Abstract
Galectin-3 (Gal-3) is a β-galactoside-binding lectin implicated in the regulation of macrophage activation and inflammatory mediator production. In the present studies, we analyzed the role of Gal-3 in liver inflammation and injury induced by acetaminophen (APAP). Treatment of wild-type (WT) mice with APAP (300 mg/kg, ip) resulted in centrilobular hepatic necrosis and increases in serum transaminases. This was associated with increased hepatic expression of Gal-3 messenger RNA and protein. Immunohistochemical analysis showed that Gal-3 was predominantly expressed by mononuclear cells infiltrating into necrotic areas. APAP-induced hepatotoxicity was reduced in Gal-3-deficient mice. This was most pronounced at 48–72 h post-APAP and correlated with decreases in APAP-induced expression of 24p3, a marker of inflammation and oxidative stress. These effects were not due to alterations in APAP metabolism or hepatic glutathione levels. The proinflammatory proteins, inducible nitric oxide synthase (iNOS), interleukin (IL)-1β, macrophage inflammatory protein (MIP)-2, matrix metalloproteinase (MMP)-9, and MIP-3α, as well as the Gal-3 receptor (CD98), were upregulated in livers of WT mice after APAP intoxication. Loss of Gal-3 resulted in a significant reduction in expression of iNOS, MMP-9, MIP-3α, and CD98, with no effects on IL-1β. Whereas APAP-induced increases in MIP-2 were augmented at 6 h in Gal-3−/− mice when compared with WT mice, at 48 and 72 h, they were suppressed. Tumor necrosis factor receptor-1 (TNFR1) was also upregulated after APAP, a response dependent on Gal-3. Moreover, exaggerated APAP hepatotoxicity in mice lacking TNFR1 was associated with increased Gal-3 expression. These data demonstrate that Gal-3 is important in promoting inflammation and injury in the liver following APAP intoxication.
Keywords: acetaminophen, macrophages, inflammation, galectin-3, liver, TNFR1
Acetaminophen (APAP)-induced hepatotoxicity is the major cause of acute liver failure in the United States (Lee et al., 2008). Tissue injury is initiated by covalent binding of the reactive intermediate, N-acetyl-p-benzoquinoneimine (NAPQI), to critical cellular proteins in the liver (Dahlin et al., 1984; Jollow et al., 1973). Evidence suggests that proinflammatory/cytotoxic mediators released by activated macrophages play a role in promoting APAP-induced hepatotoxicity (reviewed in Laskin, 2009). The factors that induce macrophage activation and inflammatory mediator production in the liver after APAP intoxication have not been clearly established. In previous studies, we demonstrated that mediators released from injured hepatocytes, including high-mobility group box-1 (HMGB1), are important in the activation process (Dragomir et al., 2011; Laskin et al., 1986). A question arises, however, about the role of macrophages themselves as a source of activating factors. Macrophages are potent secretory cells, releasing a myriad of mediators known to be important in nonspecific host defense and adaptive immunity, as well as inflammation and wound repair. These diverse activities are mediated by distinct subpopulations that develop in response to mediators macrophages encounter in their microenvironment (reviewed in Laskin et al., 2011). Two major phenotypically distinct subpopulations have been identified: classically activated proinflammatory macrophages and alternatively activated anti-inflammatory/would repair macrophages. The ability of macrophages to release activating factors that act in an autocrine and paracrine manner to induce classical and alternative activation represents an important mechanism regulating inflammatory responses to tissue injury.
Galectins comprise a family of lectins with affinity for β-galactoside-containing carbohydrates. Gal-3 is the only member of this family with a chimeric structure consisting of a conserved carbohydrate recognition domain and a nonlectin domain (Henderson and Sethi, 2009). Whereas Gal-3 is expressed at low levels in monocytes, it is upregulated during their maturation into macrophages (Liu et al., 1995). Proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), further upregulate Gal-3 in macrophages and also stimulate its release into the extracellular environment (Nishi et al., 2007). Gal-3 binds to macrophages via CD98, stimulating the production of additional proinflammatory cytokines and chemokines (Norling et al., 2009). Intra-articular administration of Gal-3 has been reported to induce inflammation in mice (Janelle-Montcalm et al., 2007). Moreover, mice with a targeted mutation of Gal-3 exhibit an impaired ability to mount an acute inflammatory response to thioglycollate (Hsu et al., 2000) or ovalbumin (Zuberi et al., 2004). These data suggest that Gal-3 is important in promoting inflammation. Because macrophage-derived inflammatory mediators contribute to liver injury following APAP intoxication (Laskin, 2009; Laskin et al., 1995), we speculated that Gal-3 may be involved in the pathogenesis of hepatotoxicity and this was investigated.
MATERIALS AND METHODS
Animals.
Male specific pathogen-free C57Bl/6J wild-type (WT) mice, Gal-3−/− mice, and TNFR1−/− mice (8–12-weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in microisolation cages and allowed free access to food and water. All animals received humane care in compliance with the institution's guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Mice were fasted overnight prior to ip administration of APAP (300 mg/kg) or pyrogen-free PBS control. After 3–72 h, mice were euthanized with nembutal (200 mg/kg) and blood collected from the abdominal vena cava for determination of aspartate and alanine transaminases using diagnostic assay kits (ThermoFisher Scientific, Waltham, MA). Liver samples (100 mg aliquots) were collected and stored at −20°C in RNAlater (Sigma-Aldrich, St Louis, MO) until RNA isolation. The remaining tissue was snap frozen in liquid nitrogen.
Preparation of liver microsomes and measurement of cytochrome P450 2e1 (Cyp2e1) activity.
Frozen liver samples (1–2 g) were homogenized at 4°C in four volumes of buffer (50mM Tris-hydrochloride, 1.15% potassium chloride, and 0.5mM phenylmethylsulfonylfluoride, pH 7.4) and then centrifuged at 12,000 × g for 20 min. Supernatants were collected and centrifuged at 105,000 × g for 90 min. Microsomes were then washed in buffer containing 1.15% potassium chloride and 10mM EDTA (pH 7.4), resuspended in 10mM potassium phosphate buffer containing 0.25M sucrose, and stored at −80°C until analysis. Cyp2e1 activity was measured by the generation of p-nitrocatechol from p-nitrophenol (Chang et al., 1998). Microsomes were incubated with 100μM p-nitrophenol and 500μM β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt at 37°C for 20 min. The reaction was stopped by the addition of trichloroacetic acid. The mixture was then centrifuged (13,000 × g, 5 min, 4°C), supernatants collected, and mixed with 2M NaOH. Changes in absorbance were measured spectrophotometrically at 535 nm. Concentrations of p-nitrocatechol in the samples were determined based on a standard curve generated with authentic p-nitrocatechol.
Measurement of hepatic glutathione.
Frozen livers (50 mg) were homogenized in ice-cold 5% metaphosphoric acid (1:10) and centrifuged at 3000 × g for 10 min. Supernatants were collected and reduced glutathione determined using a colorimetric assay kit (OxisResearch, Portland, OR). Glutathione concentrations in the samples were calculated based on a standard curve and expressed as μmol/g wet liver.
Histology and immunohistochemistry.
Livers were collected, and 5 mm samples of the left lateral lobes immediately fixed overnight at 4°C in 3% paraformaldehyde/2% sucrose. Tissue was washed three times in PBS/2% sucrose and then transferred to 50% ethanol. After embedding in paraffin, 5 μm sections were prepared and stained with hematoxylin and eosin (Goode Histolabs, New Brunswick, NJ). Histopathological evaluation was performed by a board certified veterinary pathologist (L.B. Hall). Findings were graded on a scale of 0–4, where 0 = none, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe changes. For immunohistochemistry, sections were rehydrated and stained with antibody to Gal-3 (1:25,000; R&D Systems, Minneapolis, MN) or IgG control (ProSci, Poway, CA). Binding was visualized using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Three to five random sections of each liver were examined.
Immunofluorescence.
Livers were collected and 5 mm samples of the left lateral lobes were immediately snap frozen in liquid nitrogen–cooled isopentane and embedded in OCT medium (Sakura Finetek, Torrance, CA). Six micrometer sections were prepared and fixed in 90% acetone/10% methanol. Sections were stained with antimyeloperoxidase antibody (1:100) (Dako, Carpinteria, CA), followed by isotype-specific Alexa Fluor488-conjugated secondary antibody (Molecular Probes, Carlsbad, CA). Images were acquired using a Leica SP 5 confocal microscope.
