Summary
We describe a carotid-cavernous fistula (CCF) in a middle aged woman with Ehlers-Danlos syndrome (EDS) type IV, which manifested with a left-sided ophthalmoplegia. The CCF was diagnosed on magnetic resonance imaging. To prevent potential lethal arterial wall injury, the CCF was treated endovascularly under local anesthesia and exclusively by a transvenous approach. The fistula was successfully closed with Guglielmi Detachable Coils. Notwithstanding these precautionary measures, the patient suffered an intraperitoneal and a small retroperitoneal bleed during the procedure and died suddenly ten days after intervention in hemorrhagic shock. A review of recent literature focussing on the technique of transvenous approach and the catheterization risks of CCF in Ehlers-Danlos syndrome is presented.
Key words: carotido-cavernous fistula, collagen disease, transvenous approach, ophtalmoplegia
Introduction
Ehlers-Danlos syndrome (EDS) is a group of autosomal dominant diseases of connective tissue characterized by abnormal collagen synthesis. The latest classification describes six subtypes depending on clinical and genetic presentation 1,2. Thin and translucent skin, easy bruising, intestinal or uterine fragility and vascular fragility with aneurysms and arterial ruptures are the characteristic features of EDS type IV (vascular type). Generalized vascular fragility, usually in the third or fourth decade, largely dominates the clinical picture 2. Affected adults are characterized by abnormal electrophoretic mobility and abnormal efficiency of secretion of type III procollagen in cultured dermal fibroblasts. About 50% of the affected individuals have inherited the mutant COL3A1 gene. The diagnosis can be made clinically using the diagnostic criteria1 formalized by a medical advisory group in 1997 (Table 1).The presence of two or more of the major criteria is highly indicative for EDS and laboratory testing is strongly recommended.
Table 1.
Nosology of Ehlers-Danlos Syndromes (reference 2)
Major diagnostic criteria
|
Minor diagnostic criteria
|
Spontaneous carotid-cavernous fistula (CCF) is a well-known complication in patients with EDS type IV 2. Swelling, chemosis, pain, ophthalmoplegia and bruit are the most common symptoms. We describe a case of CCF in an EDS type IV patient, who was treated endovascularly under local anesthesia by transvenous coiling of the fistula. The diagnosis and therapy of the CCF were achieved without any arterial puncture. In this case we describe a technique to perform the pure transvenous procedure. Despite our precautions, the patient suddenly died just before discharge. Literature on transvenous embolization of CCF in EDS is reviewed.
Case Report
A 45-year-old woman, known to have Ehlers-Danlos syndrome type IV, presented with diplopia, pulsatile tinnitus and orbital pain. Clinical investigation revealed complete left sided ophthalmoplegia and a left sided hearing loss. Magnetic Resonance Imaging (MRI) of the brain and orbit was performed and a CCF was diagnosed (Figure 1A). Color Doppler Ultrasound (CDUS) of the left internal carotid artery showed a marked decrease in intracranial resistance compared to the right side.
Figure 1.
