Abstract
Organic anion transporting polypeptide 1a1 (Oatp1a1) is predominantly expressed in livers of mice and is thought to transport bile acids (BAs) from blood into liver. Because Oatp1a1 expression is markedly decreased in mice after bile duct ligation (BDL). We hypothesized that Oatp1a1-null mice would be protected against liver injury during BDL-induced cholestasis due largely to reduced hepatic uptake of BAs. To evaluate this hypothesis, BDL surgeries were performed in both male wild-type (WT) and Oatp1a1-null mice. At 24 h after BDL, Oatp1a1-null mice showed higher serum alanine aminotransferase levels and more severe liver injury than WT mice, and all Oatp1a1-null mice died within 4 days after BDL, whereas all WT mice survived. At 24 h after BDL, surprisingly Oatp1a1-null mice had higher total BA concentrations in livers than WT mice, suggesting that loss of Oatp1a1 did not prevent BA accumulation in the liver. In addition, secondary BAs dramatically increased in serum of Oatp1a1-null BDL mice but not in WT BDL mice. Oatp1a1-null BDL mice had similar basolateral BA uptake (Na+-taurocholate cotransporting polypeptide and Oatp1b2) and BA-efflux (multidrug resistance–associated protein [Mrp]-3, Mrp4, and organic solute transporter α/β) transporters, as well as BA-synthetic enzyme (Cyp7a1) in livers as WT BDL mice. Hepatic expression of small heterodimer partner Cyp3a11, Cyp4a14, and Nqo1, which are target genes of farnesoid X receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and NF-E2-related factor 2, respectively, were increased in WT BDL mice but not in Oatp1a1-null BDL mice. These results demonstrate that loss of Oatp1a1 function exacerbates cholestatic liver injury in mice and suggest that Oatp1a1 plays a unique role in liver adaptive responses to obstructive cholestasis.
Keywords: Oatp transporter, bile acids, cholestasis
Cholestasis is a reduction of bile flow, resulting in the hepatic retention of products normally excreted into bile, such as cholesterol and BAs. Acute and chronic cholestasis results in hepatocellular injury, bile duct proliferation, fibrosis, cirrhosis, and eventually liver failure (Sellinger and Boyer, 1990). Some of the bile acids (BAs), especially hydrophobic BAs such as lithocholic acid (LCA), can cause cell injury in cultured hepatocytes (Perez and Briz, 2009). Therefore, the predominant mechanistic hypothesis of the hepatocellular injury during cholestasis assumes that accumulation of BAs in hepatocytes is the main cause of cell death (Guicciardi and Gores, 2002; Perez and Briz, 2009).
BAs are synthesized from cholesterol, conjugated with taurine or glycine in liver, secreted into bile, and then delivered to the intestine. The majority of BAs are absorbed from the intestine and return to the liver, which together is known as the enterohepatic circulation of BAs (Hofmann and Hagey, 2008; Klaassen and Aleksunes, 2010). In rodents, primary BAs, which are synthesized in liver, include cholic acid (CA), chenodeoxycholic acid (CDCA), α-muricholic acid (αMCA), and βMCA. Secondary BAs are formed from primary BAs by bacterial enzymes in the intestine. The major secondary BAs in rodents are deoxycholic acid (DCA), LCA, and murideoxycholic acid (MDCA), which are 7-dehydroxylation products of CA, CDCA, and α/βMCA, respectively.
Adaptive responses in liver during cholestasis include suppression of BA uptake, induction of BA efflux, inhibition of BA synthesis, and acceleration of BA detoxification (Boyer, 2007; Copple et al., 2010). For example, bile duct ligation (BDL) has been shown to: (1) decrease BA-uptake transporters (such as Na+-taurocholate cotransporting polypeptide [Ntcp], Organic anion transporting polypeptide 1a1 [Oatp1a1], and Oatp1b2), (2) increase BA-efflux transporters (such as bile salt export pump [Bsep], multidrug resistance–associated protein 3 [Mrp3], Mrp4, and organic solute transporter β [Ostβ]), and (3) suppress BA-synthetic enzymes (Cyp7a1) in livers of mice (Park et al., 2008; Slitt et al., 2007; Soroka et al., 2010). Whereas BDL has been shown to increase the total BA concentrations in both serum and livers of mice, little is known about the effects of BDL on the concentrations of individual BAs in serum and livers of mice.
Mouse Oatp1a1 is implicated to be important in hepatic uptake of xenobiotics and endogenous compounds (Hagenbuch et al., 2000). Oatp1a1 has been shown to transport BAs, such as tauro-cholic acid (TCA) in vitro (Hagenbuch et al., 2000). Thus, Oatp1a1 is proposed to transport BAs from blood into liver in vivo. Because Oatp1a1 expression is markedly decreased in bile duct–ligated mice (Slitt et al., 2007; Zhang et al., 2011b), we hypothesized that Oatp1a1-null mice would be protected against liver injury during BDL-induced cholestasis resulting from reduced hepatic uptake of BAs. To evaluate this hypothesis, liver toxicity, BA composition, as well as gene expression of BA transporters and BA-synthetic genes in wild-type (WT) and Oatp1a1-null mice were evaluated following BDL. The purpose of this study is to determine the in vivo role of Oatp1a1 in BDL-induced extrahepatic cholestasis.
MATERIALS AND METHODS
Chemicals and reagents.
BA standards were purchased from Steraloids, Inc. (Newport, Rhode Island) and Sigma-Aldrich (St Louis, MO). Ntcp (K4) and Bsep (K44) antibodies were generous gifts of Bruno Steiger (University Hospital, Zurich, Switzerland). Mrp3 (M3II-2) antibody was obtained from George Scheffer (Vrije Universiteit Medical Center, Amsterdam). Polyclonal antibody of mouse Oatp1a4 was developed against mouse Oatp1a4-specific peptide (NH2-CFPGDIDSSDTDP-COOH) and was raised in rabbits by Covance Research Products, Inc. (Berkeley, CA). The β-actin antibody (ab8227) was purchased from Abcam, Inc. (Cambridge, MA). Goat anti-rabbit IgG horseradish peroxidase–linked secondary antibody was purchased from Sigma-Aldrich. All other chemicals were purchased from Sigma-Aldrich, unless otherwise noted.
Animals and breeding.
All animal studies were approved by the institutional animal care and use committee at the University of Kansas Medical Center. Eight-week-old adult male C57BL/6 WT mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Oatp1a1-null mice were bred to homozygosity on the C57BL/6 background as described previously (Gong et al., 2011). All mice were housed in American Animal Associations Laboratory Animal Care accredited facilities with a 12:12-h light:dark cycle and provided chow (Teklad Rodent Diet #8604; Harlan Teklad, Madison, WI) and water ad libitum.
Bile duct ligation.
