Abstract
Humans with depression show impaired endothelium-dependent vasodilation, one recent demonstration of which was in the form of a reduced acetylcholine (ACh)-induced relaxation of adrenergically-precontracted small arteries biopsied from older depressed patients. Results from such uses of ACh in general have been validated as the most predictive marker of endothelium-related cardiovascular diseases. Accordingly, we examined vascular reactivity to ACh in the socially isolated prairie vole, a new animal model relevant to human depression and cardiovascular disease. Thoracic aortas were carefully dissected from female prairie voles after one month of social isolation (versus pairing with a sibling). Only aortas that contracted to the adrenergic agent phenylephrine (PE) and then relaxed to ACh were evaluated. Among those, ACh-induced relaxations were significantly reduced by social isolation (p<0.05), with maximum relaxation reaching only 30% (of PE-induced precontraction) compared to 47% in aortas from paired (control) animals. Experimental removal of the endothelium from an additional set of aortic tissues abolished all ACh relaxations including that difference. In these same tissues, maximally-effective concentrations of the nitric oxide-donor nitroprusside still completely relaxed all PE-induced precontraction of the endothelial-free smooth muscle, and to the same degree in tissues from isolated versus paired animals. Finally, in the absence of PE-induced precontraction ACh did not relax but rather contracted aortic tissues, and to a significantly greater extent in tissues from socially isolated animals if the endothelium was intact (p<0.05). Thus, social isolation in the prairie vole may 1) impair normal release of protective anti-atherosclerotic factors like nitric oxide from the vascular endothelium (without altering the inherent responsiveness of the vascular smooth muscle to such factors) and 2) cause the endothelium to release contracting factors. To our knowledge this is the first demonstration of this phenomenon in an animal model of depression induced solely by social isolation. These findings have implications for understanding mechanisms involved in depression and cardiovascular disease.
Keywords: Acetylcholine, Endothelial dysfunction, Cardiovascular disease, Depression, Prairie vole, Social isolation
1. Introduction
Evidence obtained from both epidemiological and clinical investigations in humans and of experimental investigations in non-human animals suggests that there is a bidirectional association between mood and cardiovascular dysfunction. Cardiovascular pathophysiology significantly increases the likelihood of developing depressive disorders; and conversely depressive disorders are risk factors for cardiac morbidity and mortality [1–5]. The association between altered mood and cardiovascular disease has been observed in individuals both with and without a history of cardiac pathophysiology, and is independent of traditional cardiovascular risk factors such as high cholesterol, increased body mass index, family history, and disease severity [2, 4, 6, 7].
Despite evidence that a significant relationship exists between mood and cardiovascular regulation, the precise behavioral and neurobiological mechanisms underlying this association remain unclear. One proposed mechanism of interaction includes arterial endothelial dysfunction, an early precursor of atherosclerosis [8, 9]. Further, dysfunction of the vascular endothelium may interact with behavioral mechanisms, including inappropriate responses to environmental and social stressors, to mediate the relationship between depression and cardiovascular disease. Social isolation and perceived loneliness are associated with maladaptive grief, mood disorders, and autonomic dysfunction, as well as altered interactions among these variables [10–14]. For example, individuals with smaller social networks and fewer meaningful social connections show increased depressive symptomatology [15], cardiovascular risk factors (including coronary artery calcification, increased blood glucose levels, hypertension, and diabetes) [16, 17], and increased cardiovascular mortality [15, 16, 18–20]. Experimental investigations with rodents and non-human primates also demonstrate that negative social experiences (such as acute social stressors, long-term isolation, and social subordination stress) produce several behavioral and physiological consequences including depressive behaviors, autonomic dysregulation, atherosclerosis, immune system activation, oxidative stress, and exaggerated behavioral and cardiac reactivity to stressors [21–27].
