Summary
The Leo stent is a cranial self-expanding stent recently developed for the treatment of wide-neck aneurysms. We report the first case with total occlusion of a cavernous aneurysm nine months after stent placement without complementary coiling.
Key words: Leo stent, cerebral aneurysm, cerebral stenting
Case Report
A 48-year-old woman with severe headaches and left facial pain was referred to our neuroradiology unit. Magnetic resonance imaging (MRI) showed a left internal carotid artery (ICA) cavernous segment aneurysm. Cerebral digital subtraction angiography revealed a giant wide-necked aneurysm of the left ICA. After a discussion of management options, vessel preservation was attempted by means of stent-assisted coiling of the aneurysm.
The patient was pretreated for three days with aspirin 325 mgr/day and plavix 75 mgr/day. Following induction of general anaesthesia, a 6-French Envoy guiding catheter (Cordis-Johnson & Johnson, Miami Lakes, FL) was placed in the ICA. The patient was anticoagulated with i.v. administered heparination maintaining the activated clotting time at 2.5 times the basal level. The aneurysm was bypassed with a Prowler microcatheter distal to the aneurysm neck. A 300-cm 0.0014-in exchange wire (Terumo) was placed through the microcatheter. The microcatheter was removed and then a Vasco microcatheter 25 was advanced over the wire until the place intended for the distal end of stent and the wire was then removed. The Leo stent delivery system was then advanced inside the Vasco and a Leo stent (4.5x25) was deployed easily over the neck of the aneurysm in a satisfactory position. Given that the patient had minimal transient neurological complications, the lesion was to be packed with coils in a future session.
The patient was maintained on daily aspirin and clopidogrel for three months after stenting, after which the patient switched to a single antiplatelet agent. Follow-up MRI was performed at six months revealing a thrombus formation within the giant aneurysm. Angiography nine months after stenting demonstrated the complete occlusion of the aneurysmal sack.
Discussion
One of the recent advances in the vascular treatment of broad-based intracranial aneurysms is the use of a cranial stent deployed across the aneurysm neck in the parent artery in combination with intra-aneurysmal filler materials, such as metallic coils or liquid polymer Onyx 3. However, a considerable rate of incomplete occlusion is associated with the stent and coil combination, resulting in recanalization of 20% in the follow-up studies after treatment of complicated aneurysms 4. The use of artificial endoluminal vessel grafts has been reported in anecdotal cases as a therapeutic alternative to exclude giant cerebral aneurysms 1. This technique has associated difficulties and risks because stent grafts have not been especially designed for use in cerebral vasculature.
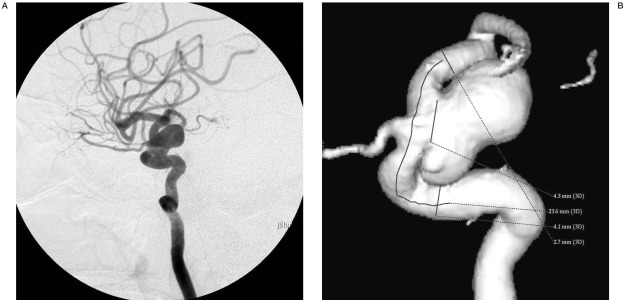
Figure 1.
A,B) Lateral angiogram and 3D reconstruction showing a giant aneurysm of ICA within the cavernous portion with a wide-neck.
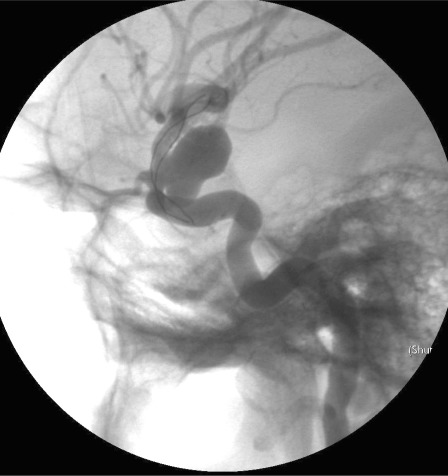
Figure 2.
4 x 2.5 cm Leo stent placed across the aneurysm neck.
The Leo Stent, a new self-expanding stent designed specifically for the treatment of patients with wide-neck post carotidal siphon aneurysms, consists of a body with twisted nitinol wires and two platinum markers that offer benefits: a higher radial force than other intracranial self-expanding stents, continuous surface coverage, and ORX visualization of both diameter and length. The stent, with its open-cell design, is similar to the Neuroform, but contains a modified geometry with three connectors between adjacent segments providing a 39% decrease in the area of the open cells, with the advantage of increased aneurysm neck coverage.
Repeated angiography in our patient over the course of nine months after stenting demonstrated complete regression of the cavernous aneurysm. Previously, spontaneous regression of cerebral aneurysms has seldom been reported 2,8 in relation to changes in flow patterns, delayed aneurysm thrombosis, similar to that in our experience, following coronary metallic stent placement 6. To our knowledge, however, our case represents the first description of aneurysmal occlusion induced by self-expanding intracranial stent placement without coiling.
