Abstract
Over the last two decades, there has been an increasing awareness regarding the potential impact of indoor air pollution on human health. People working in an indoor environment often experience symptoms such as eye, nose, and throat irritation. Investigations into these complaints have ascribed the effects, in part, to compounds emitted from building materials, cleaning/consumer products, and indoor chemistry. One suspect indoor air contaminant that has been identified is the dicarbonyl 4-oxopentanal (4-OPA). 4-OPA is generated through the ozonolysis of squalene and several high-volume production compounds that are commonly found indoors. Following preliminary workplace sampling that identified the presence of 4-OPA, these studies examined the inflammatory and allergic responses to 4-OPA following both dermal and pulmonary exposure using a murine model. 4-OPA was tested in a combined local lymph node assay and identified to be an irritant and sensitizer. A Th1-mediated hypersensitivity response was supported by a positive response in the mouse ear swelling test. Pulmonary exposure to 4-OPA caused a significant elevation in nonspecific airway hyperreactivity, increased numbers of lung-associated lymphocytes and neutrophils, and increased interferon-γ production by lung-associated lymph nodes. These results suggest that both dermal and pulmonary exposure to 4-OPA may elicit irritant and allergic responses and may help to explain some of the adverse health effects associated with poor indoor air quality.
Keywords: dicarbonyls, oxygenated reaction products, hypersensitivity, LLNA, indoor air
On average, U.S. citizens spend 80% or more of their daily lives indoors whether at home, work, or in other commercial buildings (USEPA, 2008), and over the last two decades, there has been an increasing awareness regarding the potential impact of indoor air pollution on health. The term “sick building syndrome” (SBS) has been used to describe situations in which no specific illness or cause, aside from time spent indoors, explains adverse health effects experienced by building occupants (USEPA, 1991). Indoor air quality–related health issues cost businesses billions of dollars annually due to factors including decreases in worker productivity and time off from work. Affected individuals often complain of associated symptoms such as headache; eye, nose, or throat irritation; dry cough; dry or itchy skin; dizziness and nausea; difficulty in concentrating; fatigue; and sensitivity to odors. The increasing complaints by indoor occupants, along with an increased number of potential indoor pollutants, have prompted investigations to determine if these symptoms result from exposures to indoor pollutants.
Volatile organic compounds (VOCs) are receiving considerable attention. VOCs are introduced indoors by emission from building materials and furnishings, application of chemicals (paints, cleaners, pesticides, glues, and adhesives), or indoor chemistry (e.g., ozonolysis of VOCs). Concentrations of many VOCs have been found to be consistently higher indoors (up to 10 times higher) than outdoors (USEPA, 2007). Although adverse health effects have been correlated to VOC exposure, no specific culprit(s) have been identified (Arif and Shah, 2007; Jang et al., 2007).
In addition, the secondary pollutants resulting from reactive indoor air chemistry may also be responsible for some of the health effects associated with indoor air exposures. Consumer cleaning products and air fresheners contain large amounts of VOCs, which can react with OH• (hydroxyl radicals), ozone, and/or (nitrate radicals) to form secondary oxidation products or secondary pollutants. Investigations into the specific health effects associated with exposure to these secondary pollutants are limited. Epidemiological investigations have suggested a potential link between exposure to chemicals used in indoor cleaning agents and asthma. In addition, results from our laboratory have shown that dermal exposure to indoor air reaction products can be both irritating and sensitizing when evaluated in a murine model (Anderson et al., 2007). However, identification of the presence of these secondary pollutants in indoor environments is challenging because conventional methods have not been developed for the detection of these compounds.
4-Oxopentanal (OPA) is a secondary reaction product that can be formed through a number of oxidation processes, involving the commonly used, high production volume (USEPA, 1990) indoor fragrances geraniol (Forester et al., 2007), and α-terpineol (Wells, 2005), with the indoor reactants OH• and O3. Although these types of reactions have led to measurable outdoor concentrations of 4-OPA up to 384 ppt in forestal Japan (Matsunaga et al., 2004), its presence in the indoor environment is often overlooked. 4-OPA has been detected in indoor air environments using novel methods resulting from both gas-phase and surface-phase reactions (Ham and Wells, 2008; Wells et al., 2008).
Squalene (2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene), a non-volatile alkene that constitutes ∼5 to 15% of skin oil or sebum (Greene et al., 1970) present in the outermost layer of the skin, is an important precursor to 4-OPA. Studies conducted in a simulated aircraft cabin have shown that ozone (61–77 ppb) can react with squalene on skin and clothing to produce several oxygenated organic compounds including 4-OPA (3–7 ppb) (Weschler et al., 2007; Wisthaler et al., 2005). More recent work has shown that a simulated indoor air office environment produces 4-OPA at levels up to 2 ppb dependent upon the concentration of ozone and the number of occupants present (Wisthaler and Weschler, 2010).
The toxicological effects associated with 4-OPA exposure have not been thoroughly evaluated despite the potential for its ubiquitous presence indoors. Recent work in our laboratory has demonstrated that 4-OPA and other oxygenated reaction products are capable of inducing proinflammatory responses after exposure of the gas-phase chemicals to pulmonary lung epithelial cells (Anderson et al., 2010). These results suggest that exposure to 4-OPA in the indoor environment may contribute to some of the observed adverse health effects. The present studies describe the presence of 4-OPA in an indoor environment and begin to define the immunotoxic effects by examining the irritancy and allergic potential after both dermal and pulmonary exposures using a murine model.
MATERIALS AND METHODS
Preliminary Field Sampling
Field sampling to detect the presence of 4-OPA in an indoor environment was conducted on the seventh floor wing of a health care facility (hospital). For 4-OPA detection, air was sampled for 1 h at a flow rate of 18 l/min (1080 l total sample) onto a five channel, 400 mm length denuder (URG-2000-30B5; URG, Chapel Hill, NC) coated with ground XAD-4 resin (average particle size 0.7 μm) and a filter pack (URG-2000-30FG-3; URG) with 47 mm 0.45 micron polytetrafluoroethylene filter (Fluoropore membrane; Millipore, Billerica, MA). The sampler was located at the end of a hallway to reduce workplace disruption; however, during sampling, there was routine floor cleaning with water. Reaction products were analyzed as their oxime described below. After sampling, the denuder was extracted with 40 ml of dichloromethane using a previously described technique (Lane and Gundel, 1996; Lane et al., 2000). A single extraction removed more than 95% of the collected reaction products (Arey et al., 2009). To identify oxygenated reaction products (i.e., aldehydes, ketones, and dicarbonyls), 400 μl of O-(2,3,4,5,6-pentafluoro-benzyl)hydroxylamine hydrochloride (PFBHA) (20mM in acetonitrile) was added to the dichloromethane extract and stored overnight for completion of the derivatization reaction. The vials were then blown down to approximately 4 ml, transferred and filtered into 4-ml vials and then blown down to complete dryness using ultrahigh purity N2, and finally reconstituted in 100 μl of methanol (Forester and Wells, 2009; Yu et al., 1998). All samples were then analyzed using a Varian (Palo Alto, CA) 3800/Saturn 2000 gas chromatography/mass spectrometer (GC/MS) system operated in the electron impact (EI) mode. Compound separation was achieved using a J&W Scientific (Folsom, CA) DB-5MS (0.25 mm I.D., 30-m long, 1 μm film thickness) column and the following GC oven parameters: 60°C for 1 min, then 20°C/min to 170°C, and then 3°C/min to 280°C and held for 5 min. Each sample (1 μl) was injected in the splitless mode, and the GC injector was held at 250°C and then returned to split mode 1 min following the sample injection. The Saturn 2000 ion trap mass spectrometer was tuned using perfluorotributylamine (FC-43). Full-scan EI spectra were collected from m/z 40–650. Each sample was analyzed in duplicate.