Experimental protocols with valid and reliable animal model systems will promote an increased understanding of the interactions among negative social experiences, vascular endothelial dysfunction, and the risk of cardiovascular disease. Recent studies have shown that the prairie vole (Microtus ochrogaster) may be a useful animal system for the study of these inter-relationships. The prairie vole is a highly social rodent species that is dependent on social interactions for the regulation of behavior, endocrine function, and cardiovascular regulation. This socially monogamous species shares with humans several physiological and behavioral characteristics including the capacity to form social bonds and to develop extended families, coupled with a high level of parasympathetic regulation of the heart, therefore offering a powerful translational model for understanding the mechanisms through which social experiences influence behavior and cardiovascular function [28, 29]. For instance, in this species social isolation from family members or opposite-sex partners induces depression-relevant behaviors that mimic those observed in humans, including anhedonia (i.e., reduced responsiveness to pleasurable stimuli) and learned helplessness (i.e., behavioral “despair”) [21, 30, 31]. Social isolation also sensitizes prairie voles to several autonomic and cardiovascular disturbances, including increased heart rate, reduced heart rate variability, cardiac arrhythmias, and sympathovagal imbalance [22, 32, 33].
Given its relevance to the study of social experiences, mood, and cardiovascular function, the prairie vole is a useful model system in which to investigate interactions of social stressors and vascular endothelial dysfunction. One well established marker of vascular endothelial dysfunction is impaired endothelium-dependent vasodilation [8, 34–41]. The first evidence of this phenomenon in depressed patients was observed in the form of impaired flow-mediated dilation of the brachial artery in the forearm [36–39]. More recently, this same phenomenon was demonstrated in the form of impaired acetylcholine (ACh)-induced relaxation of adrenergically-precontracted small arteries biopsied from depressed patients [40, 41] and thoracic aortas removed from a mouse model of depression induced by exposure to chronic unpredictable stressors [42]. In humans, results from such uses of ACh have been validated as the most prognostic marker of endothelium-related cardiovascular diseases [34].
In the present study, we sought to evaluate vascular reactivity to ACh in the abovementioned socially isolated prairie vole, because of its current status as a newer animal model relevant to clinical depression and cardiovascular disease [43]. We tested arterial tissues from these animals not only for impaired ACh-induced relaxation of adrenergically-induced precontraction as a measure of impaired endothelial release of relaxing substances, but also for enhanced ACh-induced contractions (detectable only in the absence of adrenergic or any other form of precontraction) as a measure of abnormal endothelial release of contracting substances. As a control measure, these same tests were performed in additional arterial tissues in which the endothelium was deliberately removed. We also examined all tissues for the ability of the nitric oxide-donor nitroprusside (NP) to relax adrenergically-induced precontraction, another widely-used control measure in ACh tests (for approximating endothelium-independent relaxation). Blood samples were collected and analyzed for elevated cholesterol, a long-recognized risk factor for endothelial dysfunction [35], and heart-to-body weight ratios were quantified. All these tests were performed after first exposing the animals to social isolation for four weeks, which has been shown to induce the depressive signs and cardiac dysfunction described above [22, 31].
2. Methods
2.1 Animals
Fifty-five adult (73 ± 2 days of age) female prairie voles were used for the experiments described here. All animals were descendants of a wild stock caught near Champaign, Illinois, and maintained on a 14/10 h light/dark cycle (lights on at 0630 h) with a temperature of 25 ± 2° C and relative humidity of 30 ± 5 %. All animals were allowed food (Purina rabbit chow) and water ad libitum. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs until the commencement of the experimental procedures. For all procedures described herein, only one animal from each sibling pair was studied. All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the local university Institutional Animal Care and Use Committees.
2.2 Experimental Treatment
Prairie voles were randomly divided into two independent groups of either paired (control; n=28) or isolated (n=27) conditions. Isolated animals were separated from their respective siblings and housed individually for four weeks, while paired animals were continually housed with the same siblings that they had lived with since weaning. This time period was chosen to be consistent with previous studies demonstrating that four weeks of social isolation in female prairie voles results in a disruption of affective behaviors (e.g., depression-relevant behaviors) [31, 44] and resting cardiac function [22]. All handling, cage changing, collection and preparation of samples, and testing of tissues and plasma (described below) were matched between the two groups.
