Abstract
Purpose
Approximately 13% of patients lacking an HLA-identical sibling have a 1-antigen-mismatched related donor (MMRD). Historically, outcomes using a 1-antigen MMRD were considered equivalent to a matched unrelated donor (UD). Recent improvements in unrelated donor (UD) stem cell transplantation (SCT) due to better molecular HLA-matching justifies investigating if UD should be preferred to MMRD in adult patients with acute leukemia.
Patients and Methods
The outcomes of MMRD (n=89) and HLA-A, B, C, DRB1 allele matched UD (n=700) SCT reported to the CIBMTR between 1995 and 2005 were compared. Patients were transplanted for acute myeloid leukemia (AML) or acute lymphoid leukemia (ALL) in first or second complete remission.
Results
Donor type was not associated with hematological recovery. Univariate and multivariate comparisons of MMRD vs. HLA-matched UD transplants showed no statistically significant differences in overall survival, disease free survival, transplant related mortality, relapse, and 100-day grade III–IV acute graft-versus-host disease (GVHD). MMRD SCT was associated with a lower rate of chronic GVHD at 1-year, 35% vs 47% p=0.03, which was confirmed in multivariate analysis (RR 0.58, 95% CI 0.39-0.85, p<0.01).
Conclusion
HLA-matched UD and MMRD SCT are associated with comparable survival. Since less chronic GVHD was observed in MMRD, this option when available remains the first choice in acute leukemia patients without an HLA-identical sibling in need of allogeneic transplantation.
Keywords: HLA-match, allogeneic transplantation, acute myeloid leukemia, acute lymphoid leukemia
INTRODUCTION
Human leukocyte antigen (HLA) identical siblings are considered the best donors, but they are available for only one third or less of patients with acute leukemia for whom allogeneic transplantation is recommended. The probability of finding a one HLA-A, B,-DR antigen mismatched related donor is around 3% between siblings and 10% with other relatives.1 Another option for these patients is to undergo an unrelated donor (UD) search. The overall probability of identifying an HLA-compatible unrelated volunteer in the international registries is approximately 10-75% depending on the race and ethnicity of the patient (http://www.marrow.org).
In recent years, survival after UD allo-SCT has improved mostly due to a better selection of donor-recipient pairs based on molecular typing of HLA class I and II loci.2-5 Moreover, recent reports show that allo-SCT outcomes of patients with with matched UD-SCT are similar to HLA identical sibling donors.6-11
The progress in the UD allo-SCT setting provides the rationale to reexamine whether this option should be recommended to patients who have a MMRD available. This question warrants investigation because it is well recognized that HLA-mismatch increases graft failure and GVHD after transplantation. Since published comparisons between UD and MMRD transplants were reported before the introduction of HLA-typing at the allele level12-17, we re-evaluated this question in recent transplants for patients with acute leukemia included in the Center for International Blood and Marrow Transplant Research (CIBMTR) database.
PATIENTS, MATERIALS AND METHODS
Data collection
Data used in this study were obtained from the Statistical Center of the CIBMTR. The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic SCT to the Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done so with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Inclusion criteria
The study population included 89 recipients with MMRD and 700 8/8 HLA-A, B, C, and DRB1 allele matched UD transplants between 1995 and 2005. This study was restricted to adult patients (18 years or older), with a diagnosis of AML or ALL in first or second complete remission (CR), who received the first bone marrow or peripheral blood SCT with either myeloablative (MAC) or reduced intensity conditioning (RIC). T-cell depleted cases were excluded. Informed consent was obtained in accordance with the Declaration of Helsinki. All surviving UD recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. Approximately 10% of surviving patients would not provide consent for use of the research data. To adjust for the potential bias introduced by exclusion of non-consenting surviving patients, a corrective action plan modeling process randomly excluded appropriately the same percentage of deceased patients using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors19.
HLA typing
HLA typing in the UD group consisted of high resolution typing of HLA –A, B, C and DRB1 alleles and verified through the NMDP retrospective typing program as previously described18. For the purpose of this study and according to recent reports, mismatches affecting only the HLA DQ locus were considered as full matches.19, 20 In the MMRD group, HLA typing was verified by reviewing HLA typing reports and restricted to low resolution typing of HLA-A, B DRB1 loci.
Endpoints
The aim of the study was to compare the clinical outcomes among patients with acute leukemia who underwent a first SCT from a 1-antigen MMRD or from an HLA-matched UD, in order to determine which donor type was associated with better outcomes. Analyzed outcomes were overall survival (OS), disease free survival (DFS), hematological engraftment, incidence of acute and chronic GVHD, incidence of relapse, and TRM.