Animals
Female BALB/c mice were used for the murine models. This mouse strain has a Th2 bias and is commonly used to evaluate IgE-mediated sensitization (Klink and Meade, 2003; Woolhiser et al., 2000). The mice were purchased from Taconic (Germantown, NY) at 6–8 weeks of age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to treatment group, weighed, and individually identified via tail marking using a permanent marker or tattoo. A preliminary analysis of variance on body weights was performed to ensure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of five per cage in ventilated plastic shoebox cages with hardwood chip bedding, NIH-31 modified 6% irradiated rodent diet (Harlan Teklad), and tap water was provided from water bottles, ad libitum. The temperature in the animal facility was maintained between 68°F and 72°F and the relative humidity between 36 and 57%. The light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited National Institute for Occupational Safety and Health animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Chemicals
4-OPA (Table 1) was synthesized by Richman Chemical Inc. (Lower Gwynedd, PA) according to Hutton et al. (2003). In brief, pyridinium chlorochromate (16.2 g, 75.0 mmol) was added to 3-acetypropanol (5.00 g, 50.0 mmol) in dichloromethane (150 ml), and this solution was stirred for 16 h at room temperature. The reaction mixture was passed through a plug of silica and concentrated in vacuo to give the crude product. Purification by column chromatography (eluting with dichloromethane) gave 4-OPA (3.53 g, 35.3 mmol, 71%), and further purification by high performance liquid chromatography gave 98.84% 4-OPA. α-Hexylcinnamaldehyde (HCA, CAS 101-86-0), 2,4-dinitrofluorobenzene (DNFB, CAS 70-34-8), methacholine chloride (MCh, CAS 62-51-1), PFBHA (98+%), and toluene 2, 4-diisocyanate (TDI, CAS 584-84-9) were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI).
TABLE 1.
Structure and Predicted Toxicity of Oxygenated Reaction Products and/or Dicarbonyls
| Chemical | Structure EC3 (%) | Jarvis valuea |
| 4-OPA | 0.08 | 0.52 |
| Glutaraldehyde (Azadi et al., 2004) | 0.09 | 0.82 |
| Ortho-phthalaldehyde (Anderson et al., 2010) | 0.05 | 0.73 |
| Diacetyl (Anderson et al., 2007) | 1.9 | 0.16 |
| Methylglyoxal (Anderson et al., 2007) | 0.42 | 0.38 |
| Glyoxal (Anderson et al., 2007) | 0.74 | 0.66 |
Occupational asthma hazard resource value: Calculation based on chemical structure for the prediction of occupational asthmagens. Values closer to 1 represent greater potential hazards. http://www.coeh.man.ac.uk/asthma/login.php.
Concentration Range Finding Studies
Concentration range finding studies were performed to select the concentrations of 4-OPA to be used for dermal and pulmonary exposures. For the dermal exposures, mice were exposed topically to acetone vehicle or increasing concentrations of 4-OPA (12.5, 25, and 50%) in acetone on the dorsal surface of each ear (25 μl per ear) for three consecutive days. For the pulmonary exposures, following light anesthesia with isoflurane (Abbott Laboratories, 99.9%), mice were exposed by pharyngeal aspiration using the method described by Rao et al. (2003) to saline vehicle or increasing concentrations of 4-OPA (50 μl per mouse; up to 10%) in diluted in sterile PBS every 3 days for three exposures. Animals were allowed to rest for 2 days following the last exposure and then weighed and examined for signs of overt toxicity, including loss of body weight, fatigue/lack of activity, and ungroomed fur.
Dermal Exposures
Combined irritancy and local lymph node assay.
To determine the irritancy and sensitization potential of 4-OPA, a combined local lymph node assay (LLNA) was conducted as previously described (Anderson et al., 2007). 4-OPA dosing concentrations (0.01–25%) and vehicle (acetone) were selected based on the results from the concentration range finding studies. The concentration of chemical required to induce a threefold increase over the vehicle control (EC3) was calculated based on the equations from Basketter et al. (1999). The positive control for the irritancy portion of the experiment was 0.3% DNFB. Irritancy measurements were performed as previously described (Woolhiser et al., 1999).
Phenotypic analysis of draining lymph node cells.
To further evaluate the mechanisms of the hypersensitivity response, the number of IgE + B220 + cells in the auricular draining lymph nodes (DLNs) was quantitated using flow cytometry. For the phenotypic analysis, mice (5 per group) were exposed to 25 μl per ear of the acetone vehicle, increasing concentrations of 4-OPA (6.25, 12.5, and 25%) or positive control (2.5% TDI) once daily for four consecutive days. Animals were weighed and examined for gross pathology at the end of the experiment (day 10). DLNs were collected (two nodes per animal per 3 ml PBS) and dissociated using the frosted ends of two microscope slides, and phenotypes were analyzed using flow cytometry as described by Manetz and Meade (1999). The following organs were also removed, cleaned of connective tissue, and weighed: liver, spleen, kidneys, and thymus. Serum was collected for total IgE analysis (see below).
Mouse ear swelling test.
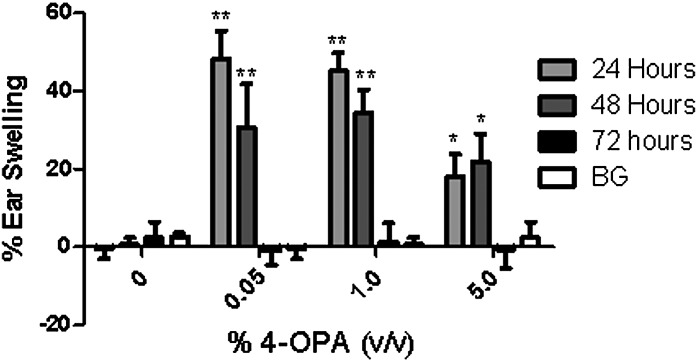
The mouse ear swelling test (MEST) followed the original procedure outlined by Gad et al. (1986) with minor modifications. Mice (5 per group) were exposed to 50 μl of the acetone vehicle, increasing concentrations of 4-OPA (0.05, 1, and 5%) or positive control (0.15% DNFB) on the dorsal clipped thorax once daily for three consecutive days (days 1–3). Concentrations evaluated were nonirritating based on the results from the irritancy assay (Fig. 2A). The animals were rested on days 4–7. On day 8, the ears of the animals were premeasured in duplicate using a modified engineer’s micrometer and then the animals were challenged with 25 μl of acetone, 10% 4-OPA, or 0.5% DNFB on the dorsal surface of the right ear pinna. Post-treatment ear measurements were taken at 30-min, 24-, 48-, and 72-h time points. Mice were sacrificed after the 72-h ear measurement. A percent ear swelling for each animal was calculated as described for the irritancy assay. Mean percent ear swelling for each dose group was compared with the mean percent ear swelling for the background control group to control for irritation of the challenge dose. The background control group (Student’s t-test) was compared with unexposed vehicle control to assess swelling caused by nonspecific irritation.
FIG. 2.
MEST. Analysis of ear swelling at 24, 48, and 72 h post-4-OPA challenge (10%). Sensitization concentration is indicated on the graph. Measurements were taken on the right ear, whereas the left ear was included as a negative control. Bars represent means ± SE of 5 mice per group. Levels of statistical significance are denoted as *p ≤ 0.05 and ** p ≤ 0.01 as compared with background (BG) control.
Cytokine messenger RNA analysis.
To evaluate Th1 and Th2 cytokine involvement in 4-OPA sensitization, the DLNs were analyzed for messenger RNA (mRNA) expression after dermal application. Mice were exposed to acetone, increasing concentrations of 4-OPA or 2.5% TDI (Th2-positive control) topically on the dorsal surface of each ear (25 μl per ear) once a day for four consecutive days. Animals were euthanized by CO2 inhalation 24 h after the last exposure, weighed, and the DLNs from each animal were collected in 1 ml of TRI Reagent (Molecular Research Center). RNA was isolated using TRI Reagent as specified by the manufacturer. To further purify the RNA, the RNeasy Mini Kit (Qiagen) with DNase treatment was used following the manufacturer’s protocol. The concentration and purity (260/280) of RNA were determined using an ND-1000 spectrophotometer (Thermo Scientific NanoDrop). Two micrograms of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) as directed by the manufacturer. Relative fold changes in gene expression were assessed using real-time PCR (RT-PCR) on a 7500 Fast Real-Time PCR System (Applied Biosystems) using TaqMan PCR Master Mix (Applied Biosystems) as specified in the manufacturer’s protocol. RT-PCR data were collected and expressed as relative fold increase over control, calculated by the following formula: 2−ΔΔCt = ΔCtSample − ΔCtControl. ΔCt = CtTarget − CtGAPDH, where Ct = cycle threshold as defined by manufacturer’s instructions. Th1 cytokines assessed were interferon-gamma (INF-γ) and interleukin (IL)-12 and the Th2 cytokines were IL-4, IL-5, and IL-10. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control.