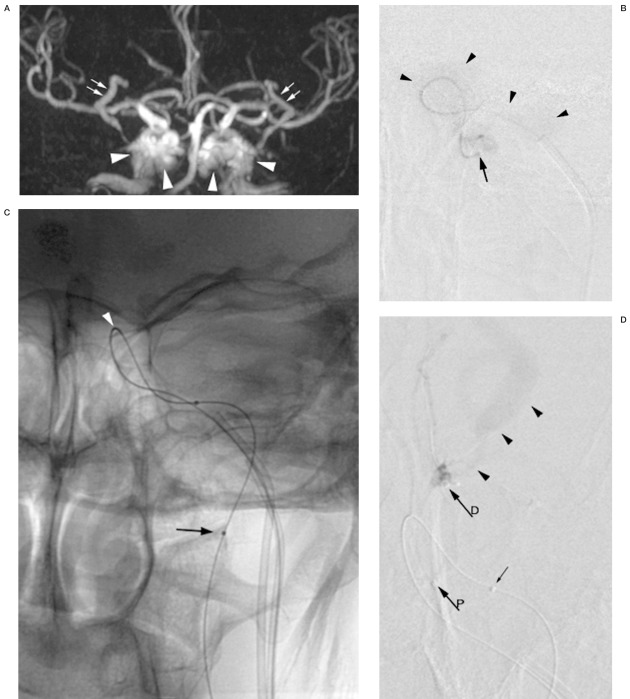
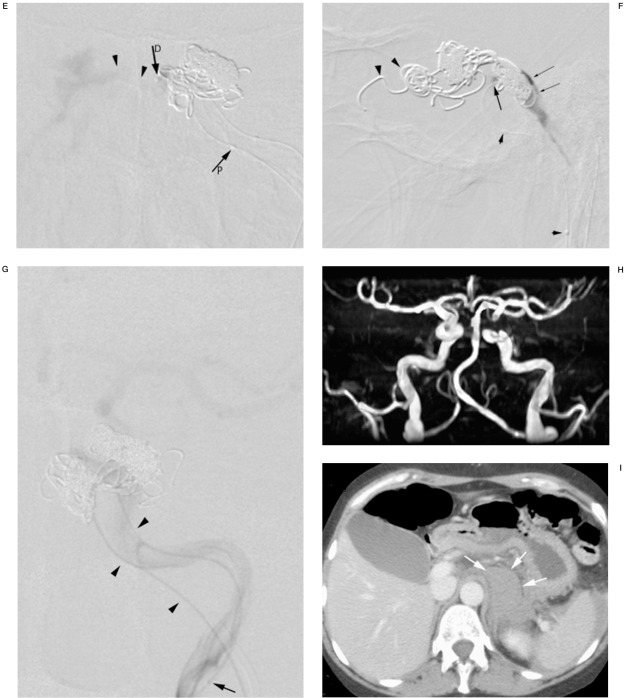
A) Time-of-flight MR angiography revealing arterialization of both cavernous sinus (arrowheads) and the superior ophthalmic veins (arrows) as an indication of a CCF. Whether the CCF is unilateral left or right-sided or bilateral cannot be determined. B) DSA of the left internal carotid via the microcatheter (arrow) introduced through the inferior petrous sinus and positioned across the fistula hole. Contrast medium is vanishing into the cavernous sinus (arrowheads). No intracranial circulation is visualized. C) Digital image of the microcatheter stabilized with a microguidewire in the left internal carotid artery. Radiopaque marker (arrow) indicates tip of the microcatheter. Bend in the microcatheter indicating the fistula hole (arrowhead). D) DSA (frontal view) of the superior ophthalmic vein showing dilatation and flow reversal (arrowheads). Coiling starts at the outlet of the ophthalmic vein to prevent persistent flow and orbital hypertension in case occlusion of the CCF would be incomplete. Proximal marker (arrowP) and distal marker (or tip) of the coiling microcatheter (arrowD) are shown. Small arrows indicate the two markers on the microcatheter in the internal carotid artery. E) DSA of the left cavernous sinus showing high outflow to the contralateral sinus. The microcatheter is positioned adjacent to the delivered coils in the superior anterior part at the entry of the intermedial part (arrowD). Coiling will continue from this position. F) Control DSA of the cavernous sinus showing stagnation of contrast medium (small arrows). Coils are covering the fistula hole (arrow), which is landmarked by the bend in the microcatheter (small arrows) in the internal carotid artery. Coils in the outlet of the superior ophthalmic vein (arrowheads). G) Control DSA of the internal carotid artery via the microcatheter (arrowheads) showing intracranial circulation and confirming occlusion of the CCF, tip of the microcatheter (arrow), and petrous sinus track (two arrowheads). H) Time-of-flight MRI angiography after coiling only depicting arterial anatomy compatible with occlusion of the CCF. Short interruption of vessel continuity in the left carotid sinus is due to susceptibility artefacts of the coils. I) Contrast-enhanced CT scan of the abdomen shows retroperitoneal hemorrhage (white arrows). Blood around the spleen is also observed.
