Abstract
Meier–Gorlin syndrome (MGS) is an autosomal recessive disorder characterized by microtia, patellar aplasia/hypoplasia, and short stature. Recently, mutations in five genes from the pre-replication complex (ORC1, ORC4, ORC6, CDT1, and CDC6), crucial in cell-cycle progression and growth, were identified in individuals with MGS. Here, we report on genotype–phenotype studies in 45 individuals with MGS (27 females, 18 males; age 3 months–47 years). Thirty-five individuals had biallelic mutations in one of the five causative pre-replication genes. No homozygous or compound heterozygous null mutations were detected. In 10 individuals, no definitive molecular diagnosis was made. The triad of microtia, absent/hypoplastic patellae, and short stature was observed in 82% of individuals with MGS. Additional frequent clinical features were mammary hypoplasia (100%) and abnormal genitalia (42% predominantly cryptorchidism and hypoplastic labia minora/majora). One individual with ORC1 mutations only had short stature, emphasizing the highly variable clinical spectrum of MGS. Individuals with ORC1 mutations had significantly shorter stature and smaller head circumferences than individuals from other gene categories. Furthermore, compared with homozygous missense mutations, compound heterozygous mutations appeared to have a more severe effect on phenotype, causing more severe growth retardation in ORC4 and more frequently pulmonary emphysema in CDT1. A lethal phenotype was seen in four individuals with compound heterozygous ORC1 and CDT1 mutations. No other clear genotype–phenotype association was observed. Growth hormone and estrogen treatment may be of some benefit, respectively, to growth retardation and breast hypoplasia, though further studies in this patient group are needed.
Keywords: Meier–Gorlin syndrome, ear-patella-short stature syndrome, origin recognition complex, pre-replication complex, genotype–phenotype
Introduction
Meier–Gorlin syndrome (MGS) (MIM 224690) is a form of primordial dwarfism, characterized by microtia, short stature, and absent or hypoplastic patellae. Furthermore, pulmonary emphysema, feeding problems, various skeletal abnormalities, genitourinary anomalies, and mammary hypoplasia frequently accompany this autosomal recessive disorder. Characteristic facial features, which gradually change with age, are frequently described. Infants typically have a small mouth with full lips and micrognathia, whereas in adults, a high forehead and a more prominent, narrow nose with a broad nasal bridge are distinguishable.
The first patient was reported by Meier in 1959.1 Gorlin reported the second patient with a similar phenotype.2 In total, only 53 cases have been described in the literature thus far.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Recently, mutations in ORC1, a pre-replication complex member gene, were identified in 5 out of 204 individuals with microcephalic primordial dwarfism.13
As individuals with mutations in ORC1 showed overlapping features with MGS, mutation analysis of ORC1 was performed in 33 individuals with MGS and revealed mutations in 4 individuals from three families.13 Mutation analysis of other genes of this pre-replication complex showed mutations in ORC4, ORC6, CDT1, and CDC6 in 14 individuals from nine families with MGS.14 Simultaneously, beginning with a family-based mapping approach in individuals with MGS with a founder effect, Guernsey et al15 identified mutations in ORC1, ORC4, and CDT1 in eight individuals from five families with MGS. The pre-replication complex forms at origins of DNA replication and is essential to initiate genome replication.22, 23 The complex consists of the origin recognition complex (encompassing the subunits ORC1 to ORC6), two regulatory proteins (CDC6 and CDT1), and the putative helicase complex (minichromosome maintenance (MCM) proteins). The origin recognition complex is loaded onto the chromatin during M and G1 phases. Afterwards, other proteins (including CDT1 and CDC6) bind to the pre-replication complex, facilitating repeated loading of the MCM helicase. The MCM helicase unwinds DNA and recruits additional replication proteins at the beginning of the S phase, thereby initiating replication. Mutations in genes from the pre-replication complex are expected to disturb this process of DNA replication. In vitro studies using lymphoblastoid cell lines or skin fibroblast cell lines from two individuals with mutations in ORC1 showed reduced levels of ORC1, ORC2, and MCM2 in chromatin-enriched cell fractions.13 Furthermore, the S-phase entry/progression in both individuals was delayed.
The five genes known to cause MGS are part of the same pre-replication complex. We posed the question whether mutations in the different genes from the pre-replication complex have a different effect on the MGS phenotype. To answer that question, we performed genotype–phenotype studies in 45 individuals with MGS, including all individuals with a molecular diagnosis of MGS thus far known.
Methods
Patients
Of the 45 individuals included in this study, 35 carried mutations in one of the five known genes for MGS. Ten individuals with a clinical diagnosis of MGS without a known molecular cause are described, including one individual carrying a monoallelic mutation in ORC1 and two carrying a monoallelic mutation in CDT1. Molecular testing showed no abnormalities in five other individuals with a clinical diagnosis of MGS, but the authors had insufficient clinical information to include them in this study.
Thirty-five individuals were described previously in literature.2, 3, 4, 5, 8, 11, 13, 14, 15, 16, 17, 21 The remaining 10 individuals were not yet described in literature. These 10 individuals were referred to the Human Genetics departments of the Radboud University Nijmegen Medical Centre, the Netherlands and the Western General Hospital in Edinburgh, UK for molecular analysis from the Netherlands, the UK, Ireland and India. The medical history and most recent clinical data of all individuals were obtained by sending a questionnaire to the referring physicians or by physical examination by the authors. The growth data were converted to standard deviations (SD) from the mean, using two different growth charts for growth around birth and postnatal growth.24, 25 Intra-uterine growth retardation was defined as weight for gestational age more than 1.3 SD (10th centile) under the mean, postnatal growth retardation as height for age more than 2 SD under the mean, and microcephaly as head circumference for age more than 3 SD under the mean.26, 27, 28 Informed consent to perform molecular investigations and to publish the medical data and photographs of the patients was obtained.
Molecular data
Sequence analysis of the ORC1, ORC4, ORC6, CDT1, and CDC6 genes was performed on DNA isolated from peripheral blood from the affected children and the parents as previously described.13, 14, 15 Copy number variation analysis of ORC4 was performed in eight individuals.15 In the individual with monoallelic mutations in ORC1, DNA was screened for intragenic deletions/duplications of ORC1, using Multiplex Ligation-dependent Probe Amplification.