Western blotting.
Liver samples (50 mg) were lysed in four volumes of buffer Real-Time PCR System containing 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid pH 7.4, 150mM NaCl, 10% glycerol, 1% Triton X-100, 1.5mM MgCl2, 1mM diethylene triamine pentaaacetic acid, 1mM phenylmethylsulfonylfluoride, 10mM sodium pyrophosphate, 50mM sodium fluoride, 2mM sodium orthovanadate, and protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were measured using the Bradford assay (Bio-Rad, Hercules, CA). Proteins were separated on 10.5–14% Tris-glycine polyacrylamide gels (Bio-Rad) and then transferred to nitrocellulose membranes. Nonspecific binding was blocked by incubation of the blots for 1 h at room temperature with buffer containing 5% nonfat milk, 10mM Tris-base, 200mM sodium chloride, and 0.1% polysorbate 20 (pH 7.6). Membranes were then incubated overnight at 4°C with anti-inducible nitric oxide synthase (iNOS; 1:4000; BD Biosciences, San Jose, CA), anti-cyclooxygenase-2 (COX-2; 1:2000; Abcam, Cambridge, MA), or anti-glyceraldehyde-3-phosphate dehydrogenase (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies, followed by incubation with isotype-specific horseradish peroxidase–conjugated secondary antibodies (1:10,000) for 1 h at room temperature. Binding was visualized using an ECL Plus chemiluminescence kit (GE Healthcare, Piscataway, NJ).
Real-time PCR.
Total RNA was isolated from liver tissue using an RNeasy kit (Qiagen, Valencia, CA). RNA purity and concentration were measured using a NanoDrop spectrophotometer (ThermoFisher Scientific, Wilmington, DE). RNA was converted into complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's directions. Standard curves were generated using serial dilutions from pooled randomly selected cDNA samples. Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on a ABI Prism 7900HT Sequence Detection System (Applied Biosystems). All PCR primer pairs were generated using Primer Express 2.0 (Applied Biosystems) and synthesized by Integrated DNA Technologies (Coralville, IA). For each sample, gene expression changes were normalized relative to 18S rRNA. Data are expressed as fold change relative to control. Primer sequences were: Gal-3, CACAATCATGGGCACAGTGAA and TTCCCTCTCCTGAAATCTAGAACAA; lipocalin 2 (24p3), AGGAACGTTTCACCCGCTTT and TGTTGTCGTCCTTGAGGCC; macrophage inflammatory protein-2 (MIP-2), AGGCTTCCCGATGAAGAG and CAGGATAAGAGCGAGAGCCTACA; interleukin (IL)-1β, AGTTGACGGACCCCAAAAGAT and GGACAGCCCAGGTCAAAGG; MIP-3α, TGGCCGATGAAGCTTGTGA and AGCGCACACAGATTTTCTTTTCT; matrix metalloproteinase-9 (MMP-9), CAAGTGGGACCATCATAACATCA and CTCGCGGCAAGTCTTCAGA; IL-10, AGGCAGCCTTGAGAAAAGA and AGTAAGAGCAGGCAGCATAGCA; CD98, GAAGCTCTGAGTTCTTGGTTGCA and CTTTCCCACATCCCGGAAT; TNF receptor-1 (TNFR1), CAGACTTGCATGGTGAGCTCTT and AGCCCAGTTACCCAACAGACA; 18S rRNA, CGGCTACCACATCCAAGGAA and GCTGGAATTACCGCGGCT.
Statistical analysis.
Experiments were repeated two to three times. Data were analyzed using Student's t-test or one-way ANOVA followed by Dunn's post hoc analysis. A p value of ≤ 0.05 was considered statistically significant.
RESULTS
Effects of APAP on Expression of Gal-3 in the Liver
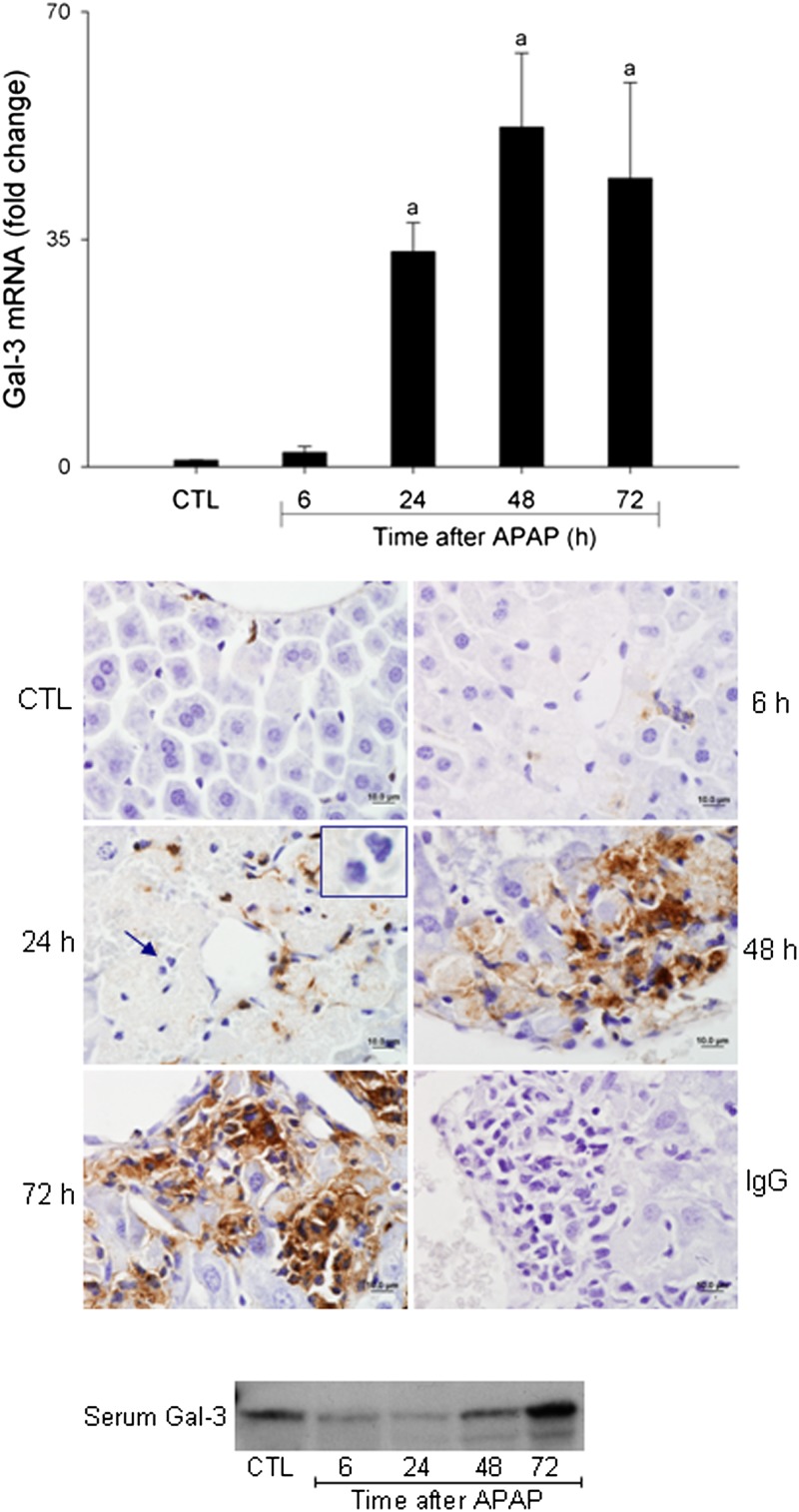
In initial studies, we analyzed the effects of APAP intoxication on Gal-3 expression in the liver. Treatment of WT mice with APAP resulted in a time-dependent increase in hepatic Gal-3 messenger RNA (mRNA) expression, which was evident at 24 h, becoming more pronounced at 48 and 72 h (Fig. 1, upper panel). This was correlated with an increase in hepatic Gal-3 protein expression, which was predominantly localized in inflammatory macrophages infiltrating into necrotic areas (Fig. 1, middle panel). No Gal-3 expression was observed in neutrophils (Fig. 1, middle panel inset). In contrast, a transient decline in serum Gal-3 levels was noted 6–24 h after APAP administration; subsequently, serum levels began to increase and by 72 h were at or above control levels (Fig. 1, lower panel).
