Summary
Perforation of intracranial aneurysms during endovascular treatment with platinum microcoils is a well-known and serious complication reported to occur in 2-4% of patients.
Inflation of a remodelling balloon across the aneurysm neck or within the proximal parent vessel is an additional technique that theoretically might be useful to reduce flow within the aneurysm and achieve hemostasis.
In the case reports that follow, we present our experience using this technique for managing intraprocedural aneurysm rupture.
Key words: brain aneurysm, complications, embolization
Introduction
Perforation of intracranial aneurysms during endovascular treatment with platinum microcoils is a well-known and serious complication. Iatrogenic rupture of an intracranial aneurysm during coil embolization results in a 30% to 40% rate of combined morbidity and mortality 1. Perforation of an aneurysm during endovascular coiling has been reported to occur in 2-4% of patients 2-6. Perforation of previously ruptured aneurysms occurs more frequently than unruptured aneurysm perforation 1. Once an aneurysm has been perforated during coiling, there are several techniques that can be employed to arrest further hemorrhage from the aneurysm.
Previously described techniques for managing intraprocedural rupture include induction of hypotension, reversal of heparinization using protamine, continued coiling of the aneurysm through the existing microcatheter, continued coiling after placement of a second microcatheter and emergent placement of a ventricular drainage catheter 7-12. Inflation of a remodelling balloon across the aneurysm neck or within the proximal parent vessel is an additional technique that theoretically might be useful to reduce flow within the aneurysm and achieve hemostasis.
In the case reports that follow, we present our experience using this technique for managing intraprocedural aneurysm rupture.
Case 1
Clinical Information: A 51-year-old female presented to our institution with a new onset severe headache and a head CT revealed subarachnoid hemorrhage over the right frontal and parietal convexities. She had no focal neurological deficit.
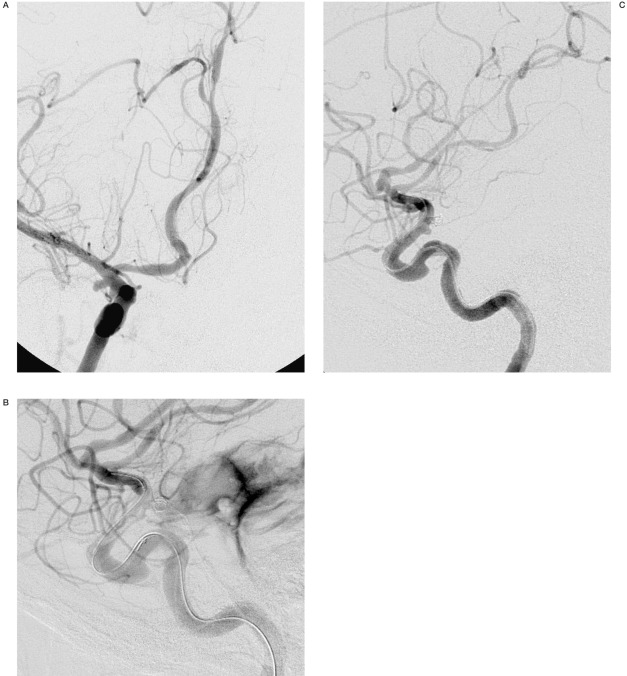
Intervention: A four-vessel cerebral angiogram including 3D rotational angiography was performed revealing a 10 mm basilar tip aneurysm with a 6mm neck. Additionally, a 2.5 mm x 2 mm right anterior choroidal artery aneurysm was discovered (Figure 1A). Given the hemorrhage pattern on CT, the anterior choroidal aneurysm was thought to be the cause of her hemorrhage and endovascular treatment was undertaken. She was placed under general anesthesia and a 6 Fr Envoy catheter (Cordis Neurovascular, Miami Lakes, FL) was placed in the right internal carotid artery. Coaxially, a 4 mm x 20 mm Hyperform balloon microcatheter (MicroTherapeutics Inc. (MTI), Irvine, CA) and an Echelon 10 microcatheter (MTI) were advanced over microguidewires into the supraclinoid internal carotid artery. The aneurysm was successfully accessed and a single 2 mm microcoil (Microsphere, Micrus Corporation, Sunnyvale, CA) was placed in the aneurysm using balloon remodelling technique. Shortly thereafter, following