Editor's Highlight: The development of hemoglobin-based oxygen carriers (HBOCs) as a replacement for whole-blood transfusions has been impeded by their systemic toxicity. This paper presents data from a series of HBOCs, demonstrating one candidate that meets predetermined safety criteria. This approach may allow the development of an acceptable blood substitute for human use.
Keywords: hemoglobin, polymerized hemoglobin, red blood cell substitute, transfusion medicine, oxygen therapeutic, pharmacokinetics/pharmacodynamics, toxicity
Abstract
Hemoglobin (Hb)-based oxygen carriers (HBOCs) are being developed as resuscitative fluids for use in multiple medical applications and in lieu of blood transfusion. However, cardiovascular, central nervous system, and renal adverse events have largely impeded progress. This has prompted a need to evaluate novel down selection approaches for HBOCs prior to in-depth preclinical and clinical safety testing. In the present study, polymerized bovine Hbs (PolybHbs) were prepared with increasing ratios of glutaraldehyde to bovine Hb (10:1, 20:1, 30:1, and 40:1). The optimal PolybHb candidate selection was based on a priori determined in vivo response to include a long circulating PolybHb with no measurable renal exposure, minimal cardiovascular response, limited oxidation to metHb in vitro, or in circulation and absence of acute end organ toxicity. Guinea pigs were dosed via a 50% blood for PolybHb exchange transfusion. Data suggested that the 30:1 preparation exhibited maximum circulatory exposure (AUC0–∞) with the lowest level of oxidation (plasma metHb formation) and minimal (< 10%) blood pressure elevation. Additionally, the 30:1 preparation was absent renal iron deposition as well as abnormal glomerular/tubular histopathology or serum creatinine elevation. Clearance pathways predominantly followed those consistent with endogenous Hb clearance based pathways. Therefore, data confirmed the ability to select a single PolybHb from a small library of HBOCs based on a priori determined characteristics. Moreover, the approach to down selection described could be applied to enhance the early predictability of human safety for this class of biological therapeutics to optimize for specific indications.
Hemoglobin (Hb)-based oxygen carriers (HBOCs) represent a class of biological therapeutics that have been studied in multiple disease state clinical trials when blood volume replacement and oxygen delivery are required (Silverman and Weiskopf, 2009). Disease states studied include traumatic and surgical blood loss, cardiac bypass, stroke, myocardial infarction, and sickle cell anemia. Additionally, these agents are approved in certain countries for use in lieu of blood transfusion due to either shortage or donor blood safety concerns.
In the United States, several HBOCs have been evaluated clinically under regulatory supervision; however, none have been approved for licensure. The primary regulatory issues with HBOCs stem from unacceptable safety profiles in clinical trials that indicate an increased risk to benefit ratio (Silverman and Weiskopf, 2009). The primary safety signals observed suggest increased severe adverse events associated with the cardiovascular system, central nervous system, and kidneys. The mechanisms of these toxicities are not well defined; however, the primary focus has been on the nitric oxide (NO)–depleting effects of Hb, which can lead to hypertension and vascular dysfunction (Yu et al., 2009). An equally important mechanism of Hb toxicity involves oxidation of heme within the protein, which can lead to free radical generation and finally heme and iron release (Buehler et al., 2007; Butt et al., 2010). The primary effects can be acute and localized tissue injury, particularly in organ systems experiencing extensive exposures.
HBOCs were often developed as single entity molecules for intended use in multiple and diverse indications. Development strategies have focused on chemical and recombinant modification of Hb, however, limited examples exist where in vivo studies were employed in a multicandidate down selection process. In a series of papers focused on recombinant-based heme pocket re-engineering intended to minimize heme iron's interaction with NO, one can find a description of extensive recombinant and biochemical efforts followed by a limited in vivo screening analysis (Doherty et al., 1998; Dou et al., 2002; Olson et al., 2004). As part of these studies, libraries of modified proteins were evaluated and down selected based on hemodynamic response in rats. Particular focus was placed on systemic vasoconstriction, and the choice of engineered Hb was based on the rate of NO dioxygenation determined in vitro and vascular resistance determined in vivo.
The down selection of an appropriate product candidate for preclinical and later clinical trial evaluation may further benefit from initial evaluation of libraries of product candidates to include early assessment of pharmacokinetics (PKs), hemodynamics, oxidation, and toxicological responses in vivo. We have developed and evaluated a library of four molecular sized bovine hemoglobins (bHb) polymerized with glutaraldehyde and collectively termed PolybHb. PolybHbs were synthesized with cross-link densities (i.e., molar ratio of glutaraldehyde to bHb) of 10:1, 20:1, 30:1, and 40:1 according to a previously described methodology reported in the literature (Zhou et al., 2011). Within the context of this study, the PolybHbs function as a group of identically prepared HBOCs with the single variable in their preparation being the glutaraldehyde to bHb polymerization ratio. This allowed us to study the effects of modified Hbs with identical starting material and modifying agent on a priori determined in vivo characteristics. In the present set of experiments, the four PolybHbs were evaluated for PK characteristics (metabolism, distribution, and excretion), safety pharmacology (systemic blood pressure response), in vitro and in vivo oxidation, and potential for tissue-based toxicity (acute renal toxicity). Our criteria for meeting selection included a long circulating modified Hb with minimal breakdown in circulation, no renal excretion, limited hypertensive response, minimal potential for oxidation, and no initial evidence of renal tissue toxicity (renal iron deposition, histological changes, or increases in serum creatinine). We additionally suggest that this type of approach could serve as a novel method to down select HBOCs early in their development using a priori determined in vivo attributes that may be applicable to use specific indications.
MATERIALS AND METHODS
Materials
Glutaraldehyde (70%), NaCl, KCl, NaOH, Na2S2O4, NaCl (USP), KCl (USP), CaCl2-2H2O (USP), NaOH (NF), sodium lactate (USP), N-acetyl-l-cysteine (USP), NaCNBH3, and NaBH4 were purchased from Sigma-Aldrich (Atlanta, GA). One hundred and five hundred kilodalton hollow fiber cartridges were purchased from Spectrum Labs (Rancho Dominguez, CA). KCN, K3Fe(CN)6, and all other chemicals were purchased from Fisher Scientific (Pittsburgh, PA). In preparation for experiments, all glassware and plasticware were immersed in 1 mol/l NaOH solution for more than 6 h to degrade any endotoxin present, followed by thorough rinsing with high performance liquid chromatography (HPLC) grade water (the mean conductivity value being 18.2 × 106 Ω·cm). bHb was prepared from fresh bovine red blood cells (RBCs) stored in 3.8% sodium citrate solution at a final concentration of 90:10 vol/vol (bovine blood:sodium citrate solution). Bovine RBCs were purchased from Quad Five (Ryegate, MO). bHb was purified from lysed bovine RBCs via tangential flow filtration (TFF) as described in the literature (Palmer et al., 2009).The endotoxin level of bHb was measured using a Limulus amebocyte lysate (LAL) test kit (Pyrogent Plus, Lonza, Walkersville, MD). When diluted for polymerization with glutaraldehyde, bHb demonstrated acceptable endotoxin levels (≤ 0.5 EU/ml). These data are also consistent with a previous report from our laboratory (Elmer et al., 2009).
Polymerization of bHb
PolybHbs were synthesized according to a previously described methodology in the literature (Zhou et al., 2011). In brief, four different molecular sized PolybHbs were prepared using increasing molar ratios of glutaraldehyde:bHb (10, 20, 30, and 40:1). To generate fully deoxygenated bHb, 30 g of purified bHb was diluted with phosphate buffer (PB) (20mM, pH 8.0) to yield 1200 ml of bHb solution. The bHb solution was placed inside an airtight bottle and connected to a vacuum manifold. The entire system was kept below 4°C in an ice water bath. The bHb solution was then subjected to several cycles of vacuum and argon (Ar) purging to remove the majority of O2 from solution. After 4 h of vacuum and Ar cycling, Na2S2O4 solution (1.5 mg/ml) was titrated into the bHb solution with a syringe pump (Razel Scientific, St Albans, VT), while the pO2 of the solution was simultaneously measured using a RapidLab 248 (Siemens, Malvern, PA) blood gas analyzer until the partial pressure of O2 (pO2) in the bHb solution attained a value of 0 mm Hg. At this point, an additional 30 ml of 1.5 mg/ml Na2S2O4 solution was added to the deoxygenated bHb solution to maintain the pO2 at 0 mm Hg during and after the polymerization reaction. A 30 ml syringe was used to titrate deoxygenated glutaraldehyde solution pre-equilibrated with Ar into the deoxygenated bHb solution at the following molar ratios of glutaraldehyde to bHb: 10:1, 20:1, 30:1, and the 40:1. Polymerization reaction was kept under continuous stirring at 37°C in the dark with Ar purging for 2 h, and the polymerization reaction was terminated with the addition of 20 ml of 2M NaBH4 in PB buffer (20mM, pH 8.0).