Both asymptomatic daughters of the patient had a positive dermal fibroblast test and have inherited the mutant COL3A1 gene. During a previous consultation at the Department of Genetics, the patient and her family were fully informed of the high risks of intubation anesthesia and arterial catheterization in EDS. We discussed the technique of endovascular coiling and agreed on planning a procedure under local anesthesia and via an exclusive venous access.
Both femoral veins were punctured and 80 cm long 5F sheaths (Cook Europe, Bjaeverskov, Denmark) were introduced. On the left side a 4F vertebral catheter (Terumo, Tokyo, Japan) was positioned at the estuary of the left petrous sinus. Using an Excel 14 microcatheter (Boston Scientific Target, Cork, Ireland) and a 0.010 inch Transend (Boston Scientific Target, Miami, USA) microguidewire the cavernous sinus was catheterized. The site of the fistula was sought and the fistula hole entered to reach the internal carotid artery.
The microcatheter and the guidewire were first pushed retrogradely into the internal carotid artery and then down into the common carotid artery, where its intraluminal position was confirmed by ultrasound. Then, the microcatheter was positioned in the upper cervical segment of the internal carotid. Injection of contrast medium showed no intracranial opacification as the contrast medium was diluted and passed entirely through the fistula (Figure 1BC).
A second 4F vertebral catheter was placed at the same position as the first one and the petrous sinus was catheterized with a similar Excel 14 microcatheter and a 0.010 inch Transend microguidewire. The catheter was placed at the estuary of the left ophthalmic vein which showed reversed flow (Figure 1D). The ophthalmic vein was occluded using two Guglielmi Detachable Coils (GDC, Boston Scientific Target, Cork, Ireland)-18 Standard 5 mm x 20 mm. The cavernous sinus was successively coiled medioposteriorly using a GDC-18 Standard 5 mm- x 20 mm and a GDC-18 Standard 5 mm x 15 mm, simultaneously blocking the outflow into the contralateral sinus (Figure 1E). Finally the origin of the fistula was occluded with a GDC-18 Standard 8 mm x 30 mm and a GDC-18 Standard 5 mm x 15 mm. On the control arteriography performed with the coiling microcatheter, we observed stagnation of the contrast medium in the cavernous sinus (Figure 1F). On injection of contrast medium through the microcatheter in the left carotid artery, the intracranial circulation was visualized (Figure 1G). The control CDUS of the left internal carotid artery showed normalization of the intracranial resistance with values comparable to the right internal carotid artery. The venous catheter sets were removed uneventfully.
During the intervention the patient complained of pain in the left upper quadrant for three short periods, each time improving with analgesics. MRI angiography performed one day later confirmed occlusion of the fistula (Figure 1H). Sonography and contrast enhanced CT-scan of the abdomen revealed intraperitoneal blood and a small retroperitoneal haematoma without active bleeding or aneurysm formation (Figure 1I). The patient remained stable and recovered well so that a discharge from hospital was near. However, ten days after coiling of the CCF, the patient suddenly complained of severe abdominal pain. In the following minutes, the patient lost consciousness, had signs of hemodynamic shock and was intubated. Unfortunately resuscitation failed and the patient died. An ultrasound examination of the abdomen was suspect for intra-abdominal blood. The family refused an autopsy.
