Abstract
The GluN1 subunit of the NMDA receptor shows age-related changes in its expression pattern, some of which correlate with spatial memory performance in mice. Aged C57BL/6 mice show an age-related increase in mRNA expression of GluN1 subunit splice variants that lack the N terminal splice cassette, GluN10XX (GluN1-a). This increase in expression is associated with good performance in reference and working memory tasks. The present study was undertaken to determine if GluN10XX splice variants are required for good performance in reference memory tasks in young mice. Mice were bilaterally injected with either siRNA specific for GluN10XX splice variants, control siRNA or vehicle alone into ventro-lateral orbital cortices. A fourth group of mice did not receive any injections. Starting five days post-injection, mice were tested for their performance in spatial reference memory, associative memory and cognitive flexibility tasks over 4 days in the Morris water maze. There was a 10 –19% reduction in mRNA expression for GluN10XX splice variants within the ventro-lateral orbital cortices in mice following GluN10XX siRNA treatment. Declines in performance within the first half of reference memory testing were seen in the mice receiving siRNA against the GluN10XX splice variants, as compared to the mice injected with control siRNA, vehicle and/or no treatment. These results suggest a role for the GluN10XX splice variants in orbital regions for early acquisition and/or consolidation of spatial reference memory.
Keywords: NMDA receptor, NR1, siRNA, Zeta1, splice variant, memory
1. Introduction
The aging process has been shown to cause functional declines in many different processes [1]. Memory is one function that is affected early in the aging process. One type of memory, spatial memory, which is important for navigation of organisms within their environment, is particularly affected by the aging process [2, 3]. Brain regions that are important for acquisition, consolidation and retrieval of spatial memory include the prefrontal cortex and hippocampus [2, 4, 5]. Animals, such as rodents and primates, have been used to model different aspects of spatial memory involving both the hippocampus and prefrontal cortex [6–8]. The prefrontal cortex has also been shown to be important for flexibility of learning within different memory tasks [9, 10]. The aging process is known to hamper performance in reversal tasks, which are used to assess flexibility [11, 12].
Prefrontal cortex and hippocampus have a high concentration of N-methyl-D-aspartate (NMDA) receptors, a type of glutamate receptor involved in learning and memory [13, 14]. NMDA receptors are particularly important in spatial memory [15–17]. NMDA receptors are heteromeric tetramers composed of subunits from one or more of three different families of subunits, identified as GluN1, GluN2 and GluN3 [18]. There exist eight splice variants in the GluN1 subunit family due to the presence of one N-terminal and two C-terminal splice cassettes [18]. These eight splice variants are heterogeneous in their expression patterns, both during development [19] and aging [20, 21]. The presence or absence of splice cassettes N1, C1 and C2 in individual splice variants will be indicated throughout this article by a series of three subscripts following GluN1, with 0 indicating absence; 1 indicating presence and X indicating either presence or absence of the cassettes [18]. For example, GluN1X10 indicates presence or absence of N1 cassette, presence of C1 cassette and absence of C2 cassette. The absence of the C2 cassette also implies the presence of a new terminal sequence, C2' [18].
Evidence shows that NMDA receptor binding density and the expression of some of the subunits decline with increasing age in both the hippocampus and prefrontal cortex [22–24]. The GluN1 subunit of the NMDA receptor has been shown to decline in expression of both protein and mRNA during aging in the prefrontal cortex of C57BL/6 mice [25, 26]. The individual splice forms of this subunit, representing only N or C terminal sequences, are heterogenous with respect to changes in their expression pattern during aging. Our earlier studies have shown that the mRNA expression of sequences found in GluN1X11 (GluN1-1) and GluN1X10 (GluN1-3) splice variants of the GluN1 subunit in the prefrontal/frontal cortex and hippocampus decline during aging [20], but there is an increase in the mRNA of the GluN10XX (GluN1-a) splice variants in response to behavioral testing experience in the prefrontal cortex of old mice [21]. Significant and near significant associations have been seen between higher expression of GluN1X10 and GluN10XX mRNAs within the orbital cortex and better performance in reference and working memory tasks in aged mice [21]. It is however, not known how important these splice forms are to memory. It is also not known how much influence the GluN1 subunits of the NMDA receptor have on observed cognitive flexibility in animals. The present paper utilized in vivo siRNA administration to determine whether decreased expression of GluN10XX splice variants in orbital cortices play a significant role in spatial memory and/or flexibility in young C57Bl/6 mice. The use of young animals has the advantage of not having all of the confounds of other changes that can occur during aging.
2. Methods
2.1 Animals
A total of 48 male three-month-old C57BL/6 mice (The Jackson Laboratory, Maine) were used for the study. They were fed ad libitum and housed in cages under 12 hr light and 12 hr dark cycle. The animals were randomly divided into four treatment groups of twelve animals each; siRNA specific for GluN10XX splice variants (GluN10XX siRNA), control siRNA, vehicle and no treatment. After behavioral testing was performed, all animals were euthanized with exposure to CO2, followed by decapitation. The brains were then harvested, frozen rapidly with dry ice and stored at −80 °C until further processing. One animal brain was lost after harvesting, hence only 47 animal brains were used for further processing.
2.2 Injection solutions
A custom designed GluN10XX siRNA and predesigned control siRNA (Catalog # 4404021) were purchased from Applied Biosystems. GluN10XX siRNA was specifically designed not to interfere with any of the other GluN1 subunit mRNA sequences or other known mouse mRNAs. The sequence used for GluN10XX siRNA sense strand was 5'-CGUGAGUCCAAGGCAGAGAtt-3' and for antisense was 5'-UCUCUGCCUUGGACUCACGct-3'. The GluN10XX siRNA and control siRNAs were diluted to a concentration of 500μM with RNAse free water and stored in separate aliquots. On surgery days, an aliquot of both the GluN10XX siRNA and control siRNA were diluted with an equal volume of transfection reagent (siLentFect Lipid Reagent for RNAi, Bio-Rad, Hercules, CA) to give a final concentration of 250μM. Animals meant for vehicle alone were injected with equal parts of transfection reagent and sterile water.