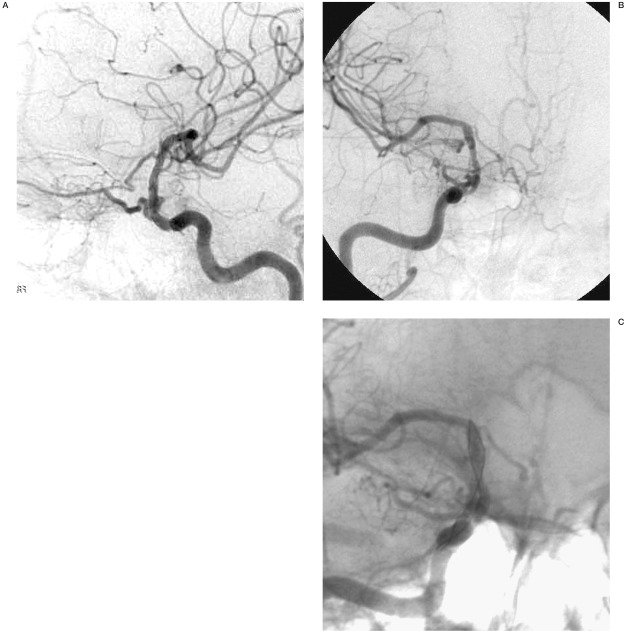
Figure 3.
A-C) Lateral and AP angiogram obtained nine months after stenting reveals exclusion of the giant aneurysm and the reconstructed internal carotid artery.
In all likelihood post-stenting regression was due to flow reversal and the consequent change in flow dynamics as well as shear stress forces that may have led to intraaneurysmal thrombosis with subsequent vessel remodelling. The neck orifice, or inflow zone, is a surface area which is proportional to the square of the radius of the neck. Because of this exponential relationship, a small change in the diameter of the neck can dramatically change the area of the inflow zone promoting thrombosis of wide-necked aneurysms 7. Placement of a porous stent across the inflow zone alters the dynamics of blood flow, often reducing flow into the aneurysm 8.
The stent acts as a new flow conduit within that vessel, hemodynamically uncoupling the vessel.The result is stagnation, i.e. reduced flow vortices within the aneurysm. A new intima may form across the stent over time, reinforcing the likelihood of a recurrence.
Neointimal hyperplasia is histologically characterized by smooth muscle cell proliferation and excessive extracellular matrix production. This process typically peaks between three and six months and then stabilizes.
Stent implantation leads to flow reduction in aneurysmal cavities and may result in complete thrombosis of the aneurysmal sack and definitive wall repair without any additional treatment. The exclusion of the aneurysm from the circulation is a result of successive rheological events induced by stenting 8. The change in the vortex characteristics and intrasaccular flow reduction can lead to complete and permanent arterial wall reconstruction and the consequent exclusion of the aneurysm from the circulation.
It appears that flow reversal and the thereby changed flow dynamics and shear stress forces might have led to intraaneurysmal thrombosis with subsequent vessel remodelling. Vascular remodelling is an active and adaptive process of structural alteration that is dependent on the dynamic interaction between locally generated growth factors, vasoactive substances and hemodynamic stimuli 9. The stent induces changes in the luminal diameter in a way that it is reshaped to maintain a constant predetermined level of shear stress.
This case report demonstrates that the Leo stent produces cerebral hemodynamic changes that may lead to plastic alterations in vessel architecture inducing aneurysmal changes in the vortex characteristics and intrasaccular flow reduction resulting in complete thrombosis of the aneurysmal sack and definitive wall repair without any additional treatment.
References
- 1.Mawad ME, Cekirge S, Ciceri E, Saatci I. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg. 2002;96:474–482. doi: 10.3171/jns.2002.96.3.0474. [DOI] [PubMed] [Google Scholar]
- 2.Lylyk P, Cohen JE, Ferrario A, Miranda C. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg. 2002;97:1306–1313. doi: 10.3171/jns.2002.97.6.1306. [DOI] [PubMed] [Google Scholar]
- 3.Saatchi I, Cekirge HS, et al. Treatment of internal carotid artery aneurysms with a covered stent: Experience in 24 patients with mid-term follow-up results. Am J Neuroradiol. 2004;25:1742–1749. [PMC free article] [PubMed] [Google Scholar]
- 4.Senn P, Krauss JK, et al. The formation and regression of a flow-related cerebral artery aneurysm. Clin Neurol Neurosurg. 2000;102:168–172. doi: 10.1016/s0303-8467(00)00085-8. [DOI] [PubMed] [Google Scholar]
- 5.Hans FJ, Krings T, et al. Spontaneous regression of two supraophthalmic internal cerebral artery aneurysms following flow pattern alteration. Neuroradiology. 2004;46:469–473. doi: 10.1007/s00234-004-1204-4. [DOI] [PubMed] [Google Scholar]
- 6.Vanninen R, Manninen H, Ronkainen A. Broad-Based Intracranial Aneurysms. Thrombosis Induced by Stent Placement. Am J Neuroradiol. 2003;24:263–266. [PMC free article] [PubMed] [Google Scholar]
- 7.Han PP, Albuquerque FC, et al. Percutaneous intracranial stent placement for aneurysms. J. Neurosurg. 2003;99:23–30. doi: 10.3171/jns.2003.99.1.0023. [DOI] [PubMed] [Google Scholar]
- 8.Baráth K, Cassot F, et al. Anatomically shaped internal carotid artery aneurysm in vitro model for flow analysis to evaluate stent effect. Am J Neuroradiol. 2004;25:1750–1759. [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons GH, Dzau VJ. The emerging concept of vascular remodelling. N Engl J Med. 1986;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]