The date of engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) equal or more than 0.5×109/L. Platelet engraftment was defined as the achievement of a platelet count equal or more than 20×109/L without platelet transfusions in the previous 7 days. The acute GVHD end-point referred to the development of grades II-IV and grades III-IV according to the Glucksberg criteria 21. Chronic GVHD was diagnosed following the older definitions22. Relapse consisted of leukemia recurrence, whereas TRM was death resulting from any cause other than relapse. DFS was defined as survival in complete remission after SCT. For OS, death from any cause was considered an event. All living patients were censored at last follow-up. Disease was classified according to cytogenetic risk. For AML, MRC23 and SWOG24 classification systems were used; in cases where there was a discrepancy, the classification system that resulted in the higher risk status was used. For ALL, the MRC/ECOG criteria were used25. Statistical analysis
Patient-, disease-, and transplant-related variables were compared between the two groups using the χ2 statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Hematopoietic recovery, occurrence of GVHD, TRM and disease relapse were calculated using cumulative incidence estimates, taking into account the competing risk structure26, 27. Probabilities of DFS and OS were estimated from the time of transplantation using Kaplan-Meier curves28. Groups were compared using the 2-sided log-rank-test26, 27.
For the multivariate analysis, Cox proportional hazards regression models were applied. The proportional hazards assumption was assessed for each covariate by using a time-dependent covariate approach. Covariates which violate the proportional hazard assumption were adjusted by stratification. Stepwise forward-backward selection was used to build the models from the prognostic factors under consideration. A threshold of 0.05 was used for selection of covariates. SAS software version 9.1 (SAS Institute, Cary, NC) was used in all the analysis.
RESULTS
Patient, disease, transplant characteristics
Detailed patient characteristics are shown in Table 1. Compared to HLA-matched UD group, recipients of MMRD transplants differed in the following characteristics that have been associated with better outcomes: younger age, more favorable cytogenetics in ALL cases, higher ABO matching between donor and patient, and more frequent use of methotrexate. Some differences suggested that MMRD would have worse outcomes, including: more frequent transplants from female donor to male patient, older donor age, less common low-risk CMV donor-recipient serologic status, and lower percentage of patients receiving the transplant during the later period 2001-2005 The median follow-up for survivors in the MMRD was 54 (range 3-135) months and 38 (range 10-149) months in the HLA-matched UD group.
Table 1.
Patient, disease, and transplant characteristics
| MMRD | HLA-matched UD | ||||
|---|---|---|---|---|---|
| Characteristics | N Eval | N (%) | N Eval | N (%) | P-value |
| Number of patients | 89 | 700 | |||
| Age, median (range), years | 89 | 35 (18-64) | 700 | 43 (18-74) | <0.01 |
| Sex | 89 | 700 | 0.50 | ||
| Male | 50 (56) | 367 (52) | |||
| Female | 39 (44) | 333 (48) | |||
| Donor/Recipient race match | --- | 700 | N/A | ||
| Mismatched | 42 ( 6) | ||||
| Matched | 616 (88) | ||||
| Unknown | 42 ( 6) | ||||
| Karnofsky score prior to transplant | 89 | 700 | 0.29 | ||
| <90 | 24 (27) | 158 (23) | |||
| ≥90 | 60 (67) | 469 (67) | |||
| Unknown | 5 ( 6) | 73 (10) | |||