Pulmonary Exposures
Pulmonary exposures.
For the short-term exposure studies, mice (7 per group) were sensitized on the dorsal surface of both ears with 25 μl per ear of acetone vehicle control or a concentration of 4-OPA ranging from 0.05 to 1% on days 1–4 and challenged (day 7) via pharyngeal aspiration with 1% 4-OPA. Mice were sacrificed 24-h post-final pharyngeal aspiration (day 8). A 4-OPA aspiration control (no dermal sensitization and only single 4-OPA aspiration) was included in the study. For the long-term exposure studies, mice (7 per group) were sensitized on the dorsal surface of both ears with 25 μl per ear of acetone vehicle control or a concentration of 4-OPA ranging from 1 to 10% for 4 days and challenged with 1% 4-OPA in 50 μl by pharyngeal aspiration on days 6, 10, 14, and 18. A 4-OPA aspiration control (no dermal sensitization and four 4-OPA aspirations) and 4-OPA dermal control (no 4-OPA aspirations and only dermal exposures) were included in the study. Mice were sacrificed 24-h post-final pharyngeal aspiration (day 19).
Airway hyperreactivity.
For both the short- and the long-term exposure studies, analysis of airway hyperreactivity (AHR) was performed as described previously (Howell et al., 2002) with minor modifications. Briefly, 24 h following the last exposure, mice were placed in Buxco whole body plethysmography chambers and baseline enhanced pause (Penh) values were obtained over a 5-min period. Mice were then challenged by nebulizing increasing concentrations (10, 25, and 50 mg/ml) of MCh into the chambers. At each concentration of MCh, Penh was assessed over a 5-min period with MCh exposure occurring for the first 3 min followed by 2 min of fresh air. Immediately following assessment of AHR, mice were sacrificed with a lethal dose of pentobarbital (0.1 ml pentobarbitone sodium, 200 mg/ml), and blood was collected by transection of the abdominal aorta for analysis of serum IgE. Lungs were collected for mRNA analysis, lung-associated lymph nodes (LALN) were collected for analysis of protein expression, and bronchoalveolar lavage (BAL) fluid was collected for analysis of cell infiltrates (described below).
Analysis of BAL fluid after pulmonary exposure to 4-OPA.
Lungs collected from animals in the short- and long-term exposure studies were perfused with 10 ml of PBS to eliminate neutrophils present in the blood, and the trachea was cannulated to perform BAL. PBS (1 ml) was introduced into the lungs via the tracheal cannula and carefully withdrawn. This was repeated two additional times to collect remaining cells. The recovered fluid (75–80% of the injected volume) was centrifuged at 800 × g for 10 min at 4°C, and the cells were resuspended in 200 μl of flow staining buffer (1% bovine serum albumin and 0.1% sodium azide in PBS, pH 7.4) containing the following combinations of fluorochrome-conjugated antibodies to identify infiltrating eosinophil, neutrophil, and alveolar macrophage [anti-CD45-APC (30-F11), anti-Siglec-F-PE (E50-2440), anti-CD11c-biotin (HL3), and anti-LY6G-FITC(1A8)] or B and T cell [CD45-APC (30-F11), CD45R/B220-PE (RA3-6B2), and CD3-FITC (145-2C11)] populations. All antibodies were purchased from BD Biosciences (San Jose, CA). Cell suspensions were incubated with labeled antibodies on ice in the dark for 30 min and washed by centrifuging cells for 5 min at 486 × g. Samples stained with biotin-labeled anti-CD11c were resuspended in flow staining buffer containing streptavidin PercP and incubated for an additional 30 min on ice in the dark. All samples were then fixed by resuspending cells in 0.1 ml BD cytofixation buffer (BD Bioscience) and incubating for 15 min. Cells were then washed, resuspended in flow staining buffer, and enumerated using a FACScaliber Flow Cytometer (Becton Dickinson, San Jose, CA). Lung cellular infiltrates were identified phenotypically based upon the distinct expression of the following cell surface markers: eosinophils (CD45+, LY6G-, Siglec F+, CD11c low), neutrophils (CD45+, LY6G+), and alveolar macrophages (CD45+, LY6G-, Siglec F+, CD11c high).
Lung cytokine mRNA analysis.
To evaluate the cytokine profile involved in 4-OPA exposure, the lungs were collected in 3 ml of PBS from animals in the short-term exposure study (BAL collection was not preformed on these animals), dissociated using the frosted ends of two microscope slides, and analyzed for mRNA expression according to the procedure described previously for the dermal exposure studies. The following cytokines were analyzed: INF-γ, CCL2, CCL3, CCL5, CCR2, CCL19, TNF-α, GM-CSF, IL-12 IL-4, IL-5, and IL-10.
Analysis of cytokine production by DLN cells.
LALNs were collected in 2 ml PBS from mice in the long-term exposure study and dissociated using the frosted ends of two microscope slides. Cell counts were performed using a Coulter Counter (Z1 model; Beckman Coulter), and cells were adjusted to 1 × 106 cells/ml using sterile RPMI media containing 10% fetal calf serum and gentamycin (50 mg/ml). Cells were added to a 48-well plate in a 500 μl volume, stimulated with α-CD3 and α-CD28 (2 μg/ml of each; BD Pharmingen), and incubated for 24 h at 37°C and 5% CO2. Supernatants were analyzed for IL-4, IL-5, and INF-γ production using an OptEIA ELISA kit purchased from BD Biosciences according to the manufacturer’s instructions. Supernatant samples collected from each culture (two stimulated and two unstimulated for each mouse) were added to the plates in triplicate along with serial dilutions of the standards. Plates were read at 450 nm (optical density [OD] values for standards ranging from 0.77 to 1.93) using a SpectraMax M2 spectrophotometer (Molecular Devices). Cytokine concentration was extrapolated from the standard curve. The final concentration is based on the adjusted OD value (OD value from unstimulated cultures subtracted from the OD value generated from the stimulated cultures for each mouse).
IgE Antibody Levels
Following euthanasia of animals included in the dermal or pulmonary exposure study, blood samples were collected via cardiac puncture or transaction of the abdominal aorta, respectively. Sera were separated by centrifugation and frozen at −20°C for subsequent analysis of IgE by ELISA. A standard colorimetric sandwich ELISA was performed as previously described (Anderson et al., 2007).
Statistical Analysis
For analysis, data were first tested for homogeneity using the Bartlett’s chi square test. If homogeneous, a one-way ANOVA was conducted. If the ANOVA showed significance at p < 0.05 or less, the Dunnett’s multiple range t-test was used to compare treatment groups with the control group. For the AHR data, 4-OPA-related effects were determined for 10, 25, and 50 mg/ml MCh individually. Linear trend analysis was performed to determine if 4-OPA had exposure concentration–related effects for the specified endpoints. Statistical analysis was performed using Graph Pad Prism version 5.0 (San Diego, CA). Statistical significance is designated by *p ≤ 0.05 and **p ≤ 0.01.
RESULTS
Preliminary Field Results
Three 1-h samples were collected in a health care facility using a denuder/filter/pump apparatus. Several carbonyl compounds were detected as their respective oximes by GC/MS. The chromatographic peak areas characteristic of the 4-OPA oxime appeared to increase over time (Supplementary figure S1) suggesting an increase in 4-OPA concentration during the sampling interval (Wells, 2005). This increase is likely due to oxidation of both VOCs and squalene (Wells, 2008; Wisthaler and Weschler, 2010). Although these data are preliminary and not quantitative, it does demonstrate the presence of 4-OPA in indoor air.
Toxicity and Concentration Range Finding Studies
Dermal exposure to concentrations of 4-OPA greater than 25% was found to be toxic with animals exhibiting weight loss greater than 10% of original body weight and ruffled fur. For these reasons, 25% was the highest concentration of 4-OPA used in subsequent dermal exposure studies. Pulmonary exposure to concentrations of 4-OPA greater than 5% resulted in greater than 10% loss in body weight, air-filled intestines, and lethargy. Therefore, 1% 4-OPA was chosen as the highest concentration used for aspirations in subsequent studies.