deflation of the balloon, the anesthesia team reported the patient was hypertensive and bradycardic. An angiogram through the guiding catheter revealed contrast extravasation into the subarachnoid space (Figure 1B). The heparinization was reversed with 50 mg Protamine (Eli Lilly, Indi-anapolis, IN) intravenously and a temporary burst suppression of 2.5mg/kg propofol (Diprivan, Astra Zeneca, London, UK) was given intravenously. The first coil was detached and attempt was made at placing a second 2 mm coil (Ultipaq, Micrus Corporation) in the aneurysm. However, this coil was noted to extend through the dome of the aneurysm. The second coil was then removed because it appeared that the coil was well outside the aneurysm sac within the adjacent subarachnoid space. The remodelling balloon was subsequently inflated across the neck of the aneurysm for 10-15 seconds and then deflated. A subsequent angiogram revealed no further contrast extravasation and the procedure was terminated (Figure 1C). She did well clinically over the next few days. A ventricular drain was placed three days after the coiling for development of mild hydrocephalus. Twelve days following the attempted choroidal aneurysm coiling the basilar tip aneurysm was successfully coiled without incident. Right internal carotid angiography at that time revealed persistent filling of the anterior choroidal artery aneurysm. The previously placed coil was noted near the dome of the aneurysm and the opacified portion of the aneurysm appeared identical to its precoiled configuration, suggesting that the coil was partially or completely extravascular. Three days later the patient underwent uneventful clipping of the anterior choroidal artery aneurysm. She left the hospital three days later without any clinical deficits.
Figure 1.
A) 51-year-old female with subarachnoid hemorrhage. Working projection right internal carotid angiogram demonstrates the anterior choroidal artery aneurysm (arrow) prior to endovascular intervention. B) Right internal carotid angiography following placement of the first coil demonstrates extravasation of contrast into the subarachnoid space (arrow). C) Following inflation of the remodelling balloon across the aneurysm neck for 10-15 seconds, angiography demonstrates cessation of contrast extravasation. The aneurysm is completely occluded (arrow).
Case 2
Clinical Information: A 70-year-old male was referred for evaluation and possible endovascular treatment of an unruptured, incidentally discovered vertebrobasilar junction aneurysm. It was during the workup for his left carotid stenosis that the vertebrobasilar junction aneurysm was discovered and he was referred to our institution for additional evaluation and treatment.
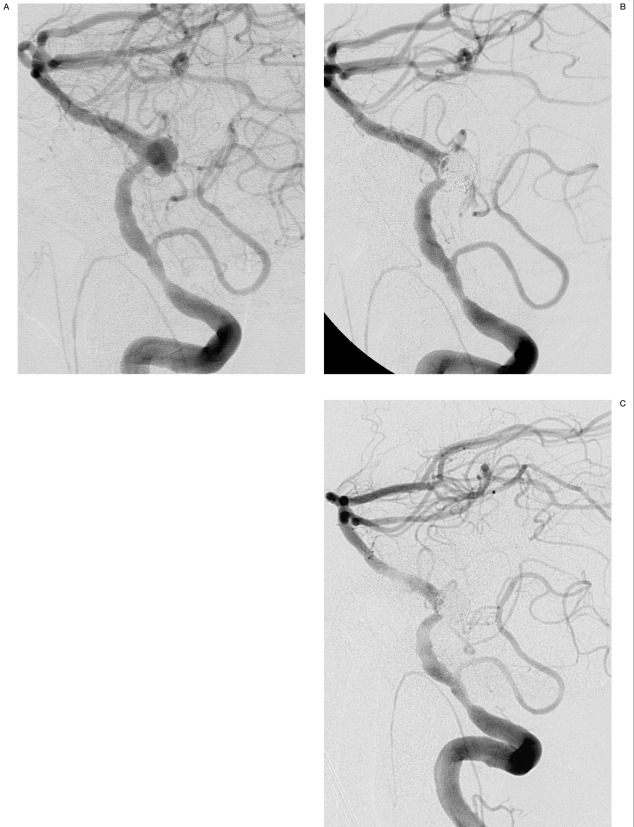