2.3 Stereotaxic surgery
Injection of either vehicle, control siRNA or GluN10XX siRNA into the ventro-lateral orbital cortex of the brain was performed by stereotaxic surgery, as described by Yoon and coworkers [27]. Briefly, twenty-four hours before surgery animals were provided with 120 mg of acetominophen (Tylenol, McNeil-PPC Inc., Skillman, NJ) per 100 ml of drinking water. Anesthesia was induced with 4% isoflurane (Vet One, distributed by MWI, Meridian, ID) and maintained with 1.5–2.25%. Mice were placed in the stereotaxic apparatus. Eyes were lubricated with sterile Puralube (Fougera & Co., Melville, NY). The scalp was shaved and surgically scrubbed. A longitudinal skin incision (7 to 9 mm) was made to expose the dorsal surface of the skull over the prefrontal cortex. Holes were drilled in the skull over the ventro-lateral orbital cortices 2.3 mm rostral to Bregma and 1.6 mm to the left and right of the longitudinal suture [28]. Mice were injected with 5μl of either GluN10XX siRNA, control siRNA or vehicle at a depth of 2.7 mm from the surface of the skull at a rate of 500 nl/min with an electric syringe pump (UltraMicroPump 3 with SYS-Micro4 controller, World Precision Instruments, Sarasota, FL). The syringe remained in place for 3 minutes following the injection prior to removal. The skin incision was sealed with glue (Super Glue Corp., Rancho Cucamonga, CA). Mice received 0.1cc of 0.03μM buprenorphine intraperitoneally immediately post-recovery, acetaminophen plus codeine phosphate solution (1ml per 20ml of drinking water; Pharmaceutical Associates Inc., Greenville, SC) for three days, and then acetominophen alone (120 mg in 100 ml water) until euthanasia. Animals in the no treatment group received no surgery, anesthesia or analgesia.
2.4 Behavioral testing
Behavioral testing of the animals started on the 5th day following surgery. Spatial reference memory, cognitive flexibility and associative memory (cued control task) were tested with the use of the Morris Water Maze. A 1.2m diameter metal tank was covered with white contact paper and filled with water that was made opaque white with nontoxic tempera paint (Prang, Dixon Ticonderoga Company, Heathrow, FL). A platform was placed 1 cm below water level. Spatial cues consisted of figures of geometric shape and other items such as toys and pieces of cloth. The cues were placed high on the walls of both the room and the tank. There were seven different platform positions available at five different distances from the tank wall. Trials were videotaped using a video camera (Sony Corp., Tokyo, Japan) placed above the center of the tank on the ceiling of the room. The animals' path was tracked with the “SMART” video tracking system (San Diego Instruments, San Diego, CA). There were different entry points for each trial and the mice were placed in the tank facing the wall.
2.4.1 Acclimation
Three days prior to surgery, mice were acclimated to swimming and platform sitting for two consecutive days. Each acclimation session consisted of each mouse swimming for 60 seconds in the tank without the platform and then being trained to remain on the platform for 30 seconds each day. This platform position was different from the one used for reference memory and flexibility testing. No spatial cues were positioned in the room during acclimation. There was a one-day gap between acclimation and surgery.
2.4.2 Spatial reference memory and cognitive flexibility testing
On days 5 through 7 post-surgery, mice underwent spatial reference memory testing. The task consisted of 8 place trials per day and probe trials at the end of each day. There was also a probe trial at the beginning of the first day of reference memory testing. The platform was kept in the same quadrant for each place trial. Place trials consisted of 60 seconds maximum in the water searching for the platform, 30 seconds on the platform and 2 minutes of cage rest. If a mouse failed to find the platform within the designated 60 second swim time, it was led to the platform by the experimenter. Probe trials were performed to assess the animal's ability to show a bias for the platform location [29]. During the probe trial, the platform was removed and the mouse was allowed to search in the water for 30 seconds. On the 8th day post-surgery, a reversal task was performed, in which the platform was placed in the opposite quadrant in the tank, to assess cognitive flexibility. This task also consisted of 8 place and 1 probe trials and was similar to the reference memory task.
2.4.3 Associative memory (cued control task)
Cued trials were designed to test motivation, visual acuity, and physical ability for the task. On the 9th day following surgery, mice underwent 6 cued trials. The platform was kept submerged but was marked by a 20.3 cm support with a flag. For each cued trial, the platform was changed to a different position and the mouse was placed into the tank facing the wall at one of the entry points and was allowed to search for the platform for 60 seconds. All mice were tested at one platform position before the platform was moved to a new position.
2.5 Brain sectioning
To visualize and quantitate any changes in specific mRNA expression following treatments, the brains were prepared for in situ hybridization. Each brain was sectioned coronally and sagittally, 12μm thick, with the use of a Leica CM1850 UV Cryostat (Leica Microsystems Inc., Bannockburn, IL). Coronal sections were obtained from both frontal lobes and included the center of the injection site. One half of each remaining brain was then sectioned sagittally. Brain sections representing at least one animal from each experimental group were placed on each slide, with positions varied between cutting groups, and were kept frozen at −80 °C until further processing. Coronal sections were used for measuring the oligonucleotide probe density and determining the medial-lateral extent of the injection effects. Sagittal sections were used for determining the caudal extent of injection effects. The brain of one uninjected control was lost prior to in situ hybridization.