In Vivo Studies Identify 4-OPA to Be an Irritant and Sensitizer After Dermal Exposure
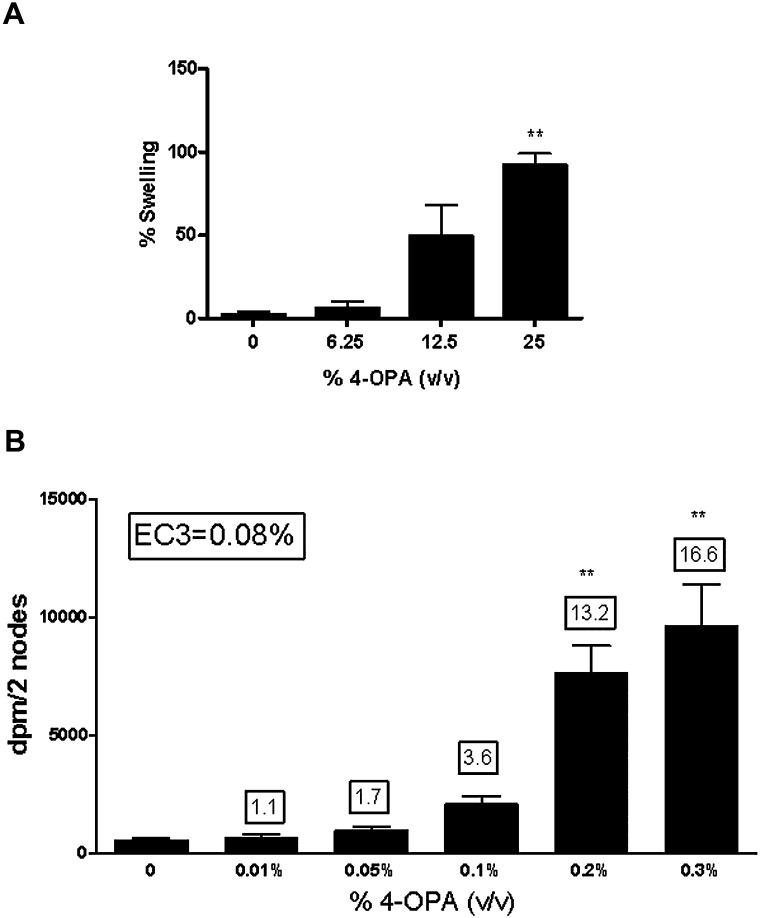
Dermal exposure to 4-OPA was found to be irritating based on a dose-responsive increase in ear swelling (linear trend test; p < 0.01) observed 24-h post-final 4-OPA exposure (Fig. 1A). Statistical significance was reached after exposure to 25% (92 ± 7% ear swelling). The positive control for irritancy studies (0.3% DNFB) resulted in an average increase of 60% ear swelling post exposure. A dose-responsive (linear trend test; p < 0.01) increase in lymphocyte proliferation was also observed after dermal exposure to 4-OPA. Initial 4-OPA concentrations tested (3.125–25%) in the LLNA generated a stimulation index (SI) greater than threefold at all concentrations; therefore, the experiment was repeated using concentrations between 0.01 and 2% (Fig. 1B, Table 2). Statistical significance was obtained after treatment with concentrations of 0.2% 4-OPA and higher. Exposure to 4-OPA resulted in a calculated EC3 value of 0.08% (Fig. 1B) classifying it as an extreme sensitizer (Loveless et al., 2010). HCA (30%) was used as a positive control for the LLNA and resulted in an average SI value of 23.4.
FIG. 1.
Ear swelling and allergic sensitization potential as a result of dermal exposure. Analysis of irritation (A) and sensitization potential (B) after exposure to 4-OPA. Bars represent means ± SE of 5 mice per group. Numbers above the bars represent SIs. Levels of statistical significance are denoted as **p ≤ 0.01 as compared with vehicle control.
TABLE 2.
Phenotypic and Total IgE Analysis
| Dose group | LLNA (DPM) | IgE + B220 + (% lymphocyte population) | B220 + (% lymphocyte population) | Total IgE (ng/ml) | |
| 4-OPA (%) | |||||
| 0a | 455 ± 99 | 2.04 ± 0.9 | 18.2 ± 0.9 | 618 ± 14 | |
| 6.25 | 9394 ± 896** | 16.3 ± 2.0** | 33.8 ± 1.0** | 705 ± 157 | |
| 12.5 | 14604 ± 1491** | 15.9 ± 1.9** | 37.1 ± 1.2** | 906 ± 50 | |
| 25 | 26912 ± 1447** | 30.8 ± 3.5** | 39.9 ± 3.6** | 1350 ± 166* |
Note. Values represent the mean ± SE derived from 5 animals per group.
Represents acetone vehicle.
Significantly different from acetone controls: *p ≤ 0.05, **p ≤ 0.01.
Experiments Investigating the Mechanism of Dermal Sensitization by 4-OPA Produce Contradictory Results
Immune phenotyping.
The mechanisms of 4-OPA sensitization were further investigated using phenotypic analysis of B220+ and IgE + B220+-expressing cells in the DLNs. Consistent with the LLNA results, statistically significant increases in percent of B220+ and IgE + B220+-expressing cells were identified in the DLN of mice treated with all concentrations (6.25–25%) of 4-OPA (Table 2). Exposure to the highest concentration of 4-OPA (25%) resulted in increases of 30.8 ± 3.5% (B220+) and 39.9 ± 3.6% (IgE + B220+). TDI (2.5%) was used as a positive control for phenotyping experiments and resulted in significant mean elevations of the percent IgE + B220+ (33 ± 2.7%) and B220+ (40 ± 1.9%) lymphocyte populations. No significant changes in organ or body weights were observed in these animals (Supplementary Table S1), although there was a concentration dose-responsive increase in spleen weights (linear trend test; p < 0.01).
Total IgE analysis.
Supporting the observed increase in IgE producing cells in the phenotyping study, exposure to 4-OPA produced dose-dependent (linear trend test; p < 0.01) modest elevations in total serum IgE levels (Table 2) reaching statistical significance at the 25% treatment (1350 ± 166 ng/ml). TDI (2.5%) was also used as a positive control for the total IgE ELISA and resulted in a significant elevation of total IgE (1903 ± 210 ng/ml) when compared with vehicle (618 ± 101 ng/ml).
Cytokine expression.
Cytokine mRNA expression in the DLNs was also analyzed to further evaluate the mechanism of 4-OPA-induced hypersensitivity. Significant relative fold increases in INF-γ mRNA expression (4.6–12.6) compared with the acetone control animals were observed following dermal exposure to 4-OPA at concentrations ranging from 0.5 to 25%. Fold changes (fold change following exposure to 25% 4-OPA indicated in parentheses) of IL-4 (1.32), IL-5 (0.96), IL-10 (1.04), or IL-12 (1.12) mRNA were not significantly modulated following treatment with any of the 4-OPA concentrations tested.
Mouse ear swelling test.
After prior sensitization to 4-OPA (0.05–5%), significant increases in ear swelling were at 24- and 48-h post 4-OPA (10%) challenge (Fig. 2) with the peak increase occurring at 24 h. The lowest concentration tested (0.05%) induced the greatest increase in ear swelling at 24-h post-challenge (48% ± 7.1), whereas the smallest increase (18% ± 5.9) was observed after sensitization to the highest concentration of 4-OPA (5%). No increases were observed at 30 min or 72 h post-challenge. No swelling was observed for the background control animals when compared with the unexposed vehicle control. DNFB (The Th1-positive control) induced significant increases in ear swelling at 24-, 48-, and 72-h post-challenge (108 ± 14.4, 152 ± 13.7, and 134 ± 17.1%).