Age-matched male WT and Oatp1a1-null mice (n = 5 per group) were individually housed in mouse cages. All surgeries were conducted under aseptic conditions. For induction of anesthesia, mice were placed into a closed-circuit chamber with an inflow of 3% isoflurane and an oxygen flow rate of 1 l/min. After induction of anesthesia, the head of each mouse was positioned into a face mask connected to an anesthesia machine with an inflow of 1% isoflurane and an oxygen flow rate of 1 l/min. Depth of anesthesia, heart rate, and respiration rate were monitored, and body temperatures were maintained at 37°C by means of heating pads. The abdomen of each mouse was shaved and swabbed with Betadiene and 70% alcohol. Surgical silk thread (7-0) was placed around the common bile duct using micro-dissection forceps. The gallbladder was removed, and the common bile duct was ligated doubly, close to the beginning of its intrapancreatic portion. Sham surgeries were performed similarly without BDL. The abdominal cavity was closed with an interlocking running stitch with 5-0 silk. The skin was then be sutured (5-0 nylon suture), and after closing the abdomen, the incision was disinfected with Betadiene. All surgeries were performed between 8:30 A.M. and 1:30 P.M. Twenty-four hour after surgery, mice were anesthetized with 4% isoflurane, blood was collected by orbital bleeding into heparinized tubes, and serum was obtained by centrifuging blood at 6000 × g for 15 min. Livers were washed with saline, frozen in liquid nitrogen, and stored at −80°C until time of analysis.
Antibiotic treatment.
Cephalosporin and neomycin combination treatment has been shown to result in nearly a complete suppression of microbial population in the intestine (Goris et al., 1986). Age-matched WT and Oatp1a1-null mice (n = 6 per group) were individually housed in mouse cages. Cephalothin (2 g/l) and neomycin (2 g/l) were dissolved in the drinking water and given to mice. Drinking water was changed daily with fresh antibiotics. On day 10, sham and BDL surgeries were performed as described above. Twenty-four hours after the surgeries, tissues were collected from the mice as described above.
Serum alanine aminotransferase, alkaline phosphatase, and total bilirubin.
Serum samples were analyzed by standard enzymatic colorimetric assays using kits for alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin in accordance with the manufacturer's protocols (Pointe Scientific Inc., Canton, MI). The absorption of each sample was assessed by spectrophotometry at wavelengths of 340, 405, and 555 nm, respectively.
Histopathology.
Liver samples were fixed in 10% formalin prior to routine processing and paraffin embedding. Liver sections (5 μm in thickness) were stained with hematoxylin and eosin for the evaluation of hepatocellular necrosis.
BA quantification.
BA concentrations were quantified by a recent method using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) (Zhang and Klaassen, 2010). BA stock solutions were diluted with 50% methanol and spiked with internal standards (2H4-glyco-chenodeoxycholic acid and 2H4-CDCA) to construct standard curves between 5 and 20,000 ng/ml. All standard curves were constructed using a 1/concentration2 weighted quadratic regression, and the correlation coefficient (r2) for all BAs was above 0.99. The limit of detection (signal/noise ratio = 3) for the various BAs was in the range of 5–10 ng/ml, which equals 0.01–0.02 nmol/ml.
Total RNA isolation.
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. Total RNA concentrations were quantified spectrophotometrically at 260 nm. Integrity of RNA samples was determined by formaldehyde-agarose gel electrophoresis with visualization by ethidium bromide fluorescence under ultraviolet light.
Multiplex suspension array.
The messenger RNA (mRNA) of various genes was quantified by multiplex suspension array (Panomics/Affymetrix, Fremont, CA). Individual gene accession numbers can be accessed at www.panomics.com (sets #21021 and #21151). Samples were analyzed using a Bio-Plex 200 System Array reader with Luminex 100 xMAP technology, and the data were acquired using Bio-Plex Data Manager version 5.0 (Bio-Rad, Hercules, CA). Assays were performed according to the manufacturer's protocol. mRNA data were normalized to Rpl13a mRNA and presented as relative light units.
Preparation of total liver protein.
Livers were homogenized in ST buffer (0.25M sucrose, 10mM Tris HCl, pH 7.4) containing protease inhibitors and centrifuged at 10,000 × g for 15 min at 4°C. After centrifuging the supernatant, which contains the membrane proteins, protein concentrations were determined using a Bradford protein assay kit from Sigma-Aldrich.
Western blot analysis.
Proteins were electrophoretically resolved after heating by use of SDS-polyacrylamide gels (4% stacking and 8–12% resolving) and transblotted overnight at 4°C onto Polyvinylidene-Fluoride-Plus membranes (Micron Separations, Westboro, MA). Membranes were blocked for 2 h in 5% nonfat dry milk with 0.1% Tween-20 in Tris-buffered saline (TBS-T). All primary and secondary antibodies were diluted in 2% nonfat dry milk in TBS-T. Primary antibody dilutions were as follows: Ntcp (K4, 1:2000), Oatp1a4 (1:500), Bsep (K44, 1:2000), and Mrp3 (M3II-2, 1:500). Blots were subsequently incubated with a species-appropriate horseradish peroxidase–conjugated secondary antibody for 1 h. Membranes were stripped and reprobed with a dilution of 1:2000 β-actin antibody (ab8227, Abcam) to confirm equal protein loading. Protein-antibody complexes were detected by use of an enhanced chemiluminescent kit (Amersham Life Science, Arlington Heights, IL) and exposed to X-ray film (Denville Scientific, Metuchen, NJ). Intensities of protein bands were determined by use of the ImageJ Analysis software (National Institutes of Health, http://rsbweb.nih.gov/ij/).
Statistical analysis.
Data are expressed as mean ± SE (n = 5–6). Data were analyzed by one-way ANOVA, followed by Duncan's post hoc test. Statistical significance was set at p < 0.05.
RESULTS
Liver Injury 24 h After BDL
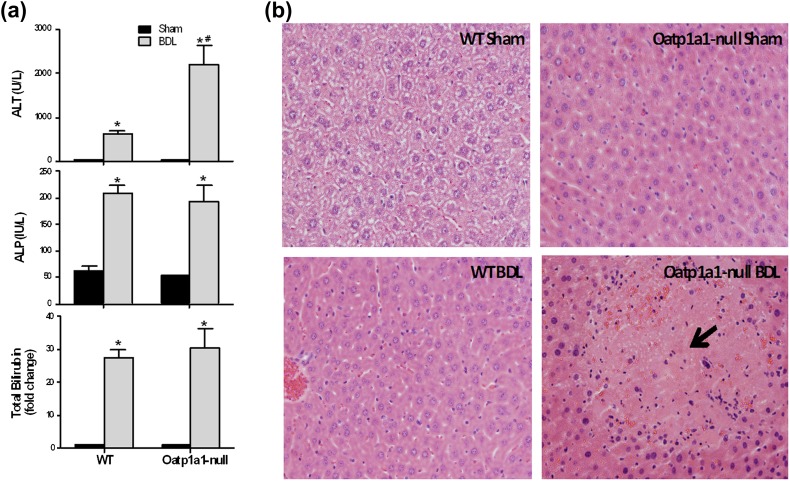
In preliminary studies, Oatp1a1-null mice became sick and stopped eating food at 48 h after BDL and started to die at about 72 h after BDL. All three Oatp1a1-null mice died within 4 days after BDL, whereas all three WT mice survived (data not shown). At 24 h after BDL, Oatp1a1-null mice did not show obvious clinical signs associated with poor health and food consumption. Therefore, to evaluate the early effects of BDL on liver, 24-h BDL surgeries were performed in both WT and Oatp1a1-null mice. BDL increased serum ALT in Oatp1a1-null BDL mice, and this transaminase was about 2.5-fold higher than that in WT BDL mice (Fig. 1a). In contrast, Oatp1a1-null BDL mice had similar ALP and total bilirubin in serum as WT BDL mice (Fig. 1a). Twenty-four hours after BDL, no obvious damage was observed in the livers of WT mice, whereas severe multifocal necrosis was observed throughout the livers in Oatp1a1-null mice (Fig. 1b). Taken together, even 24 h after BDL, Oatp1a1-null mice had higher serum ALT and more severe liver injury than WT mice.