Discussion
Vascular interventions in patients with EDS type IV are hazardous. Arteriography is not indicated in patients known to have EDS because of the high incidence of arterial dissection and the development of pseudoaneurysms at the puncture sites 3. In a review of all CCF cases in EDS published before 1991, Schievink et Al calculated a mortality rate of 58% (ten out of 17 patients). Four (23%) deaths were directly therapy related, the others died of complications of EDS 4. Surgical ligation of the internal or common carotid artery was the initial palliation proposed for CCF in EDS. In the eighties, transarterial balloon embolization became the therapy of choice for CCF. However, due to the vessel fragility in EDS, procedures were often complicated by devasting dissections along the catheterized arteries 5,6. To circumnagivate the vulnerable arteries, a transvenous approach for balloon embolization was proposed, but did not seem very successful 7-9.
In the first years after Schievink's review, transarterial balloon embolization remained well accepted with a reported success rate of 70% (five out of seven treated CCFs in EDS) and, most remarkably, no procedure-related complications (Table 2) 8-10. However, since the report of Horowitz et Al in 2000 on remote vascular catastrophes after neurovascular interventions in EDS, transarterial balloon embolization seems to have lost its adherents 11. It is very likely that in daily practice more technical difficulties and vascular complications were encountered than expected from the published, mostly successful, cases. Furthermore, with the introduction of the detachable coils, interventionalists were eager to use this easy-to-handle embolic agent, which could be delivered via the less risky transvenous approach 11-13. Nonetheless, reported periprocedural complications with transvenous coil embolization were substantial including retroperitoneal hematoma, clostridium diarrhea, colonic rupture and one (20%, 1/5 CCFs) fatal iliac artery perforation. In all of these cases with a transvenous approach at least one arterial catheterization was performed for diagnostic and monitoring purposes. Therefore, some of the vascular complications might have been due to arterial wall injury.
Table 2.
Reported treated cases of CCF in EDS type IV since 1991
| Patient# | Reference | Publication date |
Sex | Age | Complaint | Diagnosis | Intervention | Number of Punctures Arterial |
Complication | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 1993 | M | 22 | Headache, pulsatile noise |
Arteriography | Failed transvenous balloon, transarterial balloon |
3 | None | Good |
| 2 | 8 | 1996 | F | 39 | Proptosis, pulsating noise |
Arteriography | Transarterial balloon | 1 | None | Recurrence after a few hours |
| 2b | 8 | F | 39 | Proptosis, headache |
Arteriography | Failed transvenous balloon, transarterial balloon |
1 | None | Good, (died 1 year later of intestinal hemorrhage) |
|
| 3 | 8 | 1996 | F | 39 | Proptosis, chemosis |
Arteriography | Transarterial balloon | 2 | None | Good, (collaps 5 months later and death) |
| 4 | 8 | 1996 | F | 39 | Pain, pulsating noise |
Arteriography | Transarterial balloon | 2 | None | Recurrence after a few hours |
| 4b | 8 | F | 39 | Pain, pulsating noise |
Arteriography | Transarterial balloon | 2 | None | Good | |
| 5 | 9 | 1996 | F | 40 | Reduced visus, pulsatile bruit |
Arteriography | Transarterial balloon | 1 | None | Good |
| 6 | 10 | 2000 | F | 18 | Proptosis, chemosis |
Arteriography | Transarterial coiling | 4 | ICA occlusion, cervical ICA dissection, ruptured splenic artery |
Died of rupture papillary muscle |
| 7 | 10 | 2000 | F | 40 | ? | Arteriography | Transvenous coiling | 1 | Retroperitoneal hematoma |
Good (other side 7 years later) |
| 7b | 10 | F | 47 | Rushing sound, diplopia |
Arteriography | Transvenous coiling | 5 | Iliac artery perforation |
Died of blood loss | |
| 8 | 11 | 2000 | F | 33 | Pain, ophtalmoplegia |
Arteriography | Transvenous coiling | 2 | Clostridium diarrhea |
No improvement of visus |
| 9 | 12 | 2002 | F | 48 | Pain, diplopia, tinnitus |