| Disease | 89 | 700 | 0.66 | ||
| AML | 59 (66) | 480 (69) | |||
| ALL | 30 (34) | 220 (31) | |||
| Disease status | 89 | 700 | 0.26 | ||
| CR1 | 61 (69) | 437 (62) | |||
| CR2 | 28 (31) | 263 (38) | |||
| Cytogenetics - AML | 59 | 480 | 0.63 | ||
| Low | 5 ( 8) | 46 (10) | |||
| Intermediate / high | 40 (68) | 345 (72) | |||
| Unknown | 14 (24) | 89 (19) | |||
| Cytogenetics - ALL | 30 | 220 | <0.01 | ||
| Low | 13 (43) | 40 (18) | |||
| Intermediate / high | 8 (27) | 102 (46) | |||
| Unknown | 9 (30) | 78 (36) | |||
| Graft type | 89 | 700 | 0.35 | ||
| Bone Marrow | 35 (39) | 312 (45) | |||
| Peripheral Blood | 54 (61) | 388 (55) | |||
| Conditioning regimen | 89 | 700 | 0.24 | ||
| Ablative | 73 (82) | 535 (76) | |||
| RIC / Non-myeloablative | 16 (18) | 165 (24) | |||
| Use of ATG in conditioning | 87 | 700 | 0.16 | ||
| No | 72 (83) | 616 (88) | |||
| Yes | 15 (17) | 84 (12) | |||
| GVHD prophylaxis | 89 | 700 | 0.01 | ||
| CSA/FK506±others (no MTX) | 10 (11) | 158 (23) | |||
| CSA/FK506 +MTX | 79 (89) | 542 (77) | |||
| Time from diagnosis to transplantation, months, median (range) |
89 | 7 (2-183) | 699 | 7 (1-171) | 0.12 |
| Donor relationship | 89 | 700 | N/A | ||
| Sibling | 54 (61) | --- | |||
| Parent | 15 (17) | --- | |||
| Child | 13 (15) | --- | |||
| Other relative | 7 ( 8) | --- | |||
| Unrelated | --- | 700 (100) | |||
| HLA Difference | 88 | 700 | |||
| HLA-A | 39 (44) | --- | |||
| HLA-B | 25 (28) | --- | |||
| HLA-DRB1 | 25 (28) | --- | |||
| ABO match | 89 | 700 | <0.01 | ||
| Matched | 51 (57) | 300 (43) | |||
| Minor mismatch | 13 (15) | 183 (26) | |||
| Major mismatch /bi-directional | 19 (21) | 217 (31) | |||
| Unknown | 6 ( 7) | 0 | |||
| Donor/Recipient sex match | 89 | 700 | 0.02 | ||
| Male/Male | 26 (29) | 266 (38) | |||
| Male/Female | 22 (25) | 198 (28) | |||
| Female/Male | 24 (27) | 101 (14) | |||
| Female/Female | 17 (19) | 135 (19) | |||
| CMV match | 89 | 700 | <0.01 | ||
| D−/R− | 20 (22) | 217 (31) | |||
| D−/R+ | 14 (16) | 230 (33) | |||
| D+/R− | 14 (16) | 96 (14) | |||
| D+/R+ | 41 (46) | 141 (20) | |||
| Unknown | 0 | 16 ( 2) | |||
| Donor age, year, median (range) | 88 | 38 (9-71) | 700 | 34 (18-60) | 0.05 |
| Year of transplant | 89 | 700 | <0.01 | ||
| 1995-2000 | 55 (62) | 169 (24) | |||
| 2001-2005 | 34 (38) | 531 (76) | |||
| Median follow-up of survivors (range), months |
37 | 54 (3-135) | 299 | 38 (10-149) | |
| Number of death | 52 | 401 | N/A | ||
| Primary disease | 12 (23) | 145 (36) | |||
| New malignancy | 0 | 3 ( 1) | |||
| GVHD | 9 (17) | 48 (12) | |||
| IPN | 7 (13) | 18 ( 4) | |||
| Infection | 9 (17) | 78 (19) | |||
| Organ failure | 8 (15) | 84 (21) | |||
| Graft Failure | 1 ( 2) | 1 (<1) | |||
| Hemorrhage | 1 ( 2) | 14 ( 3) | |||
| Accidental death | 0 | 2 (<1) | |||
| Unknown | 5 (10) | 8 ( 2) | |||
N=number; HLA=Human leukocyte antigen; Ag=Antigen; AML=Acute myeloid leukemia; ALL=Acute lymphoblastic leukemia; CR=Complete remission; RIC=Reduced intensity conditioning; GVHD=Graft versus host disease; CSA=Cyclosporine; FK506=Tacrolimus; MTX=Methotrexate; CMV=Cytomegalovirus; D/R=Donor/Recipient; IPN=Idiopatic pneumonia.
Engraftment
The data of hematological engraftment are shown in Table 2. The incidences of neutrophil engraftment at 28 days were 89% (95%CI 81-95%) for MMRD and 93% (95% CI 91-95%) for HLA-matched UD (p=0.21). Among those who engrafted, the median times to neutrophil engraftment (0.5×109/L) were 16 days after MMRD and 15 days after HLA-matched UD transplantation and the median times to platelet engraftment (20×109/L) were 18 and 20 days, respectively.
Table 2.