FIG. 1.
Liver toxicity 24 h after BDL in WT and Oatp1a1-null mice. (a) Serum ALT, ALP, and total bilirubin from sham and BDL mice (n = 5 per group) were quantified with analytical kits (Pointe Scientific). All data are expressed as fold change (mean ± SE) for five mice in each group. *Statistically significant difference between sham and BDL groups (p < 0.05). (b) Histological analysis of liver sections from WT and Oatp1a1-null mice 24 h after BDL. Liver sections (5 μm) were stained with hematoxylin-eosin.
Total BA Concentrations in Serum and Livers 24 h After BDL
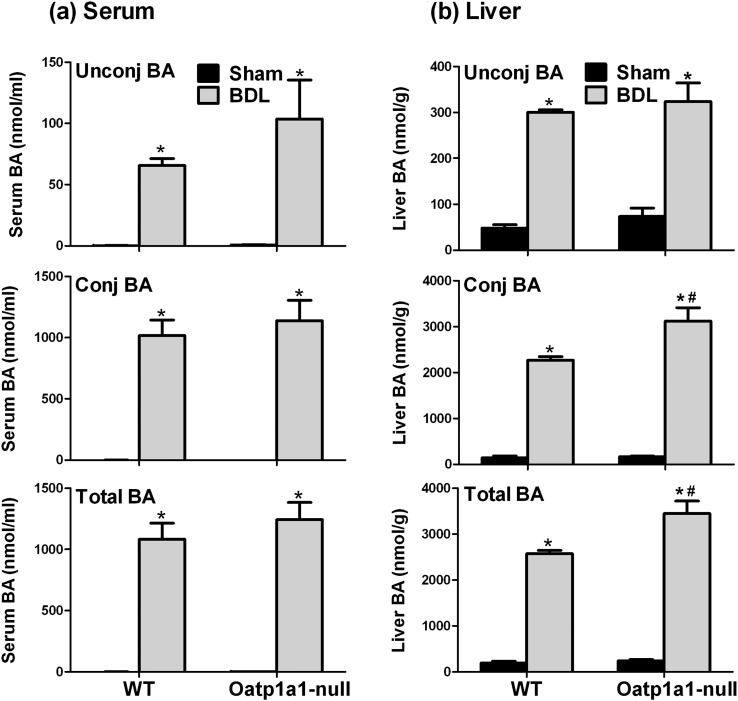
To investigate whether BAs contribute to the differences in BDL-induced liver injury between WT and Oatp1a1-null mice, the concentrations of BAs in serum and livers of WT BDL and Oatp1a1-null BDL mice were quantified. Figure 2a illustrates the concentrations of unconjugated BAs, conjugated BAs, and total BAs in serum of WT and Oatp1a1-null BDL mice. BDL in both WT and Oatp1a1-null mice resulted in a marked increase in serum unconjugated and conjugated BAs. Mainly due to the increase in conjugated BAs, the concentrations of total BAs in serum of WT BDL and Oatp1a1-null BDL mice were increased about 910- and 750-fold, respectively. Taken together, total BAs in serum were not significantly different between WT BDL and Oatp1a1-null BDL mice, although total unconjugated BAs in serum of Oatp1a1-null BDL mice (103.7 nmol/ml) tended to be higher than that in WT BDL mice (65 nmol/ml).
FIG. 2.
Unconjugated, conjugated, and total BA concentrations in serum (a) and livers (b) of WT and Oatp1a1-null mice 24 h after BDL. All data are expressed as mean ± SE for five mice in each group. *Statistically significant difference between the sham-operated and BDL mice (p < 0.05). #Statistically significant difference between the WT BDL and Oatp1a1-null BDL mice (p < 0.05).
Figure 2b illustrates the concentrations of unconjugated BAs, conjugated BAs, and total BAs in livers of WT BDL and Oatp1a1-null BDL mice. Twenty-four hours after BDL, total unconjugated BAs were increased similarly (about fivefold–sixfold) in livers of WT and Oatp1a1-null mice, whereas total conjugated BAs were about 35% higher in livers of Oatp1a1-null mice than that in WT mice. In addition, Oatp1a1-null BDL mice (240→3450 nmol/g) also had about 35% higher concentration of total BAs in livers than WT BDL mice (190→2570 nmol/g). Taken together, Oatp1a1-null BDL mice had slightly higher concentrations of total BAs in livers than WT BDL mice.
Individual BA Concentrations in Serum and Livers 24 h After BDL
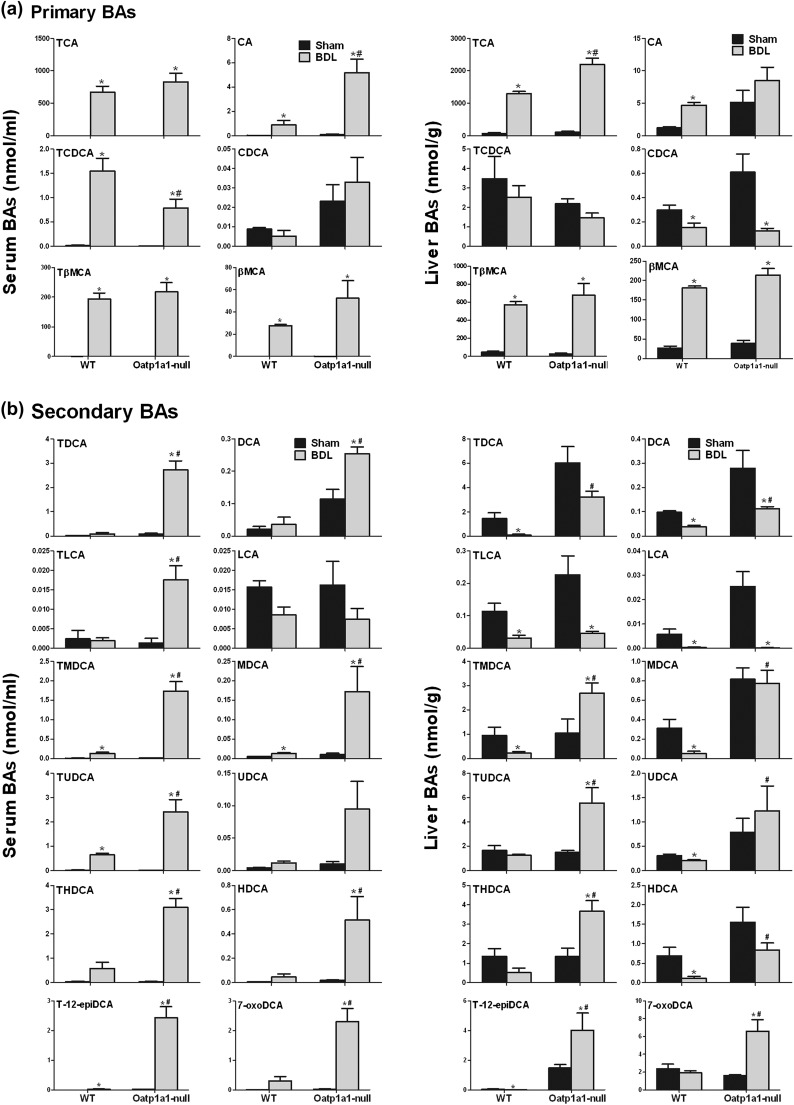
The concentrations of total BAs in serum and livers cannot explain the dramatic difference in BDL-induced liver injury between WT and Oatp1a1-null mice. During UPLC-MS/MS analysis, it was noted that tauro-dihyroxy BAs in serum and livers were markedly different between WT BDL and Oatp1a1-null BDL mice (Supplementary fig. 1). Tauro-12epi-DCA (T-12epiDCA) was identified by the retention time and mass spectrum of authentic standards. The secondary BAs such as T-12epiDCA and tauro-deoxycholic acid (TDCA) were virtually nondetectable in serum and livers of WT BDL mice but markedly increased in Oatp1a1-null BDL mice.