FIG. 1.
Effects of APAP intoxication on Gal-3 expression. Livers were collected 6–72 h after treatment of WT mice with APAP (300 mg/kg, ip) or control (CTL). Upper panel: Gal-3 expression was analyzed by real-time PCR. Data were normalized to 18S rRNA. Each bar represents the mean ± SE (n = 3–10 mice). aSignificantly different (p < 0.05) from CTL. Middle panel: Sections were stained with anti-Gal-3 antibody or IgG control, as described in the Materials and Methods section. One representative section from three independent experiments is shown. Original magnification, ×100. Inset, neutrophils. Lower panel: Serum was collected 6–72 h after treatment of WT mice with APAP or control (CTL). Gal-3 expression was analyzed by Western blotting. One representative blot from five independent experiments is shown.
Role of Gal-3 in APAP-Induced Hepatotoxicity
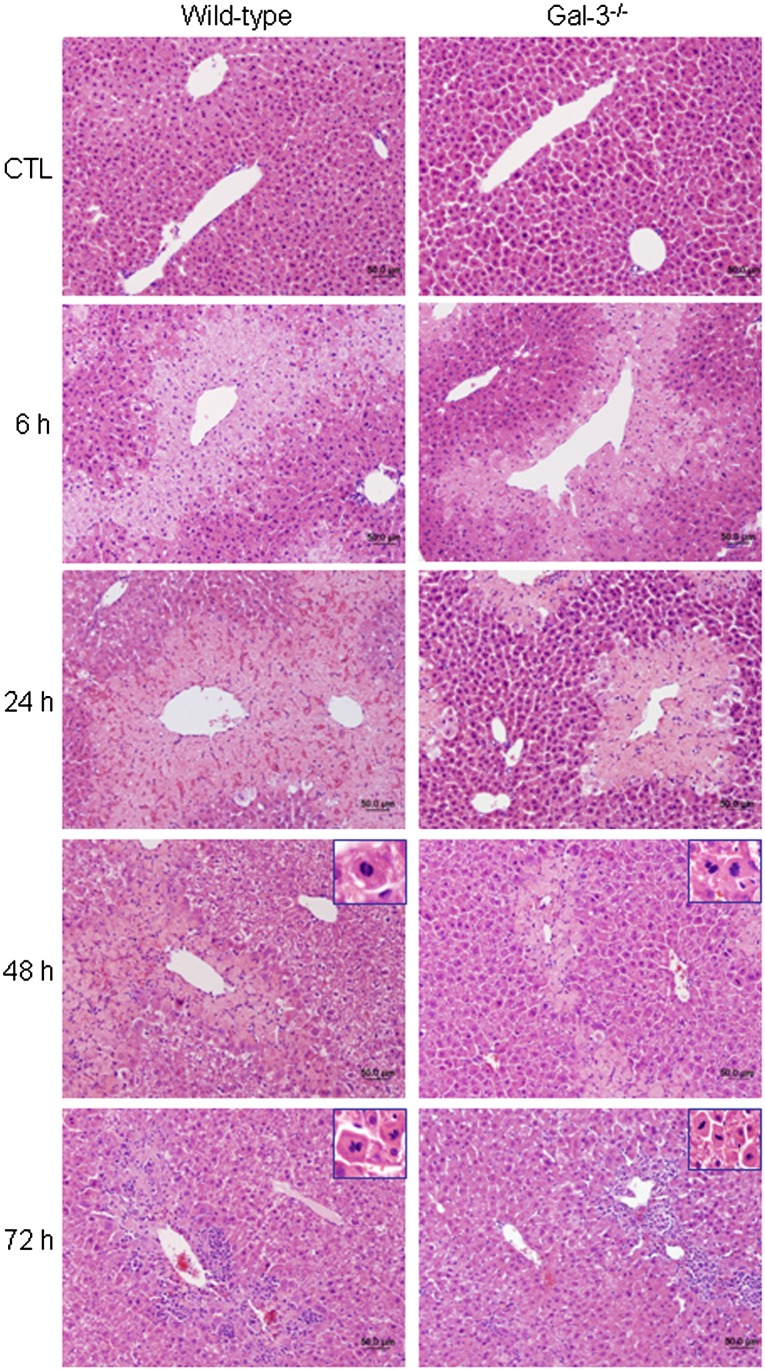
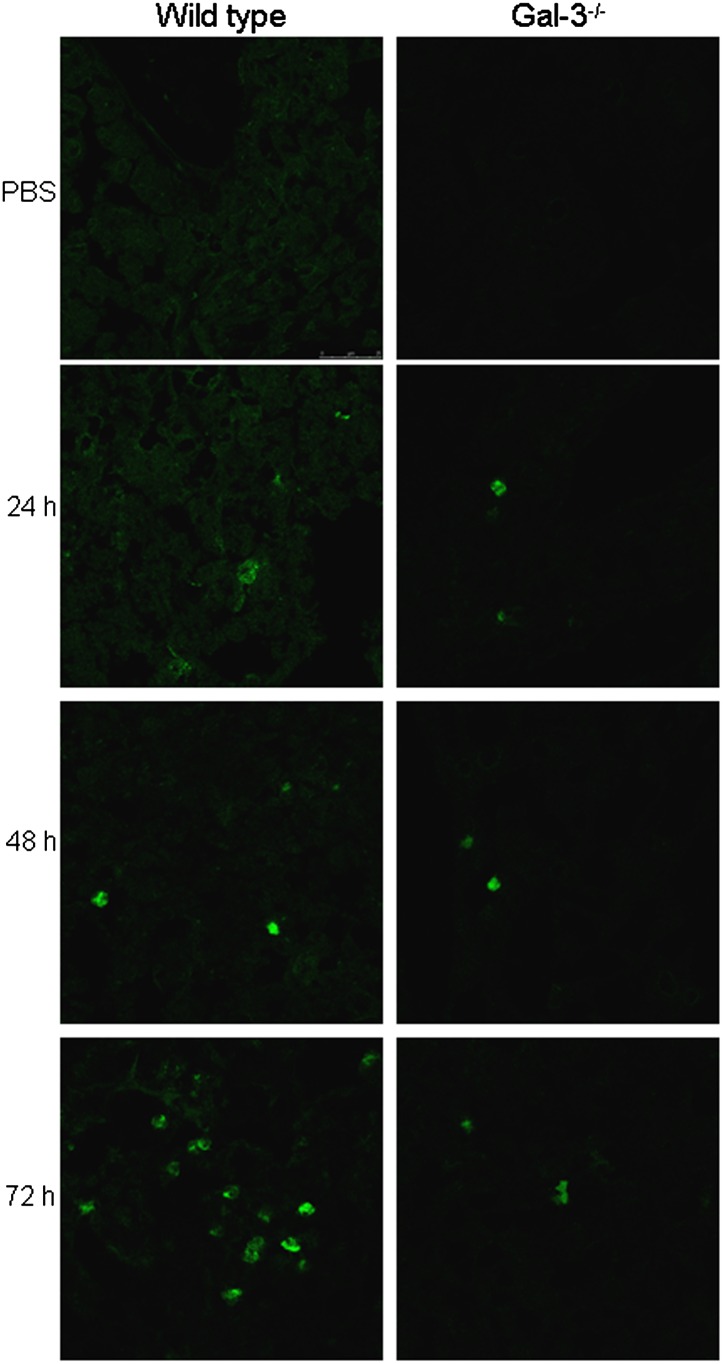
To investigate the role of Gal-3 in the pathogenesis of APAP-induced hepatotoxicity, we used mice with a targeted deletion of the lgals3 gene. In WT mice, APAP administration resulted in centrilobular hepatic necrosis, which was evident within 3 h (Fig. 2 and Gardner et al., 2010). At 6 h post-APAP, mild degeneration, necrosis, and hemorrhage were noted in centrilobular areas, with no evidence of neutrophils (Table 1). By 24 h, moderate coagulative centrilobular necrosis was present, as well as minimal to mild hemorrhage within the necrotic areas; minimal infiltration of neutrophils was also noted in centrilobular areas. Moderate coagulative necrosis persisted in centrilobular regions of the liver for 48 h and was accompanied by mild neutrophil infiltration. At 72 h post-APAP, mild necrosis and moderate neutrophil infiltration were evident (Figs. 2 and 3, Table 1). Mitotic figures were also observed in surviving hepatocytes surrounding necrotic areas at 48–72 h, indicating liver regeneration (Fig. 2, inset). Histopathologic changes in the liver were associated with a time-related increase in serum transaminases, which peaked 6–24 h post-APAP treatment (Table 2). APAP-induced hepatotoxicity was significantly blunted in Gal-3−/− mice when compared with WT mice, as evidenced by more rapid decreases in serum transaminases and attenuated histologic alterations. Thus, by 72 h post-APAP, only minimal necrosis was observed in livers of Gal-3−/− mice relative to mild/moderate necrosis in WT mice (Fig. 2, Tables 1 and 2). Although the extent of neutrophil infiltration into the liver was similar in Gal-3−/− and WT mice 24–48 h post-APAP, as determined histologically and by myeloperoxidase immunostaining (Table 1 and Fig. 3), by 72 h, there were significantly fewer neutrophils in livers of Gal-3−/− mice when compared with WT mice.