2.6 In situ hybridization
The sequence used for the oligonucleotide probe specific for GluN10XX and GluN11XX splice variants were 5'-AACTGCAGCACCTTCTCTGCCTTGGACTCCCGTTCCTCCA-3' and 5'-GCGCTTGTTGTCATAGGACAGTTGGTCGAGGTTTTCATAG-3' respectively [19] (Macromolecular Resources, Colorado State University, Fort Collins, CO); and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was 5'-TGGGCCCTCAGATGCCTGCTTCACCACCTTCTTGATGTCA-3' (Invitrogen Corp., Carlsbad, CA). Probes were labeled with 33P-dATP (Perkin Elmer, Waltham, MA; specific activity: 3257 to 3839 Ci/mM) using terminal deoxyribonucleotidyl transferase (Invitrogen Corp., Carlsbad, CA) and purified with Microspin G-25 columns (Amersham Bioscience, Piscataway, NJ). The specific activities for the labeled oligonucleotides were calculated to be 17–69 dpm/fmol for GluN10XX, 28dpm/fmol for GluN11XX and 30–58 dpm/fmol for GAPDH probe, depending on the labeling experiment.
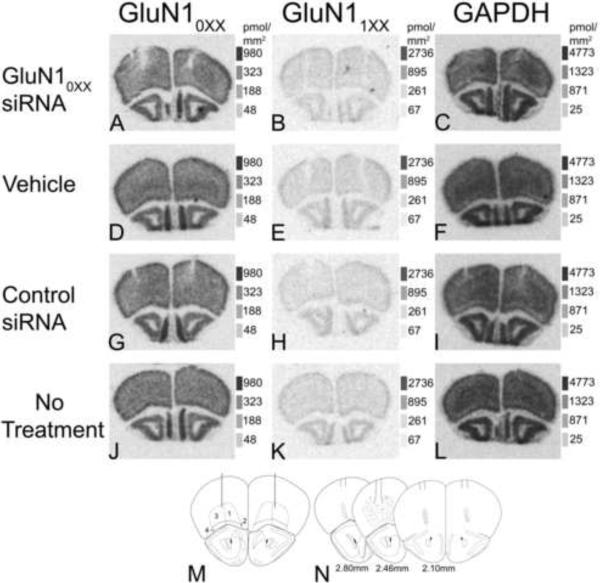
In situ hybridization was performed as described by Watanabe and coworkers [30] and previous studies in our lab [20, 21]. Briefly, each solution step was performed with gentle rotation on a rotating table except for the fixation and hybridization steps. Slides with sections were thawed, air-dried, fixed in 4% paraformaldehyde-PBS (pH 7.2; 25 °C) for 15 min, and treated with 2 mg/ml glycine in PBS (pH 7.2; 25 °C) for 20 min and 0.25% acetic anhydride-0.1M triethanolamine (pH 8.0; 25 °C) for 10 min. Slides were incubated for 2 hr at 25 °C in a prehybridization solution consisting of 50% formamide, 0.1M Tris-HCl (pH 7.5), 4× SSC (1× SSC = 150mM NaCl and 15mM sodium citrate), 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin, 2% sarkosyl, and 250 μg/ml salmon testes DNA. Slides were then successively washed for 5 min each in 2X SSC, 70 and 100% ethanol, and air-dried for 15 min. Hybridization was performed by placing 150 μl of prehybridization solution containing 10% dextran sulfate and 0.33 pmoles of 33P-labeled oligonucleotide probe onto the slides, covering the slides with parafilm, and incubating them for 18 hr in a 42 °C oven, humidified with 5× SSC. After incubation, slides were rinsed for 40 min in 2× SSC and 0.1% sarkosyl (25°C) and for 2°40 min in 0.1× SSC and 0.1% sarkosyl (55 °C) and air-dried. Nonspecific hybridization was determined by addition of 50-fold excess non-radiolabelled oligonucleotide to the hybridization solution on some slides. Slides were exposed to Kodak Biomax films for 8 days for the GluN10XX probe, 7 days for the GluN11XX probe, and 1 day for the GAPDH probe along with a slide containing 14C standards. Brain and standard images were captured using a Macintosh G4 computer with a Powerlook 2100 XL scanner (UMAX, Taiwan) and NIH Image software. Quantitative densitometry was performed on the images of coronally cut sections from four slides for total hybridization and two slides for nonspecific hybridization from each animal with the use of NIH Image software (Version 1.63). Analyzed sections were within 0.36 to 0.46mm of the center of the injection site. The prefrontal cortical regions analyzed for mRNA expressions were deep (cortical layers IV–VI) and superficial (cortical layers II–III) layers of ventral and lateral orbital cortex from both left and right sides of the brain (Fig. 2M). Hybridization densities from both sides of the brain were averaged for each animal to give one value per brain region per animal. Specific signal was determined by subtracting nonspecific hybridization from total hybridization. The 14C standards were used to convert optical density to pmol of labeled 33P-dATP/mm2 tissue [31].
Fig. 2.