Clarification and Diafiltration of PolybHb Solution
The resulting PolybHb solutions were initially clarified by filtering them through a column packed with autoclaved glass wool in order to remove large particles. The clarified PolybHb solution was then diluted from 1500 to 2000 ml and then subjected to four cycles of diafiltration via TFF against an ice-cold modified lactated Ringer's solution (NaCl 115 mmol/l, KCl 4 mmol/l, CaCl2-2H2O 1.4 mmol/l, NaOH 13 mmol/l, sodium lactate 27 mmol/l, and N-acetyl-l-cysteine 2 g/l). One hundred kilodalton HF cartridges were used for diafiltration of the PolybHb solutions with a glutaraldehyde:bHb molar ratio of 10:1, whereas 500 kDa HF cartridges were employed for PolybHb solutions with glutaraldehyde:bHb molar ratios of 20:1, 30:1, and 40:1. For each diafiltration cycle, the PolybHb solution was concentrated to a volume less than 200 ml. At the end of diafiltration, the endotoxin level of PolybHb was measured using an LAL test kit (Pyrogent Plus). Increased viscosity leads to a falsely high LAL value given that the assay is based on measurement of clot formation. As a result, each solution required dilution to prepolymerization (25 mg/ml) levels. When diluted to prepolymerization concentrations PolybHbs demonstrated acceptable endotoxin levels (≤ 0.5 EU/ml). At the final diafiltration step, the resulting concentrated PolyHb solution was immediately stored at −80°C for future use.
MetHb Level and Protein Concentration of PolybHb Solutions
The metHb level of PolybHb solutions was measured via the cyanomethemoglobin method (Oser, 1965). Total protein concentration was measured using the Bradford (1976) method using the Coomassie Plus protein assay kit (Pierce Biotechnology, Rockford, IL).
Size Exclusion Chromatography Coupled With Multiangle Static Light Scattering Analysis of PolybHb Solutions
The absolute molecular weight distribution of bHb/PolybHb solutions was measured using a size exclusion chromatography (SEC) column (Ultrahydrogel linear column, 10 μm, 7.8 × 300 mm; Waters Corp., Milford, MA) driven by a 1200 HPLC pump (Agilent, Santa Clara, CA), controlled by Eclipse 2 software (Wyatt Technology, Santa Barbara, CA) connected in series to a DAWN Heleos (Wyatt Technology) light scattering photometer and an OptiLab Rex (Wyatt Technology) differential refractive index detector. The mobile phase consisted of 20mM PB (pH 8.0), 100 ppm NaN3, and 0.2M NaCl (Fisher Scientific) in HPLC grade water that was filtered through a 0.2 μm membrane filter. PolyHb solutions were diluted to 1 mg/ml with the mobile phase, and 60 μl of sample was injected onto the column via a 1200 Autosampler (Agilent). All data were collected and analyzed using Astra 5.3 (Wyatt Technology) software.
In Vitro Autoxidation of PolybHb Solutions
All PolybHb samples were 95% ferrous (Fe2+) or oxy-PolybHb immediately prior to autoxidation experiments. Experiments were carried out with 20–25μM heme in sealed cuvettes with room air equilibrated with 50mM Chelex-treated potassium phosphate buffer at 37°C. Absorbance changes in the range of 450–700 nm due to spontaneous oxidation of Hb were recorded in a temperature-controlled photodiode array spectrophotometer (Hewlett Packard 8453, Palo Alto, CA). Similar oxidation assays were also performed in the presence of superoxide dismutase (4.6 U/ml) and catalase (414 U/ml). Autoxidation reactions were followed to near completion (∼24 h), at which time 22μM potassium ferricyanide (K3Fe(CN)6) was added to completely oxidize the remaining ferrous PolybHb. A multicomponent analysis was performed to calculate the ferrous and ferric species based on their individual extinction coefficients. Autoxidation rates were obtained from plots of the loss of ferrous to ferric PolybHb versus time using nonlinear least-squares curve fitting (single-exponential, two parameter decay) techniques in Sigma-Plot (SPSS, Chicago IL).
Animals, Surgical Preparation, and Experimental Design
Male Hartley guinea pigs were purchased from Charles Rivers Laboratories (Wilmington, MA) and acclimated for 1 week upon arrival to the Food and Drug Administration (FDA)/Center for Biologics Evaluation and Research (CBER) animal care facility. All animals were fed normal diets throughout the acclimation period and weighed 350–450 g at the time of study. Animal protocols were approved by the FDA/CBER Institutional Animal Care and Use Committee with all experimental procedures performed in adherence to the National Institutes of Health (NIH) guidelines on the use of experimental animals. Surgical preparation was performed as described previously in the literature (Buehler et al., 2007). Fully conscious and freely moving guinea pigs were randomized to receive a 50% blood for 10:1, 20:1, 30:1, or 40:1 PolybHb (n = 5–6 guinea pigs per group) volume exchange transfusion (ET) based on the equation for determining blood volume in the species (Ancill, 1956) (50% ET (ml) = (0.07 (ml/g) × body weight (g))/2). Plasma from blood in the heparinized withdrawal syringe for each transfused animal was obtained to determine the total PolybHb removed during the ET period (approximately 12 min). Each PolybHb solution was transfused as a 100 mg/ml protein solution. Blood samples (0.2 ml) were obtained from the arterial catheter prior to infusion (baseline) and at the end of ET (time 0), and at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, and 96 h, or until plasma became cleared of PolybHb. Urine was collected using standard rodent metabolic cages under ice at 3 h intervals over the initial 24 h period then for 3 h daily until PolybHb was no longer observed in plasma at designated blood sampling intervals. Plasma was used for evaluation of: (1) total PolybHb, (2) ferrous (Fe2+) PolybHb, and (3) ferric (Fe3+) PolybHb using a photodiode array spectrophotometer (Model 8453 Hewlett Packard) (Evelyn and Malloy, 1938). The circulating PolybHb polymer distribution in plasma and excreted in urine was evaluated as a function of time using a previously described method in the literature (Buehler et al., 2007). Plasma and pooled urine samples (50 μl) were additionally evaluated by SEC run on a BioSep-SEC-S3000 (600 × 7.5 mm) SEC column (Phenomenex, Torrance, CA) attached to a Waters Delta 600 pump and Waters 2499 dual-wavelength detector, controlled by a Waters 600 controller using Empower2 software (Waters Corp.).
In a separate group of animals (n = 4), the right femoral artery was also catheterized with PE10 tubing fused to PE50 tubing to allow for simultaneous administration of PolybHb and monitoring of arterial blood pressure (systolic, diastolic, and mean) and heart rate (HR). The right carotid artery catheter was connected to a Gould P23 XL pressure transducer (Gould Instrument System Inc., Valley View, OH) for recording blood pressure. Arterial blood pressure was recorded continuously at 100 Hz using a MP100A-CE data acquisition system (Biopac Systems, Inc., Santa Barbara, CA). Data were analyzed off-line using AcqKnowledge software (Biopac Systems, Inc.) to determine the mean arterial pressure (MAP) from the following formula (diastolic + 1/3 [systolic−diastolic]) with each being averaged over consecutive minutes of acquired data.
PK Analysis of PolybHb Solutions
The dose (mg) of PolybHb received by each animal at the end of ET was determined as previously described for ET based PK studies (Buehler et al., 2004, 2007). Briefly, the total amount of PolybHb in the plasma from whole blood collected in the ET syringe from the total amount of infused PolybHb according to the following equation:
Where doseinfused is the concentration of PolybHb (mg/ml) infused, Vinfused is the PolybHb infusion volume (ml), [PolybHb]total ET is the concentration of PolybHb (mg/ml) from plasma sampled out of the withdrawal syringe, and VET is the volume (ml) collected in the withdrawal syringe.
PK parameters were determined for total PolybHb, ferrous PolybHb (oxy/deoxy), and ferric PolybHb from plasma at each blood collection time point. Noncompartmental methods employed by WinNonlin version 5.2.1 (Pharsight Corp., Mountain View, CA) were used to calculate PK parameter estimates. The area under the plasma concentration time curve (AUC0–∞) was estimated using the linear trapezoidal rule to the last measurable concentration (AUC0–Clast) where Clast is the last measurable plasma concentration. Extrapolation to infinity (AUCClast–∞) was calculated by dividing Clast by the negative value of the terminal slope (k) of the log-linear plasma concentration-time curve. Thus, AUC0–∞ is equal to the sum of AUC0–Clast and AUCClast–∞. Additional parameters were calculated as follows: the plasma clearance (CL) was calculated as the dose divided by AUC0–∞, the mean residence time (MRT) was calculated as k−1, the volume of distribution (Vdss) was calculated as the product of CL and MRT, and half-life (t1/2) was calculated as ln(2) divided by k.
Western Blot Analyses
Kidney lysates were resolved on 4–12% Bis-Tris gels, transferred to polyvinylidene fluoride membranes, and blocked for 1 h in Tris-buffered saline and Tween-20 (TBS-T) with 5% nonfat dry milk. Membranes were incubated overnight at 4°C with an antibody to HO-1 (1:2000) Calbiochem (San Diego, CA). Rabbit polyclonal anti-HO-1 (cat# SPA-894) washed and then incubated with a relevant horseradish peroxidase–conjugated secondary antibody for 1 h. The signal was developed using an ECL Plus kit and detected on HyperECL film (GE Healthcare, Piscataway, NJ). Membranes were stripped and reprobed for β-actin. Densitometry analysis was performed using ImageJ software (NIH, Bethesda, MD) with normalization to actin.
Tissue Iron Measurements
Kidney, liver, and spleen tissue (100 mg) were homogenized in double deionized H2O at 1:10 wt/vol. Homogenates were mixed with 500 μl of an acid mixture containing 1mM HCl and 10% Trichloroacetic acid (TCA), and incubated at 50°C for 1 h with intermittent shaking (Carter, 1971). The samples were then centrifuged at 15,000 × g for 15 min at room temperature. The clear supernatant (90 μl) was mixed with 30 μl of 20 mg/ml ascorbic acid followed by 20 μl of ferrozine (0.85% wt/vol in hydroxylamine hydrochloride). The samples were allowed to completely develop for 30 min. The absorbance was measured at 560 nm using the Synergy 4 Hybrid Multi-Mode microplate reader (Biotek, Winooski, VT). A standard curve was generated using an iron standard (500 μg/dl).