Statistical analysis
Differences in birth weight, height, and head circumference between the different gene categories of MGS (individuals with biallelic mutations in ORC1, ORC4, ORC6, CDT1, and CDC6, and individuals with a clinical diagnosis of MGS without a definitive molecular diagnosis) were analyzed using the Student's t-test. The significance level was set at the 5% probability level. Statistical analysis was performed using standard statistical software (SPSS version 16.0, Inc., Chicago, IL, USA).
Results
Patients and molecular investigation
The clinical data of all 45 individuals from 35 families with MGS are summarized in Table 1. Thirty-five individuals from 26 families had biallelic mutations in one of the five pre-replication genes underlying MGS. In 10 individuals from nine families, no definitive underlying molecular cause could be identified.
Table 1. Clinical features of 35 individuals with Meier–Gorlin syndrome with biallelic mutations in one of the five pre-replication genes and 10 individuals with a clinical diagnosis of Meier–Gorlin syndrome without a definitive molecular diagnosis, including one individual with a monoallelic mutation in ORC1 and two with a monoallelic mutation in CDT1.
| Clinical characteristics | ORC1 (10 individuals) | ORC4 (7 individuals) | ORC6 (7 individuals) | CDT1 (10 individuals) | CDC6 (1 individual) | Total (35 individuals) | No definitive molecular diagnosis (10 individuals) |
|---|---|---|---|---|---|---|---|
| Sex ratio M/F | 4 M/6 F | 1 M/6 F | 5 M/2 F | 3 M/7 F | 1 M/0 F | 14 M/21 F | 3 M/7 F |
| Average age at examination | 13 y 1 m | 13 y 11 m | 8 y 10 m | 12 y 5 m | 15 y 6 m | 12 y 9 m | 11 y 2 m |
| Range age at examination | 3 m–47 y | 5 y–23 y | 3 y 10 m–15 y 5 m | 4 days–22 y | 3 m–47 y | 3 y 4 m–28 y 10 m | |
| Classical triad of clinical features | |||||||
| Short stature (height for age <−2 SD) | 10/10 | 6/7 | 6/7 | 8/10 | 1/1 | 31/35 (89%) | 9/10 (90%) |
| Microtia | 9/10 | 7/7 | 7/7 | 10/10 | 1/1 | 34/35 (97%) | 10/10 (100%) |
| Patellar hypoplasia/aplasia | 6/7 | 7/7 | 6/7 | 10/10 | 1/1 | 30/32 (94%) | 9/10 (90%) |
| Growth (represented in mean SD) | |||||||
| Birth weight (SD range) | −4.5 SD (<−6.5 to −1.7) | −4.1 SD (−6 to −2.3) | −3.2 SD (−3.9 to −1.9) | −3 SD (−3.9 to −0.3) | −4.1 SD | −3.8 SD (< −6.5 to −0.3) | −2.9 SD (−4.4 to −2) |
| IUGR (birth weight <−1.3 SD) | 9/9 | 7/7 | 6/6 | 9/10 | 1/1 | 32/33 (97%) | 10/10 (100%) |
| Height at examination (SD range) | −7.1 SD (−9.6 to −5.2) | −5.2 SD (−6.4 to −1.8) | −2.5 SD (−3.3 to −0.8) | −3.7 SD (−6 to −0.4) | −3.5 SD | −4.4 SD (−9.6 to −0.4) | −3.3 SD (−6.4 to −1.4) |
| Weight at examination (SD range) | −6.2 SD (−9.3 to 0.8) | −3.7 SD (−5.3 to −1.7) | −3 SD (−5 to −0.3) | −3.3 SD (−5.5 to −1.4) | −3.2 SD | −3.9 SD (−9.3 to 0.8) | −4 SD (−7.5 to −0.6) |
| Head circumference (SD range) | −6.7 SD (−9.8 to −4) | −2.5 SD (−3.2 to −0.7) | −2.4 SD (−3.3 to −1.6) | −1.3 SD (−5 to 1.7) | −1.8 SD | −2.9 SD (−9.8 to 1.7) | −2.6 SD (−5 to −1.3) |
| Microcephaly (OFC for age <−3 SD) | 8/8 | 2/5 | 1/6 | 2/9 | 0/1 | 13/29 (45%) | 3/10 (30%) |
| Disproportionate stature | 1/3 | 0/7 | 1/4 | 2/2 | 0/1 | 4/17 (24%) | 3/9 (33%) |
| Facial features | |||||||
| Abnormally formed ears | 6/7 | 2/5 | 3/7 | 7/9 | 1/1 | 19/29 (66%) | 8/10 (80%) |
| Low-set ears | 4/6 | 1/5 | 3/7 | 7/9 | 1/1 | 16/28 (57%) | 8/10 (80%) |
| Posteriorly rotated ears | 0/3 | 1/5 | 3/5 | 2/3 | 1/1 | 7/17 (41%) | 6/10 (60%) |
| Convex nasal profile | 2/3 | 0/2 | 4/4 | 0/4 | 1/1 | 7/14 (50%) | 6/9 (67%) |
| Narrow nose | 1/2 | 5/5 | 0/4 | 2/4 | 0/1 | 8/16 (50%) | 4/10 (40%) |
| High nasal bridge | 2/4 | 3/3 | 3/5 | 1/4 | 1/1 | 10/17 (59%) | 7/10 (70%) |
| Microstomia | 5/7 | 5/5 | 3/7 | 4/9 | 1/1 | 18/29 (62%) | 9/10 (90%) |
| Full lips | 7/8 | 2/5 | 5/7 | 6/9 | 1/1 | 21/30 (70%) | 9/10 (90%) |
| Micro-/retrognathia | 5/7 | 5/5 | 7/7 | 7/9 | 1/1 | 25/29 (86%) | 10/10 (100%) |
| Downslanted palpebral fissures | 1/3 | 0/3 | 5/6 | 2/4 | 1/1 | 9/17 (53%) | 3/10 (30%) |
| Neurological | |||||||