Figure 3a illustrates the concentrations of the primary BAs in serum and livers of WT BDL and Oatp1a1-null BDL mice. The serum of both WT BDL and Oatp1a1-null BDL mice had a prominent increase in TCA, CA, TβMCA, and βMCA. The livers of both WT BDL and Oatp1a1-null BDL mice had a similar increase in TCA, TβMCA, and βMCA. CA was increased in livers of WT BDL mice but not in Oatp1a1-null BDL mice. However, Oatp1a1-null BDL mice had fivefold higher CA and 50% lower tauro-chenodeoxycholic acid (TCDCA) in serum as well as 70% higher TCA in serum.
FIG. 3.
Individual primary and secondary BAs in serum and livers of WT and Oatp1a1-null mice 24 h after BDL. The concentrations of primary (a) and secondary (b) BAs in serum and livers of WT and Oatp1a1-null mice (n = 5 per group) were analyzed by UPLC-MS/MS. All data are expressed as mean ± SE of five mice in each group. *Statistically significant difference between WT and Oatp1a1-null mice (p < 0.05).
Figure 3b illustrates the concentrations of secondary BAs in serum and livers of WT BDL and Oatp1a1-null BDL mice. BDL in WT mice had little effect on serum TDCA, DCA, tauro-litho (TLCA), LCA, ursodeoxycholic acid (UDCA), tauro-hyodeoxycholic acid (THDCA), hyodeoxycholic acid (HDCA), or 7-oxoDCA. In contrast, BDL markedly increased TDCA, DCA, TLCA, THDCA, HDCA, and 7-oxoDCA in serum of Oatp1a1-null mice. BDL also markedly increased tauro-murideoxycholic acid (TMDCA), murideoxycholic acid (MDCA), tauro-ursodeoxycholic acid (TUDCA), and T-12epiDCA in serum of Oatp1a1-null mice. Taken together, at 24 h after BDL, secondary BAs such as DCA, TLCA, TMDCA, MDCA, TUDCA, THDCA, HDCA, and 7-oxoDCA were 3- to 12-fold higher in serum of Oatp1a1-null than WT mice, whereas TDCA and T-12epiDCA were increased by about 30- and 75-fold, respectively, in serum of Oatp1a1-null but not WT mice.
BDL decreased hepatic concentrations of TDCA, DCA, TLCA, LCA, TMDCA, MDCA, UDCA, HDCA, and T-12epiDCA in WT mice (Fig. 3b). In contrast, BDL increased TMDCA and T-12epiDCA and had little effect on TDCA, MDCA, UDCA, and HDCA in livers of Oatp1a1-null mice. In addition, BDL markedly increased TUDCA, THDCA, and 7-oxoDCA in livers of Oatp1a1-null but not WT mice. Taken together, at 24 h after BDL, secondary BAs such as DCA, TMDCA, MDCA, TUDCA, UDCA, THDCA, HDCA, and 7-oxoDCA were about 2- to 14-fold higher in livers of Oatp1a1-null than WT mice, whereas TDCA and T-12epiDCA were about 30- and 510-fold, respectively, higher in livers of Oatp1a1-null than WT mice.
mRNA Expression of BA Transporters 24 h After BDL
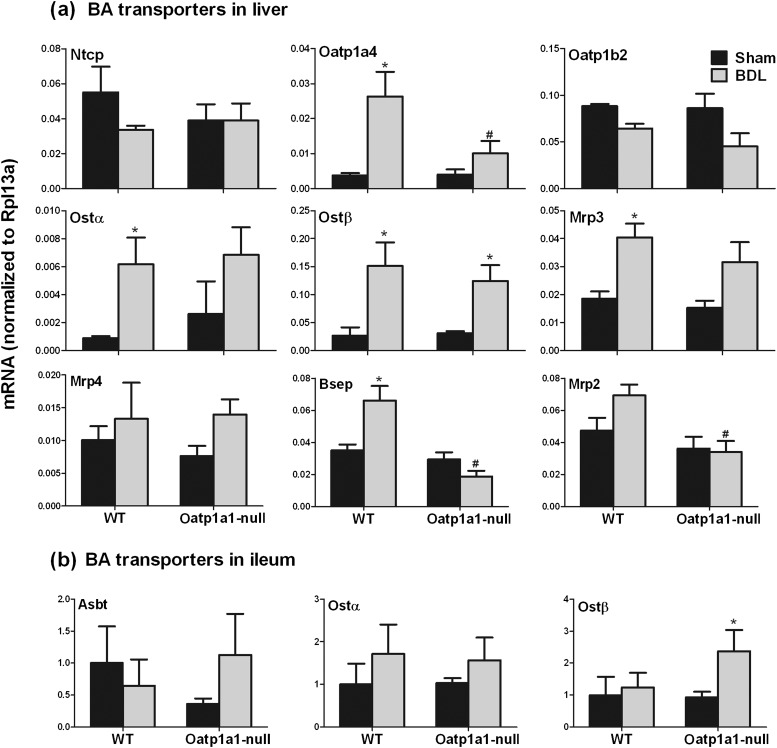
To investigate whether the increase of secondary BAs in serum of Oatp1a1-null BDL mice is due to altered BA transport, the mRNA expression of BA transporters in livers (Fig. 4a) and ilea (Fig. 4b) of WT BDL and Oatp1a1-null BDL mice were quantified. Twenty-four hours after BDL, there was little effect on Ntcp and Oatp1b2 in either WT or Oatp1a1-null mice (Fig. 4a). BDL increased Oatp1a4 about fivefold in WT mice but not in Oatp1a1-null mice. BDL significantly increased Ostα/β expression about fourfold in WT mice, whereas Ostα was unaltered, and Ostβ was increased about threefold in Oatp1a1-null mice. BDL increased Mrp3 about 120% and had little effect on Mrp4 in WT mice. In contrast, BDL had little effect on either Mrp3 or Mrp4 in Oatp1a1-null mice. Bsep and Mrp2 are canalicular BA-efflux transporters that efflux BAs from liver to bile. BDL increased Bsep about 88% and had no effect on Mrp2 in WT mice. In contrast, BDL had little effect on either Bsep or Mrp2 in Oatp1a1-null mice. Taken together, Oatp1a1-null BDL mice had about 60% lower Oatp1a4, 70% lower Bsep, and 50% lower Mrp2 in livers than WT BDL mice.