Univariate analysis.
| MMRD | HLA-matched UD | ||||
|---|---|---|---|---|---|
| Outcome event | N | Prob (95% CI) | N | Prob (95% CI) | P-value |
| ANC>0.5×109/L | 89 | 700 | |||
| @28 days | 89 (81-95)% | 93 (91-95)% | 0.21 | ||
| @100 days | 93 (87-97)% | 95 (94-97)% | 0.43 | ||
| Platelet>20×109/L | 89 | 700 | |||
| @60 days | 71 (61-80)% | 81 (78-84)% | 0.05 | ||
| @100 days | 75 (65-83)% | 85 (82-87)% | 0.04 | ||
| Acute GVHD II-IV | 86 | 695 | |||
| @ 100 days | 49 (38-60)% | 47 (43-51)% | 0.71 | ||
| Acute GVHD III-IV | 86 | 698 | |||
| @ 100 days | 22 (14-32)% | 15 (13-18)% | 0.15 | ||
| Chronic GVHD | 85 | 690 | |||
| @ 1 year | 35 (25-46)% | 47 (44-51)% | 0.03 | ||
| TRM | 86 | 698 | |||
| @ 1 year | 34 (24-44)% | 24 (21-27)% | 0.07 | ||
| @ 2 year | 38 (28-48)% | 27 (24-31)% | 0.06 | ||
| @ 3 year | 39 (29-50)% | 31 (27-34)% | 0.14 | ||
| Relapse | 86 | 698 | |||
| @ 1 year | 15 (8-23)% | 23 (20-26)% | 0.06 | ||
| @ 2 year | 19 (11-28)% | 27 (24-31)% | 0.07 | ||
| @ 3 year | 20 (12-29)% | 28 (25-32)% | 0.09 | ||
| Disease free survival | 86 | 698 | |||
| @ 1 year | 51 (41-62)% | 53 (50-57)% | 0.74 | ||
| @ 2 year | 44 (33-55)% | 46 (42-49)% | 0.74 | ||
| @ 3 year | 41 (30-52)% | 41 (37-45)% | 0.93 | ||
| Overall survival | 89 | 700 | |||
| @ 1 year | 57 (46-67)% | 61 (57-64)% | 0.51 | ||
| @ 2 year | 46 (35-56)% | 50 (46-54)% | 0.49 | ||
| @ 3 year | 42 (31-52)% | 44 (40-48)% | 0.65 | ||
HLA=Human leukocyte antigen; ANC=Absolute neutrophil count; GVHD=Graft versus host disease; TRM=Transplant related mortality.
Graft versus host disease
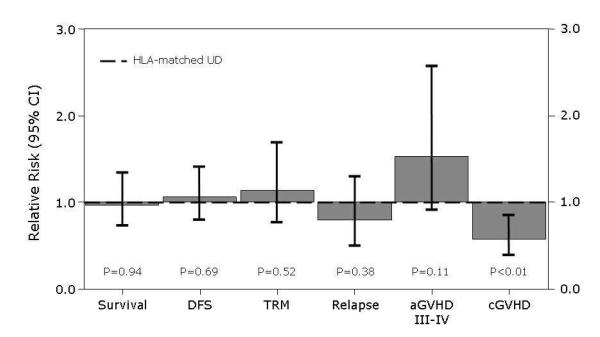
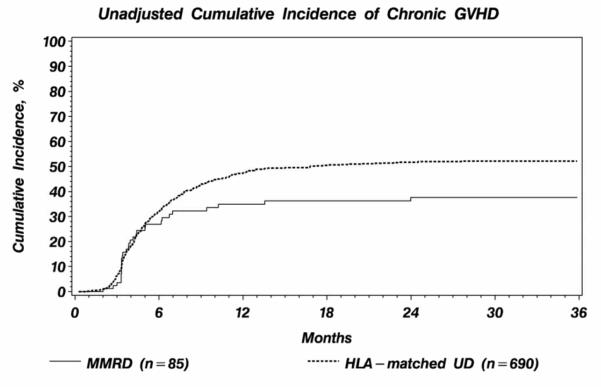
Probability of grade II-IV acute GVHD at 100 days was 49% (95% CI 38-60%) in the MMRD group and 47% (95% CI 43-51%) in HLA-matched UD (Table 2). In multivariate analysis, donor type was not associated with grade II-IV acute GVHD (RR 1.11, 95% CI 0.80-1.55, p=0.53), or with grade III-IV acute GVHD (RR 1.53, 95%CI 0.91-2.57, p=0.11) (Table 3). The 1-year probability of chronic GVHD after MMRD was 35% (95% CI 25-46%) compared to 47% (95% CI 44-51%), after HLA-matched UD transplantation. The 1-year probability of chronic extensive GVHD after MMRD was 24% (95% CI 15-34%) compared with 36% (95% CI 33-40%) after HLA-matched UD transplantation (P=0.01). Multivariate analysis also showed a significantly lower rate of chronic GVHD in MMRD than HLA-matched UD transplantation (RR 0.58, 95% CI 0.39-0.85, p<0.01) (Figure 1). Unadjusted cumulative incidence curves of chronic GVHD are shown in Figure 2.