Intervention: In anticipation of using stent-assisted coiling, the patient received a 300 mg loading dose of clopidogrel (Plavix, Bristol-Myers Squibb Company, New York, NY) the day prior to the procedure and was taking aspirin 81 mg daily. A four vessel cerebral angiogram was performed including intracranial rotational 3-D angiography following injection of the left vertebral artery. The right vertebral artery was occluded and provided no contribution to the basilar artery. There was diffuse atherosclerotic disease of the left vertebral artery with a 50% stenosis just proximal to PICA. A tri-lobed 9 mm x 8 mm x 6 mm aneurysm with a 5.5 mm neck was noted at the left vertebrobasilar junction (Figure 2A). Because of the parent vessel atherosclerotic disease and a relatively favorable neck, we elected to coil the aneurysm primarily without stent or remodelling balloon assistance. Systemic heparinization was maintained with ACTs in the low 300s. A 6 Fr Envoy guiding catheter (Cordis) was placed in the left vertebral artery. A Rebar 18 microcatheter (MTI) was placed in the aneurysm without difficulty. Initially, a 7 mm complex coil (Orbit, Cordis) was detached in the aneurysm without difficulty. Subsequently, two 5 mm x 10 cm coils (Hydrocoil 10, MicroVention, Inc., Aliso Viejo, CA), a 4 mm x 10 cm coil (Hydrocoil 10) and a 3 mm x 6 cm coil (Hydrocoil 10) were detached in the aneurysm without difficulty. Angiographic evaluation demonstrated thrombus formation at the junction of the parent vessel and aneurysm neck. A bolus of 0.25 mg/kg abciximab (ReoPro, Eli Lilly) was then administered intravenously over one minute. Thirty minutes following administration of ReoPro, the intraarterial thrombus had resolved. The microcatheter was repositioned in an attempt to access the untreated left-sided lobule of the aneurysm. This maneuver was unsuccessful, so the main body of the aneurysm was then reentered and a 2 mm x 6 cm coil (Orbit) was placed near the neck of the aneurysm without difficulty and detached. A subsequent angiogram demonstrated extravasation of contrast into the subarachnoid space (Figure 2B). The heparinization was immediately reversed with 50 mg of Protamine and a temporary burst suppression of 2.5 mg/kg Propofol was given. The anesthesia team was instructed to induce hypotension. Serial angiograms over the next fifteen minutes demonstrated persistent contrast extravasation. A 4 mm x 7 mm balloon (HyperForm, MTI) was then prepared and inflated in the vertebral artery at the C1 level. Forty-five seconds after inflation, the patient became hypotensive and bradycardic, with a heart rate in the 50s. The balloon was then deflated and angiography revealed no further extravasation (Figure 2C). The patient was admitted to the neurological intensive care unit where no hydrocephalus or vasospasm were encountered. He left the hospital eleven days following the procedure with mild confusion but no focal neurological deficit.
Figure 2.
A) 70-year-old male with an unruptured vertebrobasilar aneurysm. Working projection angiography demonstrates the vertebrobasilar aneurysm (arrow) prior to endovascular treatment. B) After placement of the final coil, vertebral angiography demonstrates extravasation of contrast into the subarachnoid space (arrow). The coil loops overlying the parent vessel (double arrow) are within a laterally projecting lobule of the aneurysm and do not compromise the parent vessel on other views (not included). C) Vertebral angiography after forty-five seconds of remodelling balloon inflation demonstrates cessation of contrast extravasation.