FIG. 4.
The mRNA expression of BA transporters in livers (a) and ilea (b) of WT and Oatp1a1-null mice 24 h after BDL. Total RNA from livers and ilea of sham-operated and BDL mice was analyzed by multiplex suspension array. The mRNAs were normalized to Rpl13a. All data are expressed as mean ± SE for five mice in each group. *Statistically significant difference between the sham-operated and BDL mice (p < 0.05). #Statistically significant difference between the WT BDL and Oatp1a1-null BDL mice (p < 0.05).
Figure 4b illustrates the mRNA expression of BA transporters in ilea of WT BDL and Oatp1a1-null BDL mice. Asbt is the major BA-uptake transporter for conjugated BAs in ileum. BDL had little effect on Asbt in either WT or Oatp1a1-null mice. Ostα/β are the major BA-efflux transporters in ileum. BDL had little effect on Ostα in either WT or Oatp1a1-null mice. BDL increased Ostβ about 120% in Oatp1a1-null but not in WT mice. Taken together, Oatp1a1-null BDL mice had about 90% higher Ostβ in ilea than WT BDL mice.
Western Blot Analysis of BA Transporters in Livers of Mice 24 h After BDL
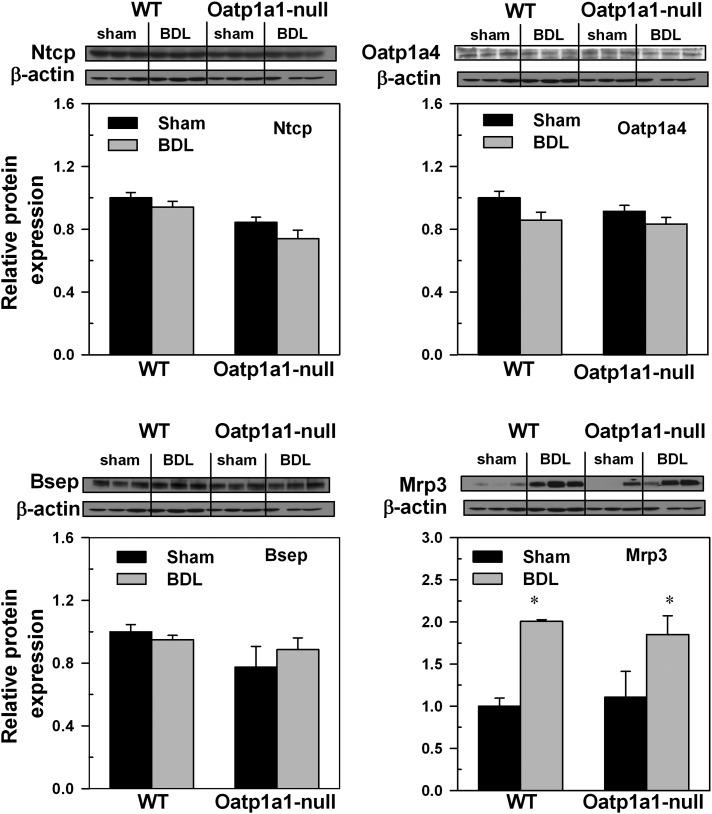
Western blot analyses were performed on four key BA transporters for which antibodies were available. As shown in Figure 5, BDL had little effect on protein levels of Ntcp in both WT and Oatp1a1-null mice. Although BDL increased the mRNA expression of Oatp1a4 and Bsep in WT mice, Western blot analyses demonstrated that BDL had little effect on the protein levels of Oatp1a4 or Bsep in either WT or Oatp1a1-null mice. In contrast, BDL increased the amount of Mrp3 protein in both WT and Oatp1a1-null mice. Taken together, Oatp1a1-null BDL mice had similar protein levels of Ntcp, Oatp1a4, Bsep, or Mrp3 in livers as WT BDL mice.
FIG. 5.
Ntcp, Oatp1a4, Bsep, and Mrp3 protein abundance in livers of mice 24 h following BDL. Western blots for Ntcp, Oatp1a4, Bsep, and Mrp3 were performed with use of liver membrane protein fraction (40 μg protein/lane) from sham-operated and BDL mice. β-Actin blot was used as a protein loading control for each transporter. Bars represent the relative Ntcp, Oatp1a4, Bsep, and Mrp3 protein amounts ± SE of three mice. The scales for mean relative protein expression differ for each transport protein. *Statistical difference (p < 0.05) from the respective values of sham-operated mice.
mRNA Expression of BA-Synthetic Enzymes 24 h After BDL
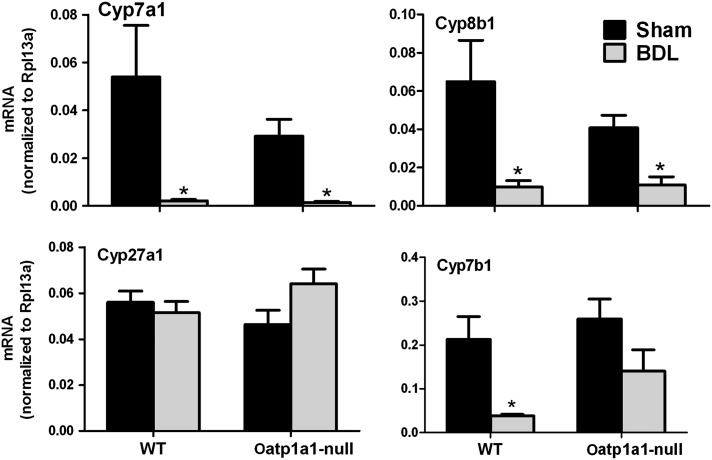
To investigate whether the increase of secondary BAs in serum of Oatp1a1-null BDL mice is due to altered BA synthesis, the mRNA expression of BA-synthetic enzymes in livers of WT BDL and Oatp1a1-null BDL mice was quantified (Fig. 6). Cyp7a1 and 8b1 are the two critical enzymes in the classic pathway of BA synthesis. BDL decreases Cyp7a1 (95%) and Cyp8b1 (85%) in WT mice. BDL also markedly decreased Cyp7a1 (95%) and Cyp8b1 (75%) in Oatp1a1-null mice. Cyp27a1 and Cyp7b1 are involved in the alternative pathway of BA synthesis. BDL had little effect on Cyp27a1 in either WT or Oatp1a1-null mice. BDL markedly decreased Cyp7b1 (82%) in WT but not in Oatp1a1-null mice. Taken together, Oatp1a1-null BDL mice had similar mRNA expression of BA-synthetic enzymes as WT BDL mice.