FIG. 2.
Effects of loss of Gal-3 on APAP-induced structural alterations in the liver. Livers were collected 6–72 h after treatment of WT and Gal-3−/− mice with APAP or control (CTL). Sections were stained with hematoxylin and eosin. One representative section from three independent experiments is shown. Original magnification, ×20. Insets, ×60.
TABLE 1.
Histopathological Evaluation of Hepatic Necrosis and Neutrophilic Infiltrates in WT and Gal-3−/− Mice After APAP Administration
| Necrosis |
Neutrophils |
|||
| Time (h) | WT | Gal-3−/− | WT | Gal-3−/− |
| CTL | 0 | 0 | 0 | 0 |
| 6 | 2.0 ± 0.0 | 2.0 ± 0.0 | 0 | 0 |
| 24 | 3.0 ± 0.0 | 2.7 ± 0.3 | 1.0 ± 0.0 | 1.3 ± 0.3 |
| 48 | 3.0 ± 0.0 | 2.0 ± 0.6 | 2.0 ± 0.0 | 2.0 ± 0.6 |
| 72 | 2.3 ± 0.3 | 0.3 ± 0.3 | 3.3 ± 0.3 | 0.7 ± 0.3 |
Notes. WT and Gal-3−/− mice were treated with 300 mg/kg APAP or PBS control (CTL). Liver sections were prepared 6–72 h later for histopathological analysis. Findings were graded on a scale of 0–4, where 0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe changes. Data are expressed as mean ± SE (n = 3 mice).
FIG. 3.
Effects of loss of Gal-3 on APAP-induced neutrophil emigration into the liver. Livers were collected 24–72 h after treatment of WT and Gal-3−/− mice with APAP or control (CTL). Sections were stained with antimyeloperoxidase antibody. One representative section from two independent experiments is shown.
TABLE 2.
Effects of APAP on Serum Transaminases in WT and Gal-3−/− Mice
| ALT (U/l) |
AST (U/l) |
|||
| Time (h) | WT | Gal-3−/− | WT | Gal-3−/− |
| CTL | 32.1 ± 2.6 | 45.4 ± 7.2 | 60.6 ± 5.6 | 82.5 ± 10.6 |
| 6 | 7797.0 ± 508.1a | 6363.1 ± 1266.9 | 7473.0 ± 481.8a | 7711.1 ±1917.4a |
| 24 | 10,905.8 ± 825.5a | 7673.6 ± 1022.9a,b | 6391.1 ± 911.4a | 3908.2 ± 723.6a |
| 48 | 1194.0 ± 126.1a | 454.2 ± 53.5b | 522.9 ± 51.8a | 265.7 ± 26.5b |
| 72 | 247.6 ± 41.4 | 116.6 ± 15.1b | 261.4 ± 29.3 | 93.8 ± 15.4b |
Notes. ALT, alanine aminotransferase; AST, aspartate aminotransferase. WT and Gal-3−/− mice were treated with 300 mg/kg APAP or PBS control (CTL). Sera were collected 6–72 h later and analyzed for ALT and AST. Data are expressed as mean ± SE (n = 5–12 mice).
Significantly different (p < 0.05) from CTL.
Significantly different from WT.
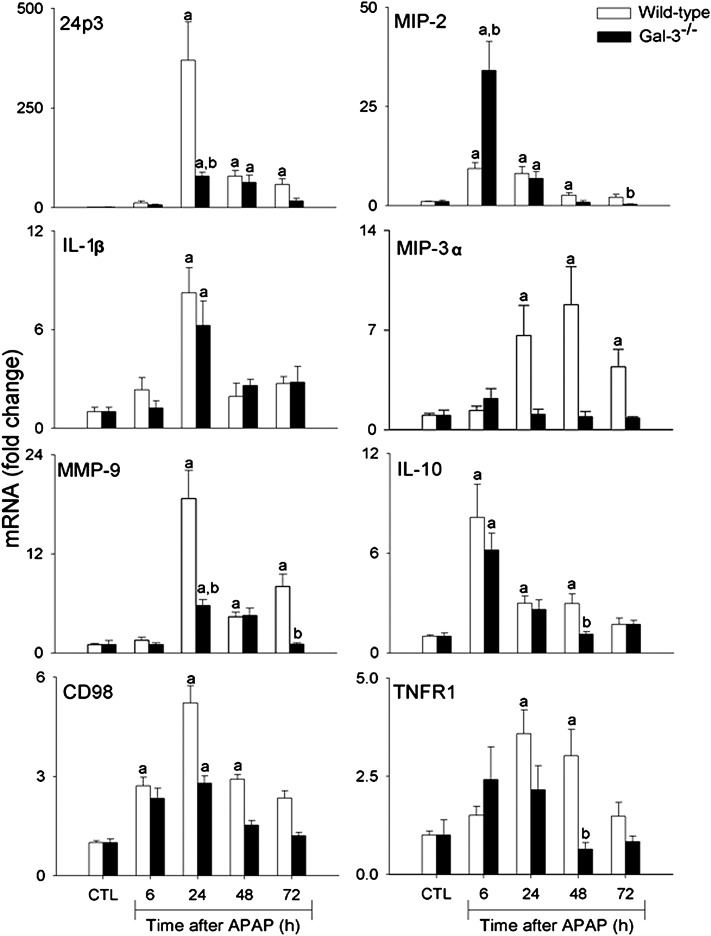
24p3 is an acute phase protein and a marker of oxidative stress and inflammation released by neutrophils, macrophages, and epithelial cells (Borkham-Kamphorst et al., 2011; Roudkenar et al., 2007; Sunil et al., 2007). In WT mice, APAP intoxication was characterized by a dramatic increase in expression of 24p3 mRNA, which was most prominent after 24 h (Fig. 4). Subsequently, levels began to decline toward control. Loss of Gal-3 resulted in a significant attenuation of this response.
FIG. 4.
Effects of loss of Gal-3 on APAP-induced expression of inflammatory markers. Livers were collected 6–72 h after treatment of WT and Gal-3-/- mice with APAP or control (CTL). Samples were analyzed by real-time PCR. Each bar represents the mean ± SE (n = 3–8 mice). aSignificantly different (p < 0.05) from CTL. bSignificantly different (p < 0.05) from WT mice.