Histopathology and Immunohistochemistry
Hematoxylin and eosin histopathology.
Kidneys were fixed in 10% formalin, embedded in paraffin, and 5 μm sections were cut and stained by standard hematoxylin-eosin (H/E) procedures. Tissues were scored using a semiquantitative grading system as follows: 0 = minimal, 1 = mild, 2 = moderate, 3 = severe (minimal indicates a detectable process but barely present, mild: small aggregates of inflammatory or necrotic cells, moderate: large aggregated inflammatory or necrotic cells at 200×, and severe: large multifocal aggregates making up greater than 50% of the tissue area).
Nonheme iron histopathology.
Kidneys, livers, and spleens were fixed in 10% formalin, embedded in paraffin, and 5 μm sections were prepared. Sections were dewaxed in xylene and rehydrated in graded ethanol and deionized water. Nonheme ferric iron deposition was detected using Perls method with diaminobenzidine (DAB) intensification (Meguro et al., 2007). Sections were incubated with Perls iron reagent containing 5% potassium ferrocyanide and 2% hydrochloric acid for 45 min at room temperature and rinsed in deionized water. Sections were then incubated with 0.3% hydrogen peroxide and 0.01M sodium azide in methanol for 30 min at room temperature. All sections were then rinsed in 0.1M PB, pH 7.4, incubated with DAB for 5 min, washed in deionized water, and lightly counterstained with Gill's II hematoxylin.
Heme oxygenase-1 immunohistochemistry.
Sections were dewaxed in xylene and rehydrated in graded ethanol and deionized water. Antigen retrieval was performed by heat treatment in a microwave oven for 15 min in 10mM sodium citrate buffer (pH 6.0), cooled for 30 min at room temperature, and rinsed with deionized water and PBS containing 0.05% Tween-20 (PBS-T). Sections were incubated with 3% H2O2 in PBS-T for 15 min at room temperature. Sections were blocked with PBS-T containing 10% normal horse or goat serum and 0.25% Triton-X for 1 h at room temperature followed by incubation with primary antibodies to HO-1 (1:400) overnight at 4°C. The signal was developed with peroxidase-conjugated avidin-biotin complexes (Vectastain Elite ABC, Vector Labs) and diaminobenzidine (SigmaFast DAB, Sigma). Slides were washed with double deionized water, counterstained with hematoxylin, rinsed, and dehydrated in graded ethanol and xylene. Images of the kidney, liver, and spleen were obtained using an Olympus IX71 inverted microscope (×40 and ×400 magnification).
Surface Plasmon Resonance
Haptoglobin binding to each of the PolybHbs was performed by coupling 10 μg of Hp 1-1 in acetate buffer pH 4.0 to a ProteOn sensor chip (Bio-Rad, Hercules, CA) in a ProteOn XPR36 system. Chip conditioning was done with the following injection sequence: 0.5% SDS, 50mM NaOH, and 100mM HCl all at a flow rate of 30 μl/min. Each PolybHb was run at a flow rate of 100 μl/min with a contact time of 60 s and dissociation time of 1800 s. Decreasing concentrations (4, 2, 1, 0.5 and 0.25 μM) of each PolybHb solution in 200 μl of ProteOn running buffer was passed over the chip. Chip regeneration was performed with 1.6M glycine/HCl.
Statistical Analysis
All data are expressed as the mean ± SEM. Statistical comparisons were performed by a one-way ANOVA with an a priori test for planned comparisons followed by a post hoc Bonferroni's test for determination of group differences. In all analyses, a value p < 0.05 was taken as the level of statistical significance.
RESULTS
Polymerized Hemoglobin Molecular Sizes
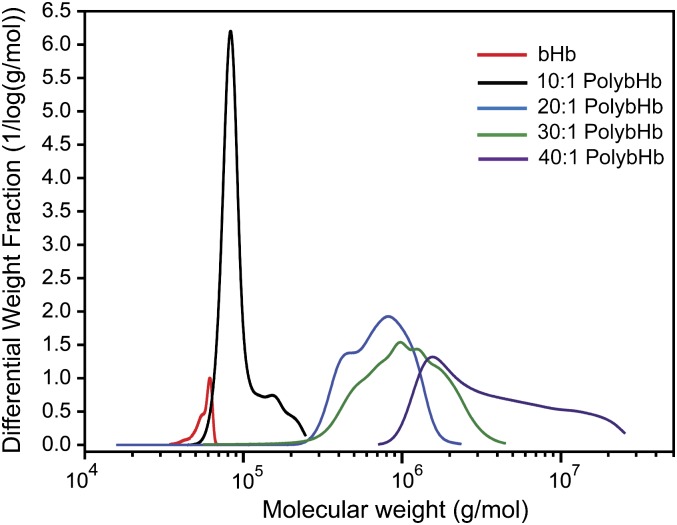
Figure 1 and Table 1 show the SEC coupled with multiangle static light scattering (MASLS) analysis of molecular sizes generated by reacting a range of glutaraldehyde concentrations with bHb. Ratios of polymerization agent to bHb are indicated over a range from 10:1 to 40:1, and molecular size is expressed as kilodalton (kDa).
FIG. 1.
Absolute molecular weight distribution of unmodified bHb and PolybHb solutions. The weight averaged molecular weight of each solution is listed in Table 1.
TABLE 1.
Weight Averaged Molecular Weight (MW) of Polymerized Hemoglobin Solutions
| Solution | MW (kDa) |
| bHb | 65.3 ± 0.6 |
| 10:1 PolybHb | 91 ± 10 |
| 20:1 PolybHb | 749 ± 43 |
| 30:1 PolybHb | 1330.3 ± 159 |
| 40:1 PolybHb | 5494.6 ± 362 |
Note. SDs represent reactions run in triplicate.
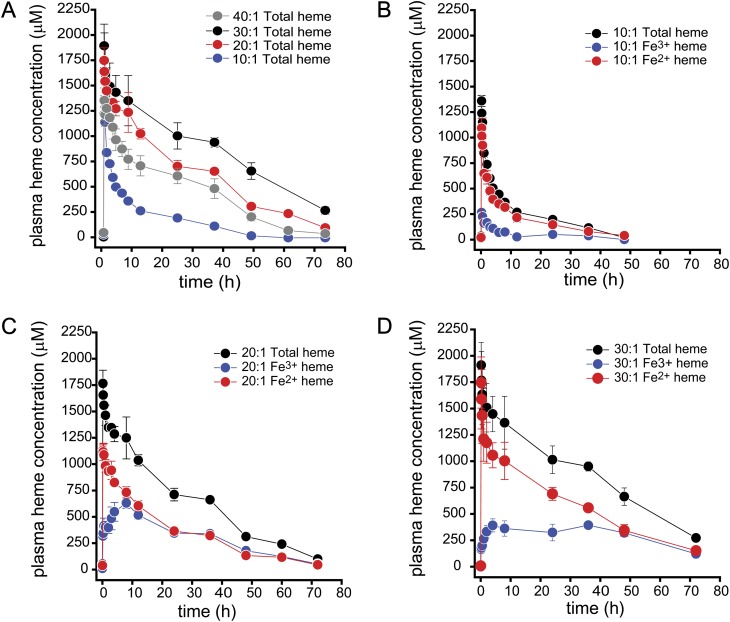
PK Analysis
Table 2 shows the PK parameter estimates for total PolybHb, ferrous (Fe2+) PolybHb, and ferric (Fe3+) PolybHb following 50% PolybHb for blood ETs. All guinea pigs received comparable doses at the end of transfusion based on our calculation described in the Materials and Methods section. Figure 2 shows the data used to define noncompartmental PK parameter estimates plotted as the mean ± SEM plasma concentration versus time. Total PolybHb (ferrous, Fe2+ and ferric, Fe3+) estimates for the terminal half-life (t½β) indicate significant differences between PolybHb 30:1 being nearly twofold greater than PolybHbs 20:1, 10:1, and 40:1. The primary exposure parameters Cmax, AUC0–∞, and total Cl demonstrated significantly lower Cmax values following ET with PolybHb 10:1. This finding is directly related to rapid renal filtration and clearance as evidenced by increased urinary Hb observed during transfusion and over the time course of urine collection. AUC0–∞ was significantly higher following ET with PolybHb 30:1 compared against PolybHbs 20:1 and 10:1. These data suggest a twofold greater circulatory exposure following transfusion with PolybHb 30:1. Total Cl was significantly slower following ET with PolybHb 30:1 consistent with a twofold slower rate compared with PolybHbs 20:1 and 10:1. Taken together, this data suggest a greater circulatory exposure following ET with PolybHb 30:1 compared with either PolybHb 10:1 or 20:1. Interestingly, dosing with the PolybHb 40:1 preparation, the largest molecular sized PolybHb, demonstrated PK parameters that were comparable to the PolybHb 20:1 preparation and therefore suggest a nonlinear PK dependence on molecular size. These data suggest that molecular size exceeding 2000 kDa may promote faster clearance and reduced exposure times. Therefore, we did not continue to extensively pursue this preparation in the remaining experiments.
TABLE 2.