Representative images of coronal frontal lobe sections showing mRNA expression of GluN10XX (A, D, G, J), GluN11XX (B, E, H, K) and GAPDH (C, F, I, L) following treatment with GluN10XX siRNA (A, B, C), vehicle (D, E, F), control siRNA (G, H, I) or no treatment (J, K, L). M). Image of the region of brain where the injections were applied. Solid vertical lines indicate needle placement. Numbers indicate regions where mRNA analysis was performed (regions 1 = deep (cortical layers IV–VI) ventral orbital, 2 = superficial (II–III) ventral orbital, 3 = deep lateral orbital and 4 = superficial lateral orbital. N) Diagramatic representation of evidence of the injection site needle tracks (vertical solid lines from top) and the spread of grossly visible reduction of mRNA for GluN10XX subunit splice variants (stippled area) from rostral to caudal. Distances rostral to Bregma are indicated below each diagram (N). Image M and N were adapted from Paxinos et al. [28] (A–L) Standard images and the equivalent pmol of labeled 33P/mm2 tissue are shown to the right of each section image.
2.7 Data Analysis
Data for behavioral testing were analyzed as described earlier with a few modifications [21]. Briefly, the distance of the animal from the platform was measured every 0.2 second by the computer for the whole duration of the trial. Cumulative proximity was calculated by adding together the distance calculated at each 0.2 second interval. Correction for start position was performed using a macro in Excel software (Microsoft Corp., Seattle). A cumulative proximity measurement for the ideal path was calculated by this macro, with the use of starting position, average swim speed and platform position. This cumulative proximity measure for the ideal path was subtracted from the cumulative proximity score for the whole track to obtain the corrected cumulative proximity scores for the place trials in reference memory and reversal tasks and the cued control tasks. For the probe trials of the reference memory and reversal tasks, the corrected cumulative proximity score for the trial was divided by the corrected sample number to obtain a corrected average proximity score. Pathlength and latency were obtained from the software and were corrected for start position. Correction of pathlength was done by subtracting the calculated ideal pathlength from the actual pathlength. For the correction of latency, ideal path latency was obtained by dividing ideal pathlength by average speed, which was then subtracted from the latency.
The density data of mRNAs from image analysis were normalized to the average of untreated mice to reduce variability between films and assays. Normalization factors were obtained by dividing the overall averages of the brain regions analyzed in all the non-injected mice by the averages for the non-injected animals within each assay group and were then multiplied by all animals' values within the assay. Differences in performance in reference memory, flexibility, and cued tasks and mRNA densities for GluN0XX splice variants and GAPDH were analyzed separately by repeated measures ANOVA followed by Fisher's protected post-hoc analysis using Statview software (SAS Institute Inc., Cary, NC).
3. Results
3.1 Spatial reference memory
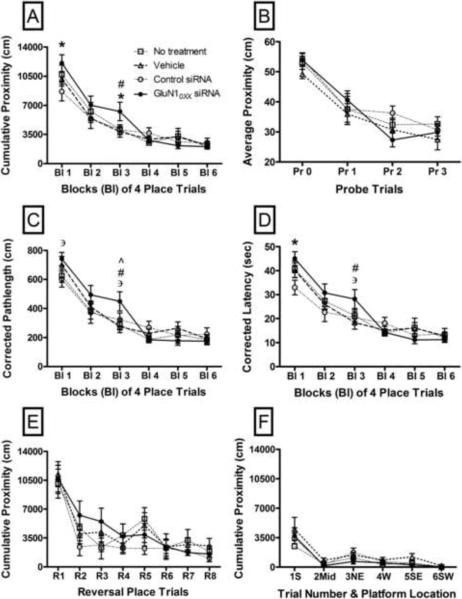
There was no overall effect of treatment on cumulative proximity in the blocks of four place trials of the reference memory task (F(3,44) = .66, p = .58), but there was a significant treatment by block interaction (F(15,220) = 1.79, p = .038). When individual blocks were analyzed separately for effects of treatment, there was a significantly higher cumulative proximity in the reference memory task in mice injected with GluN10XX siRNA as compared to mice injected with control siRNA in block 1 (p = .02, Fig. 1A) and block 3 (p = .049, Fig. 1A). There was also a significantly higher cumulative proximity for mice injected with GluN10XX siRNA than mice injected with the vehicle (transfection reagent) within the third block of four place trials in the reference memory task (p = .03, Fig. 1A). Similar patterns were observed with the more traditional pathlength (Fig. 1C) and latency (Fig. 1D) measurements in the place trials. In both the pathlength and latency measures there were no overall significant effects of treatment (F(3,44) = .67, p = .57 and F(3,44) = .77, p = .51, respectively), but there were significant interactions between treatment and blocks of place trials (F(15,220) = 1.83, p = .03 and (F(15,220) = 1.93, p = .02, respectively). Analysis of individual blocks showed a near-significant longer pathlength (p = 0.06, Fig. 1C) and a significant increase in latency (p = 0.009, Fig. 1D) in block 1of the place trials in animals injected with GluN10XX siRNA as compared to control siRNA. In block 3 of the place trials, the pathlength was significantly longer in animals injected with GLuN10XX siRNA as compared to mice injected with vehicle (p = 0.01, Fig. 1C) or left untreated (p = 0.02, Fig. 1C) and a near-significant increase over animals injected with control siRNA (p = 0.07, Fig. 1C). With respect to latency in block 3, there was a significant increase in animals injected with GluN10XX siRNA versus animals injected with vehicle (p = 0.02, Fig. 1D) and a near-significant increase over animals injected with control siRNA (p = 0.07, Fig. 1D). In the probe trials for reference memory, there was no difference in average proximities between different treatment groups (F(3,44) = .90, p = .45, Fig. 1B).
Fig. 1.