Effects of Loss of Gal-3 on APAP-induced Expression of Inflammatory Proteins
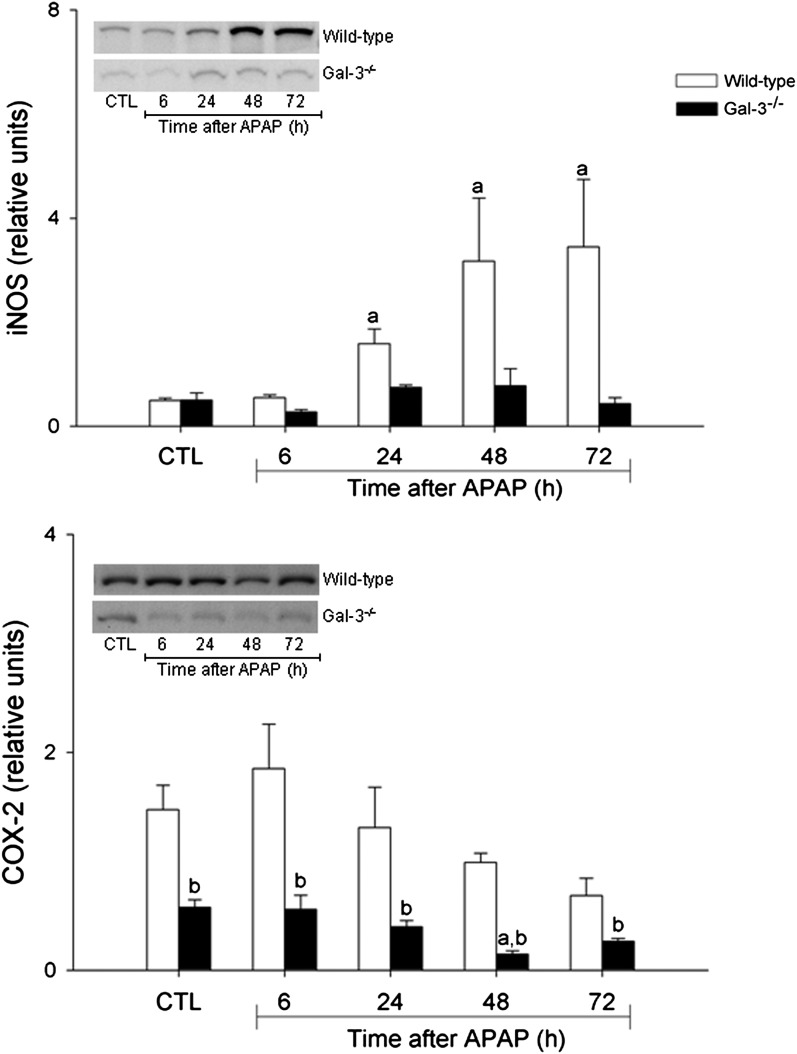
In further studies, we analyzed the effects of loss of Gal-3 on APAP-induced expression of pro- and anti-inflammatory proteins implicated in hepatotoxicity (Laskin, 2009). APAP administration to WT mice was associated with a time-related increase in mRNA expression for the proinflammatory mediators MIP-2, MIP-3α, and IL-1β. Whereas MIP-2 expression increased within 6 h and persisted for 48 h after APAP, MIP-3α upregulation was delayed until 24 h and remained elevated for 72 h. In contrast, IL-1β expression was transiently increased at 24 h post-APAP. MMP-9 mRNA expression also increased in the liver 24 h after APAP, remaining elevated for at least 72 h (Fig. 4). Protein expression of iNOS, the enzyme mediating macrophage production of nitric oxide (Laskin et al., 2010), also increased 48–72 h after APAP administration to WT mice (Fig. 5). Loss of Gal-3 blunted the effects of APAP on MIP-3α, MMP-9, and iNOS but had no effect on expression of IL-1β. MIP-2 expression was also reduced in Gal-3−/− mice, relative to WT mice, at 48–72 h post-APAP. In contrast, expression of this chemokine was significantly increased at 6 h in Gal-3−/− mice. Expression of the anti-inflammatory cytokine IL-10 was also upregulated in WT mice following APAP intoxication (Fig. 4). This was observed within 6 h and remained elevated for 48 h, although at reduced levels. Loss of Gal-3 resulted in decreased IL-10 expression at 48 h post-APAP. COX-2 is a key enzyme regulating the biosynthesis of both pro- and anti-inflammatory eicosanoids (Cook, 2005). Constitutive COX-2 protein was detected in the livers of both WT and Gal-3−/− mice; however, expression of this protein was significantly reduced in Gal-3−/− mice (Fig. 5). Whereas in WT mice, APAP had no major effect on COX-2 protein expression, a significant decrease was noted in Gal-3−/− mice 48 h after treatment.
FIG. 5.
Effects of loss of Gal-3 on APAP-induced expression of iNOS and COX-2. Livers were collected from WT and Gal-3−/− mice 6–72 h after treatment with APAP or control (CTL). iNOS (upper panel) and COX-2 (lower panel) expression were analyzed by Western blotting. Densitometric analysis was performed using ImageJ. Each bar represents the mean ± SE (n = 3–5 mice). aSignificantly different (p < 0.05) from CTL. bSignificantly different (p < 0.05) from WT mice.
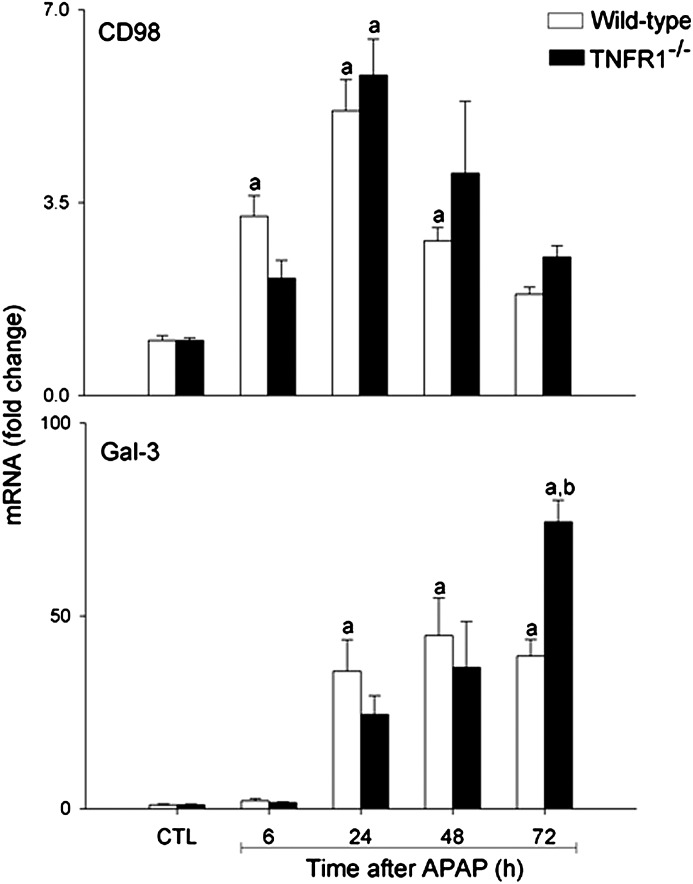
CD98 has been proposed as a macrophage receptor for Gal-3 (Dong and Hughes, 1997). In WT mice, APAP administration resulted in a time-dependent increase in CD98 mRNA expression, which was maximal after 24 h (Fig. 4). Loss of Gal-3 blunted the effects of APAP on CD98 expression.
