Summary
Reproducible animal models not only facilitate the pre-clinical assessment of aneurysm therapy but can also help in training for interventional procedures. The objective of this study was to find an animal model that can be used to test different endovascular occlusion techniques.
Aneurysms in the right common carotid artery were created in 35 NZW rabbits by distal ligation and intraluminal elastase infusion. A total of 27 aneurysms were occluded by endovascular embolization with GDC-Coils. The time needed for placement of the microcatheter into the aneurysm by a professional interventionalist, a semi-professional interventionalist and a trainee was measured. The percentage of occlusion (occlusion rate) of the aneurysms was determined angiographically after embolization and again three months later, followed by a histological examination.
Aneurysms of 2-6 mm size were reliably created in all 35 animals; mean size was 3.0 mm in height and 5.5 mm in diameter. Occlusion was achieved in 27 animals. Five animals from the group of 35 were initially planned as a control group with no embolization. We added to the control group one animal whose aneurysm could not be occluded endovascularly because of partial thrombosis and small size of the aneurysm. The angiographically determined mean occlusion rate was 89.5% ± 11.3% standard deviation. Histological evaluation of the six aneurysms of the control group showed that they remained patent. Aneurysms that underwent embolization showed organized thrombus formation with no signs of recanalization. Two animals died from anaesthesia related or embolic complications. The time needed by the professional did not significantly decrease, after a little practice the trainee was nearly as quick as the professional. The beginner showed extensive progress, reducing the time for catheter placement by more than 50%.
This paper describes the angiographic and histopathologic findings and also demonstrates possible methods for training in interventional procedures. Animal models will play a vital part in the training of future interventionalists. This model has the capability of testing different embolization devices (GDC, Onyx®) and methods. Histologic long-term prognosis and the physical effect of the coils can be evaluated with this model.
Key words: animal model, aneurysm, rabbit, embolization, coil, GDC, training model
Introduction
Reproducible animal models not only facilitate the preclinical assessment of aneurysm therapy and follow-up, but also play an important role in the training of interventionalists. They have been an integral part of the development of endovascular occlusion techniques 24. For example, Guglielmi detachable coils (GDC) were first tested on lateral aneurysms in a swine model 8.
In choosing a suitable aneurysm model, different aspects have to be considered. It is important to reproduce the haemodynamic forces in human intracranial aneurysms, which are mainly located at bifurcations 2,7,10-12.
Most existing animal models as well as the swine model used for testing GDCs do not reproduce these conditions. In these models side-wall aneurysms are often used. Here a venous patch is sewn on the carotid artery 3,14. These models have the disadvantage of being surgically demanding, and the morphology of the venous vessel wall does not correspond to the wall of an intracranial artery. Also, sidewall aneurysms are subjected to far less shear stress then bifurcation aneurysms.
In addition, a preceding surgical intervention will influence the response of the aneurysm to the coil as this operation will lead to scar formation and foreign bodies in the region of the neck. It is also important to consider the blood coagulation profile of the laboratory animal, which can differ from that in humans. There have been numerous studies on the fibrinolytic system in rabbits and it has been found that it mimics that of humans 4.
Therefore an aneurysm model in rabbits seems ideal, not only to simulate embolization but also to test different embolization devices. Anticoagulation schemes can also be used in rabbits to prevent thrombembolic complications in the treatment of intracranial human aneurysms 4,13. Also the anatomy of the vessels in the head and neck area in rabbits is well known, as it is similar to that of humans (Figure 1). Aneurysm models in rabbits have been described 3,6 and can be used as a training model for neurointerventionalists. We took these as a basis for our bifurcation model in New Zealand White Rabbits. The aim of our study was to establish a working concept to test technical innovations in the treatment of aneurysms, thus making embolization results more predictable in human intracranial aneurysms.