Graphs showing performance of mice in various tasks of memory in the Morris water maze. Performance of mice within blocks of four place learning trials (A,C,D) and each probe trial (B) for a 3-day spatial reference memory task, each reversal trial for assessment of cognitive flexibility (E) and each cued control trial for an associative memory task (F). Performance within blocks of place trials was evaluated by cumulative proximity (A), pathlength (C) and latency (D), after correcting for the start position for each. p < .05 for differences between animals injected with siRNA specific for GluN10XX subunit splice variants (GluN10XX siRNA) and either animals injected with control siRNA (*), animals injected with vehicle (#) or animals without any treatment (^). ∋ indicates p = .06 – .07 for difference between animals injected with siRNA specific for GluN10XX and control siRNA. Data indicate mean±SEM. Bl = blocks, Pr = probe trial, R = reversal trial, S = south, Mid = close to center, NE = northeast, W = west, SE = southeast, SW = southwest.
3.2 Cognitive flexibility
There were no significant differences in cumulative proximity between mice in different treatment groups (F(3,44) = .65, p = .59; Fig. 1E) overall in the reversal task (escape platform in an opposite quadrant) and no significant interaction between treatment and different reversal trials (F(3,44) = 1.043, p = .38). There was no significant difference in the reversal probe trial between different treatment groups (F(3,44) = .255, p = .86; data not shown)
3.3 Associative memory in cued control task
There were no significant differences (F(3,44) = 1.94, p = .14) in cumulative proximity scores between the different treatment groups overall in the cued control task, in which the platform position was made visible by placing a flag on top (Fig. 1F). With the exception of the first cued trial, all treatment groups had lower cumulative proximities in the cued trials than in any of the place trials in the reference memory task (Fig. 1A, F).
3.4 mRNA expression following treatments
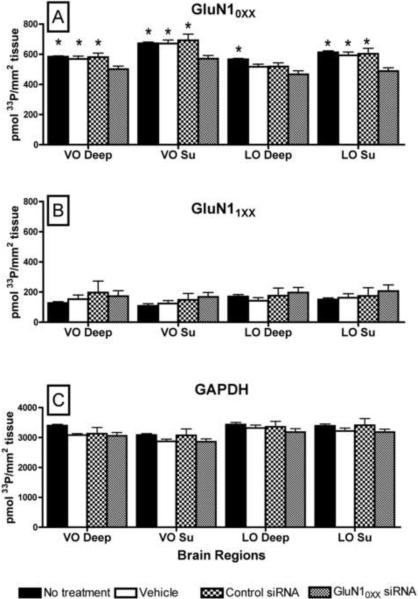
There was an overall significant effect of treatment (F(3,43) = 4.84, p = .005) on hybridization density (pmol 33P/mm2 tissue) for the GluN10XX splice variants across all the brain regions analyzed. The deep (p = .004–.01) and superficial (p = .002–.01) layers of ventral orbital and the superficial layers of lateral orbital (p = .001–.003) regions had a significantly lower hybridization density for GluN10XX splice variant mRNA in the animals treated with GluN10XX, siRNA, as compared to all other controls (Fig. 2A, D, G, J, 3A). In the deep layers of lateral orbital cortex, GluN10XX splice variant mRNA in the animals treated with the GluN10XX, siRNA had a trend for lower hybridization density as compared to animals injected with control RNA (p =.06) or vehicle (p = .07) and a significantly lower expression than the animals left untreated (p < 0.001, Fig. 3A). The animals treated with the GluN10XX siRNA exhibited declines in GluN10XX splice variant mRNA of 10–19% across the various brain regions analyzed, as compared to the animals treated with a control siRNA (Fig. 3A). There was no significant effect of treatment on hybridization density of GluN11XX (F(3,43) = .071. p = .73; Fig. 2B, E, H, K, 3B) or GAPDH mRNA (F(3,43) = 0.82, p = .49; Fig. 2C, F, I, L, 3C). The actual sites of injection appeared to be near the intended injection site and extended down to the orbital region (Fig. 2N) for all injected animals. The spread of grossly visible reduction in mRNA for GluN10XX splice variants extended from 0.25mm to 2.0mm lateral to the midline, 1.5mm to 3.0mm ventral to the surface of the skull and 1.94mm to 2.8mm rostral to the Bregma (Fig. 2N).
Fig. 3.
Graphs showing mRNA expression of GluN10XX subunit splice variants (A), GluN11XX (B) and GAPDH (C) in different regions of the prefrontal cortex of the brain. * p < .05 for difference from mRNA expression in animals injected with siRNA specific for GluN10XX subunit splice variants (GluN10XX siRNA). VO = ventral orbital, LO = lateral orbital, su = superficial cortical layers II–III, deep = crtical layers IV–VI.
4. Discussion
The present study provided evidence for a role for the GluN10XX subunit splice variants of the NMDA receptor within the prefrontal cortex of the brain in spatial reference memory in young mice. Reduction of the GluN10XX splice variant mRNA expression in the ventral and lateral orbital regions of the brain in young mice lead to poor performance in the first half of training for spatial reference memory. There was no difference in behavior between any treatments in the later phases of reference memory training. This reduction in GluN10XX splice variant mRNA did not significantly affect cognitive flexibility in the reversal trials or associative memory in cued trials.
Young mice treated with siRNA specific for GluN10XX splice variants of the NMDA receptors showed delays in learning early on in training, as compared to the mice treated with control siRNA and vehicle treated animals. There was a 10–19% decline in mRNA expression for the GluN10XX splice variants in superficial and deep layers of ventro-lateral orbital region of the brain after treatment with the specific siRNA. Specificity of knockdown of GluN10XX splice variants was verified by the absence of any alterations in hybridization density of the splice variants containing the N1 cassette, GluN11XX or a control mRNA, GAPDH, following GluN10XX specific siRNA treatment in the same brain regions. These results suggest that the delay in learning seen in the reference memory task was likely due to the reduction in expression of the GluN10XX splice variants in the ventro-lateral orbital regions of the brain.