Reciprocal Regulation of Gal-3 and TNFR1 Expression in the Liver Following APAP Intoxication
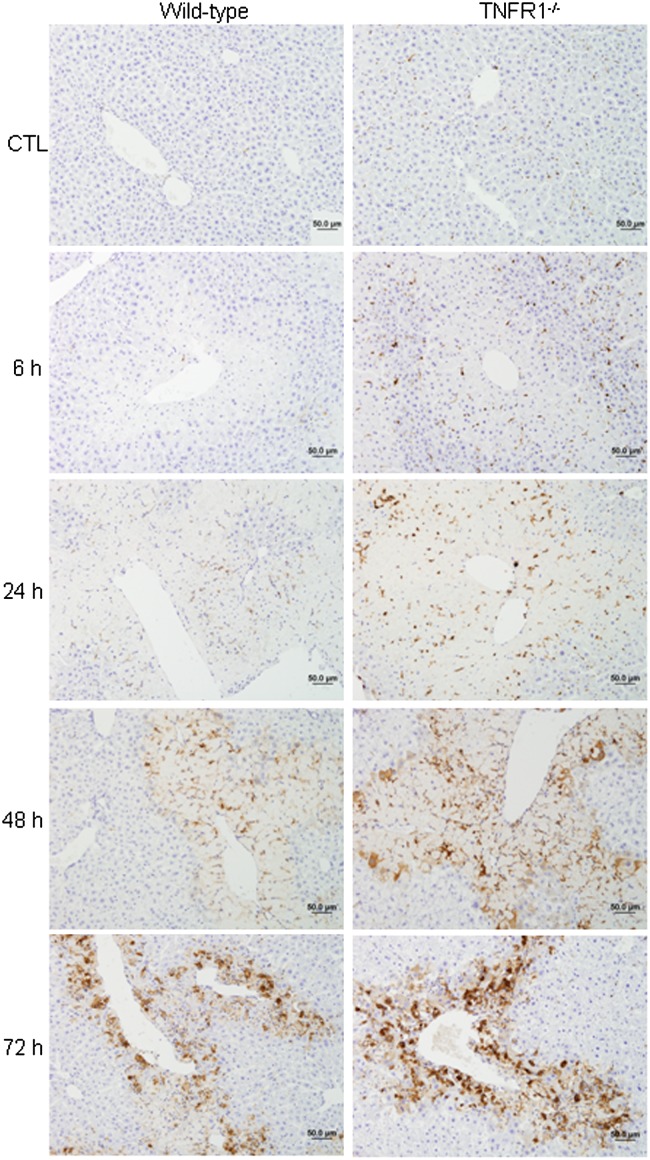
TNF-α signaling via TNFR1 has been shown to be important in the production of mediators involved in tissue repair and antioxidant defense during APAP-induced hepatotoxicity (Chiu et al., 2003a,b). In agreement with earlier studies (Ishida et al., 2004), we found that APAP administration to WT mice resulted in a significant increase in hepatic TNFR1 mRNA expression, which was maximal after 24–48 h (Fig. 4). This was reduced in APAP-treated Gal-3−/− mice. To investigate the role of TNF-α signaling via TNFR1 in APAP-induced Gal-3 expression, we used mice with a targeted deletion of the gene encoding TNFR1, which we have previously reported to be more susceptible than WT mice to APAP-induced hepatotoxicity (Chiu et al., 2003b; Gardner et al., 2003). Whereas loss of TNFR1 had minimal effects on APAP-induced CD98 expression, Gal-3 mRNA levels were significantly greater in TNFR1−/− mice relative to WT mice 72 h post-APAP (Fig. 6). Gal-3 protein expression was also increased in livers of TNFR1−/− mice when compared with WT mice (Fig. 7). This was noted within 6 h and became more prominent after 48–72 h.
FIG. 6.
Effects of loss of TNFR1 on APAP-induced Gal-3 and CD98 mRNA expression. Livers were collected 6–72 h after treatment of WT and Gal-3-/- mice with APAP or control (CTL). Samples were analyzed by real-time PCR. Each bar represents the mean ± SE (n = 3–5 mice). aSignificantly different (p < 0.05) from CTL. bSignificantly different (p < 0.05) from WT mice.
FIG. 7.
Effects of loss of TNFR1 on APAP-induced Gal-3 protein expression. Livers were collected 6–72 h after treatment of WT and TNFR1−/− mice with APAP or control (CTL). Sections were stained with anti-Gal-3 antibody as described in the Materials and Methods section. One representative section from three independent experiments is shown. Original magnification, ×20.
Effects of Loss of Gal-3 on Hepatic Glutathione and Cyp2e1 Activity
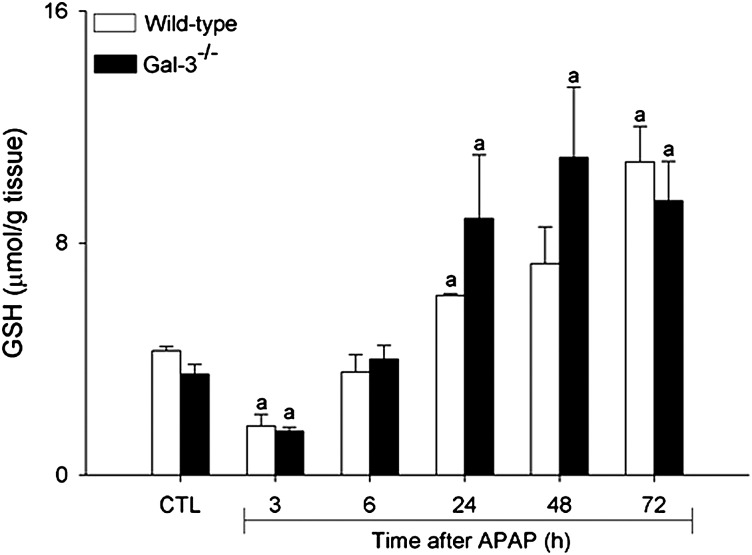
To determine if reduced hepatotoxicity in Gal-3−/− mice was due to altered APAP metabolism, we measured the activity of hepatic Cyp2e1, the major enzyme mediating the generation of the reactive APAP metabolite NAPQI (Lee et al., 1996). No significant differences were noted in the activity of Cyp2e1 between WT and Gal-3−/− mice (0.91 ± 0.05 versus 0.97 ± 0.05 nmol/min/mg protein, respectively). We also measured hepatic glutathione levels. APAP administration to mice resulted in a rapid and transient decline in hepatic-reduced glutathione levels in WT, which was evident within 3 h; subsequently, glutathione levels increased and by 72 h were above control levels (Fig. 8). Loss of Gal-3 had no significant effect on APAP-induced alterations in hepatic glutathione levels.
FIG. 8.
Effects of APAP on hepatic glutathione levels. Livers were collected 3–72 h after treatment of WT and Gal-3-/- mice with APAP or control (CTL) and total glutathione levels assayed. Each bar represents the mean ± SE (n = 3–6 mice). aSignificantly different (p < 0.05) from CTL.
DISCUSSION
The present studies demonstrate that Gal-3 plays a role in promoting late proinflammatory responses and perpetuating injury in the liver following APAP intoxication. This is based on our findings that Gal-3 is markedly upregulated in macrophages infiltrating into the liver 48–72 h after APAP administration and that loss of Gal-3 results in reduced hepatotoxicity at these times and decreased expression of the proinflammatory proteins, 24p3, MMP-9, MIP-3α, iNOS, and CD98. Neutrophil influx into the liver is also suppressed. These findings are novel and may have therapeutic implications for developing new approaches to treating APAP overdose.
APAP administration resulted in a dramatic increase in Gal-3 expression in the liver, which was most prominent after 48–72 h. Moreover, the major cell population expressing Gal-3 consisted of mononuclear cells accumulating in necrotic areas, which have previously been shown to display features of classically activated macrophages (Laskin and Pilaro, 1986). These findings are in agreement with previous studies showing that Gal-3 is upregulated in leukocytes infiltrating the liver during carbon tetrachloride-induced fibrosis and Toxoplasma gondii infection (Bernardes et al., 2006; Henderson et al., 2006). Our observation that serum Gal-3 levels declined rapidly following APAP administration is consistent with increased Gal-3 accumulation in the liver and suggests a local, intrahepatic role for Gal-3 in this model of injury. Blood monocytes have been shown to synthesize Gal-3 (Weber et al., 2009); reduced serum levels after APAP may also be due to increased emigration of these cells into the liver.
Gal-3 has been reported to be increased in various models of tissue injury where it functions to stimulate macrophage production of proinflammatory mediators (Norling et al., 2009). Consistent with this activity are findings that loss of Gal-3 is protective in antigen-induced arthritis (Forsman et al., 2011), renal ischemia-reperfusion injury (Fernandes Bertocchi et al., 2008), neonatal hypoxic-ischemic brain injury (Doverhag et al., 2010), streptozotocin-induced diabetes (Mensah-Brown et al., 2009), diet-induced steatohepatitis (Iacobini et al., 2011), and concanavalin A–induced liver injury (Volarevic et al., forthcoming). Moreover, protection in each of these models correlates with decreased levels of proinflammatory mediators. Similarly, we found that APAP-induced hepatotoxicity, as assessed histologically, by serum transaminases and by expression of 24p3, was significantly reduced in Gal-3−/− mice at 48–72 h, a time coordinate with the accumulation of Gal-3-positive macrophages in livers of WT mice. These findings together with the observation that APAP-induced expression of iNOS, MIP-3α, and MMP-9, proinflammatory proteins implicated in tissue injury (Gardner et al., 2002; Ito et al., 2005; Schutyser et al., 2003), was reduced at these times in Gal-3−/− mice, suggest that Gal-3 plays a role in promoting late inflammatory responses and the persistence of hepatic injury. This is supported by our finding that hepatotoxicity resolved more rapidly in Gal-3−/− mice relative to WT mice. However, the observation that APAP-induced hepatotoxicity was not completely prevented by loss of Gal-3 indicates that factors released early after injury, including damage-associated molecular patterns such as HMGB1 and heat shock protein 70, contribute to the pathogenic response to APAP (Dragomir et al., 2011; Martin-Murphy et al., 2010).