Long-Term Pulmonary Exposure to 4-OPA Induces Enhanced AHR
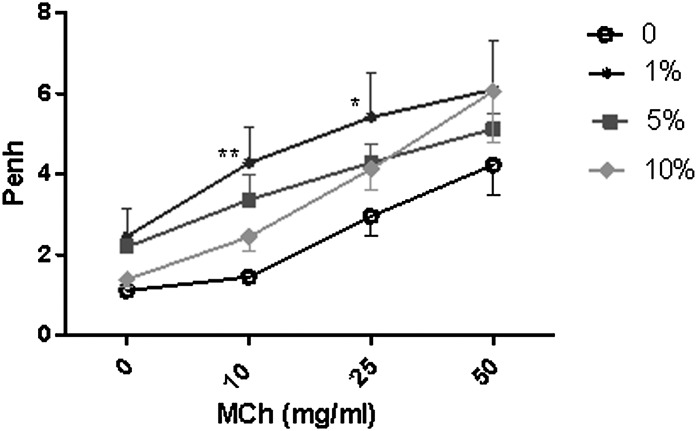
No alterations in AHR were observed after exposure to 4-OPA for the short-term exposure studies (Supplementary Table S2). Repeated dermal (1%) and pulmonary exposure (4 doses; 1%) significantly enhanced airway responsiveness to aerosolized MCh (Fig. 3B; Table 3) with average Penh values of 4.27 ± 0.67, 5.41 ± 1.36, and 6.05 ± 1.34 compared with the vehicle control values of 1.45 ± 0.13, 2.95 ± 0.54, and 4.27 ± 0.87, respectively, for mice exposed to 1% 4-OPA (dermal and pulmonary). Penh values were enhanced for the 5 and 10% 4-OPA treatment groups compared with the unexposed controls; however, they were not significant. No significant elevations were observed for the dermal control (no aspiration; Table 3); however, the 4-OPA aspiration control (no dermal sensitization) also resulted in significantly elevated Penh values of 5.44 ± 0.76 (25 mg/ml MCh) and 7.49 ± 1.05 (50 mg/ml MCh). A dose-responsive (linear trend test; p < 0.01) increase in the cellularity of the LALN was also observed following 4-OPA treatment with statistical significance at all concentrations (Table 3). No alterations in total serum IgE levels were detected in these animals (Table 3).
FIG. 3.
Increased AHR after long-term 4-OPA exposure. Analysis of AHR after nonspecific challenge in BALB/c mice following dermal and pulmonary exposure to 4-OPA. Mice received 4 days of dermal exposure (concentration indicated on graph) followed by four pulmonary challenges of 4-OPA (1%). Data presented represent nonspecific challenge with MCh. Bars represent means ± SE of 7 mice per group. Levels of statistical significance are denoted as *p ≤ 0.05 and **p ≤ 0.01 as compared with vehicle control (no dermal or pulmonary exposure to 4-OPA).
TABLE 3.
AHR, IgE, and LALN Cellularity Following Long-Term Pulmonary Exposure to 4-OPA
| AHR (Penh) |
||||||
| 4-OPA (vol/vol) | Total IgE (ng/ml) | LALN cellularity (cells/node × 106) | 0 mg/ml MCh | 10 mg/ml MCh | 25 mg/ml MCh | 50 mg/ml MCh |
| 0%a | 393 ± 107 | 0.57 ± 0.10 | 1.11 ± 0.04 | 1.45 ± 0.13 | 2.95 ± 0.54 | 4.27 ± 0.87 |
| 1%/1% | 738 ± 50 | 2.24 ± 0.49** | 2.46 ± 0.70 | 4.27 ± 0.67* | 5.41 ± 1.36** | 6.05 ± 1.34 |
| 5%/1% | 866 ± 322 | 1.80 ± 0.13* | 2.19 ± 0.18 | 3.36 ± 0.53 | 4.29 ± 0.40 | 5.12 ± 0.31 |
| 10%/1% | 950 ± 207 | 2.85 ± 0.62** | 1.39 ± 0.19 | 2.44 ± 0.35 | 4.13 ± 0.53 | 6.05 ± 1.18 |
| 10% Dermalb | 627 ± 69 | 1.17 ± 0.11 | 1.11 ± 0.70 | 1.45 ± 2.48 | 2.96 ± 2.43 | 4.23 ± 0.72 |
| 1% aspirationc | 592 ± 112 | 2.40 ± 0.42** | 1.54 ± 0.22 | 2.59 ± 0.22 | 5.44 ± 0.76 | 7.49 ± 1.05 |
Note. Values represent the mean ± SE derived from 7 animals per group; dermal exposure concentration/pulmonary exposure concentration.
Represents vehicle control (no dermal or pulmonary exposure)
Represents dermal control (10% dermal 4-OPA).
Represents aspiration control (1% aspiration of 4-OPA).
Significantly different from vehicle controls: *p ≤ 0.05, **p ≤ 0.01.
Pulmonary Exposure to 4-OPA Increased Neutrophil Influx
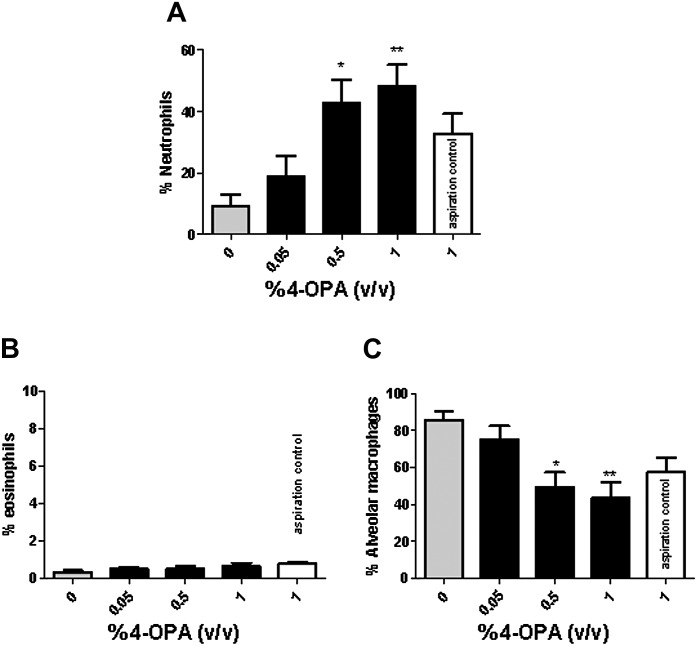
Exposure to 4-OPA produced significant elevations in BAL neutrophils for both the short- and the long-term studies. For the short-term study, a dose-dependent (linear trend test; p < 0.01) increase in BAL neutrophils (Fig. 4A) reaching statistical significance after treatment with 0.5 and 1% 4-OPA (48 ± 7%, 1% 4-OPA) was observed. Although not statistically significant, an increase in neutrophils was also observed following treatment with the 4-OPA aspiration control (33 ± 6%) when compared with the vehicle control (9 ± 4%). No significant changes were observed in percent eosinophils (Fig. 4B). The statistically significant decrease in alveolar macrophages observed following 4-OPA exposure was attributed to the compensation for the increase in percent neutrophils and therefore determined to not be of biological significance (Fig. 4C). Similar results were observed following long-term exposure to 4-OPA (Supplementary Figure S3).
FIG. 4.
BAL infiltrates after short-term exposure to 4-OPA. Analysis of percent neutrophils (A), eosinophils (B), and alveolar macrophages (C) after exposure to 4-OPA. Mice received 4 days of dermal exposure (concentration indicated on graph) followed by a pulmonary challenges of 4-OPA (1%). Aspiration controls (no dermal exposure) were also included on the graphs. Bars represent means ± SE of 7 mice per group. Levels of statistical significance are denoted as *p ≤ 0.05 and **p ≤ 0.01 as compared with vehicle control (no dermal or pulmonary exposure to 4-OPA).