Arteriography | Transvenous coiling | 1 | None | Good |
| 10 | 12 | 2002 | M | 57 | Pain, diplopia | Arteriography | Transvenous coiling | 2 | Rupture colon | Good |
| 11 | 14 | 2005 | F | 49 | Pulsatile headache, left sixth nerve palsy |
Arteriography | Transarterial coiling | 4 | Left frontal hematoma, intraventricular clot |
Died after 7d |
| 12 | Our case | 2004 | F | 46 | Tinnitus, diplopia | MRI | Transvenous coiling | None | Retroperitoneal hematoma |
Sudden death after 10 days |
| Note: Patient 2 anil 4 had are-treatment of the same CCF within a couple of days a fter the first failed transarterial balloon embolization | ||||||||||
To maximize safety, we refrained from general anesthesia and arterial punctures, and relied on a transvenous approach under local anesthesia. The unilateral ophthalmoplegia and the flow pattern difference in the carotid arteries on CDUS strongly suggested that the CCF was only left-sided. Contrast injection via the microcatheter in the intermediate part of the cavernous sinus supported this view as outflow from the left to the right cavernous sinus could be observed. Arterial catheterization was performed from the petrous sinus through the fistula hole into the internal carotid artery. Although this was only achievable with a microcatheter, injection of contrast medium and DSA produced an acceptable image of the flow through the left internal carotid and the intracranial circulation. Moreover, the bend downwards made by the microcatheter can be used as a landmark for the fistula hole. The procedure can be monitored by contrast medium injection via both microcatheters and by CDUS. Occlusion of the CCF should be confirmed by appearance of intracranial circulation (injection via the microcatheter in the internal carotid), stagnation of contrast medium in the cavernous sinus (injection via the coiling microcatheter), CDUS of both carotid arteries (normalization of arterial flow patterns) and by MR angiography after the procedure (Figure 1H).
For a transvenous embolization detachable coils are more practical than detachable balloons. Upstream navigation of a microcatheter carrying a detachable balloon into the inferior petrosal and cavernous sinus is a technical challenge. Although thrombembolic or bleeding complications are rarely encountered in venous sampling in Cushing's disease 14, in the precarious condition of EDS, all catheter or guidewire manoeuvring that could jeopardize vessel wall integrity should be avoided at all costs.
Despite all these precautionary measures, the patient did suffer a minor retroperitoneal bleed during the procedure. It seems unlikely that the retroperitoneal bleeding was caused by an iatrogenic vein injury as both 80 cm long 5F sheaths were placed in the caval vein under permanent fluoroscopic control without difficulties. We can exclude inadvertently entering of side-branches, which would have precipitated a venous retroperitoneal haemorrhage. Contrast enhanced CT-scan did not reveal vascular anomalies such as a pseudoaneurysm. As the patient's condition evolved favourably, we did not want to take the risk of exploration by arterial catheterization. The dramatic course later on suggests a profuse arterial bleeding, an abdominal organ rupture (spleen, liver) 15 or a yet to be reported large-calibre vein rupture (e.g. caval vein). Surgery in EDS seems to increase collagenase activity ensuing complications in rapid succession 16,17. Remote and delayed arterial rupture during or after endovascular procedures in EDS is much dreaded and unfortunately seems to have occurred in our case as well 11,18. The case will retain its mystery, as the family refused autopsy.
Conclusions
We described a technique to diagnose and monitor endovascular embolization of CCF in EDS by a minimally invasive and exclusively transvenous approach. Despite the proposed precautionary measures to prevent arterial injury, our patient died just before discharge. This case shows that each intervention in EDS remains hazardous and renders the prognosis of CCF in EDS uncertain.