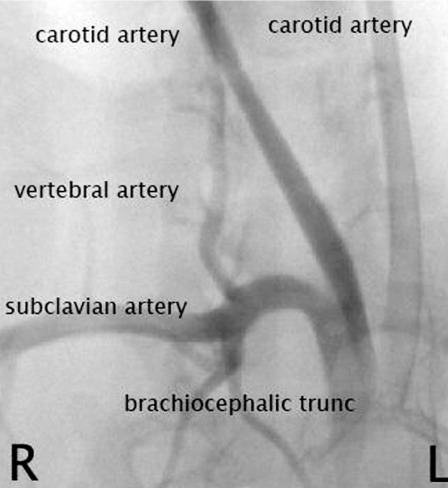
Figure 1.
DSA of right common carotid artery before elastase incubation.
Materials and Method
The university's ethics commission approved the animal experiment. The animals were of mixed sex and were maintained on a standard laboratory diet. Aneurysms were induced in 35 New Zealand White rabbits (body weight 3-4 kg). Anaesthesia was induced using an intramuscular injection of ketamine and xylazine. Maintenance anaesthesia was conducted with a mixture of ketamine and xylazine, administered by a continuous intravenous infusion through the ear vein.
For the induction of the aneurysm we first removed the fur at the neck and disinfected the operation area. The right common carotid artery was surgically exposed over a length of two cm.
First we permanently ligated the cranial part of the artery and introduced a 4F sheath into the lumen. We used a second suture of the same kind to tie the sheath to the vessel. Next a 3F balloon catheter was introduced under radioscopic guidance to the origin of the right common carotid artery in the brachiocephalic trunk. The balloon was gently inflated with a mixture of 50/50 contrast medium and saline 0.9%. Then a Prowler 10 microcatheter was placed upon the inflated balloon and 100 U of porcine elastase were injected via the microcatheter (Figure 2). The elastase was incubated for up to 20 minutes within the lumen of the CCA. After that the balloon catheter, microcatheter and sheath were removed, a third suture was used for ligation of the vessel a few centimeters above the brachiocephalic trunk to form the aneurysmal sac. The fascias and the skin were closed with a running suture and glue. Using glue has the advantage that no suture ends stick out of the wound so the animal cannot reopen it. It took the animals about 15 minutes to awake after anaesthesia was stopped. The whole procedure took about 6070 minutes. Three different interventionalists (professional, trainee, beginner) performed the embolization.
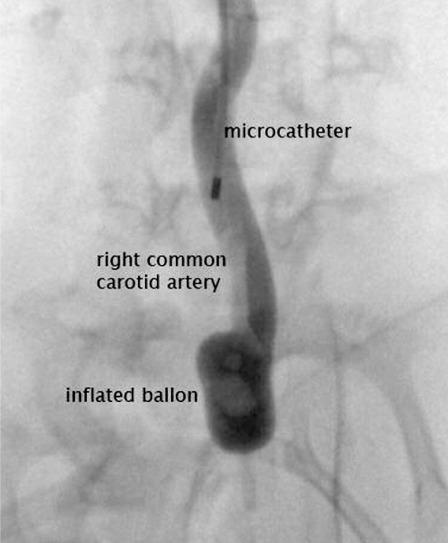
Figure 2.
Inflated ballon in brachiocephalic trunc, microcatheter in common carotid artery after applied elastase.
The professional was experienced in interventions, the trainee had assisted interventions and performed about 200 intracranial DSA himself. The beginner was a novice to interventions but had performed about 50 DSA. Each one embolized nine animals. The time from introduction of the microcatheter through the sheath until placement in the aneurysm was measured.
Control angiography
An increase in aneurysm size after three to six weeks is unlikely. Kallmes 12 observed that aneurysms show an increase in size only during the first three weeks after creation. To verify this, we performed venous control angiograms in the first ten animals after three weeks of aneurysm induction and after six weeks. As the aneurysms had not increased in size during this period we did not do controls in the other animals.
As a DSA angiography via the femoral artery is not possible in rabbits without surgical exposure of the vessel and in addition requires subsequent ligation of the artery, we only performed venous controls. For this, the animals were anaesthetized using an intramuscular injection of ketamine and xylazine as described above.