Reactive nitrogen species generated via iNOS have been shown to contribute to APAP-induced oxidative stress and tissue injury (reviewed in Laskin, 2009). APAP intoxication resulted in increased iNOS expression in the liver at 24–72 h. Findings that decreased hepatotoxicity in Gal-3−/− mice correlated with reduced iNOS expression suggest that Gal-3-positive macrophages may be a source of reactive nitrogen species. This is supported by reports that Gal-3 upregulates iNOS expression in brain macrophages (Jeon et al., 2010).
MMP-9 is an extracellular matrix–degrading enzyme that plays a role in microvascular injury induced by APAP (Ito et al., 2005). As previously reported (Gardner et al., 2003), MMP-9 expression increased in the liver following APAP administration, a response most notable at 24 h. This was significantly attenuated in Gal-3−/− mice and may contribute to reduced toxicity in these animals. These data are in agreement with reports that protection from hypoxic-ischemic brain injury in the absence of Gal-3 was associated with lower MMP-9 levels (Doverhag et al., 2010).
MIP-3α (chemokine [C-C motif] ligand 20) is a chemokine produced by macrophages and epithelial cells in response to proinflammatory stimuli such as TNF-α, interferon-γ, and lipopolysaccharide, as well as Gal-3 (Papaspyridonos et al., 2008; Schutyser et al., 2003). Expression of MIP-3α has also been reported to increase in vivo during liver and brain inflammation (Sugita et al., 2002; Utans-Schneitz et al., 1998). Similarly, we found that expression of MIP-3α increased in the liver following APAP intoxication and that this correlated with increased numbers of Gal-3-positive macrophages. The fact that loss of Gal-3 blunted the effects of APAP on MIP-3α expression provides additional support for a role of Gal-3 in promoting inflammation in the liver (Iacobini et al., 2011; Volarevic et al., forthcoming).
In agreement with previous studies, we found that APAP administration to WT mice resulted in increased expression of MIP-2 and IL-1β in the liver (Dambach et al., 2006; Imaeda et al., 2009; Liu et al., 2004). MIP-2 is a potent neutrophil chemokine (Clarke et al., 2009). Whereas in WT mice, increases in MIP-2 expression correlated with increased numbers of neutrophils in the liver, this was not observed in Gal-3−/− mice; in these mice, a marked increase in MIP-2 expression was noted 6 h after APAP intoxication, with no neutrophil emigration into the liver at this time. These data suggest that MIP-2 does not contribute significantly to early neutrophilic responses to APAP. It has been suggested that MIP-2 may play a protective role in the liver following APAP intoxication by promoting hepatocyte regeneration (Hogaboam et al., 1999). Our findings that MIP-2 expression is upregulated in Gal-3−/− mice relative to WT mice and that this was associated with reduced hepatotoxicity are in accord with this idea. The observation that MIP-2 levels decline more rapidly in Gal-3−/− mice is most likely due to reduced need for tissue repair processes. In contrast to MIP-2, APAP-induced IL-1β expression was unaffected by the loss of Gal-3. These results are consistent with recent studies suggesting that IL-1β does not play a role in the pathogenic response to APAP (Williams et al., 2010). Gal-3 has been reported to upregulate IL-1β expression in microglia (Jeon et al., 2010); moreover, tissue IL-1β levels are reduced in Gal-3−/− mice after renal ischemia-reperfusion injury (Fernandes Bertocchi et al., 2008). It may be that IL-1β plays distinct roles in different models of tissue injury.
The anti-inflammatory cytokine IL-10 has previously been shown to be upregulated in the liver following APAP intoxication and to play a hepatoprotective role in the pathogenic response (Bourdi et al., 2007; Dambach et al., 2006; Gardner et al., 2002). Increases in IL-10 mRNA levels were observed in both WT and Gal-3−/− mice 24 h after APAP administration. Surprisingly, by 48 h, IL-10 levels were reduced in Gal-3−/− mice relative to WT mice. This may be a consequence of decreased inflammation and hepatotoxicity in these mice, resulting in a reduced requirement for IL-10.
COX-2 catalyzes the biosynthesis of both pro- and anti-inflammatory eicosanoids (Stables and Gilroy, 2011). We found that COX-2 was constitutively expressed in livers of WT and Gal-3−/− mice. This is likely due to continuous exposure to endotoxin in the portal circulation (Ahmad et al., 2002; Naito et al., 2004). Previous studies have shown that mice lacking COX-2 are hypersensitive to APAP, suggesting that COX-2 is important in limiting hepatotoxicity (Reilly et al., 2001). This is thought to be due to increased generation of anti-inflammatory prostaglandins. Interestingly, hepatic COX-2 expression was reduced in Gal-3−/− mice relative to WT mice, suggesting a positive regulatory role for Gal-3 in hepatic prostanoid production. This is supported by our findings that constitutive COX-2 levels declined in the livers of Gal-3−/− mice treated with APAP. In contrast, Gal-3 appears to be negatively regulated by COX-2 as evident from reports that Gal-3 is downregulated in mice overexpressing COX-2 (Shen et al., 2007). COX-2 mRNA has been reported to be upregulated in the liver following APAP intoxication (Reilly et al., 2001). Conversely, we found no major effects of APAP on COX-2 protein expression in WT mice. These differences may be due to strain-specific responses and/or analysis of protein expression in our studies versus mRNA expression in earlier reports.
CD98 is a transmembrane glycosylated protein thought to function as a receptor for Gal-3 on macrophages (Dong and Hughes, 1997). Like Gal-3, CD98 is upregulated during tissue injury and inflammation and contributes to disease pathogenesis (Nguyen et al., 2011). Following APAP intoxication, CD98 increased in livers of WT mice; moreover, loss of Gal-3 blunted this response. This may contribute to the decreased sensitivity of Gal-3−/− mice to APAP.
TNF-α signaling via TNFR1 plays a key role in limiting the production of proinflammatory mediators and promoting antioxidant generation and tissue repair in the liver following APAP-induced toxicity (Chiu et al., 2003a,b; Gardner et al., 2003). Consistent with this activity is our finding that APAP administration to WT mice resulted in increased expression of TNFR1, which paralleled the development of hepatotoxicity. Surprisingly, reduced hepatotoxicity in Gal-3−/− mice was correlated with decreased levels of TNFR1, potentially reflecting the reduced need for activation of protective signaling pathways in these mice. We previously reported that loss of TNFR1 results in an exaggerated hepatotoxic response to APAP (Chiu et al., 2003a,b; Gardner et al., 2003). The present studies show that this is associated with increased Gal-3 expression in the liver. These data suggest that Gal-3 contributes to the increased susceptibility of TNFR1−/− mice to APAP. This is likely due to increased production of cytotoxic and proinflammatory mediators by Gal-3 positive–activated macrophages. In contrast to loss of Gal-3, loss of TNFR1 had no effect on APAP-induced expression of CD98. These results indicate that Gal-3 availability, and not receptor expression levels, is critical for the development of toxicity. It may also be that a different receptor mediates Gal-3-dependent responses in these mice.
Cyp2e1-dependent metabolism of APAP to NAPQI is a critical step in the onset of hepatotoxicity (Lee et al., 1996; Potter et al., 1973). Glutathione plays an important role in limiting toxicity by forming a nonreactive conjugate with NAPQI (Mitchell et al., 1973). Our data demonstrate that loss of Gal-3 did not alter hepatic Cyp2e1 activity or result in changes in hepatic glutathione levels in response to APAP. Taken together, these findings indicate that reduced susceptibility of Gal-3−/− mice to APAP-induced hepatotoxicity is not due to effects on NAPQI formation or inactivation.