| Intellectual disability | 1/8 | 0/7 | 0/7 | 0/8 | 0/1 | 1/31 (3%) | 1/10 (10%) |
| Delayed motor development | 1/8 | 1/7 | 3/7 | 1/9 | 0/1 | 6/32 (19%) | 4/10 (40%) |
| Delayed speech development | 2/8 | 1/7 | 2/7 | 0/9 | 0/1 | 5/32 (16%) | 3/10 (30%) |
| Respiratory tract | |||||||
| Respiratory problems during infancy | 2/4 | 2/5 | 4/7 | 2/6 | 1/1 | 11/23 (48%) | 5/10 (50%) |
| Pulmonary emphysema | 3/5 | 1/7 | 0/5 | 7/10 | 1/1 | 12/28 (43%) | 1/10 (10%) |
| Tracheomalacia | 1/4 | 2/7 | 2/5 | 3/7 | 0/1 | 8/24 (33%) | 3/10 (30%) |
| Laryngomalacia | 2/4 | 1/7 | 1/5 | 2/7 | 0/1 | 6/24 (25%) | 1/10 (10%) |
| Bronchomalacia | 1/4 | 1/7 | 1/5 | 1/7 | 0/1 | 4/24 (17%) | 1/10 (10%) |
| Cardiac anomalies | 0/9 | 1/7 | 0/5 | 1/8 | 0/1 | 2/30 (7%) | 0/10 (0%) |
| Gastrointestinal | |||||||
| Feeding problems during infancy | 6/8 | 7/7 | 5/7 | 7/9 | 1/1 | 26/32 (81%) | 10/10 (100%) |
| Nasogastric feeding/gastrostomy | 4/8 | 3/6 | 2/7 | 2/9 | 0/1 | 11/31 (35%) | 6/10 (60%) |
| Failure to thrive | 2/8 | 1/6 | 3/7 | 4/9 | 1/1 | 11/31 (35%) | 2/10 (20%) |
| Gastroesophageal reflux | 4/8 | 5/6 | 2/7 | 1/9 | 1/1 | 13/31 (42%) | 2/10 (20%) |
| Urogenital anomalies | |||||||
| Abnormal genitalia | 2/10 | 3/7 | 6/7 | 3/10 | 1/1 | 15/35 (43%) | 4/10 (40%) |
| Hypospadias | 0/4 | 0/1 | 1/5 | 0/3 | 0/1 | 1/14 (7%) | 0/3 (0%) |
| Cryptorchidism/small testes | 2/4 | 0/1 | 4/5 | 2/3 | 1/1 | 9/14 (64%) | 2/3 (67%) |
| Micropenis | 1/4 | 0/1 | 0/5 | 0/3 | 1/1 | 2/14 (14%) | 0/3 (0%) |
| Clitoromegaly | 0/6 | 2/6 | 0/2 | 0/7 | 0/0 | 2/21 (10%) | 1/7 (14%) |
| Hypoplastic labia minora/majora | 0/6 | 3/6 | 1/2 | 1/7 | 0/0 | 5/21 (24%) | 2/7 (29%) |
| Renal anomalies | 1/10 | 0/7 | 0/7 | 2/10 | 0/1 | 3/35 (9%) | 0/10 (0%) |
| Secondary sexual characteristics | |||||||
| Mammary hypoplasia | 2/2 | 4/4 | 1/1 | 3/3 | 0/0 | 10/10 (100%) | 3/3 (100%) |
| Absent/sparse axillary hair | 3/3 | 1/1 | 1/2 | 1/1 | 1/1 | 7/8 (88%) | 2/4 (50%) |
| Absent/sparse pubic hair | 0/1 | 0/1 | 1/2 | 0/1 | 0/1 | 1/6 (17%) | 0/4 (0%) |
| Growth hormone treatment | 2/7 | 5/7 | 1/7 | 1/3 | 1/1 | 10/25 (40%) | 2/8 (25%) |
| Musculoskeletal anomalies | |||||||
| Delayed bone age | 3/4 | 4/7 | 4/5 | 2/5 | 1/1 | 14/22 (64%) | 5/8 (63%) |
| Genu recurvatum | 4/6 | 2/7 | 0/7 | 3/7 | 0/1 | 9/28 (32%) | 0/7 (0%) |
| Contractures/club feet | 0/4 | 2/7 | 2/6 | 1/9 | 0/1 | 5/27 (19%) | 1/10 (10%) |
| Other | 1 Bifid uvula 2 Dislocated joints 1 Craniosynostosis | 1 Hemivertebrae 1 Asymmetric limbs | 1 Dislocated joints | 1 Osteochondroma | 1 Cleft palate 1 Bifid uvula 1 Craniosynostosis 1 Facial asymmetry 1 Spina bifida occulta 1 Choanal atresia 2 Polycystic ovaries 1 Dislocated joints | ||
Of the 35 individuals with mutations, 14 were male (40%), 21 were female (60%). The average age at most recent examination was 12 years and 9 months (ranging from 3 months to 47 years). Seventeen individuals (49%) had reached puberty or adulthood. Four individuals were deceased: two siblings with mutations in ORC1, of which one passed away at the age of 3 months with a severe cortical dysplasia, pachygyria and ventricular enlargement, cranial suture stenosis, congenital emphysema of the lung, and absence of the pancreatic tail, in addition to the classical triad of MGS (microtia, patellar anomalies, and short stature) (individual P1, Table 2).14 His brother, deceased at 17 weeks of gestation, also exhibited microtia and severe growth retardation (individual P2, Table 2). The other two individuals were siblings with mutations in CDT1 (I6 and I7 respectively, Table 2). One died after a sudden cardiac arrest and had congenital lobar emphysema for which he required surgery. His sister succumbed after 3 months of severe respiratory problems due to a tracheobronchomalacia with progressive pulmonary emphysema. Both showed clinical features of the classical triad of MGS.
Table 2. Overview of mutations detected in one of the five pre-replication genes in individuals with Meier–Gorlin syndrome: biallelic mutations in 35 individuals, monoallelic mutations in three individuals.
