148. [DOI] [PubMed] [Google Scholar]
- 21.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, et al. Weight-Independent Changes in Blood Glucose Homeostasis after Gastric Bypass or Vertical Sleeve Gastrectomy in Rats. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracy AL, Clegg DJ, Johnson JD, Davidson TL, Benoit SC. The melanocortin antagonist AgRP (83–132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav. 2008;89(3):263–71. doi: 10.1016/j.pbb.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson-Perez HE, Seeley RJ. The effect of vertical sleeve gastrectomy on a rat model of polycystic ovarian syndrome. Endocrinology. 2011;152(10):3700–5. doi: 10.1210/en.2011-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue K, Kiriike N, Fujisaki Y, Kurioka M, Yamagami S. Effects of fluvoxamine on food intake during rebound hyperphagia in rats. Physiol Behav. 1997;61(4):603–8. doi: 10.1016/s0031-9384(96)00510-0. [DOI] [PubMed] [Google Scholar]
- 25.Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167(1):103–11. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber R, Selbach K, Asmussen M, Hesse D, de Vry J. Effects of serotonin(1/2) receptor agonists on dark-phase food and water intake in rats. Pharmacol Biochem Behav. 2000;67(2):291–305. doi: 10.1016/s0091-3057(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 27.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18 (2):207–11. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 28.Thouzeau C, Le Maho Y, Larue-Achagiotis C. Refeeding in fasted rats: dietary self-selection according to metabolic status. Physiol Behav. 1995;58(6):1051–8. doi: 10.1016/0031-9384(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 29.Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol. 1994;266(2 Pt 2):R426–33. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- 30.Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez I, Tordoff MG, Friedman MI. Satiety from fat? Adverse effects of intestinal infusion of sodium oleate. Am J Physiol. 1997;273(5 Pt 2):R1779–85. doi: 10.1152/ajpregu.1997.273.5.R1779. [DOI] [PubMed] [Google Scholar]
- 32.Peters CT, Choi YH, Brubaker PL, Anderson GH. A glucagon-like peptide-1 receptor agonist and an antagonist modify macronutrient selection by rats. J Nutr. 2001;131(8):2164–70. doi: 10.1093/jn/131.8.2164. [DOI] [PubMed] [Google Scholar]
- 33.Aziz A, Anderson GH. Exendin-4, a GLP-1 receptor agonist, modulates the effect of macronutrients on food intake by rats. J Nutr. 2002;132(5):990–5. doi: 10.1093/jn/132.5.990. [DOI] [PubMed] [Google Scholar]
- 34.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6(6):1205–11. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 35.Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, et al. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett. 2004;369(1):75–9. doi: 10.1016/j.neulet.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 15(3):304–11. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Zhang G, Liang J, Ding X, Cheng Z, Hu S. Sleeve gastrectomy provides a better control of diabetes by decreasing ghrelin in the diabetic Goto-Kakizaki rats. J Gastrointest Surg. 2009;13 (12):2302–8. doi: 10.1007/s11605-009-0997-1. [DOI] [PubMed] [Google Scholar]
- 38.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 39.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95(6):666–70. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 40.Scruggs DM, Buffington C, Cowan GS., Jr Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes Surg. 1994;4(1):24–28. doi: 10.1381/096089294765558854. [DOI] [PubMed] [Google Scholar]
- 41.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502–7. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17(9):1671–7. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asztalos BF, Swarbrick MM, Schaefer EJ, Dallal GE, Horvath KV, Ai M, et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51(8):2405–12. doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zlabek JA, Grimm MS, Larson CJ, Mathiason MA, Lambert PJ, Kothari SN. The effect of laparoscopic gastric bypass surgery on dyslipidemia in severely obese patients. Surg Obes Relat Dis. 2005;1(6):537–42. doi: 10.1016/j.soard.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R, et al. Sleeve Gastrectomy in Rats Improves Post-Prandial Lipid Clearance by Reducing Intestinal Triglyceride Secretion. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen NT, Varela E, Sabio A, Tran CL, Stamos M, Wilson SE. Resolution of hyperlipidemia after laparoscopic Roux-en-Y gastric bypass. J Am Coll Surg. 2006;203(1):24–9. doi: 10.1016/j.jamcollsurg.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Singer LK, York DA, Bray GA. Macronutrient selection following 2-deoxy-D-glucose and mercaptoacetate administration in rats. Physiol Behav. 1998;65(1):115–21. doi: 10.1016/s0031-9384(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 48.Ritter S, Koegler FH, Wiater M. Effects of Metabolic Blockade on Macronutrient Selection. In: Berthoud H-R, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. Chapter 13. CRC Press; 2000. [Google Scholar]
- 49.Langhans W, Leitner C, Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R554–65. doi: 10.1152/ajpregu.00610.2010. [DOI] [PubMed] [Google Scholar]
- 50.Berger K, Winzell MS, Mei J, Erlanson-Albertsson C. Enterostatin and its target mechanisms during regulation of fat intake. Physiol Behav. 2004;83(4):623–30. doi: 10.1016/j.physbeh.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 51.Okada S, York DA, Bray GA, Erlanson-Albertsson C. Enterostatin (Val-Pro-Asp-Pro-Arg), the activation peptide of procolipase, selectively reduces fat intake. Physiol Behav. 1991;49(6):1185–9. doi: 10.1016/0031-9384(91)90349-s. [DOI] [PubMed] [Google Scholar]
- 52.Erlanson-Albertsson C, Mei J, Okada S, York D, Bray GA. Pancreatic procolipase propeptide, enterostatin, specifically inhibits fat intake. Physiol Behav. 1991;49(6):1191–4. doi: 10.1016/0031-9384(91)90350-w. [DOI] [PubMed] [Google Scholar]
- 53.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115(11):3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1823–32. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Rats from Cohort B which received Sham surgery (n=17) or VSG surgery (n=16) were tested for food choice using a macronutrient selection paradigm 8 weeks after surgery (A). A separate cohort of male (sham n=5, VSG n=6) and female (sham n=5, VSG n=6) rats were tested for food choice 2 weeks following sham or VSG surgery (B). ** p<0.01.
Rats from Cohort C which received RYGB surgery were tested for formation of a conditioned taste aversion to a 1-ml intra-gastric load of water or LiCl, using unsweetened grape Kool-Aid as the novel flavor. ** p<0.01 compared to water-conditioned RYGB rats.