PK Parameter Estimates of PolybHbs (10:1, 20:1, 30:1, and 40:1) Following a 50% Exchange Transfusion
| Estimate | Total Hb | Hb(Fe2+) | Hb(Fe3+) |
| PolybHb (10:1) | |||
| Dose (mg) | 1282 ± 96 | 1252 ± 89 | 31 ± 11 |
| Cmax (mg/ml) | 22.0 ± 1.8 | 17.8 ± 1.4 | 6.9 ± 2.6 at Tmax = 1.0 h |
| AUC0–∞ (mg h/ml) | 206 ± 5.9 | 159 ± 9.6 | 46 ± 4.9 ( 22 ± 2.8% total AUC0–∞) |
| Cltotal (ml/h) | 6.3 ± 0.6 | 8.0 ± 1.0 | |
| Vd (ml) | 108 ± 14 | 128 ± 15 | |
| t1/2 β (h) | 10.9 ± 0.3 | 10.6 ± 0.6 | |
| PolybHb (20:1) | |||
| Dose (mg) | 1375 ± 17 | 1341 ± 21 | 47 ± 5.0 |
| Cmax (mg/ml) | 28.9 ± 1.7 | 18.9 ± 1.3 | 11.4 ± 5.0 at Tmax = 5.5 h |
| AUC0–∞ (mg h/ml) | 682 ± 86 | 271 ± 40 | 358 ± 20 (46 ± 1.3% total AUC0–∞) |
| Cltotal (ml/h) | 2.0 ± 0.10 | 3.7 ± 0.01 | |
| Vd (ml) | 54 ± 3.2 | 89 ± 1.7 | |
| t1/2 β (h) | 19 ± 2.0 | 13.5 ± 0.30 | |
| PolybHb (30:1) | |||
| Dose (mg) | 1076 ± 80 | 1036 ± 82 | 40 ± 5.0 |
| Cmax (mg/ml) | 31.9 ± 4.0 | 29.1 ± 4.1** | 6.7 ± 0.96 at Tmax = 4.5 h |
| AUC0–∞ (mg h/ml) | 1126 ± 74**,* | 750 ± 62**,* | 375 ± 56 (33 ± 3.9% total AUC0–∞) |
| Cltotal (ml/h) | 0.90 ± 0.2**,* | 1.4 ± 0.2**,* | |
| Vd (ml) | 39 ± 6.8**,* | 50 ± 11**,* | |
| t1/2 β (h) | 31.1 ± 1.7**,* | 23.5 ± 1.8**,* | |
| PolybHb (40:1) | |||
| Dose (mg) | 1259 ± 20 | 1244 ± 37 | 43 ± 2.8 |
| Cmax (mg/ml) | 22.2 ± 0.10*** | 19 ± 0.41*** | 6.8 ± 0.87 at Tmax = 7.3 h |
| AUC0–∞ (mg h/ml) | 526.5 ± 42*** | 276.9 ± 22*** | 249 ± 26 (47 ± 2.1% total AUC0–∞) |
| Cltotal (ml/h) | 2.5 ± 0.17**,*** | 4.5 ± 0.28**,*** | |
| Vd (ml) | 59 ± 7.9**,*** | 98 ± 11**,*** | |
| t1/2 β (h) | 15.3 ± 1.2**,*** | 13.1 ± 0.77**,*** |
* = Significant difference between 20:1 PolybHb parameter estimates (p < 0.05).
** = Significant difference between 10:1 PolybHb parameter estimates (p < 0.05).
*** = Significant difference between 30:1 PolybHb parameter estimates (p < 0.05).
FIG. 2.
Plasma concentration versus time data following 50% blood volume for PolybHb transfusion (A–D). Data are presented for total, ferrous, and ferric heme as the mean ± SEM. The PK parameter estimates for these plots are listed in Table 2.
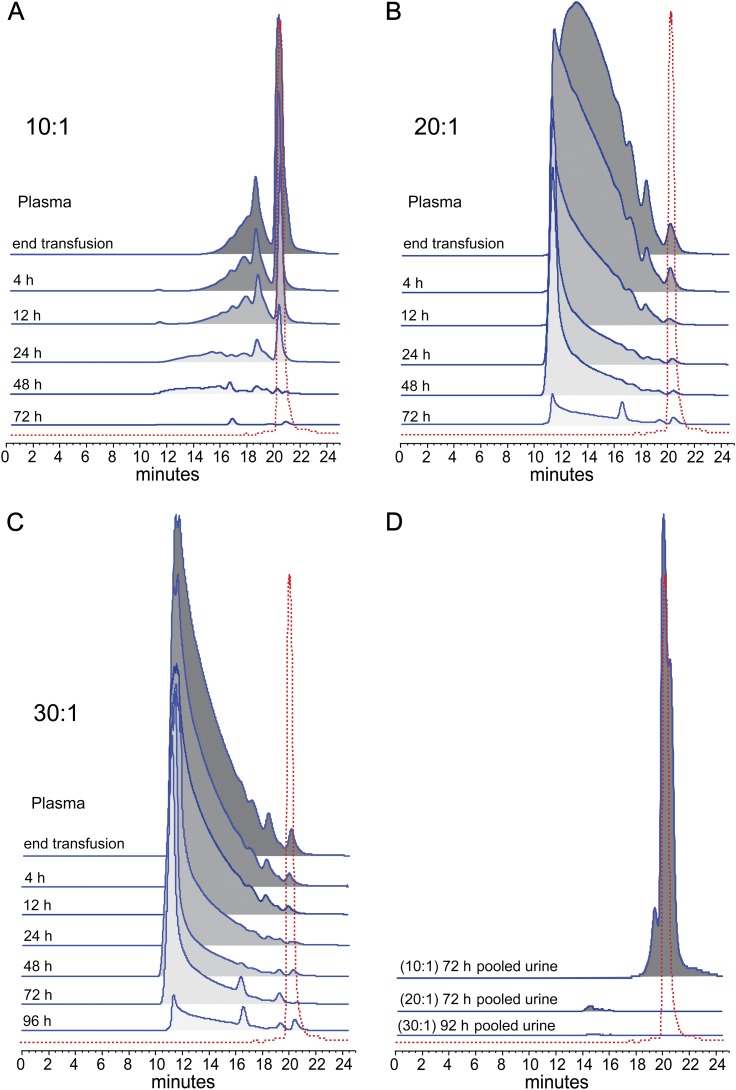
PolybHb Component Plasma Distribution
SEC chromatographs of plasma from guinea pigs are shown in Figures 3A–C. The time course of blood collection shows a rapid loss of Hb tetramer from the plasma of 10:1 PolybHb-transfused animals (Fig. 3A). The 72-h pooled urine collection suggest that a large quantity of this component is excreted renally (Fig. 3D). The multitetrameric PolybHb components within the 20:1 and 30:1 PolybHb preparations appear to have remained in circulation for an extended period of time (Figs. 3B–C). Gradual metabolism/clearance clearly occurred over 72–96 h with the largest fractional components circulating at significant plasma levels for as long as 48 and 72 h for the 20:1 and 30:1 PolybHb preparations, respectively. From the SEC data shown in Figure 3D, pooled urine samples suggest minimal excretion of globin from either the 20:1 or 30:1 PolybHb preparations. Taken together with the pharamacokinetic data, this evaluation suggests that optimal circulatory retention of PolybHb in this study occurs in the 20–30:1 polymerization range.
FIG. 3.
Size exclusion chromatographic distribution of polymeric components for each PolybHb preparation in plasma following blood collections (A–C). (D) The 24 h urinary appearance of Hb following transfusion with each PolybHb preparation.
Safety Pharmacology
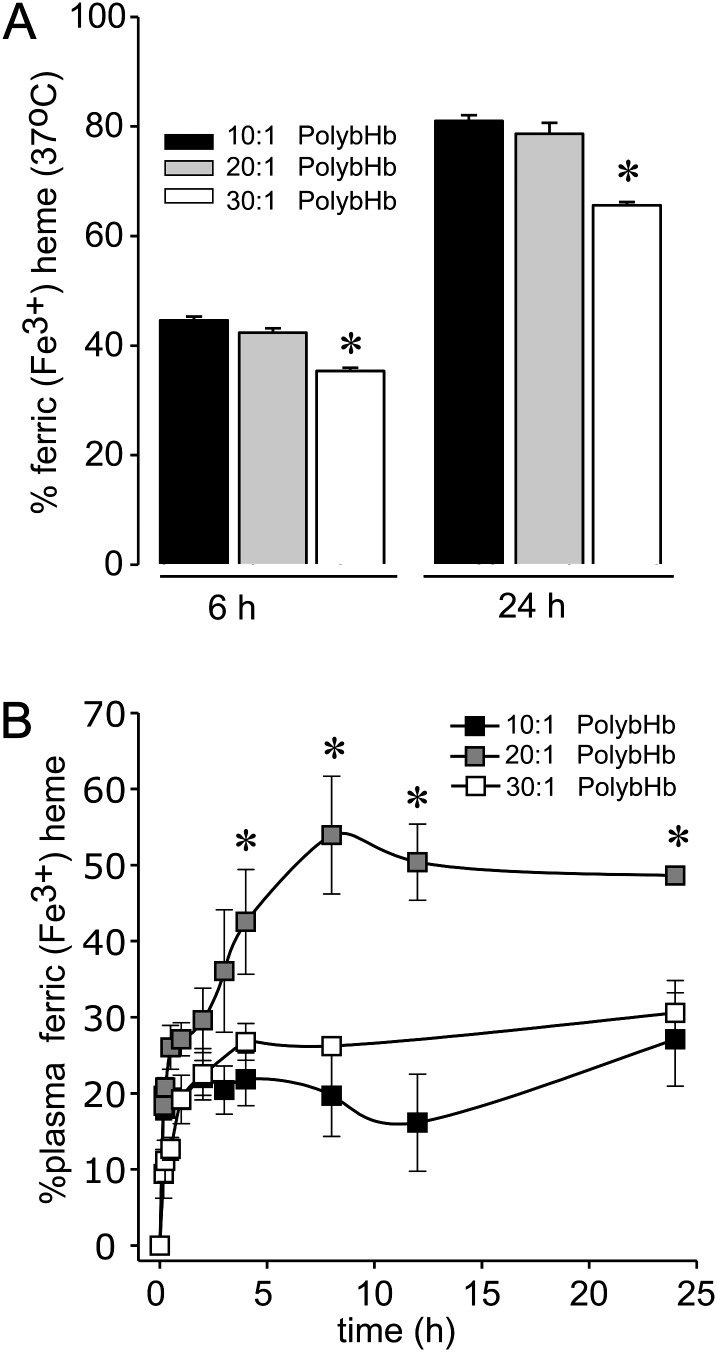
In vitro and in vivo oxidation.