A 22 G venflow (Optiva 2) was placed in the left ear vein, 6-7 ml non-ionic Omnipaque 300 (Nycomed Amersham, Princeton, NJ) were injected followed by 6-7 ml sodium chloride to produce a better bolus effect, whilst digital subtraction imaging was performed at a rate of two frames per second. Imaging was done during the arterial phase. Then the size of the aneurysm was determined by comparing it with a standard scale.
Before sacrifice, a venous control angiogram was done to determine the occlusion rate of the aneurysm 5.
Embolization
Embolization was performed after six weeks. The animals were anaesthetized as described above. The right femoral artery was surgically exposed and distally ligated. A small arteriotomy was performed with microscissors. A 3F sheath was introduced into the vessel and fixated with a suture, then a 0.10 Transend wire was coaxially delivered through a Tracker Excel 14 2 tip microcatheter into the aortic arch. A control series was done to get an impression of the anatomy of the vessels and of the aneurysm. Then the catheter was placed in the neck of the aneurysm and the animal was given 1000IE heparin i.v. Depending on the size of the aneurysm, one to several coils were placed inside the lumen of the aneurysm.
After each coil a control angiogram was done to determine the occlusion rate angiographically. In case of incomplete occlusion we placed more coils until complete occlusion or until no further coils could be safely placed in side the lumen of the aneurysm. After retrieval of the microcatheter, the femoral artery was ligated. The wound was closed with a running suture and skin glue.
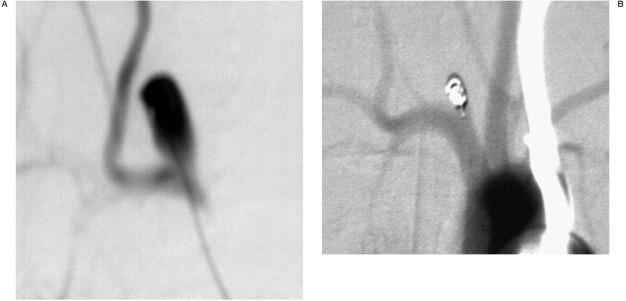
(Figure 3A,B show the microcatheter inside the aneurysm lumen / the control series after successful occlusion)
Figure 3.
A) control angiogram before embolization. B) venous control angiogram after successful embolization.
Sacrifice of the animals
The animals were sacrificed three months after coiling. At the time of sacrifice, the subjects were deeply anesthetized (Narcoren, Merial GmbH, Halbergmoos, Germany). The mediastinum was dissected. After thoracotomy, the aortic arch and the proximal great vessels with the aneurysm were exposed and dissected from the surrounding tissue (Figure 4).
Figure 4.
Coils are visible through the thinned vessel wall.
Histologic evaluation
The samples of the aneurysm, vessels and brain were fixed in 3% neutral buffered formalin at room temperature. To survey reactions of the surrounding tissue towards the elastase or coils, the samples were embedded in paraffin and sectioned at 1Hm increments. Afterwards staining was performed with hematoxylin-eosin and with Enthellan (Merk, Darmstadt/DE).
Results
Aneurysms of 2-6 mm size were reliably created in all 35 animals. All but three animals tolerated the operations well. One animal died from anaesthesia complications. Two animals showed a hemiparesis, one of them died shortly after the procedure; the other one fully recovered and its aneurysm could be embolized later. Occlusion was achieved in 27 animals. Five animals were initially planned as a control group with no embolization. We added to the control group one animal with an aneurysm which could not be occluded endovascularly because of partial thrombosis and small size of the aneurysm. The occlusion rate, which was angiographically determined, represents the percentage of embolized aneurysm lumen compared to the contrast filled lumen in the angiogram before embolization. The occlusion rate was evaluated in all GDC embolized aneurysms. The mean occlusion rate of all embolized aneurysms was 89.5% with a standard deviation of 11.3% right after embolization. The minimal occlusion rate was 70% and the maximal occlusion rate was 100%.