FIG. 6.
The mRNA expression of BA-synthetic enzymes in livers of WT and Oatp1a1-null mice 24 h after BDL. Total RNA from livers of sham-operated and BDL mice was analyzed by multiplex suspension array. The mRNAs were normalized to Rpl13a. All data are expressed as mean ± SE for five mice in each group. *Statistically significant difference between the sham-operated and BDL mice (p < 0.05). #Statistically significant difference between the WT BDL and Oatp1a1-null BDL mice (p < 0.05).
mRNA Expression of FXR/LXR/SHP/LRH-1 24 h After BDL
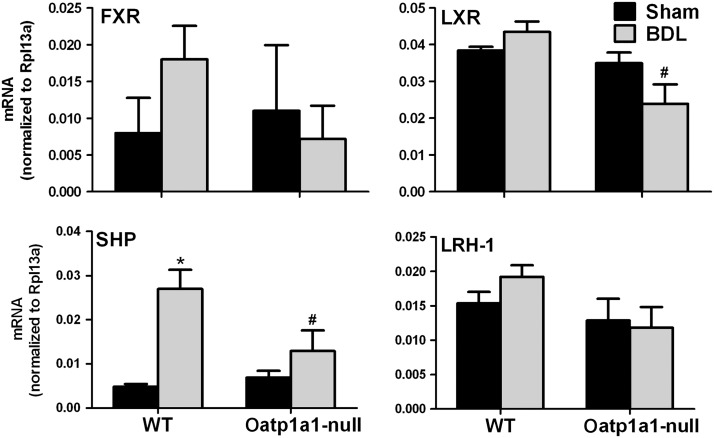
To investigate the mechanism for BDL-induced suppression of Cyp7a1, the mRNA expression of farnesoid X receptor (FXR), liver X receptor (LXR), small heterodimer partner (SHP), and liver receptor homolog-1 (LRH-1), which have been shown to regulate Cyp7a1, was quantified in livers of both WT BDL and Oatp1a1-null BDL mice (Fig. 7). BDL tended to increase FXR in WT mice but not in Oatp1a1-null mice. BDL had little effect on LXR in either WT or Oatp1a1-null mice, but Oatp1a1-null BDL mice had significantly lower LXR than WT BDL mice. BDL significantly increased SHP about 3.5-fold in WT but not in Oatp1a1-null mice. BDL had little effect on LRH-1 in either WT or Oatp1a1-null mice. Fgf15 in the ilea also plays an important role in the regulation of Cyp7a1. BDL had little effect on ileal Fgf15 in either WT or Oatp1a1-null mice (Supplementary fig. 2). Taken together, Oatp1a1-null BDL mice had about 45% lower LXR and 50% lower SHP in livers than WT BDL mice.
FIG. 7.
The mRNA expression of FXR, LXR, SHP, and LRH-1 in livers of WT and Oatp1a1-null mice 24 h after BDL. Total RNA from livers of sham-operated and BDL mice was analyzed by multiplex suspension array. The mRNAs were normalized to Rpl13a. All data are expressed as mean ± SE for five mice in each group. *Statistically significant difference between the sham-operated and BDL mice (p < 0.05). #Statistically significant difference between the WT BDL and Oatp1a1-null BDL mice (p < 0.05).
PXR/CAR/PPARα/Nrf2 Target Genes 24 h After BDL
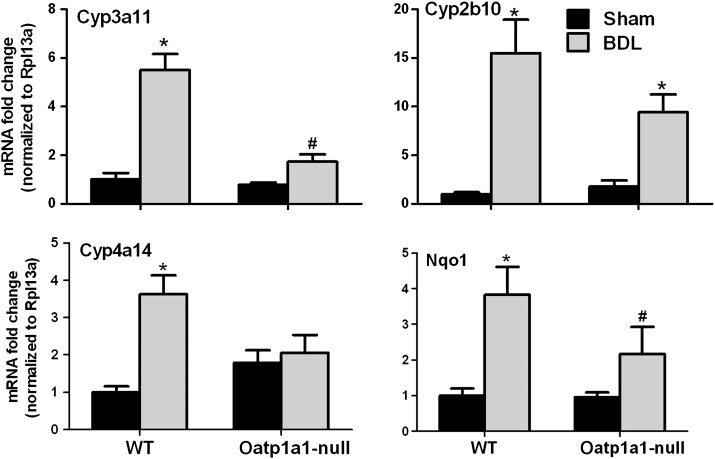
Activations of pregnane X receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor alpha (PPARα), and/or NF-E2-related factor 2 (Nrf2) are possible adaptive responses in mice to protect against cholestatic liver injury. Figure 8 illustrates the mRNA expression of PXR/CAR/PPARα/Nrf2 target genes, namely Cyp3a11, Cyp2b10, Cyp4a14, and NAD(P)H quinone oxidoreductase 1 (Nqo1), respectively, in livers of WT BDL and Oatp1a1-null BDL mice. BDL increased Cyp3a11 about 4.5-fold in WT but not in Oatp1a1-null mice. BDL increased Cyp2b10 in both WT (14-fold) and Oatp1a1-null mice (8-fold). BDL increased Cyp4a14 about 2.5-fold in WT but not in Oatp1a1-null mice. In addition, BDL increased Nqo1 about threefold in WT but not in Oatp1a1-null mice. Taken together, Oatp1a1-null BDL mice had about 70% lower Cyp3a11 and 45% lower Nqo1 than WT BDL mice.
FIG. 8.
The mRNA expression of Cyp3a11, Cyp2b10, Cyp4a14, and Nqo1 in livers of WT and Oatp1a1-null mice 24 h after BDL. Total RNA from livers of sham-operated and BDL mice was analyzed by multiplex suspension array. The mRNAs were normalized to Rpl13a. All data are expressed as mean ± SE for five mice in each group. *Statistically significant difference between the sham-operated and BDL mice (p < 0.05). #Statistically significant difference between the WT BDL and Oatp1a1-null BDL mice (p < 0.05).
Liver Inflammation in WT and Oatp1a1-Null Mice 24 h After BDL
To investigate whether inflammation contributes to the difference in BDL-induced liver injury between WT and Oatp1a1-null mice, the mRNA expression of two inflammation markers, namely tumor necrosis factor α (TNFα) and interleukin (IL)-6, was quantified in livers of WT and Oatp1a1-null BDL mice. As shown in Supplementary figure 3, BDL tended to, but not significantly, increase TNFα in livers of both WT and Oatp1a1-null mice. BDL increased IL-6 significantly in WT mice (about 120%) but not significantly in Oatp1a1-null mice. Taken together, Oatp1a1-null BDL mice had similar mRNA expression of TNFα and IL-6 in livers as WT BDL mice.