Table 3.
Multivariate analysis for acute GVHD and chronic GVHD.
| Main Effect | Acute GVHD II-IVa. | Acute GVHD III-IVb. | Chronic GVHDc. | |||
|---|---|---|---|---|---|---|
| RR (95%CI) |
P-value | RR (95%CI) |
P-value | RR (95%CI) |
P-value | |
| HLA-matched UD | 1.00 | 1.00 | 1.00 | |||
| MMRD | 1.11 (0.80-1.55) |
0.53 | 1.53 (0.91-2.57) |
0.11 | 0.58 (0.39-0.85) |
<0.01 |
HLA=Human leukocyte antigen; RR=Relative risk; CI=Confidence interval; GVHD=Graft vs. host disease.
Acute GVHD II-IV model was stratified on conditioning regimen. It was adjusted for Cytomegalovirus match (p=0.02); and graft type (p<0.01).
Acute GVHD III-IV was adjusted for CMV match (p<0.01); Karnofsky score (p<0.01); ABO match (0.04); and time from diagnosis to transplantation (p=0.03).
Chronic GVHD was adjusted for ATG use in conditioning (p<0.01); Graft type (p<0.01); and patient gender (0.04).
Figure 1.
Relative risks of MMRD SCT versus HLA-matched UD SCT (RR=1.0) from multivariate analysis. Whiskers are 95% confidence intervals.
Figure 2.
Unadjusted cumulative incidence of chronic graft vs. host disease by donor type.
Relapse
Cumulative incidence of relapse at 1, 2 and 3 years were 15% (95% CI 8-23%), 19% (95% CI 11-28%) and 20% (95% CI 12-29%) after MMRD and 23% (95% CI 20-26%), 27% (95% CI 24-31%) and 28% (95CI 25-32%) after HLA-matched UD transplantation (P-values 0.06, 0.07 and 0.09 at the mentioned time-points, respectively). Table 4 shows the results of the multivariate analysis for relapse; of note, the type of donor was not significant (RR 0.81, 95%CI 0.50-1.30, p=0.38), whereas the variables associated with higher relapse were second CR at transplant (RR 1.78, 95% CI 1.13-2.81, p=0.01), reduced intensity/non-myeloablative conditioning (RR 1.50, 95% CI 1.12-2.01, p<0.01), and time from diagnosis to transplantation less than 12 months (RR 0.56, 95% CI 0.35-0.90, p=0.02). Relapse was the primary cause of death in both groups (Table 1).
Table 4.
Multivariate analysis for relapse, TRM, DFS and OS.
| Main Effect | Relapsea. | TRMb. | DFSc. | OSd. | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95%CI) |
P-value | RR (95%CI) |
P-value | RR (95%CI) |
P-value | RR (95%CI) |
P-value | |
| HLA-matched UD | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| MMRD | 0.81 (0.50-1.30) |
0.38 | 1.14 (0.77-1.69) |
0.52 | 1.06 (0.80-1.41) |
0.69 | 0.99 (0.73-1.34) |
0.94 |
HLA=Human leukocyte antigen; RR=Relative risk; CI=Confidence interval; TRM=Treatment related mortality; DFS=Disease free survival; OS=Overall survival.
Relapse model was adjusted for karnofsky score at transplant (p=0.03); conditioning regimen (p<0.01); disease status (p=0.01); and time from diagnosis to transplantation (p=0.02).
TRM model was stratified by graft type and donor/recipient gender match. It was adjusted for patient age (p=0.05); and year of transplant (p<0.01).
DFS model was adjusted for karnofsky score at transplant (p<0.01).
OS model was stratified by graft type. It was adjusted for patient age at transplant (p<0.01); and year of transplant (p=0.02).
Transplant-related mortality
The cumulative incidence of TRM at 1, 2 and 3 years were 34% (95% CI 24-44%), 38% (95% CI 28-48%) and 39% (95% CI 29-50%) after MMRD and 24% (95% CI 21-27%), 27% (95% CI 24-31%) and 31% (95% CI 27-34%) after HLA-matched UD transplantation (P-values 0.07, 0.06 and 0.14 for each comparison at the mentioned time points). Table 4 shows the results of the multivariate analysis for TRM. There was no significant difference in TRM between different donor types (RR 1.14, 95% CI 0.77-1.69, p=0.52). The only two variables associated with increased TRM were older patient age (31-50 vs 18-30 RR=1.47, 95% CI 1.08-2.01, p=0.02) and transplantation before year 2001 (RR 1.64, 95% CI 1.19-2.27, p<0.01).