References
- 1.Beighton P, De Paepe A, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.De Paepe A, Malfait F. Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. Br J Haematol. 2004;127:491–500. doi: 10.1111/j.1365-2141.2004.05220.x. [DOI] [PubMed] [Google Scholar]
- 3.Citron SJ, Wallace RC, et al. Quality improvement guidelines for adult diagnostic neuroangiography: cooperative study between ASITN, ASNR, and SIR. J Vasc Interv Radiol. 2003;14:S257–262. [PubMed] [Google Scholar]
- 4.Schievink WI, Piepgras DG, et al. Spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome Type IV. Case report. J Neurosurg. 1991;74:991–998. doi: 10.3171/jns.1991.74.6.0991. [DOI] [PubMed] [Google Scholar]
- 5.Lach B, Nair SG, et al. Spontaneous carotid-cavernous fistula and multiple arterial dissections in type IV Ehlers-Danlos syndrome. Case report. J Neurosurg. 1987;66:462–467. doi: 10.3171/jns.1987.66.3.0462. [DOI] [PubMed] [Google Scholar]
- 6.Farley MK, Clark RD, et al. Spontaneous carotid-cavernous fistula and the Ehlers-Danlos syndromes. Ophthalmology. 1983;90:1337–1342. doi: 10.1016/s0161-6420(83)34384-0. [DOI] [PubMed] [Google Scholar]
- 7.Halbach VV, Higashida RT, et al. Transvenous embolization of direct carotid cavernous fistulas. Am J Neuroradiol. 1988;9:741–747. [PMC free article] [PubMed] [Google Scholar]
- 8.Kashiwagi S, Tsuchida E, et al. Balloon occlusion of a spontaneous carotid-cavernous fistula in Ehlers-Danlos syndrome type IV. Surg Neurol. 1993;39:187–190. doi: 10.1016/0090-3019(93)90180-9. [DOI] [PubMed] [Google Scholar]
- 9.Debrun GM, Aletich VA, et al. Three cases of spontaneous direct carotid cavernous fistulas associated with Ehlers-Danlos syndrome type IV. Surg Neurol. 1996;46:247–252. doi: 10.1016/0090-3019(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 10.Forlodou P, de Kersaint-Gilly A, et al. Ehlers-Danlos syndrome with a spontaneous caroticocavernous fistula occluded by detachable balloon: case report and review of literature. Neuroradiology. 1996;38:595–597. doi: 10.1007/BF00626107. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz MB, Purdy PD, et al. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. Am J Neuroradiol. 2000;21:974–976. [PMC free article] [PubMed] [Google Scholar]
- 12.Kanner AA, Maimon S, Rappaport ZH. Treatment of spontaneous carotid-cavernous fistula in Ehlers-Danlos syndrome by transvenous occlusion with Guglielmi detachable coils. Case report and review of the literature. J Neurosurg. 2000;93:689–692. doi: 10.3171/jns.2000.93.4.0689. [DOI] [PubMed] [Google Scholar]
- 13.Chuman H, Trobe JD, et al. Spontaneous direct carotid-cavernous fistula in Ehlers-Danlos syndrome type IV: two case reports and a review of the literature. J Neuroophthalmol. 2002;22:75–81. doi: 10.1097/00041327-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Miller DL, Doppman JL, et al. Neurologic complications of petrosal sinus sampling. Radiology. 1992;185:143–147. doi: 10.1148/radiology.185.1.1523298. [DOI] [PubMed] [Google Scholar]
- 15.Ng SC, Muiesan P. Spontaneous liver rupture in Ehlers-Danlos syndrome type IV. J R Soc Med. 2005;98:320–322. doi: 10.1258/jrsm.98.7.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepin M, Schwarze U, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 17.Barabas AP. Ehlers-Danlos syndrome type IV. Letter to the editor. N Engl J Med. 2000;343:366–368. doi: 10.1056/NEJM200008033430513. [DOI] [PubMed] [Google Scholar]
- 18.Desal HA, Toulgoat F, et al. Ehlers-Danlos syndrome type IV and recurrent carotid-cavernous fistula: review of the literature, endovascular approach, technique and difficulties. Neuroradiology. 2005;47:300–304. doi: 10.1007/s00234-005-1378-4. [DOI] [PubMed] [Google Scholar]