BDL-Induced Cholestasis in WT and Oatp1a1-Null Mice Treated With Antibiotics
Secondary BAs, which are produced by intestinal bacteria, were increased markedly in Oatp1a1-null BDL mice. To investigate whether intestinal bacteria contribute to the difference in BDL-induced liver injury between WT and Oatp1a1-null mice, WT and Oatp1a1-null mice were treated with antibiotics for 10 days before BDL. Twenty-four hours after BDL, large areas of necrosis were also observed in livers of antibiotic-treated Oatp1a1-null mice but not in antibiotic-treated WT mice (Supplementary fig. 4). Antibiotic treatment decreased unconjugated (Supplementary fig. 5a, black bars) but increased conjugated BAs (Supplementary fig. 5b, black bars) in serum of Oatp1a1-null sham mice. In contrast, antibiotics increased both unconjugated (Supplementary fig. 5a, gray bars) and conjugated BAs (Supplementary fig. 5b, gray bars) in serum of Oatp1a1-null BDL mice. Interestingly, antibiotic treatment had little effect on the unconjugated (Supplementary fig. 5c, black bars) or conjugated BAs in livers of both Oatp1a1-null sham and BDL mice (Supplementary fig. 5c and 5d). After antibiotic treatment, Oatp1a1-null sham mice had markedly higher TωMCA (7-fold), TβMCA (13-fold), TCA (17-fold), TMDCA (28-fold), TUDCA (9-fold), THDCA (6-fold), TCDCA (69-fold), and TDCA (2-fold) in serum than WT sham mice (Supplementary fig. 6). In addition, after antibiotic treatment, most of the BAs in serum of Oatp1a1-null BDL mice were higher than those in WT BDL mice (Supplementary fig. 6). Taken together, antibiotic treatment did not prevent BDL-induced liver injury in Oatp1a1-null mice.
DISCUSSION
Cholestasis is a clinical syndrome that is characterized as decreased formation and/or flow of bile. This syndrome is manifested by the hepatic retention of products normally excreted into bile, in particular BAs. Limiting hepatic uptake of BAs has been proposed to be one of the adaptive responses during cholestasis (Boyer, 2007). One important question is whether therapy for cholestasis can be devised to inhibit BA-uptake transporters and thus augment this potentially beneficial adaptive response. The determinants of hepatic uptake of BAs include NTCP/Ntcp and OATPs/Oatps (Meier and Stieger, 2002). In mice, Oatp1a1, 1a4, and 1b2 are predominantly expressed in liver and are all able to transport BAs in vitro (Meier and Stieger, 2002). In the present study, we used Oatp1a1-null mice to investigate whether inhibition of Oatp1a1, a proposed BA-uptake transporter, protects against cholestatic liver injury.
The present results indicate that Oatp1a1-null mice are highly sensitive to BDL. In the acute evaluations carried out, there was more liver injury in Oatp1a1-null mice than WT mice. Compared with WT BDL mice, the total BA concentrations in Oatp1a1-null BDL mice were similar in serum, but higher in livers, suggesting that loss of Oatp1a1 function does not prevent BA accumulation in liver. Although BDL increased the mRNA expression of Oatp1a4, Mrp3, and Bsep in livers of WT BDL mice, there was no difference in the protein levels of these transporters between WT BDL and Oatp1a1-null BDL mice. The similar concentrations of total BAs in serum and livers appear to be consistent with this similar expression of the major BA transporters in livers of WT BDL and Oatp1a1-null BDL mice. A previous study from this laboratory demonstrated that accumulation of BAs in liver during cholestasis results in inflammation-mediated liver injury (Zhang et al., 2011b). However, the available data in the present study suggest that the exacerbated BDL-induced liver injury in Oatp1a1-null mice may be not attributed to inflammation because Oatp1a1-null BDL mice had similar expression of inflammation markers as WT BDL mice. It should be noted that Oatp1a1-null mice may establish liver injury earlier than 24 h after BDL, and thus further time-course studies between 0 and 24 h after BDL are required to evaluate the contribution of inflammation to BDL-induced liver injury in Oatp1a1-null mice.
Secondary BAs are more hydrophobic and thus more toxic than primary BAs. A previous study from this laboratory illustrated that the majority of BAs in WT BDL mice are conjugated primary BAs (Zhang et al., 2011b). However, BDL markedly increased conjugated secondary BAs, such as TDCA, TMDCA, TUDCA, and THDCA, in both serum and livers of Oatp1a1-null mice. This increase is not likely due to altered BA transport or BA synthesis in liver because Oatp1a1-null BDL mice had similar BA-uptake transporters (Ntcp and Oatp1b2), BA-efflux transporters (Mrp3, Mrp4, and Ostα/β), and BA-synthetic enzymes (Cyp7a1, 8b1, 27a1, and 7b1). Instead, this increase seems to be due to the enhanced intestinal absorption because Oatp1a1-null mice have been shown to have increased intestinal permeability (Zhang et al., 2011a). In addition, Oatp1a1-null BDL mice tended to have an increase in ileal BA transporters such as Asbt and Ostβ. Oatp1a1-null BDL mice had more TCA in livers than WT BDL mice. The CA-derived secondary BAs, such as TDCA, T-12epiDCA, and 7-oxoDCA, were almost undetectable in serum of WT BDL mice but markedly increased in serum of Oatp1a1-null BDL mice. A previous study illustrated that TCA in livers of BDL mice was markedly increased at 6 h and thereafter decreased from 6 h to 1 day after surgery (Zhang et al., 2011b). This suggests that loss of Oatp1a1 function may impair the metabolism of TCA during cholestasis. Therefore, both a decrease in liver BA detoxification and an increase in intestinal absorption of secondary BAs may contribute to the exacerbated BDL-induced liver injury in Oatp1a1-null mice.
FXR, PXR, CAR, PPARα, and Nfr2 are key nuclear receptors that participate in adaptive responses to cholestasis (Guo et al., 2003; Hays et al., 2005; Liu et al., 2003; Okada et al., 2009; Stedman et al., 2005). FXR plays a key role in the regulation of BA synthesis and transport (Sinal et al., 2000). PXR and CAR play important roles in BA-detoxifying enzymes in mice, such as the regulation of Mrp4 (Assem et al., 2004; Wagner et al., 2005). PPARα and Nrf2 have also been shown to regulate BA-detoxifying enzymes, such as Mrps and Ugts (Barbier et al., 2003; Tanaka et al., 2009). The present study shows that BDL had little effect on Mrp4 in either WT or Oatp1a1-null mice. This may be due to the early time point (1 day) of BDL in mice because Mrp4 was found to be only induced after 2 days of BDL in C57BL/6 mice (Zhang et al., 2011b). BDL markedly increased SHP, Cyp3a11, Cyp2b10, Cyp4a14, and Nqo1 in WT BDL mice, suggesting activation of FXR, PXR, CAR, PPARα, and Nrf2, respectively. In contrast, the mRNA expression of these target genes was not changed (Cyp3a11, Cyp4a14, and Nqo1) or lower (Cyp2b10) in livers of Oatp1a1-null BDL mice. These evidences suggest that Oatp1a1-null mice have an impaired cytoprotective response to BDL-induced cholestasis.