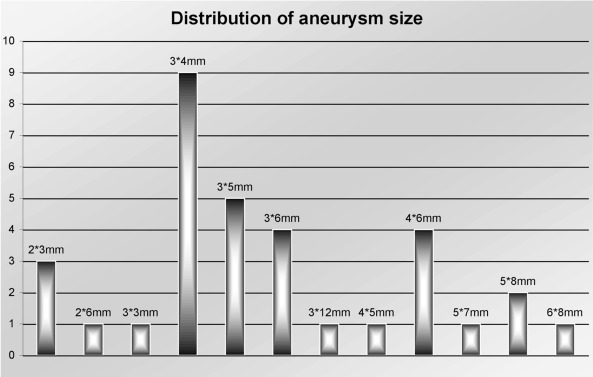
The mean width of the 33 aneurysms was 3.2 mm ± 0.8 mm. The mean height of the aneurysm was 5.1 mm ± 1.8 mm. Figure 5 shows the size distribution of all aneurysms.
Figure 5.
Distribution of size in all aneurysms.
Technically all aneurysms but two were successfully embolized, which means we achieved an occlusion rate of the aneurysms of more than 95%. In one case occlusion was incomplete (70%) as due to the wide neck shape of the aneurysm no further coils could be safely placed inside the lumen. In the other case no coils could be placed due to the small size and partial thrombosis of the aneurysm. Angiographic control was conducted eight weeks after creation of the aneurysms as well as 8-12 weeks after embolization.
In these intravenous digital subtraction control arteriographies the occlusion rate did not differ from the one determined immediately after embolization (p>0.05).
Concerning embolization time the professional interventionalist took on average one minute three seconds in the first three animals, one minute 18 seconds in the next three animals and one minute one second in the last three animals. The trainee in interventions took on average three minutes 53 seconds in the first three animals, two minutes 37 seconds in the following three animals and 1 minute 45 seconds in the last three.
The trainee in diagnostic angiography took eight minutes seven seconds on average for the first three animals, seven minutes 56 seconds for the next lot and five minutes 31 seconds for the last three animals.
Histology
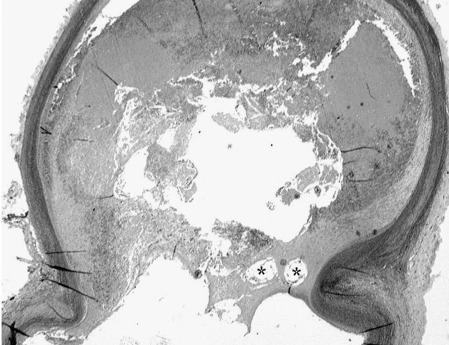
The histologic samples show mostly thrombosed lumen of the aneurysm. Some of the hollow spaces are due to removal of platinum coils (Figure 6). But there are still signs of recanalization, especially in the middle of the aneurysm lumen.
Figure 6.
Histologic sample shows mostly thrombosed lumen of aneurysm. Some of the hollow spaces are due to removal of platinum coils (*).
Discussion
Reproducible animal models have always been an indispensable part of the development of endovascular occlusion devices and techniques 19.
Ideally, experimental aneurysms should be subjected to similar haemodynamic forces as found in human cerebral bifurcation aneurysms. The physical dimensions and radiographic appearance should also be similar. If no embolization is performed, it should remain patent indefinitely 16. Rat, rabbit, pig, sheep, dog and primate species have been considered for the testing of endovascular devices, some requiring exquisite surgical skills 22. Masaryk 15 created 12 vein-pouch sidewall aneurysms in a canine model. Murayama 20 created 40 lateralwall aneurysms in pigs by using the external jugular vein. However, studies using experimental models in dogs or pigs are not necessarily applicable to humans.
Carter et Al 4 outlined the similarity of the human fibrinolytic system to that of the rabbit and concluded that thrombus formation in aneurysms can be simulated in a rabbit model. Local surgery should be avoided to minimize the healing response that might impair studies on anticoagulation or biologic activity of coils.
Vein-pouch bifurcation aneurysms in rabbits have been used previously1. This procedure has a high mortality rate, is lengthy and often leads to parent artery occlusion. In addition, if the wall is of venous origin rather than arterial, and if suture material is present in the aneurysmal neck, this can influence the biologic milieu of the aneurysm.