The rates of in vitro autoxidation (kox, per hour) are shown in Table 3. In Figure 4A, the percent of sample autoxidized after 6 and 24 h at 37°C is shown. The data demonstrate a significantly lower in vitro autoxidation at both 6 and 24 h of incubation for the 30:1 PolybHb group compared with the 10:1 and 20:1 PolybHb groups. This in vitro data were consistent with in vivo data evaluating heme iron oxidation and suggesting maximum plasma ferric (Fe3+) PolybHb occurring between 8 and 12 h posttransfusion (Fig. 4B). Levels reached as high 52 ± 3.0% of the total circulating plasma PolybHb concentration in the 20:1 PolybHb group. Maximal in vivo oxidized plasma PolybHb concentrations occurred at approximately 1 h in the 10:1 PolybHb group with plasma PolybHb levels reaching 20 ± 2%. In the 30:1 PolybHb group, maximal plasma oxidized PolybHb levels occurred at approximately 4 h with levels reaching 27 ± 2%. Total exposures, as determined by AUC0–∞ (mg h/ml), to oxidized PolybHb in the 10, 20, and 30:1 PolybHb groups are shown in Table 2 and suggest the highest total exposure to ferric PolybHb occurred following transfusion with the 10:1 and 20:1 PolybHb preparations.
TABLE 3.
Fast (α) and Slow (β) Phase Autoxidation Rate Constants at pH 7.4 and 37°C
| Hb | kox (per hour) α | kox (per hour) β |
| Bovine | 0.0088 ± 0.00003 | 0.00063 ± 0.00009 |
| PolybHb (10:1) | 0.0071 ± 0.00003 | 0.00098 ± 0.00003 |
| PolybHb (20:1) | 0.0080 ± 0.00006 | 0.00090 ± 0.00006 |
| PolybHb (30:1) | 0.0089 ± 0.00006 | 0.0006 ± 0.00001 |
FIG. 4.
(A) Autoxidation of PolybHb in vitro at 37°C, * indicates a significant difference (p < 0.05) versus 10:1 and 20:1. (B) In vivo oxidation over the initial 24 h of blood sampling posttransfusion, * indicates a significant difference (p < 0.05) versus 10:1 and 30:1.
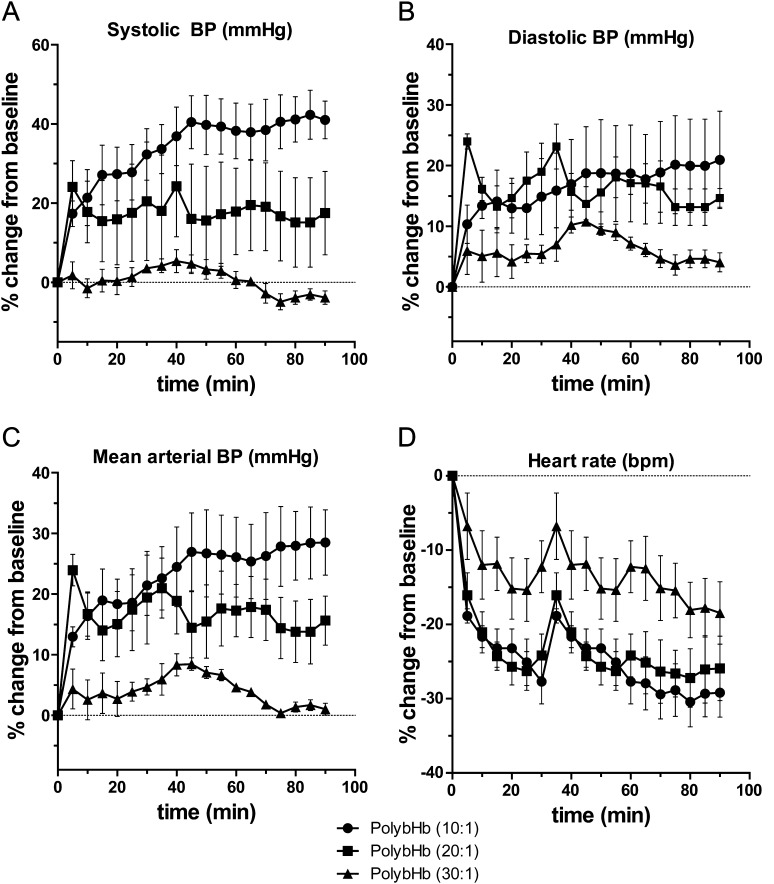
Blood pressure response to polybHbs.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), MAP, and HR are shown in Figures 5A–D. The most dramatic rise in blood pressure was observed in animals transfused with 10:1 PolybHb. The maximal SBP, DBP, and MAP observed in this group were 41 ± 5.7% (AUC, 3014 mm Hg), 21 ± 8.0% (AUC, 1467 mm Hg), and 28 ± 5% (AUC, 2050 mm Hg) above basal values. HR declined to a minimum of −30 ± 3.0% consistent with peripheral arterial vasoconstriction or a direct myocardial depressant effect. Conversely, animals transfused with 30:1 PolybHb demonstrated maximal SBP, DBP, and MAP of 5.4 ± 2.9% (AUC, 227 mm Hg), 11 ± 0.7% (AUC, 553 mm Hg), and 8.4 ± 1.0% (AUC, 361 mm Hg) above basal values. The HR declined to a minimum of −18 ± 4.2% again consistent with some degree of peripheral arterial vasoconstriction or a direct myocardial depressant effect. Animals transfused with 20:1 PolybHb demonstrated a moderate effect on blood pressure parameters and HR, consistent with a molecular size between that of 10:1 and 30:1 PolybHb. For all parameters measured (SBP, DBP, MAP, and HR) and at each time point (every 5 min over 90 min), animals in the 10:1 PolybHb group demonstrated significant changes compared with baseline (p < 0.05) and significant changes compared with the 30:1 PolybHb group (p < 0.05). The most substantial rise in blood pressure in all PolybHb transfusion groups was reflected in the DBP and hence a rise in MAP reflective of this response. Following transfusion with 30:1 PolybHb, the SBP, DBP, and MAP response was generally mild and transient returning to basal values within 90 min postexposure. Conversely, the 10:1 and 20:1 transfused animals did not return SBP or DBP to basal values over the time course of evaluation.
FIG. 5.
(A) SBP percentage change from baseline, (B) DBP percentage change from baseline, (C) MAP percentage change from baseline, and (D) HR percentage change from baseline. For all data points, there was a significant difference (p < 0.05) between PolybHb 10:1 and PolybHb 30:1.
PolybHb Molecular Size and the PK-Pharmacodynamic Relationship
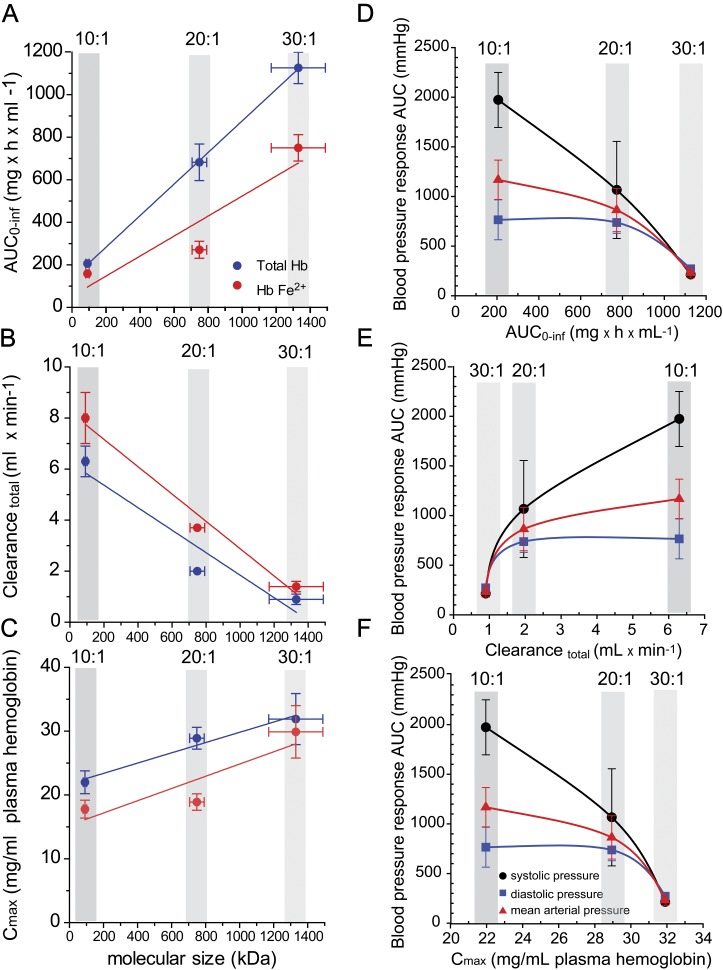
The primary physicochemical property of PolybHb preparations evaluated in this study was molecular size. Previous work has suggested that molecular size is one of several physicochemical determinants that influence PK (Matheson et al., 2000) and physiological responses to HBOCs (Cabrales et al., 2009; Faivre-Fiorina et al., 1999). Three primary exposure parameters (AUC0–∞, CLtotal, and Cmax) were all highly correlated to the molecular size of PolybHb. The regression analysis of total and ferrous PolybHbs for each of the three PK parameters is shown in Figures 6A–C. AUC0–∞ values increased linearly with increasing molecular sized PolybHbs, and these parameters were highly correlated, r2 = 0.9998 (Fig. 6A). Total clearance was inversely related to molecular size (Fig. 6B) with a correlation coefficient of r2 = 0.9161, suggesting longer circulatory retention of physically larger PolybHbs within the range of sizes studied here. Doses of PolybHbs were not different between groups, as shown in Table 2, however, Cmax values, which occur immediately following transfusion increased with larger size PolybHbs, r2 = 0.9651 (Fig. 6C). These data suggest that the initial distribution of PolybHb to nonvascular compartments, for example, the kidneys, lymphatics, and subvascular spaces may occur more readily with smaller size PolybHbs. This is supported by a molecular size–dependent effect on renal excretion of PolybHbs and subsequently renal tubular iron deposition (see Fig. 7). Moreover, the present data analysis suggests that extending circulatory retention can be achieved by optimizing PolybHb molecular size for our library of PolybHbs.