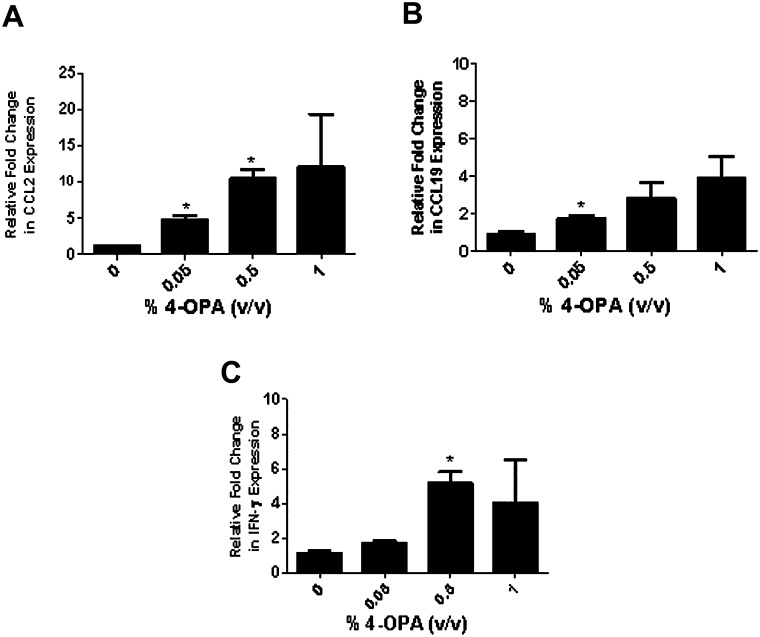
Pulmonary Exposure to 4-OPA Increased Expression of IFN-γ mRNA and Protein Expression
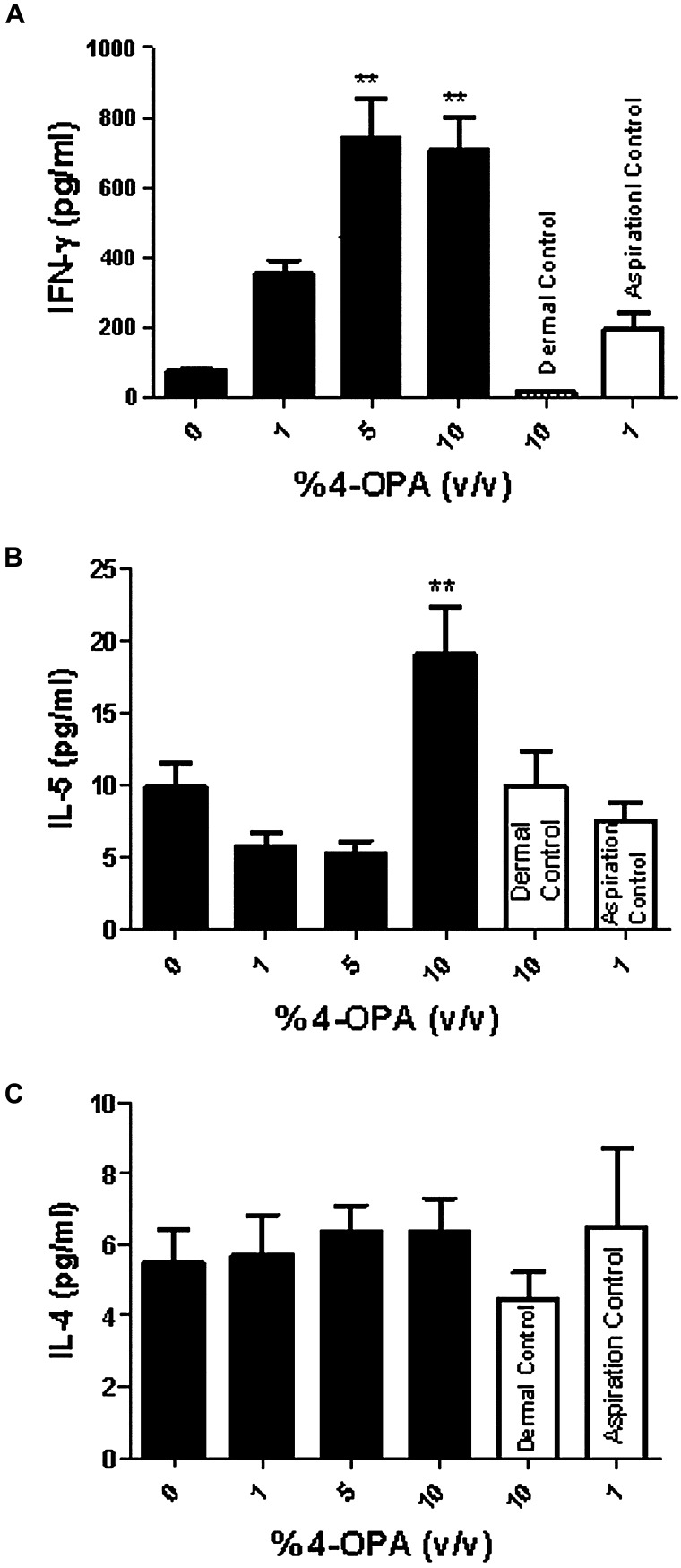
Cytokine mRNA expression in the lung was analyzed to further evaluate the effect of 4-OPA sensitization (Fig. 5). Statistically significant dose-responsive fold increases in gene expression greater than 5 were observed for CCL2 (Fig. 5A), CCL19 (Fig. 5B), and IFN-γ (Fig. 5C) for the short-term exposure study. The 4-OPA aspiration alone (no dermal exposure) did not significantly elevate any of these cytokines (CCL2 [3.3], CCL19 [1.9], and IFN-γ [0.8]) above fivefold. No changes in expression were observed for CCL3, CCL5, CCR2, TNF-α, GM-CSF, IL-12, IL-4, IL-5, and IL-10 (data not shown). Consistent with the mRNA results, a dose-responsive (linear trend test; p < 0.01) increase in IFN-γ protein production by the LALN was observed following long-term exposure to all concentrations of 4-OPA (Fig. 6A). The maximum increase in INF-γ protein expression was calculated to be 739 ± 114 pg/ml compared with 73 ± 7 pg/ml for the vehicle control. A significant increase in IL-5 production was also observed after exposure to 10% 4-OPA (dermal) with 1% challenge; however, this increase was not dose responsive (Fig. 6B). No changes were detected in IL-4 expression (Fig. 6C). No significant increases were observed for the 4-OPA aspiration control (no dermal sensitization) or dermal exposure controls (no 4-OPA aspiration) for any cytokine tested (Figs. 6A–C).
FIG. 5.
Lung mRNA expression after 4-OPA exposure measured by quantitative real-time PCR. Quantitative real-time PCR analysis of CCL2 (A) CCL19 (B) or IFN-γ (C) mRNA expression. Mice received 4 days of dermal exposure (concentration indicated on graph) followed by a pulmonary challenge of 4-OPA (1%). Bars represent means ± SE of 7 mice per group. Levels of statistical significance are denoted as *p ≤ 0.05 and **p ≤ 0.01 as compared with vehicle control (no dermal or pulmonary exposure to 4-OPA).
FIG. 6.
4-OPA sensitization results in increased cytokine production in the DLN. Analysis of INF-γ (Α), IL-5 (B), and IL-4 (C) protein expression generated by stimulated LALN after long-term exposure to 4-OPA. Mice received 4 days of dermal exposure (concentration indicated on graph) followed by four pulmonary challenges of 4-OPA (1%). Aspiration controls (no dermal exposure) and dermal control (no aspiration exposure) were also included on the graphs. Bars represent mean fold change ± SE of 7 mice per group. Levels of statistical significance are denoted as *p < 0.05 as compared with vehicle control (no dermal or pulmonary exposure to 4-OPA).
DISCUSSION
Field measurements taken in an indoor workplace detected the presence of 4-OPA in the indoor environment, and this preliminary data inferred that the concentration increased over the course of sampling (See Supplementary Figure S1). This observation prompted this hazard evaluation of 4-OPA using a murine model. Although results from the dermal exposure study suggest a possible IgE-mediated allergic response based on elevations in IgE, the pulmonary studies do not fully corroborate this finding. It is important to note that there were major differences between the dermal and pulmonary studies, including route of exposure, concentration, and duration of exposure. High concentrations of 4-OPA (up to 25%) were evaluated in the dermal studies, although due to route specific toxicity, much lower concentrations were tested in the pulmonary studies (1%). In addition, serum IgE levels are based on total IgE because specific IgE could not be measured in these studies. Therefore, it is possible that nonspecific responses could also be influencing the observed elevations in the dermal exposure studies.