Biliary obstruction causes bacterial overgrowth and translocation in the small intestine (Ding et al., 1993). Secondary BAs, which are produced by intestinal bacteria, markedly increase in the Oatp1a1-null BDL mice. In another study, it appears that Oatp1a1-null mice have 10-fold higher bacteria in the small intestine than do WT mice (Zhang et al., 2012). This may contribute to the marked increase in secondary BAs in serum and livers of Oatp1a1-null BDL mice. In particular, T-12epiDCA, which is virtually nondetectable in WT BDL mice, markedly increases in Oatp1a1-null BDL mice, suggesting that BDL alters the intestinal bacteria composition in Oatp1a1-null mice. However, antibiotic treatment did not protect against liver injury in Oatp1a1-null BDL mice. Although these results seem to suggest that intestinal bacteria do not have a role in the increased sensitivity of Oatp1a1-null mice to BDL, it should be noted that antibiotic treatment markedly increases serum conjugated BAs and alters the hepatic mRNA expression of BA transporters in Oatp1a1-null sham control mice (data not shown). Originally, the use of antibiotics was proposed to be an obvious, direct method to assess the contribution of the intestinal bacteria to the marked sensitivity noted in the Oatp1a1-null BDL mice. However, the available data suggest that antibiotic treatment is not the ideal method to investigate the effect of intestinal bacteria on BDL-induced liver injury in Oatp1a1-null mice, and additional studies with germ-free animals are warranted as possible alternatives to explore the role of the intestinal flora in the toxicity associated with BDL in Oatp1a1-null mice.
The present study provides a new perspective on the in vivo functions of OATPs/Oatps. Human OATP1B1 and 1B3 are highly expressed at the hepatocyte basolateral plasma membrane and have been shown to transport BAs in vitro. However, the transport direction of OATP1B1 and 1B3 is controversial. For example, OATP1B3 may mediate the extrusion of organic anions (such as BAs) by symporting with glutathione (Briz et al., 2006). Mouse Oatp1b2 is orthologous to human OATP1B1 and OATP1B3. Our previous study illustrated that Oatp1b2-null mice have an increase in unconjugated but not conjugated BAs in serum (Csanaky et al., 2011), indicating that Oatp1b2 plays important roles in the hepatic uptake of unconjugated BAs. van de Steeg et al. (2010) also demonstrated that mice null for Oatp1a/1b transporters (Oatp1a1,1a4, 1a5, 1a6, and 1b2) have increased unconjugated BAs in plasma. In contrast, Oatp1a1-null mice do not have dramatic alterations in serum BAs or bilirubin (Gong et al., 2011; Zhang et al., 2011a). Thus, Oatp1a1 may have a different in vivo function than Oatp1b2, which may have the predominant contribution to the phenotype observed in Oatp1a/1b-null mice. It has been demonstrated that Oatp1a1 and 1a4 can be potentially bidirectional (Li et al., 2000). Given the exacerbated liver injury in Oatp1a1-null BDL mice, the role of Oatp1a1 as a BA efflux mechanism during cholestasis remains a possibility.
In summary, the present data demonstrate that Oatp1a1 plays a unique and essential role during obstructive cholestasis. The downregulation of Oatp1a1 during BDL suggests that inhibition of Oatp1a1 may be beneficial for the cholestatic liver disease. However, dysfunction of Oatp1a1 increases intestinal bacteria and intestinal permeability and may also impact the normal function of important transcription factors in the liver. Therefore, the long-term deficiency of Oatp1a1 may sensitize the livers of mice for future insults such as cholestasis. The findings in the present study provide new perspectives on the roles of OATPs/Oatps in BA metabolism, homeostasis of intestinal bacteria, and liver disease.
FUNDING
National Institutes of Health (grant number ES009649 and RR021940).
Supplementary Material
Acknowledgments
The authors would like to thank the members in Dr Klaassen's lab for their assistance in tissue collection and manuscript review. Dr I.L.C. made almost equal contributions as the first author.
References
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J. Biol. Chem. 2004;279:22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- Barbier O, Duran-Sandoval D, Pineda-Torra I, Kosykh V, Fruchart JC, Staels B. Peroxisome proliferator-activated receptor alpha induces hepatic expression of the human bile acid glucuronidating UDP-glucuronosyltransferase 2B4 enzyme. J. Biol. Chem. 2003;278:32852–32860. doi: 10.1074/jbc.M305361200. [DOI] [PubMed] [Google Scholar]
- Boyer JL. New perspectives for the treatment of cholestasis: Lessons from basic science applied clinically. J. Hepatol. 2007;46:365–371. doi: 10.1016/j.jhep.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz O, Romero MR, Martinez-Becerra P, Macias RI, Perez MJ, Jimenez F, San Martin FG, Marin JJ. OATP8/1B3-mediated cotransport of bile acids and glutathione: An export pathway for organic anions from hepatocytes? J. Biol. Chem. 2006;281:30326–30335. doi: 10.1074/jbc.M602048200. [DOI] [PubMed] [Google Scholar]
- Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53:10. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JW, Andersson R, Soltesz V, Willen R, Loft S, Poulsen HE, Parsson H, Olsson K, Bengmark S. The effect of biliary decompression on bacterial translocation in jaundiced rats. HPB Surg. 1993;7:99–110. doi: 10.1155/1993/69283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LL, Aranibar N, Han YH, Zhang Y, Lecureux L, Bhaskaran V, Khandelwal P, Klaassen CD, Lehman-McKeeman LD. Characterization of organic anion transporting polypeptide (Oatp) 1a1 and 1a4 null mice reveals altered transport function and urinary metabolomic profiles. Toxicol. Sci. 2011;122:587–597. doi: 10.1093/toxsci/kfr114. [DOI] [PubMed] [Google Scholar]
- Goris H, de Boer F, van der Waaij D. Oral administration of antibiotics and intestinal flora associated endotoxin in mice. Scand. J. Infect. Dis. 1986;18:55–63. doi: 10.3109/00365548609032307. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig. Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Adler ID, Schmid TE. Molecular cloning and functional characterization of the mouse organic-anion-transporting polypeptide 1 (Oatp1) and mapping of the gene to chromosome X. Biochem. J. 2000;345:115–120. [PMC free article] [PubMed] [Google Scholar]
- Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26:219–227. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: A role for intracellular glutathione. Mol. Pharmacol. 2000;58:335–340. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Investig. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier PJ, Stieger B. Bile salt transporters. Annu. Rev. Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Sugimoto H, Utsunomiya H, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem. Biophys. Res. Commun. 2009;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- Park YJ, Qatanani M, Chua SS, LaRey JL, Johnson SA, Watanabe M, Moore DD, Lee YK. Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology. 2008;47:1578–1586. doi: 10.1002/hep.22196. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellinger M, Boyer JL. Physiology of bile secretion and cholestasis. Prog. Liver Dis. 1990;9:237–259. [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim. Biophys. Acta. 2007;1768:637–647. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol. Sci. 2009;108:247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J. Clin. Investig. 2010;120:2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Csanaky IL, Lehman-McKeeman LD, Klaassen CD. Loss of organic anion transporting polypeptide 1a1 increases deoxycholic acid absorption in mice by increasing intestinal permeability. Toxicol. Sci. 2011a;124:251–260. doi: 10.1093/toxsci/kfr236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CR, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2011b;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J. Lipid Res. 2010;51:13. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Lehman-McKeeman LD, Klaassen CD. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PLoS One. 2012;7:e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.