FIG. 6.
(A–C) PolybHb molecular size-PK relationships for exposure parameters: AUC0–∞, total clearance, and maximum plasma concentration (Cmax). (D–F) PK-pharmacodynamic relationships with AUC of blood pressure from 0 to 4 h used as the primary pharmacodynamic endpoint.
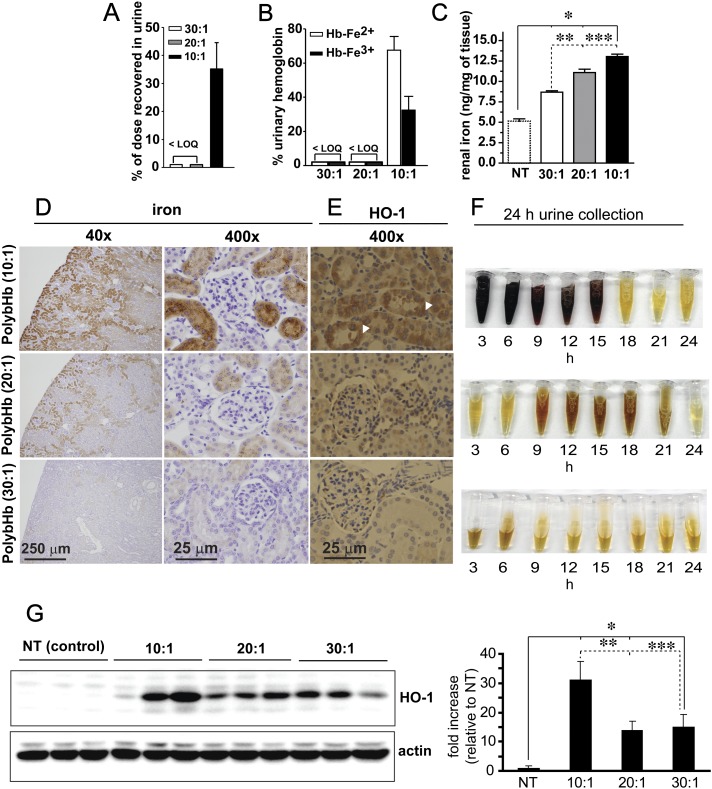
FIG. 7.
(A) Percentage of dose excreted in urine, < LOQ (less than the assay limits of quantitation). (B) Percentage of excreted Hb in the ferrous (Hb-Fe2+) and ferric (Hb-Fe3+) oxidation states, < LOQ (less than the assay limits of quantitation). (C) nanograms (ng) of kidney tissue iron per mg of tissue, *significant difference (p < 0.05) compared with nontreated animals, **significant difference (p < 0.05) between 30:1 and 20:1 PolybHb and between 30:1 and 10:1 PolybHb. (D) Perl's iron staining with DAB intensification of kidney and (E) heme oxygenase (HO-1) immunohistochemistry of renal cortical tubular cells, white arrow heads indicate HO-1 positive regions. Scale bars equal to 1 cm. (F) Urine collected over eight 3 h intervals for 24 h. (G) Western blot analysis of HO-1 expression in renal cortical tissue (left) and densitometry of band intensity (right). *Significant difference (p < 0.05) compared with nontreated animals, **significant difference (p < 0.05) between 10:1 and 20:1 PolybHb, and ***between 10:1 and 30:1 PolybHb.
The PK-blood pressure relationship between AUC0–∞, CLtotal, and Cmax of each PolybHb preparation and their respective posttransfusion blood pressure responses are shown in Figures 6D–F. SBP, DBP, and MAP are expressed as AUC0–4 h such that maximal blood pressures returned to basal values. From these data, it can be suggested that PolybHb exposure parameters are most closely related to linear elevations in SBP as observed in curve fitting of the data. PK-pharmacodynamic relationships demonstrated a minimum blood pressure effect following transfusion with the PolybHb preparation exhibiting the highest exposure (i.e., greatest AUC0–∞ and Cmax and least CLtotal). Taken together, these data suggest that increasing molecular size beyond 1300 kDa would not meaningfully influence the SBP, DBP, or MAP any further.
Renal Exposure to PolybHb Preparations
As shown in Figure 7A, 10:1 PolybHb total dose excreted in the urine was 35 ± 9.4% (704.9 ± 158μM heme), and of this concentration, 67 ± 8.0% and 32 ± 8.0% were excreted as ferrous and ferric (oxidized) Hb, respectively (Fig. 7B). No Hb was detected in the urines of 20:1 and 30:1 PolybHb-transfused animals (Fig. 7A).
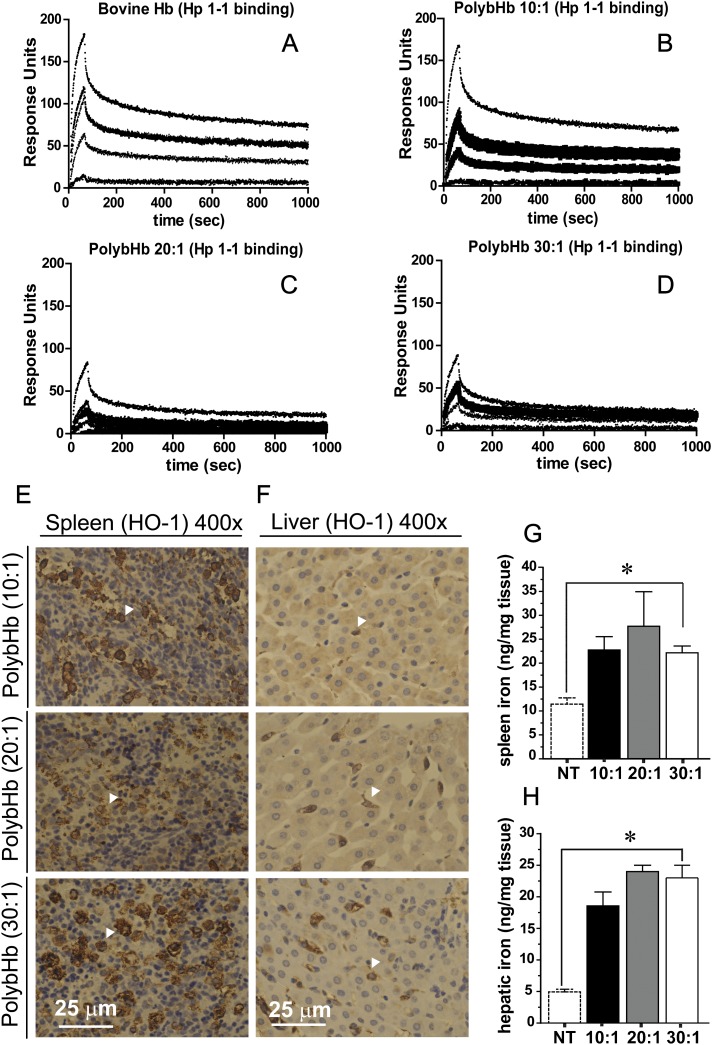
Renal tissue iron was quantified by the ferrozine method in lieu of morphometric analysis. Basal tissue iron in the kidneys was 5.1 ± 0.58 ng/mg and this increased significantly at 24 h posttransfusion with 10:1 PolybHb (13 ± 0.6 ng/mg), 20:1 PolybHb (11 ± 0.8 ng/mg), and 30:1 PolybHb (9 ± 0.5 ng/mg) as shown in Figure 7C. Additionally, kidney tissue was stained using the Perl's iron method with DAB intensification. This data show a clear PolybHb size effect on brown granular staining in the renal cortex (Fig. 7D). This is supported by increased heme oxygenase immunoreactivity (white arrow heads) in similar cortical regions where iron deposition was observed to be greatest (Fig. 7E). This was also confirmed by an additional Western blot analysis of tissue for heme oxygenase as shown in Figure 7F. Densitometry (Fig. 7G, right panel) indicates a dramatic increase in the 10:1 PolybHb-transfused animal group compared with the control, 20:1 PolybHb-, and 30:1 PolybHb-transfused animal groups. Urine collections obtained over 24 h demonstrate hemoglobinuria in the 10:1 PolybHb-transfused animal group, potential latent excretion of heme within the 9–21 h collection range in the 20:1 PolybHb-transfused animal group. Animals transfused with 30:1 PolybHb demonstrated no effects consistent with renal filtration of heme-globin, heme, or free iron based on the quantitative and qualitative analyses.