Mice treated with GluN10XX specific siRNA exhibited a worse performance in the very beginning of training in the reference memory task, as compared to control siRNA injected animals. However, the lack of difference from the vehicle-injected animals in the same block of trials made this difficult to interpret. At the beginning of the second day (Block 3), the group treated with siRNA specific for the GluN10XX splice variants performed worse than each of the control groups in one or more measures of place trial performance. These GluN10XX siRNA injected mice then showed improved performance, similar to all other groups, by block 4, suggesting that GluN10XX splice variants may play a role primarily in early learning. The results were similar between the more traditional measures of pathlength and latency and the proximity score, although not always reaching significance, with p values ranging from .009–.07. The proximity measure has been shown to be the most sensitive at measuring spatial bias for mice in the water maze [32]. It is less affected by swim speed or floating behavior observed in mice and better reflects spatial bias than the traditional measures [29]. The near-significant differences at p = .07 between the control siRNA and GluN10XX siRNA-treated mice in pathlength and latency measures may suggest that this is a modest effect for those measures of performance, but it does appear that the GluN10XX siRNA-treated mice were impaired in their search for the platform as compared to those receiving control siRNA.
Reference memory tasks involving several trials per day for several days in the Morris water maze involve both acquisition and consolidation of memory and it was not possible to determine which was affected in this study. Memory consolidation in mice has been shown to occur within several hours to days after training for a task [33]. Leon and coworkers have shown, with the use of protein kinase inhibitors, that memory consolidation can occur 2 hours after acquisition of single day learning in the Morris water maze [34]. With the use of inhibitors of protein synthesis, Artinian and co-workers [35] observed initiation of memory consolidation as early as 4 hours after training in the Morris water maze. The major differences in performance following treatment with the GluN10XX siRNA in this study were seen during the first half of training, particularly in the beginning of day 2, and further improvements in performance occurred later. Based on the above findings, it is possible that the deficit in memory seen in animals treated with GluN10XX specific siRNA during the first half of training may be due to problems with early acquisition and/or early consolidation.
In the present study, we observed 10–19% declines in mRNA expression for the GluN10XX subunit splice variants across the ventro-lateral orbital region of the prefrontal cortex following treatment with GluN10XX specific siRNA. The GluN1 subunit of the NMDA receptor has been identified as a necessary subunit for proper functioning of the NMDA receptor [36–38]. The GluN10XX subunit splice variants which lack the N1 cassette, have reduced affinity for agonist by almost five fold as compared to the ones with the N1 cassette [39]. These GluN10XX subunit splice variants show increased mRNA expression in orbital, insular and medial prefrontal cortices of the brain in old mice after they have been subjected to behavioral testing experience [21]. This increase in GluN10XX subunit splice variant mRNA expression in the orbital region also had a near-significant (corrected p = .08) association with performance in reference memory in old mice, with higher expressers showing better memory [21]. Inducible and region specific knockout of all GluN1 subunits has revealed involvement of the subunit in consolidation of hippocampal-independent nondeclarative taste memory [40]. The prefrontal cortex has been shown to be important for reference memory function including formation of recent memory [41, 42] and recall of stored information [43, 44]. Tests of spatial memory using Morris water maze with multiple distant cues and varied number of trials have been shown to involve prefrontal cortex for acquisition of memory [45]. The prefrontal cortex has also been shown to be involved in consolidation and recall of recent spatial memory after training in the Morris water maze [34]. Therefore, a reduction in GluN10XX subunit splice variants by GluN10XX siRNA treatment in the ventro-lateral orbital cortex might be responsible for the problem in early acquisition and/or consolidation of long term memory after training with Morris water maze.
We only observed differences in reference memory between the animals left untreated and the animals receiving GluN10XX siRNA in the pathlength measure for the present study. The mice that were left untreated did not receive any acetaminophen, codeine or isoflurane. Prolonged exposure to isoflurane during development can cause neurodegeneration in rodents [46] but does not have this same effect on aged rodents [47]. A study by Ishida and coworkers [48] on the effects of acetaminophen on memory performance in Morris water maze shows that a high dose (302.3 mg/kg) causes memory impairment, but a low dose (15.1 mg/kg) facilitates memory performance. The dose of acetaminophen in the present study was close to the low dose of acetaminophen in the Ishida et al. [48] study and so may have facilitated memory performance in mice that underwent surgery and received acetaminophen.
Reduction of the mRNA for GluN10XX subunit splice variants in the ventro-lateral orbital regions did not seem to inhibit performance in the probe trials of the reference memory task, in which the escape platform was missing. Instead there appeared to be a trend for improved performance in the mice injected with GluN10XX specific siRNA, as compared to the mice injected with control siRNA at the end of the second day of training. The probe trials were used to measure the bias towards a previously learned location of the escape platform. If there was no difference among the different treatment groups, it would indicate a similar bias among the treatment groups for the previously learned platform position. The greatest difference between injected animals in the place trials occurred at the beginning of the second training day. The measurement of bias was at the end of that day, after the mice receiving GluN10XX siRNA showed equal or better performance to the other treatment groups in place trials. Thus, the bias may have been developed by the time the probe trial was performed. Young animals possess redundant systems for different functions of the body, which deteriorates over time [3]. Better performance in place and probe trials towards the end of the second training day observed in the mice injected with GluN10XX specific siRNA could be due to use of other redundant systems that are available to these young animals.