| Gene | Nucleotide alterations | Amino-acid alterations | Hetero-/homozygous | Putative effect | Number of individuals/families | Individual reference number |
|---|---|---|---|---|---|---|
| ORC1 | c.266T>A | p.Phe89Ser | Homozygous | Missense | 1/1 | 1: P3I |
| c.314G>A | p.Arg105Gln | Homozygous | Missense | 1/1 | 1: P5 | |
| [c.314G>A]+[c.1482-2A>G] | p.Arg105Gln + intron 9 splice acceptor site | Heterozygous | Missense + splice site | 2/2 | 2: P3, P4I | |
| [c.314G>A]+[c.1999_2000delGTinsA] | p.Arg105Gln + p.Val667fsX24 | Heterozygous | Missense + frameshift | 2/1 | 2: P1, P2 | |
| [c.314G>A]+[c.1996C>T] | p.Arg105Gln + p.Arg666Trp | Heterozygous | Missense | 1/1 | 3: 1983I | |
| [c.314G>A]+[c.2159G>A] | p.Arg105Gln + p.Arg720Gln | Heterozygous | Missense | 1/1 | 1: P4 | |
| c.380A>G | p.Glu127Gly | Homozygous | Missense | 2/1 | 1: P1, P2 | |
| [c.1721C>T]*** | p.Thr57Met | Monoallelic | Missense | 1/1 | I1 (new individual)II | |
| ORC4 | c.521A>G | p.Tyr174Cys | Homozygous | Missense | 4/3 | 2: P6, P7III 3: 1652, 1768, 1769, 1899 |
| [c.521A>G]+[c.874_875insAACA] | p.Tyr174Cys + p.Ala292fsX19 | Heterozygous | Missense + frameshift | 2/2 | 2: P5; 3: 1939 | |
| [c.521A>G]+CNV del | p.Tyr174Cys + del | Heterozygous | Missense + deletion | 1/1 | 3: 1882 | |
| ORC6 | [c.2T>C]+[c.449+5G>A] | p.Met1? + p.? | Heterozygous | Missense + splice site | 4/3 | I2, I3, I4, I5 (new individuals) |
| [c.257_258delTT]+[c.695A>C] | p.Phe86X + p.Tyr232Ser | Heterozygous | Nonsense + missense | 3/1 | 2: P8, P9, P10 | |
| CDT1 | [c.196G>A] + [c.351G>C] | p.Ala66Thr + p.Gln117His (exon 2 splicing donor site) | Heterozygous | Missense + splice site | 1/1 | 2: P12 |
| [c.351G>C]+[c.1385G>A] | p.Gln117His (exon 2 splicing donor site) + p.Arg462Gln | Heterozygous | Splice site + missense | 1/1 | 2: P16 | |
| [c832G>T]+[c.1385G>A] | pGlu278X + p.Arg462Gln | Heterozygous | Nonsense + missense | 2/1 | I6, I7 (new individuals) | |
| [c.1081C>T]+[c.1357C>T] | p.Gln361X + p.Arg453Trp | Heterozygous | Nonsense + missense | 1/1 | 2: P17 | |
| [c.1385G>A]+[c1560C>A] | p.Arg462Gln + p.Tyr520X | Heterozygous | Missense + nonsense | 4/2 | 2: P11, P13, P14, P15 | |
| [c.1385G>A]*** | p.Arg462Gln | Monoallelic | Missense | 2/1 | I8, I9III; Bongers et al3 | |
| c.1402G>A | p.Glu468Lys | Homozygous | Missense | 1/1 | 3: 1627 | |
| CDC6 | c.968C>G | p.Thr323Arg | Homozygous | Missense | 1/1 | 2: P18 |
1I=Bicknell et al.13
2I=Bicknell et al.14
3I=Guernsey et al.15
IIIndividual I1 carries only one mutation in ORC1; Individuals I8 and I9 carry only one mutation in CDT1.
IIIIndividuals P6 and P7 from the article of Bicknell et al14 2011 are the same individuals as 1768 and 1769, respectively, described in the article of Guernsey et al.15
***Monoallelic.
Of the 10 individuals without a known molecular cause, one had a monoallelic missense mutation in ORC1 (I1, Table 2) and two siblings had a monoallelic missense mutation in CDT1 (I8 and I9, Table 2). The healthy fathers of these three individuals carried the same mutation. Of the 10 individuals, three were male (30%), seven were female (70%), with an average age at last examination of 11 years and 2 months. Three individuals had reached puberty or adulthood.
Homozygous or compound heterozygous mutations in ORC1, ORC4, ORC6, CDT1, and CDC6 were identified in 35 individuals. Ten had mutations in ORC1 (29%), seven in ORC4 (20%), seven in ORC6 (20%), ten in CDT1 (29%), and one in CDC6 (3%). The mutations identified are presented in Table 2. No homozygous or compound heterozygous null mutations were detected consistent with MGS mutations acting through partial loss of pre-replication complex function. Homozygous missense mutations were detected in 10 out of 35 individuals with biallelic mutations from eight families (29%), compound heterozygous missense mutations in two unrelated individuals (6%). Compound heterozygous missense and frameshift mutations were identified in four individuals from three families (11%), compound heterozygous missense and splice site mutations in eight individuals from seven families (23%), compound heterozygous missense and nonsense mutations in ten individuals from five families (29%). One individual (3%) had a heterozygous missense mutation and a partial gene deletion.
The classical triad of clinical features
The clinical diagnosis of MGS was previously based on the classical triad of microtia, absent or hypoplastic patellae, and short stature. In our cohort, all three features were generally present, although not all three features had to be present to diagnose MGS.
Seven individuals did not show all the features of the classical triad. They had mutations in ORC1, ORC4, ORC6, and CDT1. One individual without mutations had microtia, with normal stature and normal patellae. Of the 35 individuals, four with biallelic mutations (11% one with ORC4, one with ORC6, and two with CDT1 mutations) had a normal stature, but small ears and absent patellae. One individual (3%) with ORC6 mutations had microtia and short stature, without patellar aplasia/hypoplasia. One individual (3%) with ORC1 mutations had normal sized ears and normal patellae. This individual was originally diagnosed with microcephalic primordial dwarfism.13
Ears
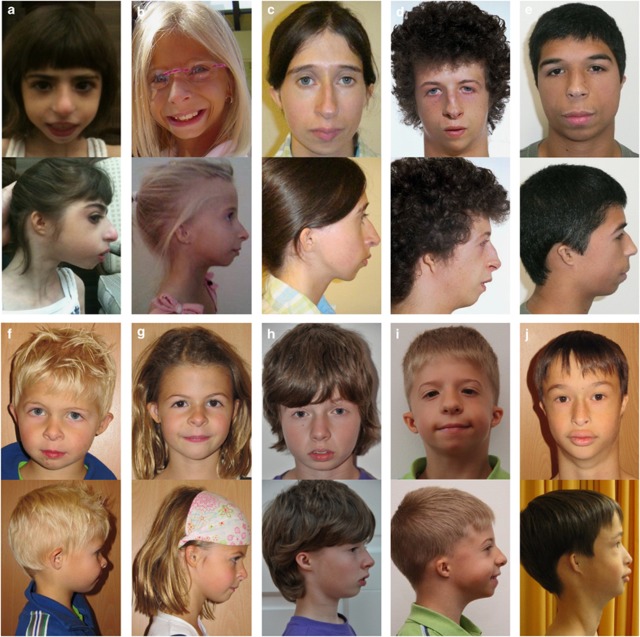
Microtia was present in 34 out of 35 individuals (97%) with mutations and all individuals clinically diagnosed with MGS. One individual with mutations in ORC1 had normal sized and shaped, though posteriorly rotated ears. Microtia ranged from slightly small, normal shaped, and positioned ears to abnormally formed (27/39; 69%), low set (24/38; 63%), and posteriorly rotated (13/27; 48%) ears (microtia grade 2) (Figure 1).29 Conductive hearing loss was detected in two individuals with mutations in ORC1 and ORC6, accompanied by a narrow ear canal in one.