Dermal exposure to 4-OPA did initiate an elicitation response in the MEST with the peak response occurring at 24-h post-challenge. Classical T-cell–mediated sensitizers generally demonstrate an increase or stable response at 48 h. For example, the peak response for DNFB was at 48 h post-challenge, with significant increases still observed at 72 h. For 4-OPA, swelling was still apparent by 48 h, although decreased, and completely resolved by 72 h. Interestingly, the highest responses were observed when sensitized to the lowest concentrations of 4-OPA (0.05%), and the lowest responses were observed when sensitized to the highest concentrations of 4-OPA (5%). Similar types of divergent responses have been observed for other sensitizing chemicals. For example, exposure to high concentrations of glutaraldehyde has been shown to induce immediate swelling (30 min), whereas lower concentrations generate more of a delayed type response (24–48 h) when tested in the MEST (Azadi et al., 2004). The inverse concentration dose-response relationship for Penh values, also observed in the long-term AHR study, could be a factor of chemical toxicity. Further support for a T-cell–mediated hypersensitivity response is the increased levels of INF-γ observed after both dermal and pulmonary exposure to 4-OPA. Although IgE-mediated sensitizers can cause elevations in IFN-γ, it is in addition to observed elevations in Th2 cytokines, such as IL-4 and IL-5 (Van Och et al., 2002). Although exposure to 4-OPA did significantly elevate IL-5 protein production in stimulated LALN, the lack of elevations in IL-4, IgE, and eosinophils suggest that the result is not biologically significant and does not support an IgE-mediated hypersensitivity response.
Other dicarbonyls are generated through reactive indoor chemistry, and it is hypothesized that these types of compounds may induce a variety of respiratory effects. A large number of studies have focused on investigations associated with exposure to VOCs; however, research investigating the potential health effects following exposure to secondary pollutants found in the indoor environment is limited. Our group has identified the dicarbonyls (glyoxal, glycolaldehyde, methylglyoxal, and glyoxylic acid) as sensitizers (Table 1) and irritants using the murine LLNA (Anderson et al., 2008, 2007). Proinflammatory responses were also observed when these structurally similar compounds, including 4-OPA, were exposed to pulmonary epithelial cells in the gas phase, further supporting the association of these types of chemicals with adverse health effects (Anderson et al., 2010). In addition, other secondary pollutants generated from reactive indoor air chemistry have been shown to affect airway responsiveness with an increase in sensory irritation in mice after inhalation exposures to mixtures of isoprene and ozone (Rohr et al., 2003; Wilkins et al., 2001). Studies conducted by Rohr et al. (2002) also found that terpene/ozone reaction products had greater effects on pulmonary function (breath frequency) in mice following inhalation exposure compared with the parent compounds. Doyle et al. (2004) observed increased cytotoxicity, IL-8, and IL-6 gene expression when cells were exposed to 1,3-butadiene, ozone, and their oxidation products in vitro. Other reaction products, such as carboxylic acids, have also been identified to be lung irritants (Nair, 1997).
Further support that 4-OPA induces a Th1-mediated hypersensitivity response was demonstrated in the long-term AHR studies. Elevations in neutrophil production (33%) were observed for the aspiration control, which might suggest chemical induced toxicity, although they were not statistically significant when compared with the vehicle control. The neutrophil population was also higher (48%) for the mice with prior dermal sensitization suggestive of a potential immune-mediated response. In addition, CCL2 and CCL19, proteins involved in immunoregulatory and inflammatory processes including the recruitment and trafficking of T cells, B cells, dendritic cells, and other immune cells (Robbiani et al., 2000; Schroder et al., 2004), were both found to be elevated after 4-OPA exposure. The observed results including increases in AHR (Penh) after nonspecific challenge, neutrophil, cytokine, and IFN-γ production support pulmonary irritation and inflammation caused by chemical exposure. Chemical irritants such as chlorine have been shown to enhance airway resistance and neutrophil numbers in the BAL fluid following pulmonary exposure in mice (McGovern et al., 2010). It is also important to note that that enhanced pause (Penh) value generated from the AHR is an indirect measurement of flows and volumes based on software algorithms, which calculate a physiological value from a measured value. Although it is important to mention that this limitation associated with penh, whole body plethysmography provides the benefits of endpoint analysis on unrestrained conscious animals.
Similar to the findings of the study described in this manuscript, inflammation of the upper respiratory tract resulting in nose and throat irritation are symptoms often linked to SBS. Studies in humans have shown that exposure to VOCs causes an influx of neutrophils into the nasal passages 4 and 18 h after exposure (Koren et al., 1992). Also, consistent with the results presented in this manuscript, recent data suggests that dermal exposure of 4-OPA in the gas phase may be responsible for certain health complaints. An increased sensation of lip and skin dryness was observed in individuals exposed to ozone in a simulated aircraft environment compared with individuals exposed in the same environment without ozone (Strom-Tejsen et al., 2007). These symptoms could potentially be explained by the increase in oxidation products, such as 4-OPA, generated on the skin. Therefore, these results support the potential contribution of 4-OPA to some of the adverse health effects associated with indoor air exposure.
Although precise concentrations of individual dicarbonyls in the indoor air environment are not known due to limitations of conventional types of detection methods, they are suspected to be low (ppb range). The exposure regimes employed in these investigations delivered a relatively high dose of 4-OPA (correlating to ∼100 to 250,000 ppm) over a short period of time to the skin of a mouse for the purposes of hazard identification. Personal exposure levels to 4-OPA in the indoor environment are estimated to be much lower than the concentrations used in these studies and additional studies would be necessary for risk assessment. However, given the similar types of observed biological response for secondary pollutants in both in vitro and in vivo models, long-term chronic exposure to individual chemicals or the summation of concentrations of several dicarbonyls may potentially be large enough to contribute to the rising number of health complaints resulting from indoor air exposure (Weschler et al., 2007). In the studies conducted by Koren et al., the concentrations for the individual VOCs present in the mixture of 22 VOCs were well below the threshold limit values; however, the authors hypothesized that the sum of the VOCs present in the mixture, rather than any single constituent chemical, may be the cause of the effects observed in the study. Further studies are needed to address the question of a cumulative health effect caused by structurally similar chemicals (e.g., glyoxal, methylglyoxal, and glutaraldehyde) when present in a mixture. Numerous reactions in the indoor environment are occurring at the same time involving structurally similar compounds (Table 1). These low–molecular weight chemicals are known to generate analogous types of biological responses and have similar predicted toxicities based on the Jarvis value (Jarvis et al., 2005).
In summary, 4-OPA was identified as an irritant and sensitizer using a combined LLNA. The results from the dermal and pulmonary exposure studies suggest that the mechanism behind 4-OPA-induced hypersensitivity may be a function of both exposure route and concentration. Independent of these discrepancies, 4-OPA was found to induce an immune-mediated response, and this finding supports the hypothesis that chemicals generated though reactive indoor air chemistry may be contributing to the health effects associated with indoor air exposure. The high production volume of the parent compound, suspected ubiquitous presence of 4-OPA in the indoor environment, suggested adverse health effects, increased time spent indoors, increases in “green” household cleaners, and increases in new building materials and more airtight buildings, raise concern for human exposure to 4-OPA through both dermal and pulmonary routes. These factors warrant additional investigations examining the health effects caused by exposure to these types of compounds. In addition, identification of 4-OPA as a potential hazard also supports investigations into risk assessment and the long-term immunological health effects associated with chronic exposure along with encouraging methods for better detection of these types of compounds.
FUNDING
This work was supported by an interagency agreement with the National Institute of Environmental Health Sciences (Y1-ES0001-06).
Supplementary Material
Acknowledgments
The findings and conclusion in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health.