In summary, the present studies identify Gal-3 as a novel regulator of late inflammatory responses in the liver following APAP intoxication and an important contributor to hepatotoxicity. Further studies are needed to identify the mechanisms involved in Gal-3-dependent inflammatory responses during the pathogenesis of APAP-induced liver injury.
FUNDING
National Institutes of Health grants R01GM034310, R01ES004738, R01CA132624, U54AR055073, and P30ES005022.
References
- Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J. Leukoc. Biol. 2002;71:1005–1011. [PubMed] [Google Scholar]
- Bernardes ES, Silva NM, Ruas LP, Mineo JR, Loyola AM, Hsu DK, Liu FT, Chammas R, Roque-Barreira MC. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am. J. Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, Drews F, Weiskirchen R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-kappaB activation. Liver Int. 2011;31:656–665. doi: 10.1111/j.1478-3231.2011.02495.x. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem. Res. Toxicol. 2007;20:208–216. doi: 10.1021/tx060228l. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Crespi CL, Waxman DJ. Spectrophotometric analysis of human CYP2E1-catalyzed p-nitrophenol hydroxylation. In: Phillips IR, Shephard EA, editors. Methods in Molecular Biology. Cytochrome P450 Protocols. Vol. 107. Totowa, NJ: Humana Press, Inc.; 1998. pp. 147–152. [DOI] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, Laskin DL. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2003a;285:G959–G966. doi: 10.1152/ajpgi.00219.2003. [DOI] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, Laskin JD, Laskin DL. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol. Appl. Pharmacol. 2003b;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M. CXC chemokines play a critical role in liver injury, recovery, and regeneration. Am. J. Surg. 2009;198:415–419. doi: 10.1016/j.amjsurg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA. Eicosanoids. Crit. Care Med. 2005;33:S488–S491. doi: 10.1097/01.ccm.0000196028.19746.42. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach DM, Durham SK, Laskin JD, Laskin DL. Distinct roles of NF-κB p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;211:157–165. doi: 10.1016/j.taap.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Dong S, Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj. J. 1997;14:267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- Doverhag C, Hedtjarn M, Poirier F, Mallard C, Hagberg H, Karlsson A, Savman K. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2010;38:36–46. doi: 10.1016/j.nbd.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Dragomir AC, Laskin JD, Laskin DL. Macrophage activation by factors released from acetaminophen-injured hepatocytes: Potential role of HMGB1. Toxicol. Appl. Pharmacol. 2011;253:170–177. doi: 10.1016/j.taap.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Bertocchi AP, Campanhole G, Wang PH, Goncalves GM, Damiao MJ, Cenedeze MA, Beraldo FC, de Paula Antunes Teixeira V, Dos Reis MA, Mazzali M, et al. A role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl. Int. 2008;21:999–1007. doi: 10.1111/j.1432-2277.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Forsman H, Islander U, Andreasson E, Andersson A, Onnheim K, Karlstrom A, Savman K, Magnusson M, Brown KL, Karlsson A. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011;63:445–454. doi: 10.1002/art.30118. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Gray JP, Joseph LB, Cervelli J, Bremer N, Kim Y, Mishin V, Laskin JD, Laskin DL. Potential role of caveolin-1 in acetaminophen-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;245:36–46. doi: 10.1016/j.taap.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, Bruno M, Gerecke DR, Gordon MK, Laskin DL. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol. Appl. Pharmacol. 2003;192:119–130. doi: 10.1016/s0041-008x(03)00273-4. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, et al. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: Potential role of tumor necrosis factor-α and interleukin-10. Toxicol. Appl. Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999;6:573–584. doi: 10.1038/sj.gt.3300858. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobini C, Menini S, Ricci C, Fantauzzi CB, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M, et al. Galectin-3 ablation protects mice from diet-induced NASH: A major scavenging role for galectin-3 in liver. J. Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Investig. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, Mukaida N. The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J. Leukoc. Biol. 2004;75:59–67. doi: 10.1189/jlb.0403152. [DOI] [PubMed] [Google Scholar]
- Ito Y, Abril ER, Bethea NW, McCuskey RS. Inhibition of matrix metalloproteinases minimizes hepatic microvascular injury in response to acetaminophen in mice. Toxicol. Sci. 2005;83:190–196. doi: 10.1093/toxsci/kfh291. [DOI] [PubMed] [Google Scholar]
- Janelle-Montcalm A, Boileau C, Poirier F, Pelletier JP, Guevremont M, Duval N, Martel-Pelletier J, Reboul P. Extracellular localization of galectin-3 has a deleterious role in joint tissues. Arthritis Res. Ther. 2007;9:R20. doi: 10.1186/ar2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J. Immunol. 2010;185:7037–7046. doi: 10.4049/jimmunol.1000154. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- Laskin JD, Heck DE, Laskin DL. Nitric oxide pathways in toxic responses. In: Ballantyne B, Marrs T, T. Syversen, editors. General and Applied Toxicology, Chapter 17, pp. 425--438. 3rd ed. Oxford, U.K: Wiley-Blackwell; 2010. [Google Scholar]
- Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol. Appl. Pharmacol. 1986;86:204–215. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pilaro AM, Ji S. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol. Appl. Pharmacol. 1986;86:216–226. doi: 10.1016/0041-008x(86)90052-9. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a β-galactoside-binding lectin, in human monocytes and macrophages. Am. J. Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol. Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Brown EP, Al Rabesi Z, Shahin A, Al Shamsi M, Arsenijevic N, Hsu DK, Liu FT, Lukic ML. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009;130:83–88. doi: 10.1016/j.clim.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Naito M, Hasegawa G, Ebe Y, Yamamoto T, et al. Differentiation and function of Kupffer cells. Med. Electron Microsc. 2004;37:16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D'Amato M, Sitaraman SV, et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J. Clin. Invest. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, et al. Role of galectin-3 in human pulmonary fibrosis. Allergol. Int. 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J. Endocrinol. 2009;201:169–184. doi: 10.1677/JOE-08-0512. [DOI] [PubMed] [Google Scholar]
- Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, Greaves DR. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Throm. Vasc. Biol. 2008;28:433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J. Pharmacol. Exp. Ther. 1973;187:203–210. [PubMed] [Google Scholar]
- Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, Pohl LR. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem. Res. Toxicol. 2001;14:1620–1628. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: Addressing its expression under the harmful conditions. J. Radiat. Res. (Tokyo) 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Shen J, Pavone A, Mikulec C, Hensley SC, Traner A, Chang TK, Person MD, Fischer SM. Protein expression profiles in the epidermis of cyclooxygenase-2 transgenic mice by 2-dimensional gel electrophoresis and mass spectrometry. J. Proteome Res. 2007;6:273–286. doi: 10.1021/pr060418h. [DOI] [PubMed] [Google Scholar]
- Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, Matsuyama T. Induction of macrophage-inflammatory protein-3α gene expression by TNF-dependent NF-κB activation. J. Immunol. 2002;168:5621–5628. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Patel KJ, Nilsen-Hamilton M, Heck DE, Laskin JD, Laskin DL. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp. Mol. Pathol. 2007;83:177–187. doi: 10.1016/j.yexmp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utans-Schneitz U, Lorez H, Klinkert WE, da Silva J, Lesslauer W. A novel rat CC chemokine, identified by targeted differential display, is upregulated in brain inflammation. J. Neuroimmunol. 1998;92:179–190. doi: 10.1016/s0165-5728(98)00204-5. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. Forthcoming doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- Weber M, Sporrer D, Weigert J, Wanninger J, Neumeier M, Wurm S, Stogbauer F, Kopp A, Bala M, Schaffler A, et al. Adiponectin downregulates galectin-3 whose cellular form is elevated whereas its soluble form is reduced in type 2 diabetic monocytes. FEBS Lett. 2009;583:3718–3724. doi: 10.1016/j.febslet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1β in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 2010;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, Apgar JR, Kawakami T, Lilly CM, Liu FT. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am. J. Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]