Figure 1.
Facial features of individuals with Meier–Gorlin syndrome. Frontal and lateral view of 10 individuals with Meier–Gorlin syndrome. Note the different grades of microtia and the microstomia with full lips at young age. In the older individuals, the nose is more prominent and narrow, with a convex profile. Individuals (a) and (b) have mutations in ORC1 (individuals P3 and P4, Table 2);13 individual (c) has mutations in ORC4 (individual P5, Table 2);14 individual (d) has mutations in CDT1 (individual P11, Table 2);14 individual E has mutations in CDC6 (individual P18, Table 2);14 individuals (f, g, and h) are new individuals with mutations in ORC6 (individuals I2-I4, Table 2). In individuals (i and j), no definitive molecular diagnosis could be made. Individual (j) was previously described by Bongers et al in 2001.3
Patellae
Patellar malformations were reported in 30 out of 32 individuals (94%) with mutations, including all individuals with mutations in ORC6, CDT1, and CDC6. The patellae were absent in 21 individuals (70%), hypoplastic in nine (30%). The patellae of the monozygotic twins with homozygous missense mutations in ORC4 were reported to be palpable in early childhood, but were not palpable at several examinations during adolescence.14, 15 However, in both twins, no radiological examinations were performed.
Two individuals were reported to have dimples over their knees, highly suggestive of patellar anomalies, at the age of 3 and 11 months, but no ultrasound examination was performed.
The two individuals without patellar anomalies had mutations in ORC1 and ORC6.
Patellar aplasia/hypoplasia was reported in nine individuals (90%) clinically diagnosed with MGS.
Growth and growth hormone treatment
No significant difference in birth weight was detected compared for the individuals with mutations in the different genes and without a known molecular cause (P>0.05).
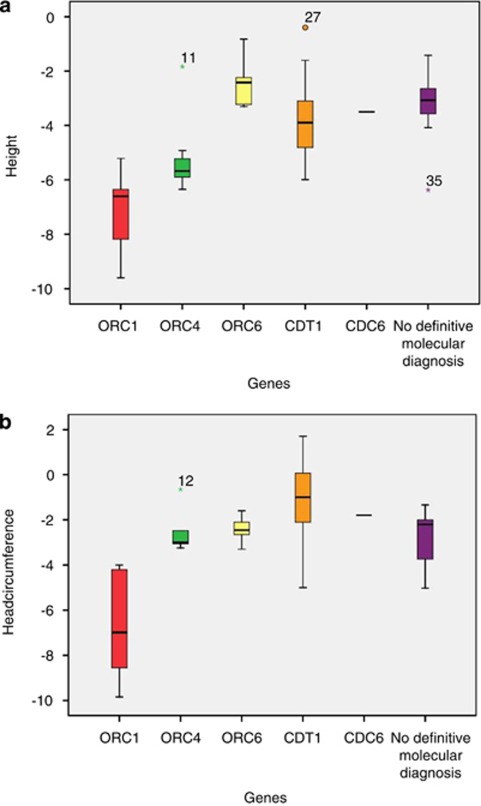
Postnatal growth (P<0.02) and head circumference (P<0.004) were significantly more delayed in individuals with ORC1 mutations, compared with individuals from other gene groups (Figures 2a and b). Microcephaly was generally more apparent in older individuals.
Figure 2.
(a) Comparison of height (SD) of individuals with Meier–Gorlin syndrome per gene category in 33 individuals with biallelic mutations and 9 individuals without definitive molecular diagnosis: n=9 ORC1; n=7 ORC4; n=7 ORC6; n=9 CDT1; n=1 CDC6; n=9 no definitive molecular diagnosis. (b) Comparison of head circumference (SD) of individuals with Meier–Gorlin syndrome per gene category 29 individuals with biallelic mutations and 10 individuals without definitive molecular diagnosis: n=8 ORC1; n=5 ORC4; n=6 ORC6; n=9 CDT1; n=1 CDC6; n=10 no definitive molecular diagnosis.
Intrauterine growth retardation (weight for gestational age <−1.3 SD25) was established in 32 out of 33 individuals (97%) with mutations in one of the five known genes. Birth weight ranged from 1580 g after 40 weeks of gestation, to 3260 g after 41 weeks of gestation. One individual had a normal birth weight and mutations in CDT1. The birth weight of two individuals was unknown. IUGR was present in all ten individuals without definitive molecular diagnosis.
Postnatal growth was delayed (height for age <−2 SD24) in 31 out of 35 individuals (89%) with mutations and in 9 out of 10 individuals (90%) clinically diagnosed with MGS.
Microcephaly (OFC <−3 SD24) at birth was present in 3 out of 14 individuals (21%) with mutations (1/1 with mutations in ORC1, 1/4 in ORC4, and 1/1 in CDC6). Postnatally, microcephaly was present in 13 out of 30 individuals (43%) with mutations (8/8 ORC1; 2/5 ORC4; 1/6 ORC6; 2/9 CDT1). Microcephaly was reported in 3 out of 10 individuals (30%) clinically diagnosed with MGS.
Growth hormone treatment was initiated in eight individuals with mutations in all five genes and pubertal development was delayed with a gonadotropin-releasing hormone analog in one individual with ORC4 mutations. Positive results of growth hormone treatment were reported in one individual with mutations in ORC4 and one with mutations in CDC6. The latter received treatment from age 2.5 years till age 7 years and from age 7.5 years till age 15.5 years. His height improved from −5 to −3 SD during the first 4 years, but showed no further improvement afterwards.
The former received growth hormone treatment from the age of 3 years till the age of 9 years. Initially, her growth velocity increased, but the positive effect wore off and the treatment was stopped.