Disease-free Survival
Disease-free survival at 1, 2 and 3 years were 51% (95% CI 41-62%), 44% (95% CI 33-55%), 41% (95% CI 30-52%) after MMRD transplantation and 53% (95% CI 50-57%), 46% (95% CI 42-49%), 41% (95% CI 37-45%) after HLA-matched UD (P-values 0.74, 0.74 and 0.93, respectively). No significant difference in disease-free survival was found in multivariate analysis between the two groups as shown in Table 4. The only factor associated with decreased DFS was a Karnofsky score below 90% (RR 1.37, 95% CI 1.10-1.69, p<0.01).
Overall Survival
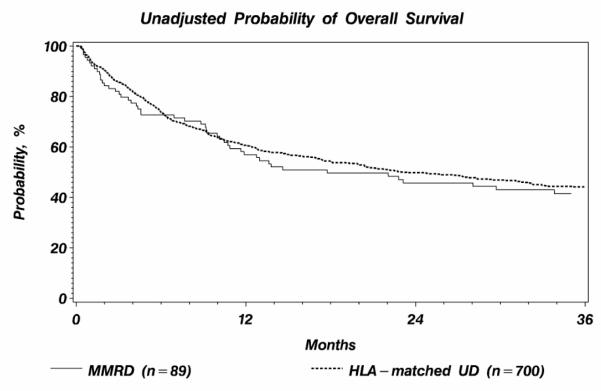
Overall survival curves are shown in Figure 3. Overall survival probability at 1, 2 and 3 years were 57% (95% CI 46-67%), 46% (95% CI 35-56%), 42% (95% CI 31-52%) after MMRD transplantation 61% (95% CI 57-64%), and 50% (95% CI 46-54%), and 44% (95% CI 40-48%) after HLA-matched UD (P-values 0.51, 0.49 and 0.65, respectively). As shown in Table 4, there was no significant association between OS and donor type. Variables associated with decreased OS were older patient age (31-50 vs. 18-30, RR 1.34, 95%CI 1.06-1.69, p=0.01) and transplant before year 2001 (RR 1.35, 95%CI 1.05-1.72, p=0.02).
Figure 3.
Unadjusted probability of overall survival by donor type.
Subset analyses
Inclusion of cell dose (total nucleated cells in BM SCT and mononuclear cells in PB SCT) was limited by missing data but analysis of available data did not change the conclusions (data not shown). No significant differences were identified between class I mismatches and class II mismatches within the 1-antigen MMRD group for all endpoints (data not shown). Finally, no significant differences in outcomes were identified when the comparison between HLA-matched UD and 1-antigen MMRD were limited to the non-myeloablative/reduced intensity conditioning subset (data not shown).
DISCUSSION
As HLA matching is the most important variable in allogeneic SCT13,16,19 and current HLA typing methods using high resolution molecular techniques have improved the results after UD-SCT over the last 10 years,2 previously reported comparisons between related and unrelated transplants may be outdated20. Some patients may have both a MMRD and a high likelihood of having an 8/8 HLA-matched UD, and for this group of patients, it is of interest to determine whether an unrelated donor search should be initiated
A key finding from this study was that the main outcomes of TRM, relapse, DFS, and OS were similar in the HLA-matched UD and MMRD groups, suggesting that the two alternatives are indeed comparable. Although more patients in the MMRD group received methotrexate as GVHD prophylaxis (89% vs 77%), GVHD prophylaxis was not statistically significant in the univariate or multivariate analysis, and thus we consider it unlikely that it contributed to the differences observed in cGVHD incidence. This finding is in agreement with previous studies comparing the two alternative donor sources15,16. The only observed difference consisted of an increased incidence of chronic GVHD after HLA-matched UD, a complication frequently leading to impaired quality of life32. Taking the latter into account, and due to the lack of benefit in terms of DFS and OS, it is reasonable to use the MMRD when available, instead of proceeding to an unrelated donor search. As this study was performed with patients with acute leukemia in first or second CR, our findings are limited to these patients, and the extension to patients with more advanced disease or other diseases need further investigation.