Cawley 5 created sidewall aneurysms in rabbits by intraluminal elastase incubation of the stump of the external carotid artery. In the ECA flow-rates and shear stress are low. In our aneurysm model in rabbits the blood flow from the aorta is directed at the orifice, i.e. the neck of the experimental aneurysm, so that we expect it will reduce thrombosis within the aneurysm and provide haemodynamic stress similar to that in intracranial bifurcation aneurysms.
The aneurysm sizes created in our study ranged within the typical size of human cerebral aneurysms. In the "International Subarachnoid Aneurysm trial" (ISAT) 53% of the ruptured aneurysms were smaller then five mm in diameter 18. The ISAT study only contained ruptured aneurysms. Animal models however are especially important in the treatment of asymptomatic, unruptured aneurysms. These are commonly treated when they are above seven mm in size, as the risk of rupture increases with size 25.
In unruptured aneurysms more therapeutic options such as extensive anticoagulation to prevent thrombi from the aneurysm or the embolization material itself, intracranial stents or liquid embolization agents (ONYX) are possible. Intracranial stents as well as liquid embolization agents require anticoagulation to prevent thromboembolism. The presented aneurysm model produces aneurysms that can be treated with all these embolization techniques.
In addition the model used has the advantage of a relatively simple surgical procedure required for the construction of aneurysms. Occlusion of the common carotid artery in rabbits can be performed with little danger of creating an ischemic infarction because of the collateral blood flow to the brain via the circle of Willis 21.
Previous studies have documented minimal inflammatory response to GDCs 17. This may result in enhancement of the aneurysm wall due to the inflammation associated with thrombosis, a mild foreign body inflammatory reaction, and in the growth of neocapillaries during the organization of the thrombus and aneurysm wall 17.
Experience plays an important role in aneurysm therapy. A previous bifurcation model in canines 26 showed that this model was useful in training for interventions, but seemed unsuitable as a test model for new embolization materials. This was because aneurysmal recurrence was not observed. The aneurysms created in our model showed signs of recanalization. In addition we could demonstrate a significant training effect in the interventionalists. We found that for the experienced interventionalist the time needed for placement of the catheter into the aneurysm did not significantly decrease.
However, there was an obvious learning curve in the trainee and the beginner. In particular, the beginner could reduce the time needed by almost 40%. After training the trainee nearly reached the time of the professional interventionalist. We conclude that the inexperienced angiographer can profit most from training on an aneurysm model.
Conclusions
This concept, based on an aneurysm model in rabbits, may help not only in training for interventional procedures but also in testing technical innovations and in the creation of new protective devices, thus, hopefully leading to better outcomes of endovascular procedures in the near future. Training on an aneurysm model helps to gain experience in aneurysm treatment, can reduce embolization time and thus the risk for the patient. A range of applications seems possible, e.g. for testing liquid embolic agents to decrease distal migration of the liquid embolic agent through intra-aneurysmal flow control with proximal balloon protection, balloon inflation across the neck of the aneurysm, or the use of metallic stents. Due to the similarity of the human fibrinolytic system to that of the rabbit, emerging biologic modification techniques as well as different anticoagulation regimes can be evaluated, thus making embolization results more predictable before use in human intracranial aneurysms.