Growth hormone therapy, with positive effects, was initiated in two males without mutations. One received growth hormone from the age of 3 years and 1 month. His height increased from −6.9 to −3.1 SD at 12 years and 7 months of age. The other male was treated with growth hormone from the age of 5 years and 5 months. His height increased from −5.8 to −4.1 SD at the age of 6 years and 9 months.
Neurological features
Most individuals with MGS had normal intellect (39/41; 95%) and showed normal motor and speech development (31/42; 74%). A mild intellectual disability was reported in one individual with mutations in ORC1 and one individual without mutations. Moderate learning difficulties were recorded in an individual with homozygous missense mutations in ORC1 who suffered from intraventricular hemorrhage due to prematurity. The delay in motor development in one individual with mutations in CDT1 might be related to congenital limb anomalies (club feet and genu recurvatum). Besides the anomalies in the individual with mutations in ORC1 described above, structural brain anomalies were rarely observed in MGS.
Facial features
All individuals with MGS (both molecularly and clinically diagnosed) had a recognizable facial appearance with microtia (44/45; 98%), microstomia (27/39; 69%), full lips (30/40; 75%), and retro-/micrognathia (35/39; 90%) (Figure 1). The profile of the nose was less consistent, but often convex (13/23; 57%) and narrow (12/26; 46%) with a high nasal bridge (17/27; 63%) and appeared to become more prominent with age (Figure 1). Less frequent findings were strabismus (3/23; 13%), a bifid uvula (2/45; 4%), and cleft palate (1/45; 2%).
Respiratory tract
Pulmonary emphysema may be a serious complication in individuals with MGS. It was present in 12 out of 28 individuals (43%) with mutations in ORC1 (3), ORC4 (1), CDT1 (7), and CDC6 (1), and 1 out of 10 individuals (10%) without molecular diagnosis. None of the seven individuals with ORC6 mutations had pulmonary emphysema. Emphysema was congenital in most individuals, except for two, in whom the diagnosis was made at 4 and 7 years of age, respectively. Structural abnormalities of the respiratory tract, comprising laryngomalacia, tracheomalacia, and bronchomalacia are a relatively frequent finding in individuals with MGS. They were reported in 10 out of 24 individuals (42%) with mutations and 3 out of 10 individuals (30%) without a known molecular cause. One individual with mutations in ORC4 had a tracheoesophageal fistula with trachea- and bronchomalacia. She developed secondary aspiration pneumonia and required nocturnal mechanical ventilation. Ten out of 25 individuals (40%) suffered from recurrent respiratory tract infections without apparent structural malformations during infancy and young childhood, improving with aging.
Cardiovascular tract
Congenital heart defects were rarely observed in MGS. They were present in 2 out of 30 individuals (7%) with mutations. One individual with mutations in ORC4 had a perimembranous ventricular septal defect causing congestive heart failure, which closed spontaneously. The other individual had mutations in CDT1 and a patent ductus arteriosus (PDA), which required interventional coiling.
Gastrointestinal tract
Feeding problems in infancy and young childhood were very common in individuals with MGS, with a prevalence of 81% (26/32) in individuals with mutations and 100% (10/10) in individuals without a known molecular cause. They may, however, partially be triggered by anxiety about the growth deficiency in these individuals. Feeding problems ranged from a small appetite (17/40; 43%) to gastroesophageal reflux (15/41; 37%), and failure to thrive (13/41; 32%). Of 41 individuals, 17 (41%) had tube feeding or gastrostomy interventions.
Urogenital tract
Minor genital anomalies were frequently described in individuals with MGS. In our cohort of 35 individuals with mutations in one of the five genes underlying MGS, 15 (43%) had genital anomalies (10/14; 71% males, 5/21; 24% females; mutations in all five genes). The genital anomalies in males encompassed small testes (2/14; 14%) and cryptorchidism (7/14; 50%), accompanied by a micropenis in two (14%). One individual had hypospadias (7%). In females, hypoplastic labia majora were present in four (19%). Two had clitoromegaly in addition, one also had hypoplastic labia minora. Hypoplastic labia minora alone was reported in one individual (5%). The same genital anomalies were seen in individuals without a molecular diagnosis: cryptorchidism (1/3; 33%), small testes (1/3; 33%), and hypoplastic labia majora (2/7; 29%).
Structural renal anomalies were uncommon in individuals with MGS: unilateral kidney aplasia was reported in two individuals (6%) with mutations in CDT1. Kidney stones were detected in one individual (3%) with mutations in ORC1.
Secondary sexual characteristics
Mammary hypoplasia was invariably present in all 13 post-pubertal females (100% 10 with mutations in ORC1, ORC4, ORC6, and CDT1; three without molecular diagnosis). Exogenous estrogen treatment in two siblings with ORC4 mutations had a positive effect on breast development. Menarche and menstrual cycles were normal in all. Hypoplastic nipples were seen in one male with mutations in CDT1. Sparse or absent axillary hair was reported in nine post-pubertal individuals (75% three males, six females; seven with mutations in all five genes, one with one mutation in CDT1 and one without mutations). One individual with ORC6 mutations also had sparse pubic hair.
Musculoskeletal features
In addition to the cardinal patellar malformation, various skeletal anomalies accompanied MGS, including a delayed bone age (14/22; 64%), slender long bones (13/20; 65%), hooked clavicles (1/15; 7%), genu recurvatum (9/28; 32%), club feet or other joint contractures (5/27; 19%), and joint subluxations (3/30; 10%). One individual with mutations in ORC1 required craniosynostosis surgery. Another individual with CDT1 mutations required surgery for scoliosis. One individual with mutations in CDC6 had a mandibular osteochondroma. Three out of 10 individuals (30%) without molecular diagnosis had a delayed bone age, one (10%) had contractures of the knees, one (10%) joint subluxations, and five (50%) showed muscle weakness at physical examination. One individual (10%) had craniosynostosis.
Discussion
We performed genotype–phenotype studies on 35 individuals from 26 families with mutations in one of the five known pre-replication genes for Meier–Gorlin syndrome (ORC1, ORC4, ORC6, CDT1, and CDC6) and 10 individuals from nine families without a definitive molecular diagnosis.13, 14, 15
Our clinical data of the 35 individuals with MGS show that all individuals but one had at least two of the three main classical characteristics (microtia, absent/hypoplastic patellae, and short stature). In the one individual with short stature only, it is questionable whether the diagnosis MGS could be made solely on a clinical basis, indicating a broader range of phenotypes in MGS than previously expected.