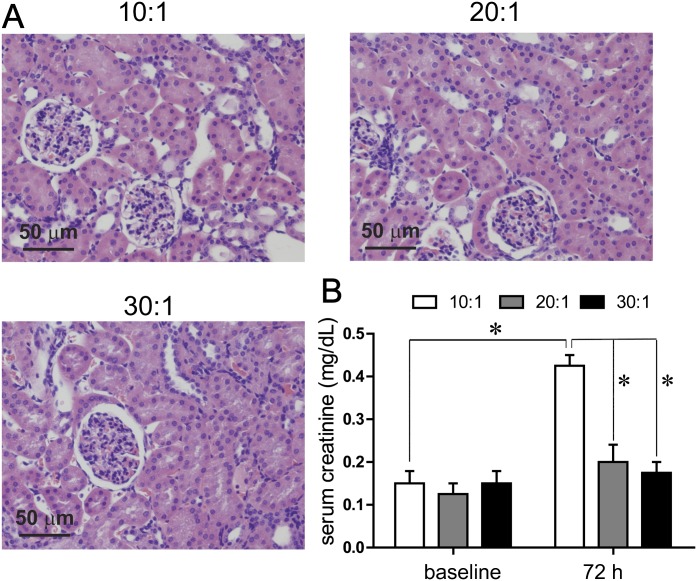
Renal Tissue Injury
The ultimate effects of renal exposure to Hb, heme, or iron can result in acute renal toxicity (Zager and Gamelin, 1989). Data in Figure 8A show hematoxylin and eosin staining for each of the PolybHb preparations. In the 10:1 PolybHb group, the proximal and distal renal cortical tubules appeared detached in localized regions, and tissue was scored as moderate injury (2 on a scale of 0–3 in four animals). This observation has been reported to be associated with cytoskeletal disruption and cell to cell adhesion and regional necrosis (Racusen, 1994). These observations were not discernible in the 20:1 and 30:1 PolybHb groups. The effects of PolybHbs on the primary marker of renal injury, serum creatinine, are shown at 72 h posttransfusion (Fig. 8B). Mean basal values for serum creatinine were 0.12 ± 0.04 mg/dl. Animals transfused with the 10:1 PolybHb preparation demonstrated a mild, but significant serum creatinine level increase to 0.43 ± 0.04 mg/dl, indicative of acute renal dysfunction when compared with baseline as well as 20:1 and 30:1 PolybHb-transfused animals. The serum creatinine levels of the animals transfused with 20:1 and 30:1 PolybHbs demonstrated 72 h serum creatinine levels of 0.20 ± 0.03 mg/dl and 0.17 ± 0.02 mg/dl and were not significantly different than basal values. These experimental observations are consistent with histopathological findings.
FIG. 8.
(A) renal cortical injury as a result of transfusion with PolybHb 10:1, 20:1, and 30:1. Scale bars equal to 1 cm. (B) serum creatinine values at baseline and 72 h posttransfusion. *Indicates significant differences (p < 0.05) comparing time points and groups.
Alternate Routes of PolybHb Clearance via the Spleen and Liver
Qualitative surface plasmon resonance data showing the extent of Hp 1-1 binding to bovine Hb, and each of the PolybHbs indicates that the 10:1 PolybHb binds to human Hp 1-1 similarly to unmodified bovine Hb (Figs. 9A and B). The extent of Hp 1-1 binding to 20:1 and 30:1 PolybHb is qualitatively less than the 10:1 PolybHb preparation. Heme oxygenase immunoreactivity in the spleen and liver are shown in tissue preparations of PolybHb-transfused animals (Figs. 9E and F). White arrows indicate regions of macrophage accumulation, particularly in the liver where stained brown, heme oxygenase positive cells are localized to intrahepatocellular regions. Total iron in the spleen and liver were quantified via the ferrozine assay and are shown in Figures 9G and H, respectively. Basal iron in the spleen was 11 ± 1.3 ng/mg and increased to 23 ± 3.0 ng/mg following 10:1 PolybHb transfusion, 27 ± 7.2 ng/mg following 20:1 PolybHb transfusion, and 22 ± 1.4 ng/mg following 30:1 PolybHb transfusion. Similarly, clearance via the hepatic route was equally relevant in PolybHb clearance with basal iron equal to 5.0 ± 0.42 ng/mg and increasing to 19 ± 2.2 ng/mg following 10:1 PolybHb transfusion, 24 ± 1.5 ng/mg following 20:1 PolybHb transfusion, and 22 ± 1.4 ng/mg following 30:1 PolybHb transfusion. These data suggest that both the spleen and liver function as significant clearance organs for all PolybHbs evaluated. Interestingly, no direct toxicity to these organs systems was observed suggesting an inherent capability for both spleen and liver to effectively clear chemically modified Hb with minimal or no tissue injury. This would suggest that optimizing clearance through these organs may be inherently safer and would limit renal exposure to globin, heme, and iron.
FIG. 9.
(A–D) Surface plasmon resonance binding of bHb, 10:1 PolybHb, 20:1 PolybHb, and 30:1 PolybHb. (E–F) Heme oxygenase-1 (HO-1) immunohistochemistry of spleen and liver. Scale bars equal to 1 cm. (G–H) Spleen and liver iron, *indicates significant differences (p < 0.05) between all PolybHb groups and nontreated (NT) animals.
DISCUSSION
To date, limited approaches have been studied to down select HBOC candidates from libraries of chemically modified Hbs intended for therapeutic purposes. The primary endpoint(s) of the most extensive studies reported to date have focused on evaluation of NO binding to modified Hb (Doherty et al., 1998). The potential results of this interaction are increased systemic vascular resistance, depressed cardiac output, and therefore an increase in systemic hypertension, which are the most well known safety signals associated with this class of therapeutics (Yu et al., 2009). As a result, the primary focus of candidate selection studies has been on NO reactivity with the Hb component of HBOCs and the hemodynamic effects. A series of studies was conducted with recombinant Hb or myoglobin analogs site specifically modified within the heme pocket to slow the rate of NO dioxygenation. Selection was based biochemically on the slowest NO reactivity with recombinant Hb (i.e., to limit NO consumption) and correlation with in vivo hemodynamic responses (Doherty et al., 1998; Dou et al., 2002; Olson et al., 2004). Although this approach down selected for optimal hemodynamic characteristics of a candidate Hb for use as an HBOC, our present work is designed to expand on these concepts and evaluate a feasible in vivo down selection process using several criteria critical to HBOC safety. The present work was aimed at expanding existing down selection techniques for small libraries of HBOCs in order to optimize (1) PKs, (2) in vitro/in vivo oxidation (defined by Hb iron transition from Fe2+ → Fe3+, metHb), (3) systemic blood pressure, and also (4) evaluate primary routes of clearance, and (5) determine the potential for acute renal toxicity.
PK parameter estimates of HBOCs determined from animal studies could effectively define the desired exposure for specific indications in humans, given that HBOC volume of distribution is largely limited to the central compartment and that clearance occurs via two primary mechanisms (renal filtration and reticulo-endothelial system). HBOCs designed to replace the use of blood should theoretically demonstrate long exposure times to mimic the duration of RBCs circulation. In the present study, data showed that the 30:1 PolybHb preparation demonstrated the most extensive circulating t1/2 and exposure related PK estimates, with an increased Cmax, AUC0–∞, and slower Cl compared with the other PolybHbs tested.
Although defining the optimal PK criteria is critical to intended clinical indication, oxidation of the HBOC in circulation, potential HBOC-mediated effects on blood pressure, routes of HBOC clearance, and HBOC-induced tissue injury are critical areas of investigation. The present study was performed in a guinea pig model of ET to approximate the plasma reductive capacity of humans. Guinea pigs, like humans, represent a species incapable of endogenous production of ascorbic acid due to an evolutionary loss of the final enzyme in its biosynthesis (Chatterjee, 1973; Nandi et al., 1997). Long circulating HBOCs are maintained in their nonoxidized form by the presence of plasma reducing agents such as ascorbic acid, which are capable of maintaining HBOC functional oxygen carrying capability and limiting pro-oxidant-induced toxicity (Buehler et al., 2007; Butt et al., 2010; Butt et al., 2011). Our in vitro oxidation studies and PK analyses of the transition states of heme iron within the circulating HBOC polymers allowed us to determine the propensity for oxidation and total circulatory exposure to oxidized HBOC. Based on our data, PolybHbs could be placed in order of most to least prone to oxidation as follows; 20:1 > 40:1 > 10:1 > 30:1. Interestingly, the experimental observations were independent of HBOC molecular size, PK characteristics, or biochemical properties. These data may further stress the importance of studies that define propensity for HBOC oxidation in human predictive species.
Evaluation of systemic blood pressure in response to PolybHb preparations suggested that molecular size was a critical attribute to minimizing the HBOC-related elevation of SBP, DBP, and MAP. The present study demonstrates that PolybHbs could be placed in order of propensity to induce a hypertensive response as follows; 10:1 > 20:1 > 40:1 = 30:1. This safety pharmacology parameter was clearly correlated to molecular size and with the exception of the 40:1 preparation correlated positively with Cmax and AUC0–∞ and inversely correlated with Cl.