Discussion
We present two patients in whom use of an endovascular balloon appeared to promote cessation of hemorrhage in the setting of iatrogenic aneurysm perforation. In the first case, rapid hemostasis was achieved after temporary balloon inflation, even with loose coil packing and persistent coil migration through the dome with additional attempts at coiling. In the second case, prolonged hemorrhage was noted, possibly because of the antiplatelet therapy administered pre-procedure as well as ReoPro administered during the procedure. The hemorrhage finally ceased after proximal balloon inflation. We propose that the use of a balloon as a mechanism for obtaining hemostasis should be considered in the case of aneurysm rupture.
Several other reports have been published regarding maneuvers to use in case of aneurysm perforation such as emergent ventriculostomy, continued coiling through the existing microcatheter or a second microcatheter, induction of hypotension, and reversal of heparinization. The use of a balloon should be considered complementary to these other techniques. The use of a balloon may increase the risk of thrombus formation, especially when performed following reversal of heparinization. Additionally, induced hypotension during inflation of a balloon could exacerbate the risk of hypoperfusion infarction. In fact, the marked change in hemodynamic status experienced in Case 2 following balloon inflation was likely a direct effect of balloon induced hypoperfusion. Therefore, inflation of a balloon should be used cautiously in conjunction with other techniques such as induced hypotension or reversal of heparinization.
This report might be considered useful by some practitioners, but still suffers from several limitations. Because it includes only two cases, generalization is difficult. We cannot prove that the inflated balloon itself enabled hemostasis, but the immediate cessation of contrast extravasation after its use leads us to think it was advantageous. This is especially true in Case 2, where extravasation had continued for fifteen minutes prior to balloon inflation despite the use of other maneuvers. While abrupt cessation of hemorrhage immediately after balloon inflation implies a direct cause, it is also possible that hemostasis was achieved simply on the basis of hydrogel swelling. The risk-benefit of this technique remains undefined. As noted above, the balloon may increase the risk of thrombus formation in the parent vessel, especially with heparinization reversed, or may increase the risk of hypoperfusion infarction, especially in the setting of induced hypotension.
References
- 1.Cloft HJ, Kallmes DF. Cerebral Aneurysm Perforations Complicating Therapy with Guglielmi Detachable Coils: A Meta-Analysis. Am J Neuroradiol. 2002;23:1706–1709. [PMC free article] [PubMed] [Google Scholar]
- 2.Levy E, Koebbe CJ, et al. Rupture of intracranial aneurysms during endovascular coiling: Management and outcomes. Neurosurgery. 2001;49:807–811. doi: 10.1097/00006123-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 3.McDougall CG, Halbach VV, et al. Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg. 1998;89:87–92. doi: 10.3171/jns.1998.89.1.0087. [DOI] [PubMed] [Google Scholar]
- 4.Ricolfi F, Le Guerinel C, et al. Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. Am J Neuroradiol. 1998;19:1653–1658. [PMC free article] [PubMed] [Google Scholar]
- 5.Valavanis A, Machado E, Chen JJ. Aneurysm rupture during GDC treatment: incidence, management and outcome. Neuroradiology. 1996;38(Suppl 2):45. [Google Scholar]
- 6.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 7.Coumans JV, McGrail KM, Watson V. Rupture of cerebral aneurysms during endovascular treatment with electrolytically detachable coils: incidence, management, and outcome. J Neurosurg. 1999;90:204A. [Google Scholar]
- 8.Doerfler A, Wanke I, et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. Am J Neuroradiol. 2001;22:1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 9.Halbach VV, Higashida RT, et al. Management of vascular perforations that occur during neurointerventional procedures. Am J Neuroradiol. 1991;12:319–327. [PMC free article] [PubMed] [Google Scholar]
- 10.Short JG, Marx WF, et al. Surgical salvage of microcatheter-induced aneurysm perforation during coil embolization. Am J Neuroradiol. 2002;23:682–685. [PMC free article] [PubMed] [Google Scholar]
- 11.Sluzewski M, Bosch JA, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome and risk factors. J Neurosurg. 2001;94:238–240. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 12.Willinsky R, terBrugge K. Use of a second microcatheter in the management of a perforation during endovascular treatment of a cerebral aneurysm. Am J Neuroradiol. 2000;21:1537–1539. [PMC free article] [PubMed] [Google Scholar]