In an infant with short stature or microtia, the diagnosis MGS should be considered and the patellae should be assessed with care, as hypoplasia of the patellae can be mild. In infants and young children, ultrasound investigations are preferred over radiographic investigations for demonstrating patellar aplasia, as the patella is completely cartilaginous and therefore radiolucent in the first few years of life.30
Follow-up of growth and development is recommended. Growth hormone treatment may be worthwhile considering, as there has been apparent benefit to some MGS individuals, however further studies need to be undertaken to determine growth trajectories and the effect of growth hormone treatment in MGS. Finally, clinicians and families should be aware that mammary hypoplasia is present in all post-pubertal females with MGS. Exogenous estrogen treatment may be beneficial to breast development, but the effects need to be studied further.
All 35 individuals had mutations in one of the five known pre-replication complex genes. Ten individuals had mutations in ORC1 (29%), seven in ORC4 (20%), seven in ORC6 (20%), ten in CDT1 (29%), and one in CDC6 (3%).
We show that individuals with ORC1 mutations have a significantly shorter stature and smaller head circumference than individuals with mutations in the other four genes and individuals without definitive molecular diagnosis.
No other clear genotype–phenotype correlation was detected between the different gene categories. Moreover, no distinct intra- and interfamilial variation was observed. However, our data suggest that compound heterozygous missense and loss-of-function mutations have a more severe effect on the phenotype, compared with homozygous and compound heterozygous missense mutations. For instance, two individuals with compound heterozygous missense and loss-of-function ORC1 mutations had a severe lethal phenotype with multiple congenital anomalies. Three individuals with each one missense and one loss-of-function mutation in ORC4 had more severe growth retardation than the other four individuals with homozygous missense mutations in ORC4 (−6.4 to −3.5 compared with −5.4 to −1.8). One of these three individuals had pulmonary emphysema, another had a congenital cardiac anomaly, and two had severe feeding problems for which they required tube feeding and gastrostomy interventions (one individual with homozygous missense ORC4 mutations also required tube feeding). Furthermore, congenital pulmonary emphysema was reported in seven out of nine individuals with compound heterozygous missense and loss-of-function CDT1 mutations. Two of these individuals had a lethal phenotype. The only individual with homozygous missense mutations in CDT1 did not have pulmonary emphysema.
So far, no homozygous or compound heterozygous null mutations were identified, suggesting that these mutations cause a lethal phenotype. Two siblings with a recurrent missense mutation (p.Arg105Gln) and frameshift mutation (p.Val667fsX24) in ORC1 had a lethal phenotype. A lethal phenotype was also seen in two siblings with compound heterozygous nonsense and missense mutations in CDT1 (p.Glu278X + p.Arg462Gln). We hypothesize that the presence of two null mutations or a combination of certain mutations has a disadvantage at conception or leads to early miscarriages, because of a severe effect of these mutations during embryological development. Results from studies in Drosophila of the gene double parked (dup), the Drosophila ortholog of CDT1, support this theory.31 Strong mutations in this gene cause embryonic lethality preceded by a failure to undergo S phase during division.
The clinical features of zebrafish with ORC1 mutations and yeast with ORC4 mutations show an overlap with the clinical features of individuals with MGS. Both zebrafish and yeast show a reduction in overall size.13, 14, 15 A slight hypoplasia of jaw cartilage, reduction in number or fusion of otolith organs, and smaller eye size was present in over 80% of zebrafish with a depletion of ORC1. The remaining zebrafish, with a more severe depletion of ORC1, had a more severe reduction in growth with an abnormal body curvature and reduced viability.13
However, individuals with MGS show additional clinical features, such as underdevelopment of the patellae and structural anomalies, such as pulmonary emphysema, brain malformations, and genitourinary anomalies. Mouse models for MGS may contribute in determining the effects of the different mutations in the pre-replication complex on embryological development.
A mutation detection rate of 78% (35 out of 45 individuals) was established. The detection rate would have been lower (70% 35 out of 50 individuals), if a clinical diagnosis of MGS could have been made in the five individuals who were excluded from the study because of insufficient clinical information.
Individuals without a molecular diagnosis had a similar phenotype to individuals with mutations. Further molecular studies, for instance parallel sequencing of pre-replication complex genes and next-generation sequencing, may be useful to diagnose MGS earlier. We suppose that the individuals without mutations in ORC1, ORC4, ORC6, CDT1, and CDC6 have mutations in other genes of the pre-replication complex or pathways linked to the pre-replication complex. Another possibility is the presence of intragenic deletions or duplications in one of the five known pre-replication genes underlying MGS.
In conclusion, MGS is a recognizable clinical phenotype, characterized by microtia, patellar aplasia/hypoplasia, and a short stature, frequently accompanied by specific facial features, respiratory and gastrointestinal problems, and skeletal and genitourinary anomalies. In three quarters of individuals with a clinical diagnosis of MGS, the diagnosis can now be confirmed by molecular analysis of ORC1, ORC4, ORC6, CDT1, and CDC6.
Individuals with ORC1 mutations have the most severe growth retardation in MGS. Therefore, testing of ORC1 first should be considered in individuals with a severe growth retardation and microcephaly. No other clear genotype–phenotype correlation was established, although compound heterozygous mutations appear to have a more severe effect on phenotype than homozygous missense mutations.
Longitudinal studies on growth/phenotype in an extended series of patients are important to investigate the effect of growth hormone and estrogen treatment on growth and mammary development, respectively.
Acknowledgments
We would like to thank the patients and their families, the late RJ Gorlin, DL Guernsey, and RM Winter for their contributions, I van Rooij for statistical advice and assistance, C Ockeloen for her help with consenting and the Medical Research Council and Lister Institute for Preventative Medicine for funding.
The authors declare no conflict of interest.