Glomerular filtration of Hb heterodimers is a primary route of clearance for both Hb and certain HBOC preparations (Caccia et al., 2009; Portoro et al., 2008). The extent of posttransfusion circulatory breakdown or pretransfusion unstable tetrameric Hb in chemically modified Hb preparations ultimately determines the extent of renal excretion (Caccia et al., 2009). The result of Hb renal excretion is observed as hemoglobinuria, which results in heme oxygenase activation, iron deposition, and oxidation of renal tissue protein and DNA (Boretti et al., 2009; Butt et al., 2010). More extensive damage to kidney tissue can result from renal tubular or glomerular injury with elevation in serum creatinine. These effects are associated with acute and potentially chronic renal failure following Hb and its breakdown products deposition within the kidneys (Zager and Gamelin, 1989). Data from the present study suggest that all PolybHbs were generally stable in circulation with the exception of the 10:1 preparation, which demonstrated extensive posttransfusion hemoglobinuria. This led to increased renal HO-1 expression suggesting high level Hb deposition in renal parenchyma and extensive iron deposition. The acute Hb exposures to kidneys lead to changes in renal proximal and distal tubule morphology and an elevation in circulating creatinine levels. These data suggest that the larger molecular size PolybHbs tested were physically more stable in circulation and directed excretion away from renal filtration.
Chemically modified Hb solutions have been shown to interact with endogenous clearance pathways associated with the reticulo-endothelial system (Buehler et al., 2008). Interaction with the Hb scavenger protein haptoglobin (Hp) is the initial event occurring in circulation to facilitate clearance of chemically modified Hb (Buehler et al., 2008). Uptake by peripheral and resident tissue macrophages functions as a primary uptake process for Hb-Hp complexes and alternatively as a secondary route of clearance for Hb and chemically modified Hb independent of Hp has been shown (Schaer et al., 2006). In the present study, the 10:1 PolybHb bound to Hp more effectively than the other PolybHbs tested. Iron deposition was highest in the spleen and liver suggesting that the 10:1 PolybHb preparation had one molecular component excreted via the kidneys and second cleared via spleen and liver, whereas the larger molecular sized PolybHbs were excreted exclusively by spleen and liver.
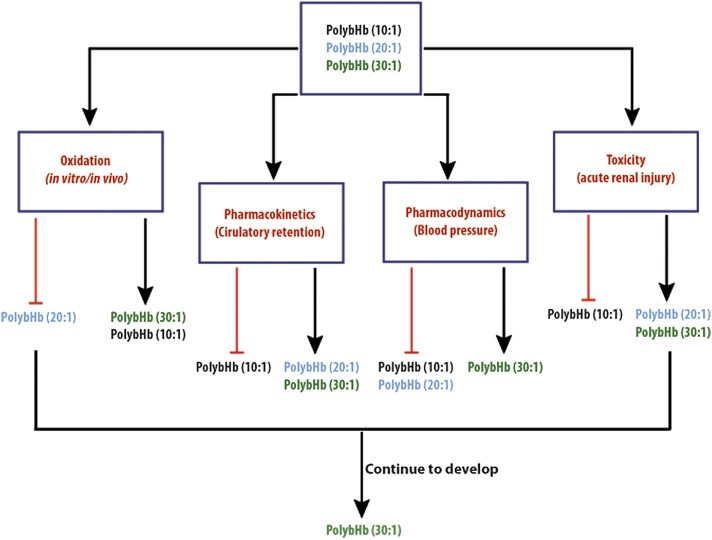
In conclusion, relevant experimental observations suggested that oxidation was a molecular size–independent event, whereas circulatory retention was molecular size dependent within a range. Most notable was that mean molecular sizes below 100 kDa promoted renal excretion, whereas very large molecular sizes (> 5000 kDa) also promoted more rapid clearance, albeit via alternate routes, but likely involving reticulo-endotheial system activation. Blood pressure elevation was found to be highly dependent on molecular size and renal toxicity was limited to the lowest molecular size preparation. Our data suggest that the 30:1 PolybHb preparation would be most optimal to pursue as a long acting HBOC. Figure 10 illustrates the rationale for selection of the 30:1 preparation for further proof of concept and good laboratory practice toxicology evaluation. Moreover, the approach illustrated in Figure 10 to HBOC down selection represents a novel method to optimize for intended indication exposure requirements and limit the potential for toxicity in later preclinical and clinical development.
FIG. 10.
Schematic depicting down selection process and hypothetical candidate for further development from this library of PolybHbs.
FUNDING
This work was supported by an FDA Critical Path grant to PWB and National Institutes of Health grants R01HL078840 and R01DK070862 to AFP. We would like to acknowledge the efforts of Dr Felice D'Agnillo in the optimization of iron and HO-1 histochemistry employed in the present work.
References
- Ancill RJ. The blood volume of the normal guinea-pig. J. Physiol. 1956;132:469–475. doi: 10.1113/jphysiol.1956.sp005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J. Clin. Investig. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buehler PW, D'Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J. Pharmacol. Exp. Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- Buehler PW, Haney CR, Gulati A, Ma L, Hsia CJ. Polynitroxyl hemoglobin: A pharmacokinetic study of covalently bound nitroxides to hemoglobin platforms. Free Radic. Biol. Med. 2004;37:124–135. doi: 10.1016/j.freeradbiomed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Buehler PW, Vallelian F, Mikolajczyk MG, Schoedon G, Schweizer T, Alayash AI, Schaer DJ. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant scavenger pathways. Antioxid. Redox Signal. 2008;10:1449–1462. doi: 10.1089/ars.2008.2028. [DOI] [PubMed] [Google Scholar]
- Butt OI, Buehler PW, D'Agnillo F. Blood-brain barrier disruption and oxidative stress in guinea pig after systemic exposure to modified cell-free hemoglobin. Am. J. Pathol. 2011;178:1316–1328. doi: 10.1016/j.ajpath.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt OI, Buehler PW, D'Agnillo F. Differential induction of renal heme oxygenase and ferritin in ascorbate and nonascorbate producing species transfused with modified cell-free hemoglobin. Antioxid. Redox Signal. 2010;12:199–208. doi: 10.1089/ars.2009.2798. [DOI] [PubMed] [Google Scholar]
- Cabrales P, Sun G, Zhou Y, Harris DR, Tsai AG, Intaglietta M, Palmer AF. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J. Appl. Physiol. 2009;107:1548–1558. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia D, Ronda L, Frassi R, Perrella M, Del Favero E, Bruno S, Pioselli B, Abbruzzetti S, Viappiani C, Mozzarelli A. PEGylation promotes hemoglobin tetramer dissociation. Bioconjug. Chem. 2009;20:1356–1366. doi: 10.1021/bc900130f. [DOI] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal. Biochem. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee IB. Evolution and biosynthesis of ascorbic acid. Science. 1973;182:1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys. Chem. 2002;98:127–148. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Elmer J, Harris DR, Sun G, Palmer AF. Purification of hemoglobin by tangential flow filtration with diafiltration. Biotechnol. Prog. 2009;25:1402–1410. doi: 10.1002/btpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelyn KA, Malloy H. Microdetermination of oxyhemoglobin, methemoglobin, and sulfhemoglobin in a single sample of blood. J. Biol. Chem. 1938;129:655–662. [Google Scholar]
- Faivre-Fiorina B, Caron A, Fassot C, Fries I, Menu P, Labrude P, Vigneron C. Presence of hemoglobin inside aortic endothelial cells after cell-free hemoglobin administration in guinea pigs. Am. J. Physiol. 1999;276:H766–H770. doi: 10.1152/ajpheart.1999.276.2.H766. [DOI] [PubMed] [Google Scholar]
- Matheson B, Razynska A, Kwansa H, Bucci E. Appearance of dissociable and cross-linked hemoglobins in the renal hilar lymph. J. Lab. Clin. Med. 2000;135:459–464. doi: 10.1067/mlc.2000.106458. [DOI] [PubMed] [Google Scholar]
- Meguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K. Nonheme-iron histochemistry for light and electron microscopy: A historical, theoretical and technical review. Arch. Histol. Cytol. 2007;70:1–19. doi: 10.1679/aohc.70.1. [DOI] [PubMed] [Google Scholar]
- Nandi A, Mukhopadhyay CK, Ghosh MK, Chattopadhyay DJ, Chatterjee IB. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997;22:1047–1054. doi: 10.1016/s0891-5849(96)00491-1. [DOI] [PubMed] [Google Scholar]
- Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic. Biol. Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Oser BL. Hawk's Physiological Chemistry. New York, NY: McGraw-Hill Book Company; 1965. [Google Scholar]
- Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol. Prog. 2009;25:189–199. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoro I, Kocsis L, Herman P, Caccia D, Perrella M, Ronda L, Bruno S, Bettati S, Micalella C, Mozzarelli A, et al. Towards a novel haemoglobin-based oxygen carrier: Euro-PEG-Hb, physico-chemical properties, vasoactivity and renal filtration. Biochim. Biophys. Acta. 2008;1784:1402–1409. doi: 10.1016/j.bbapap.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Racusen LC. Alterations in human proximal tubule cell attachment in response to hypoxia: Role of microfilaments. J. Lab. Clin. Med. 1994;123:357–364. [PubMed] [Google Scholar]
- Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: Current status and future directions. Anesthesiology. 2009;111:946–963. doi: 10.1097/ALN.0b013e3181ba3c2c. [DOI] [PubMed] [Google Scholar]
- Yu B, Bloch KD, Zapol WM. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc. Med. 2009;19 doi: 10.1016/j.tcm.2009.06.004. 103–107; S1050-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Gamelin LM. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am. J. Physiol. 1989;256:F446–F455. doi: 10.1152/ajprenal.1989.256.3.F446. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jia Y, Buehler PW, Chen G, Cabrales P, Palmer AF. Synthesis, biophysical properties, and oxygenation potential of variable molecular weight glutaraldehyde-polymerized bovine hemoglobins with low and high oxygen affinity. Biotechnol. Prog. 2011;27:1172–1184. doi: 10.1002/btpr.624. [DOI] [PubMed] [Google Scholar]