References
- Anderson SE, Ham JE, Munson AE. Irritancy and sensitization potential of glyoxylic acid. J. Immunotoxicol. 2008;5:93–98. doi: 10.1080/15476910802085681. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Jackson LG, Franko J, Wells JR. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicol. Sci. 2010;115:453–461. doi: 10.1093/toxsci/kfq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- Arey J, Obermeyer G, Aschmann SM, Chattopadhyay S, Cusick RD, Atkinson R. Dicarbonyl products of the OH radical-initiated reaction of a series of aromatic hydrocarbons. Environ. Sci. Technol. 2009;43:683–689. doi: 10.1021/es8019098. [DOI] [PubMed] [Google Scholar]
- Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int. Arch. Occup. Environ. Health. 2007;80:711–719. doi: 10.1007/s00420-007-0183-2. [DOI] [PubMed] [Google Scholar]
- Azadi S, Klink KJ, Meade BJ. Divergent immunological responses following glutaraldehyde exposure. Toxicol. Appl. Pharmacol. 2004;197:1–8. doi: 10.1016/j.taap.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Basketter DA, Lea LJ, Dickens A, Briggs D, Pate I, Dearman RJ, Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J. Appl. Toxicol. 1999;19:261–266. doi: 10.1002/(sici)1099-1263(199907/08)19:4<261::aid-jat572>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Doyle M, Sexton KG, Jeffries H, Bridge K, Jaspers I. Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells. Environ. Health Perspect. 2004;112:1488–1495. doi: 10.1289/ehp.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester CD, Ham JE, Wells JR. Geraniol (2,6-dimethyl-2,6-octadien-8-ol) reactions with ozone and OH radical: Rate constants and gas-phase products. Atmos. Environ. 2007;41:1188–1199. [Google Scholar]
- Forester CD, Wells JR. Yields of carbonyl products from gas-phase reactions of fragrance compounds with OH radical and ozone. Environ. Sci. Technol. 2009;43:3561–3568. doi: 10.1021/es803465v. [DOI] [PubMed] [Google Scholar]
- Gad SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD. Development and validation of an alternative dermal sensitization test: The mouse ear swelling test (MEST) Toxicol. Appl. Pharmacol. 1986;84:93–114. doi: 10.1016/0041-008x(86)90419-9. [DOI] [PubMed] [Google Scholar]
- Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J. Invest. Dermatol. 1970;54:240–247. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- Ham JE, Wells JR. Surface chemistry reactions of α-terpineol [(R)-2-(4-methyl-3-cyclohexenyl)isopropanol] with ozone and air on a glass and a vinyl tile. Indoor Air. 2008;18:394–407. doi: 10.1111/j.1600-0668.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- Howell MD, Weissman DN, Jean Meade B. Latex sensitization by dermal exposure can lead to airway hyperreactivity. Int. Arch. Allergy Immunol. 2002;128:204–211. doi: 10.1159/000064253. [DOI] [PubMed] [Google Scholar]
- Hutton TK, Muir KW, Procter DJ. Switching between novel samarium(II)-mediated cyclizations by a simple change in alcohol cosolvent. Org. Lett. 2003;5:4811–4814. doi: 10.1021/ol0358399. [DOI] [PubMed] [Google Scholar]
- Jang AS, Choi IS, Koh YI, Park CS. Volatile organic compounds contribute to airway hyperresponsiveness. Korean J. Intern. Med. 2007;22:8–12. doi: 10.3904/kjim.2007.22.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J, Seed MJ, Elton R, Sawyer L, Agius R. Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup. Environ. Med. 2005;62:243–250. doi: 10.1136/oem.2004.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink KJ, Meade BJ. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicol. Sci. 2003;75:89–98. doi: 10.1093/toxsci/kfg171. [DOI] [PubMed] [Google Scholar]
- Koren HS, Graham DE, Devlin RB. Exposure of humans to a volatile organic mixture. III. Inflammatory response. Arch. Environ. Health. 1992;47:39–44. doi: 10.1080/00039896.1992.9935942. [DOI] [PubMed] [Google Scholar]
- Lane DA, Gundel L. Gas and particle sampling of airborne polycyclic aromatic compounds. Polycyclic Aromat. Compd. 1996;9:67–73. [Google Scholar]
- Lane DA, Peters AJ, Gundel LA, Jones KC, Northcott GL. Gas/particle partition measurements of PAH at Hazelrigg, UK. Polycyclic Aromat.Compd. 2000;20:225–234. [Google Scholar]
- Loveless SE, Api AM, Crevel RW, Debruyne E, Gamer A, Jowsey IR, Kern P, Kimber I, Lea L, Lloyd P, et al. Potency values from the local lymph node assay: Application to classification, labelling and risk assessment. Regul. Toxicol. Pharmacol. 2010;56:54–66. doi: 10.1016/j.yrtph.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol. Sci. 1999;48:206–217. doi: 10.1093/toxsci/48.2.206. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Mochida M, Kawamura K. High abundance of gaseous and particulate 4-oxopentanal in the forestal atmosphere. Chemosphere. 2004;55:1143–1147. doi: 10.1016/j.chemosphere.2003.10.004. [DOI] [PubMed] [Google Scholar]
- McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respir. Res. 2010;11:138–151. doi: 10.1186/1465-9921-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. Final report on the safety assessment of formic acid. Int. J. Toxicol. 1997;16:221–234. [Google Scholar]
- Rao GV, Tinkle S, Weissman DN, Antonini JM, Kashon ML, Salmen R, Battelli LA, Willard PA, Hoover MD, Hubbs AF. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health A. 2003;66:1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Shore SA, Spengler JD. Repeated exposure to isoprene oxidation products causes enhanced respiratory tract effects in multiple murine strains. Inhal. Toxicol. 2003;15:1191–1207. doi: 10.1080/08958370390229870. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Wilkins CK, Clausen PA, Hammer M, Nielsen GD, Wolkoff P, Spengler JD. Upper airway and pulmonary effects of oxidation products of (+)-alpha-pinene, d-limonene, and isoprene in BALB/c mice. Inhal. Toxicol. 2002;14:663–684. doi: 10.1080/08958370290084575. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Strom-Tejsen P, Wyon DP, Lagercrantz L, Fang L. Passenger evaluation of the optimum balance between fresh air supply and humidity from 7-h exposures in a simulated aircraft cabin. Indoor Air. 2007;17:92–108. doi: 10.1111/j.1600-0668.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- USEPA. 1990 HPV Challenge Program Chemical List. Environmental Protection Agency; 1990. [Google Scholar]
- USEPA. Indoor Air Facts No. 4, Sick Building Syndrome. Research and Development (MD-56) 1991. Available at: http://www.epa.gov/iaq/pdfs/sick_building_factsheet.pdf. Accessed March 16, 2012. [Google Scholar]
- USEPA. Organic Gases (Volatile Organic Compounds—VOCs) 2007. , Environmental Protection Agency, ed. Available at: http://www.epa.gov/iaq/voc.html. Accessed March 16, 2012. [Google Scholar]
- USEPA. The Inside Story: A Guide to Indoor Air Quality. Office of Radiation and Indoor Air. 402-K-93-007; 2008. [Google Scholar]
- Van Och FM, Van Loveren H, De Jong WH, Vandebriel RJ. Cytokine production induced by low-molecular-weight chemicals as a function of the stimulation index in a modified local lymph node assay: An approach to discriminate contact sensitizers from respiratory sensitizers. Toxicol. Appl. Pharmacol. 2002;184:46–56. [PubMed] [Google Scholar]
- Wells JR. Gas-phase chemistry of alpha-terpineol with ozone and OH radical: Rate constants and products. Environ. Sci. Technol. 2005;39:6937–6943. doi: 10.1021/es0481676. [DOI] [PubMed] [Google Scholar]
- Wells JR, Morrison GC, Coleman BK. Kinetics and reaction products of ozone and surface-Bound squalene. J. ASTM Int. 2008 [Google Scholar]
- Weschler CJ, Wisthaler A, Cowlin S, Tamas G, Strom-Tejsen P, Hodgson AT, Destaillats H, Herrington J, Zhang JJ, Nazaroff WW. Ozone-initiated chemistry in an occupied simulated aircraft cabin. Environ. Sci. Technol. 2007;41:6177–6184. doi: 10.1021/es0708520. [DOI] [PubMed] [Google Scholar]
- Wilkins CK, Clausen PA, Wolkoff P, Larsen ST, Hammer M, Larsen K, Hansen V, Nielsen GD. Formation of strong airway irritants in mixtures of isoprene/ozone and isoprene/ozone/nitrogen dioxide. Environ. Health Perspect. 2001;109:937–941. doi: 10.1289/ehp.01109937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisthaler A, Tamas G, Wyon DP, Strom-Tejsen P, Space D, Beauchamp J, Hansel A, Mark TD, Weschler CJ. Products of ozone-initiated chemistry in a simulated aircraft environment. Environ. Sci. Technol. 2005;39:4823–4832. doi: 10.1021/es047992j. [DOI] [PubMed] [Google Scholar]
- Wisthaler A, Weschler CJ. Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6568–6575. doi: 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Role of sensitization routes in the development of type I hypersensitivity to natural rubber latex in mice. Am. J. Ind. Med. Suppl. 1999;1:139–141. doi: 10.1002/(sici)1097-0274(199909)36:1+<139::aid-ajim49>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Comparison of mouse strains using the local lymph node assay. Toxicology. 2000;146:221–227. doi: 10.1016/s0300-483x(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Flagan RC, Seinfeld JH. Identification of products containing -COOH, -OH, and -C=O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 1998;32:2357–2370. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.