References
- Meier Z, Poschiavo, Rothschild M. [Case of arthrogryposis multiplex congenita with mandibulofacial dysostosis (franceschetti syndrome).] Helv Paediatr Acta. 1959;14:213–216. [PubMed] [Google Scholar]
- Gorlin RJ, Cervenka J, Moller K, Horrobin M, Witkop CJ., Jr Malformation syndromes. A selected miscellany. Birth Defects Orig Artic Ser. 1975;11:39–50. [PubMed] [Google Scholar]
- Bongers EM, Opitz JM, Fryer A, et al. Meier-Gorlin syndrome: report of eight additional cases and review. Am J Med Genet. 2001;102:115–124. doi: 10.1002/ajmg.1452. [DOI] [PubMed] [Google Scholar]
- Terhal PA, Ausems MG, Van Bever Y, Kate LP, Dijkstra PF, Kuijpers GM. Breast hypoplasia and disproportionate short stature in the ear, patella, short stature syndrome: expansion of the phenotype. J Med Genet. 2000;37:719–721. doi: 10.1136/jmg.37.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev SA, Hall JG. Another adult with Meier-Gorlin syndrome--insights into the natural history. Clin Dysmorphol. 2003;12:167–169. doi: 10.1097/01.mcd.0000065052.36236.32. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Lemmerling MM, Van Mol CE, Leroy JG. The Meier-Gorlin syndrome, or ear-patella-short stature syndrome, in sibs. Am J Med Genet. 1999;84:61–67. [PubMed] [Google Scholar]
- Fryns JP. Meier-Gorlin syndrome: the adult phenotype. Clin Dysmorphol. 1998;7:231–232. doi: 10.1097/00019605-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Feingold M. Meier-Gorlin syndrome. Am J Med Genet. 2002;109:338. doi: 10.1002/ajmg.10315. [DOI] [PubMed] [Google Scholar]
- Faqeih E, Sakati N, Teebi AS. Meier-Gorlin (ear-patella-short stature) syndrome: growth hormone deficiency and previously unrecognized findings. Am J Med Genet A. 2005;137a:339–341. doi: 10.1002/ajmg.a.30899. [DOI] [PubMed] [Google Scholar]
- Dudkiewicz M, Tanzer M. Total knee arthroplasty in Meier-Gorlin syndrome. J Arthroplasty. 2004;19:931–934. doi: 10.1016/j.arth.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Cohen A, Mulas R, Seri M, et al. Meier-Gorlin syndrome (ear-patella-short stature syndrome) in an italian patient: clinical evaluation and analysis of possible candidate genes. Am J Med Genet. 2002;107:48–51. doi: 10.1002/ajmg.10083. [DOI] [PubMed] [Google Scholar]
- Boles RG, Teebi AS, Schwartz D, Harper JF. Further delineation of the ear, patella, short stature syndrome (Meier-Gorlin syndrome) Clin Dysmorphol. 1994;3:207–214. [PubMed] [Google Scholar]
- Bicknell LS, Walker S, Klingseisen A, et al. Mutations in Orc1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat Genet. 2011;43:350–355. doi: 10.1038/ng.776. [DOI] [PubMed] [Google Scholar]
- Bicknell LS, Bongers EM, Leitch A, et al. Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat Genet. 2011;43:356–359. doi: 10.1038/ng.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernsey DL, Matsuoka M, Jiang H, et al. Mutations in origin recognition complex gene orc4 cause Meier-Gorlin syndrome. Nat Genet. 2011;43:360–364. doi: 10.1038/ng.777. [DOI] [PubMed] [Google Scholar]
- Lacombe D, Toutain A, Gorlin RJ, Oley CA, Battin J. Clinical identification of a human equivalent to the short ear (Se) murine phenotype. Ann Genet. 1994;37:184–191. [PubMed] [Google Scholar]
- Bongers EM, Van Kampen A, Van Bokhoven H, Knoers NV. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68:302–319. doi: 10.1111/j.1399-0004.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- Gezdirici A, Yosunkaya E, Paydas A, Seven M, Yuksel A. Expanding the phenotypical spectrum of Meier-Gorlin syndrome with novel findings: multiple hypopigmented skin lesions and sacral dimple. Clin Genet. 2010;78 (Suppl.1:29. [Google Scholar]
- Buebel MS, Salinas CF, Pai GS, Macpherson RI, Greer MK, Perez-Comas A. A new seckel-like syndrome of primordial dwarfism. Am J Med Genet. 1996;64:447–452. doi: 10.1002/(SICI)1096-8628(19960823)64:3<447::AID-AJMG1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hurst JA, Winter RM, Baraitser M. Distinctive syndrome of short stature, craniosynostosis, skeletal changes, and malformed ears. Am J Med Genet. 1988;29:107–115. doi: 10.1002/ajmg.1320290113. [DOI] [PubMed] [Google Scholar]
- Verhallen JCTM, Van Der Lely N, Kant SG. Het syndroom van Meier-Gorlin. Tijdschr Kindergeneesk. 1999;67:32–35. [Google Scholar]
- Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license dna for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- Bell SP, Stillman B. Atp-dependent recognition of eukaryotic origins of Dna replication by a multiprotein complex. Nature. 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Prader A, Largo RH, Molinari L, Issler C. Physical growth of swiss children from birth to 20 years of age. First Zurich Longitudinal Study of growth and development. Helv Paediatr Acta Suppl. 1989;52:1–125. [PubMed] [Google Scholar]
- Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. Bmc Pediatr. 2008;8:8. doi: 10.1186/1471-2431-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Practice Bulletins--Gynecology, American College of Obstetricians and Gynecologists, Washington, DC 20090-6920, USA Intrauterine growth restriction. Clinical management guidelines for obstetrician-gynecologists. American college of obstetricians and gynecologists. Int J Gynaecol Obstet. 2001;72:85–96. [PubMed] [Google Scholar]
- Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the growth hormone research society, the Lawson Wilkins Pediatric Endocrine Society, and The European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210–4217. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Frias JL.Microcephalyin Vinken PJ, Bruyn GW (eds): Congenital Malformations of the Brain and Skull. Vol 30: Handbook of Clinical Neurology Amsterdam: Elsevier Holland Biomedical; 1977507–524. [Google Scholar]
- Hunter A, Frias JL, Gillessen-Kaesbach G, Hughes H, Jones KL, Wilson L. Elements of morphology: standard terminology for the ear. Am J Med Genet A. 2009;149a:40–60. doi: 10.1002/ajmg.a.32599. [DOI] [PubMed] [Google Scholar]
- Ogden JA. Radiology of postnatal skeletal development. Skeletal Radiol. 1984;11:246–257. doi: 10.1007/BF00351348. [DOI] [PubMed] [Google Scholar]
- Whittaker AJ, Royzman I, Orr-Weaver TL. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links dna replication with mitosis and the down regulation of s phase transcripts. Genes Dev. 2000;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]