HLA is inherited following Mendelian genetics with two mechanisms explaining the availability of a 1-antigen MMRD. The first is crossing-over in HLA genes, more frequent in class I because HLA-A loci are far from those encoding HLA-B and HLA-C. The second alternative is the presence of HLA alleles or at least one haplotype in the patient with high frequency in the overall population.29 In this circumstance, the chance of finding a related donor sharing one HLA-haplotype, with the other HLA-haplotype being identical except for one gene in the extended family (cousins, uncles, aunts, etc), is increased but the additional delay of extended family typing needs to be balanced against the low probability of finding a suitable related donor. One study estimated that more than 30 individuals need to be typed to identify a 1 antigen MMRD1. There are available tools to calculate the probability of finding a related or unrelated donor depending on the HLA typing and consulting with an HLA expert may be helpful.29,30,31 Given all these considerations and the fact that a 1-Ag MMRD was associated with similar clinical outcomes except for less chronic GVHD, one suggested approach would be to use a 1-Ag MM sibling if available but otherwise start an UD search. As only 13% will have a MMRD after extended family typing, with the aim of shortening the time to transplant, a suitable, a suitable strategy could be to perform a preliminary unrelated search while the familiar study is being performed.
The significantly lower incidence of chronic GVHD in the MMRD group observed in this study was unexpected as it is well known that HLA mismatch predisposes to acute GVHD and less strongly to chronic GVHD19. Additionally, other characteristics also favoring this complication were more frequent in the MMRD, such as female to male transplant, advanced age of the donor and/or patient, and positive CMV serologic status. In previous studies, the incidence of chronic GVHD in MMRD was similar to HLA identical sibling transplantation15. Therefore, the explanation of the higher incidence of chronic GVHD in the HLA-matched UD group may be partially explained by undetected disparities between donor and recipient other than HLA genes. Of note, most of these gene disparities may involve minor histocompatibility antigens (mHAs) that are increasingly being associated with the development of GVHD in the setting of HLA identical sibling SCT33-35. Since these antigens are frequently encoded in chromosomes different than number 6, it is likely that UD will differ in these mHAs more frequently than related donors especially if they are siblings. Nevertheless in a recent study from CIBMTR, mismatching in known mHAs was not associated with a higher incidence of GVHD in patients who underwent an Allo-SCT from a matched UD36. Although this study is the largest evaluating the role of mHAs in the unrelated setting, the small subgroup size may have limited the power to detect differences. Moreover, a recent publication has emphasized the importance of haplotype matching in the setting of UD SCT, to avoid severe GVHD37. Since most of the MMRD transplants reported here were from 1-Ag-MM siblings (and thus the HLA difference may be due to crossing over), it is likely that the degree of extended haplotype matching is likely higher than in the UD group.
This study has some limitations. The first is the small number of patients in the MMRD group that probably reflects the preference of initiating an unrelated donor search instead of an extended family search because it is time-consuming, expensive and has a low probability of success1. Nevertheless, this report contains one of the largest numbers of single antigen MMRD patients analyzed in a single report. Another drawback of the findings reported here is that, as in other retrospective registry studies, there were some differences between MMRD and UD SCT groups in aspects important for transplantation outcome, such as age (younger in MMRD) and year of transplantation (earlier in MMRD) However, this last limitation was is in part corrected by performing the multivariate analysis including these covariates and showing practically identical RR of TRM and OS in the two transplant groups. Of course, the only approach to definitively answer the question would be a randomized prospective comparison of the two transplant alternatives, what is highly unlikely.
A third limitation of the study was that HLA matching assessment of related donors was based on low resolution typing and limited to HLA-A, -B and DRB1. This is, however, the current practice in most institutions for related donor selection. Since information on HLA-C and HLA-DQ was not available it is not possible to rule out additional mismatches in the related donor group. This is unlikely in most cases affecting HLA-A (n=39, 44%), because linkage disequilibrium means that matching at HLA-B (n=25, 28%) and –DR (n=25, 28%) is generally associated with matching at HLA-C and -DQ. Nevertheless, it seems reasonable to recommend the study of at least HLA-C in patients with a mismatch in HLA-B. Although the numbers were small, the outcomes of class I mismatch vs. class II mismatch within 1-Ag MMRD transplants were similar regarding all endpoints studied which is in agreement with a previous Japanese study including 112 MMRD15. On the other hand, due to missing data we were unable to analyze if the possible impact of KIR-ligand mismatches or non-inherited maternal or paternal antigens, aspects that have been recently considered in donor selection.
In conclusion, the data from the study reported here supports that both MMRD and HLA-matched UD are acceptable for transplantation in patients who need an allogeneic procedure and lack an HLA-identical sibling. The lower incidence of chronic GVHD after MMRD transplantation and the easy and rapid access to relatives as well as the lower cost makes the MMRD modality the first option to be considered if available.
ACKNOWLEDGMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries;; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
AUTHOR CONTRIBUTIONS Designed research and drafted the manuscript (DV, JS, SJL), performed the statistical analysis (TW, FK), interpreted data and critically revised the manuscript (VG, GAH, DM, PLM, MO, EWP, OR, MS, SRS, EW, JLG, SRM, DS).