The lack of a larger deficit throughout water maze training may suggest that the GluN10XX subunit splice variants within the prefrontal cortex do not play a major role in spatial reference memory. However, a reduction in mRNA expression of only 10–19% within young animals did produce a significant decrease in performance within the early part of training. Overexpression of the GluN2B subunit within the cortex and hippocampus of transgenic mice [49] and induced within the prefrontal cortex of aged mice (unpublished observation) both showed significantly improved performance in the water maze, but only primarily in the middle of water maze training. This suggests that NMDA receptors are more essential within specific phases of learning in this task, as opposed to throughout training.
Lesions in the prefrontal cortex of rodents in some studies have been shown to cause impairment in memory performance during water maze training [45, 50]. Interestingly Hoh and coworkers have shown that the lesions are not effective if the rodents are pretrained prior to the lesion, suggesting no role of the prefrontal cortex in retrieval and storage of memory information [50]. Lesions in the prefrontal cortex in other studies failed to impair performance in water maze training [51, 52]. This might partly be due to non-uniformity of the lesions among the various studies and use of various water maze training protocols. However, this suggests that the role of prefrontal cortex in spatial reference memory is complex, which also may account for the modest changes seen in this study. Studies involving lesions to the hippocampus have been more consistent with respect to impairing performance in water maze training [45, 51–53]. The hippocampus expresses a high density of NMDA receptors and GluN1 subunits [54, 55] and these receptors are important for spatial memory in the water maze [56]. Thus, it is possible that the application of the GluN10XX siRNA to the hippocampus may produce a more robust disruption of water maze performance and provide more information about the role of these splice variants in spatial reference memory. In the current study, the focus was on the prefrontal cortex because of previous results showing that the upregulation of GluN10XX splice variant within this region in aged mice is associated with better performance in reference memory tasks of Morris water maze training [21].
There was no overall significant difference in performance of mice between different treatment groups when the escape platform was moved to the opposite quadrant. However, a trend of mice injected with GluN10XX specific siRNA to perform poorly early in the task was observed. This might suggest a role for GluN10XX splice variants within the ventro-lateral orbital region in flexibility in animals. Prefrontal cortex has been shown to be involved in reversal training in both non-human primates [57] and rodents [10, 58]. The role of the orbital prefrontal cortex in reversal learning has shown inconsistent results across different tasks. Object reversal learning was impaired in monkeys with orbital prefrontal lesions [57] and reversal learning in attentional set shifting was impaired by lesion in orbital prefrontal cortex in rats [59], but tasks involving serial reversal learning and response extinction were not affected by lesion in the same area in rats [60]. Damage to medial prefrontal cortex, however, shows compelling evidence for problems in reversal learning during spatial learning tasks in Morris water maze [10, 61]. From the above findings it may be inferred that the GluN10XX subunit splice variants in the ventro-lateral orbital region may not be important for cognitive flexibility or that a 10–19% reduction of mRNA for these splice variants was not sufficient to cause significant impairment in a young animal.
In conclusion, reducing the expression of the GluN10XX subunit splice variants of the NMDA receptor in orbital cortex appeared to interfere in the early phase of spatial reference learning. In contrast cognitive flexibility and associative memory did not appear to be altered by the reduction of the GluN10XX subunit splice variants in the ventro-lateral orbital region. Overall, this study suggested that there may be a role for GluN10XX subunit splice variants within orbital cortex in early acquisition and/or consolidation of spatial long-term memory.
Research Highlights
Injection of GluN10XX specific siRNA into mice brain showed localized reduction of its mRNA expression
Mice with reduced GluN10XX expression showed impairment in reference memory testing
GluN10XX splice variants might be involved with memory acquisition and/or consolidation
Acknowledgements
Funding sources: This research was supported in part by NIH grants AG016322 (KRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Salthouse TA. Memory aging from 18 to 80. Alzheimer Dis Assoc Disord. 2003;17:162–7. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- [2].Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp Gerontol. 2003;38:71–7. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- [3].Penner MR, Barnes CA. Memory changes with age: Neurobiological correlates. In: Kesner RP, Martinez JL, editors. Neurobiology of learning and memory. 3rd ed. Academic Press; New York: 2007. pp. 483–518. [Google Scholar]
- [4].Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–26. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- [5].Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- [6].Rapp P, Rosenberg R, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- [7].Gage F, Dunnett S, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–8. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- [8].Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–8. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- [9].Li HB, Matsumoto K, Tohda M, Yamamoto M, Watanabe H. NMDA antagonists potentiate scopolamine-induced amnesic effect. Behav Brain Res. 1997;83:225–8. doi: 10.1016/s0166-4328(97)86075-5. [DOI] [PubMed] [Google Scholar]
- [10].De Bruin JPC. Evolution of prefrontal cortex: comparative aspects of its functioning. In: Greenspan RJ, Kyriacou CP, editors. Flexibility and constraints of behavioral systems. John Wiley and Sons; Chichester: 1994. pp. 185–92. [Google Scholar]
- [11].Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, et al. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- [13].Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, et al. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [14].Bockers TM, Zimmer M, Muller A, Bergmann M, Borse N, Kreutz MR. Expression of the NMDA R1 receptor in selected human brain regions. NeuroReport. 1994;5:965–9. doi: 10.1097/00001756-199404000-00028. [DOI] [PubMed] [Google Scholar]
- [15].Pelleymounter MA, Beatty G, Gallagher M. Hippocampal 3H-CPP binding and spatial learning deficits in aged rats. Psychobiology. 1990;18:298–304. [Google Scholar]
- [16].Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. 2nd edition Oxford University Press; Oxford: 1994. pp. 340–75. [Google Scholar]