References
- 1.Abruzzo T, Shengelaia GG, et al. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. Am J Neuroradiol. 1998;19:1309–1314. [PMC free article] [PubMed] [Google Scholar]
- 2.Akiba Y, Murayama Y, et al. Guglielmi detachable coiling of wide-necked aneurysms: Part I - experimental evaluation. Neurosurgery. 1999;45:519–527. doi: 10.1097/00006123-199909000-00022. discussion 527-530. [DOI] [PubMed] [Google Scholar]
- 3.Altes TA, Cloft HJ, et al. Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. Am J Roentgenol. 2000;174:349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 4.Carter LP, Guthkelch AN, et al. Influence of tissue plasminogen activator and heparin on cerebral ischemia in a rabbit model. Stroke. 1992;23:883–888. doi: 10.1161/01.str.23.6.883. [DOI] [PubMed] [Google Scholar]
- 5.Cawley CM, Dawson RC, et al. Arterial saccular aneurysm model in the rabbit. Am J Neuroradiol. 1996;17:1761–1766. [PMC free article] [PubMed] [Google Scholar]
- 6.Cloft HJ, Altes TA, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology. 1999;213:223–228. doi: 10.1148/radiology.213.1.r99oc15223. [DOI] [PubMed] [Google Scholar]
- 7.Forrest MD, O'Reilly GV. Production of experimental aneurysms at a surgically created arterial bifurcation. Am J Neuroradiol. 1989;10:400–402. [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielmi G, Vinuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. I. Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 9.Huston J, Rufenacht DA, et al. Intracranial aneurysms and vascular malformations: comparison of time-of-flight and phase-contrast MR angiography. Radiology. 1991;181:721–730. doi: 10.1148/radiology.181.3.1947088. [DOI] [PubMed] [Google Scholar]
- 10.Kerber CW, Heilman CB. Flow dynamics in the human carotid artery. I. Preliminary observations using a transparent elastic model. Am J Neuroradiol. 1992;13:173–180. [PMC free article] [PubMed] [Google Scholar]
- 11.Kerber CW, Liepsch D. Flow dynamics for radiologists. II. Practical considerations in the live human. Am J Neuroradiol. 1994;15:1076–1086. Review. [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo S, Hashimota N, et al. Cerebral aneurysms arising at non branching sites: an experimental study. Stroke. 1997;28:398–403. doi: 10.1161/01.str.28.2.398. Discussion 403-404. [DOI] [PubMed] [Google Scholar]
- 13.Lefkowitz MA, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm: Part II clinical results. Neurosurgery. 1999;45:531–537. doi: 10.1097/00006123-199909000-00024. discussion 537-538. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald RL, Mojtahedi S, et al. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke. 1998;29:478–485. doi: 10.1161/01.str.29.2.478. discussion 485-486. [DOI] [PubMed] [Google Scholar]
- 15.Masaryk AM, Frayne R, et al. Utility of CT angiography and MR angiography for the follow-up of experimental aneurysms treated with stents or Guglielmi detachable coils. Am J Neuroradiol. 2000;21:1523–1531. [PMC free article] [PubMed] [Google Scholar]
- 16.Massoud TF, Guglielmi G, et al. Experimental Saccular Aneurysms. I. Review of Surgically-Constructed Models and their Laboratory Applications. Neuroradiology. 1994;36:537–546. doi: 10.1007/BF00593517. [DOI] [PubMed] [Google Scholar]
- 17.Mawad ME, Mawad JK, et al. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Molyneux A, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 19.Mullan S, Beckmann F, et al. An experimental approach to the problem of cerebral aneurysms. J Neurosurg. 1964;21:838–845. doi: 10.3171/jns.1964.21.10.0838. [DOI] [PubMed] [Google Scholar]
- 20.Murayama Y, Vinuela F, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II: an experimental study in a swine aneurysm model. Am J Neuroradiol. 1999;20:1992–1999. [PMC free article] [PubMed] [Google Scholar]
- 21.Neurosurg Rev. 2003 Jun 11; [Google Scholar]
- 22.Reul J, Weis J, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. Am J Neuroradiol. 1997;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JS, Masaryk TJ, et al. Intracranial aneurysms: evaluation by MR angiography. Am J Neuroradiol. 1990;11:449–455. [PMC free article] [PubMed] [Google Scholar]
- 24.Talissa A Alters, Harry J Cloft, et al. Executive Council Award Creation of Saccular Aneurysms in the Rabbit: A Model Suitable for Testing Endovascular Devices. 1999 doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 25.Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 26.Raymond J, Salazkin I, et al. Lingual artery bifurcation aneurysms for training and evaluation of neurovascular devices. Am J Neuroradiol. 2004;25:1387–1390. [PMC free article] [PubMed] [Google Scholar]