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ottinger H, Grosse-Wilde M, Scmitz A, Grosse-Wilde H. Immunogenetic marrow donor searh for 1012 patients: a retrospective analysis of strategies, outcome and costs. Bone Marrow Transplant. 1994;14(Suppl 4):S34–38. [PubMed] [Google Scholar]
- 2.Sierra J, Martino R, Sánchez B, Piñana JL, Valcárcel D, Brunet S. Hematopoietic transplantation from adult unrelated donors as treatment for acute myeloid leukemia. Bone Marrow Transplant. 2008;41:425–37. doi: 10.1038/sj.bmt.1705973. [DOI] [PubMed] [Google Scholar]
- 3.Schetelig J, Bornhäuser M, Schmid C, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–91. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 4.Arora M, Weisdorf DJ, Spellman SR, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–52. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 6.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem cell transplantation from human leukocyte antigen-identical sibling versus human leukocyte antigen-allelic-matched unrelated (10/10) in patients with standard-risk hematologic malignancy: A prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 7.Ringden O, Pavletic SZ, Anasetti C, et al. The graft versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell JA, Savoie ML, Balogh A, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 cGy total-body irradiation and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Moore J, Nivison-Smith I, Goh K, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 10.Schetelig J, Bornhäuser M, Schmitz C, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German transplant study group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 11.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: No difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 12.Petersdorf EW, Hansen JA, Martin PJ, et al. Major-Histocompatibility-Complex Class I Alleles and Antigens in Hematopoietic-Cell Transplantation. N Engl J Med. 2001;345:1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 13.Henslee-Downey PJ, Abhyankar SH, Parrish RS, et al. Use of Partially Mismatched Related Donors Extends Access to Allogeneic Marrow Transplant. Blood. 1997;89:3864–3872. [PubMed] [Google Scholar]
- 14.Hasegawa W, Lipton JH, Messner HA, et al. Influence of one human leukocyte antigen mismatch on outcome of allogeneic bone marrow transplantation from related donors. Hematology. 2003;8:27–33. doi: 10.1080/1024533031000072054. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 16.Ottinger HD, Ferencik S, Beelen DW, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood. 2003;102:1131–1137. doi: 10.1182/blood-2002-09-2866. [DOI] [PubMed] [Google Scholar]
- 17.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 18.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 20.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-Matching for Retrospective Analysis of Unrelated Donor Transplantation: Revised Definitions to Predict Survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 23.Grimwade D, Walker H, Oliver F, et al. The importance of diagnosis cytogenetics in AML. Analysis of 1612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 24.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 25.Moorman AV, Harrison CJ, Buck GAN, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 26.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28:1001–1011. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 27.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan ELMP. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Schipper RF, D’Amaro J, Oudshoorn M. The probability of finding a suitable related donor for bone marrow transplantation in extended families. Blood. 1996;87:800–804. [PubMed] [Google Scholar]
- 30.Kaufman R. HLA prediction model for extended family matches. Bone Marrow Transplant. 1995;15:279–282. [PubMed] [Google Scholar]
- 31.Mori M, Graves M, Milford EL, Beatty PG. Computer program to predict likelihood of finding an HLA-matched donor: methodology, validation and application. Biol Blood Marrow Transplant. 1996;2:134–144. [PubMed] [Google Scholar]
- 32.Baker K, Fraser C. Quality of life and recovery after graft-versus-host disease. Best Pract Res Clin Haematology. 2007;21:333–341. doi: 10.1016/j.beha.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Goulmy E, Schipper R, Pool J, et al. Mismatches of Minor Histocompatibility Antigens between HLA-Identical Donors and Recipients and the Development of Graft-Versus-Host Disease after Bone Marrow Transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 34.Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:1355–1362. doi: 10.1182/blood-2006-06-030858. [DOI] [PubMed] [Google Scholar]
- 35.Spellman S, Warden MB, Haagenson M, et al. Effects of mismatching for minor histocompability antigens on clinical outcomes in HLA-Matched unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:856–863. doi: 10.1016/j.bbmt.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao NJ. Minors come of age: minor histocompatibility antigens and graft-versus-host disease. Biol of Blood and Marrow Transplant. 2004;10:215–223. doi: 10.1016/j.bbmt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Petersdorf EW, Malkki M, Gooley TA, Martin PJ, Guo Z. MHC haplotype matching for unrelated hematopoietic cell transplantation. PLoS Med. 2007;4:59–68. doi: 10.1371/journal.pmed.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]