- [17].Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech Ageing Dev. 1998;104:227–48. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- [18].Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends in Neurosciences. 1995;18:306–13. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- [19].Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14:3180–94. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Magnusson KR, Bai L, Zhao X. The effects of aging on different C-terminal splice forms of the zeta1(NR1) subunit of the N-methyl-d-aspartate receptor in mice. Brain Res Mol Brain Res. 2005;135:141–9. doi: 10.1016/j.molbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [21].Das SR, Magnusson KR. Relationship between mRNA expression of splice forms of the zeta1 subunit of the N-methyl-D-aspartate receptor and spatial memory in aged mice. Brain Res. 2008;1207:142–54. doi: 10.1016/j.brainres.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Frontiers in Aging Neuroscience. 2010;2:11. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Magnusson KR. Influence of dietary restriction on ionotropic glutamate receptors during aging in C57B1 mice. Mech Ageing Dev. 1997;95:187–202. doi: 10.1016/s0047-6374(97)01884-8. [DOI] [PubMed] [Google Scholar]
- [24].Kito S, Miyoshi R, Nomoto T. Influence of age on NMDA receptor complex in rat brain studied by in vitro autoradiography. J Histochem Cytochem. 1990;38:1725–31. doi: 10.1177/38.12.2147708. [DOI] [PubMed] [Google Scholar]
- [25].Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–5. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- [26].Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–74. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yoon SO, Lois C, Alvirez M, Alvarez-Buylla A, Falck-Pedersen E, Chao MV. Adenovirus-mediated gene delivery into neuronal precursors of the adult mouse brain. Proc Natl Acad Sci U S A. 1996;93:11974–9. doi: 10.1073/pnas.93.21.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. Academic Press; London: 2001. [Google Scholar]
- [29].Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- [30].Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–90. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- [31].Eakin TJ, Baskin DG, Breininger JF, Stahl WL. Calibration of 14C-plastic standards for quantitative autoradiography with 33P. J Histochem Cytochem. 1994;42:1295–8. doi: 10.1177/42.9.8064137. [DOI] [PubMed] [Google Scholar]
- [32].Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Frontiers in Integrative Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–7. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- [34].Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- [35].Artinian J, McGauran AM, De Jaeger X, Mouledous L, Frances B, Roullet P. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur J Neurosci. 2008;27:3009–19. doi: 10.1111/j.1460-9568.2008.06262.x. [DOI] [PubMed] [Google Scholar]
- [36].Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992;300:39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- [37].Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–4. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- [38].Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–43. [PubMed] [Google Scholar]
- [39].Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci U S A. 1993;90:6731–5. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cui Z, Lindl KA, Mei B, Zhang S, Tsien JZ. Requirement of NMDA receptor reactivation for consolidation and storage of nondeclarative taste memory revealed by inducible NR1 knockout. Euro J Neurosci. 2005;22:755–63. doi: 10.1111/j.1460-9568.2005.04257.x. [DOI] [PubMed] [Google Scholar]
- [41].Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. NeuroReport. 2006;17:341–4. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- [42].Zhao M-G, Toyoda H, Lee Y-S, Wu L-J, Ko SW, Zhang X-H, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–72. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [43].Paradiso S, Crespo Facorro B, Andreasen NC, O'Leary DS, Watkins LG, Boles Ponto L, et al. Brain activity assessed with PET during recall of word lists and narratives. NeuroReport. 1997;8:3091–6. doi: 10.1097/00001756-199709290-00017. [DOI] [PubMed] [Google Scholar]
- [44].Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Houle S, et al. Functional brain maps of retrieval mode and recovery of episodic information. NeuroReport. 1995;7:249–52. [PubMed] [Google Scholar]
- [45].Compton DM, Griffith HR, McDaniel WF, Foster RA, Davis BK. The flexible use of multiple cue relationships in spatial navigation: a comparison of water maze performance following hippocampal, medial septal, prefrontal cortex, or posterior parietal cortex lesions. Neurobiol Learn Mem. 1997;68:117–32. doi: 10.1006/nlme.1997.3793. [DOI] [PubMed] [Google Scholar]
- [46].Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- [47].Stratmann G, Sall JW, Bell JS, Alvi RS, May LV, Ku B, et al. Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats. Anesthesiology. 2010;112:305–15. doi: 10.1097/ALN.0b013e3181ca33a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ishida T, Sato T, Irifune M, Tanaka K, Nakamura N, Nishikawa T. Effect of acetoaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol (Oxf) 2007;21:757–67. doi: 10.1177/0269881107076369. [DOI] [PubMed] [Google Scholar]
- [49].Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- [50].Hoh TE, Kolb B, Eppel A, Vanderwolf CH, Cain DP. Role of the neocortex in the water maze task in the rat: a detailed behavioral and Golgi-Cox analysis. Behav Brain Res. 2003;138:81–94. doi: 10.1016/s0166-4328(02)00237-1. [DOI] [PubMed] [Google Scholar]
- [51].Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, et al. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–84. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–26. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- [53].Pouzet B, Welzl H, Gubler MK, Broersen L, Veenman CL, Feldon J, et al. The effects of NMDA-induced retrohippocampal lesions on performance of four spatial memory tasks known to be sensitive to hippocampal damage in the rat. Eur J Neurosci. 1999;11:123–40. doi: 10.1046/j.1460-9568.1999.00413.x. [DOI] [PubMed] [Google Scholar]
- [54].Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- [55].Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–74. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Morris RGM. Synaptic plasticity and learning: Selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–57. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren J, Ekert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 219–41. [Google Scholar]
- [58].Li L, Shao J. Restricted lesions to ventral prefrontal subareas block reversal learning but not visual discrimination learning in rats. Physiol Behav. 1998;65:371–9. doi: 10.1016/s0031-9384(98)00216-9. [DOI] [PubMed] [Google Scholar]
- [59].Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol. 1974;87:772–80. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- [61].Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]