2.3 Experimental Tissues
All prairie voles were subjected to the following procedures for removal, preparation, and testing of aortic and other tissues, following 4 weeks of either social isolation or social pairing (control condition). Anesthesia was achieved with a mixture of ketamine and xylazine as described previously [31]. Blood was sampled as described previously [31], centrifuged at 4° C, at 3500 rpm, for 15 minutes to obtain plasma. Plasma was stored for later analysis of cholesterol as described below. Then the chest of each animal was opened, lungs and large veins discarded, residual blood in its cavity flushed out with cold physiological buffer, prepared as described previously [45], and the thoracic aorta removed with as much care as possible to minimize stretching. Unfortunately, some stretching was unavoidable as we found that the aorta in this animal was surrounded by considerably more connective and fat tissue than we have encountered in larger species [46]. The heart was also removed and weighed for later calculation of heart weight-to-body weight ratio. The aortas were transported on ice in cold physiological buffer to another laboratory for the vascular reactivity tests described below. There connective and fat tissue adhering to the outer wall of each aorta were removed, with as much care as possible to avoid excess stretching of the vessel (although again some was unavoidable). Each cleaned aorta was then sectioned into a pair of 3-millimeter cylindrical rings, using a bound set of evenly-spaced scalpel blades to optimize length uniformity. Each of these rings was mounted between two tungsten wire stirrups, which are strong enough not to bend during ring contraction yet thin enough not to damage the inner monolayer of endothelial cells [45, 47]. For a select number of aortas, this inner endothelial cell layer was deliberately removed from one ring of each pair (before mounting on stirrups) by rubbing it off with a roughened hypodermic needle inserted through the lumen of the vessel as described previously [47]. The mounting of each tissue ring on stirrups allowed for its suspension from a mechanical tension-measuring transducer (Grass Instruments, West Warwick, RI) down into a conventional muscle bath. Each muscle bath consisted of 40 ml of physiological buffer warmed to 37° C and gassed to a pH of 7.4 with a regulated delivery of a 95%/5% mixture of O2/CO2. Before beginning any experimental tests each ring was allowed to equilibrate in such buffer for several minutes, stretched mechanically to a basal resting (passive loading) tension of 1800 milligrams (mg); a level shown in preliminary work to optimize potassium (K)-induced contractions. All tensions for each ring (resting and induced) were recorded (in mg units) with the aid of the abovementioned tension-measuring transducer connected to a Grass paper chart recorder as described previously [45].
2.4 Vascular Reactivity Tests
The aortic tissue rings collected from each paired or isolated animal and prepared as described above were subjected to the following tests. First, each tissue ring from each animal was repeatedly exposed to a buffer containing 90 millimolar potassium (K), known to cause maximum K-induced contraction of vascular smooth muscle [45], to verify the contractile ability of the tissue. After recording these contractions, all rings were returned to buffer with normal K (6 millimolar) and next treated with multiple graded concentrations of ACh (administered cumulatively at 2-minute intervals) to evaluate their contractile responsiveness to ACh, due to stimulation of muscarinic receptors in either endothelium or smooth muscle [35]. After rinsing the same rings repeatedly with ACh-free buffer, they were treated again with multiple levels of ACh, except this time after first precontracting their smooth muscle with the selective alpha-1 adrenergic receptor agonist phenylephrine (PE). This allows evaluation of ACh-induced endothelium-dependent relaxation of such smooth muscle, due to stimulation of muscarinic receptors only in the endothelial layer [35], as widely conducted in aortic tissues from other species and usually expressed as % of the PE-induced precontraction [42, 46, 48–50]. We chose a PE concentration of 0.25 micromolar which in preliminary work caused nearly 50 % of maximum PE-induced contraction in our prairie vole aortas. We did not use the endogenous alpha adrenergic receptor agonist norepinephrine, as employed previously in biopsied arteries from depressed patients [40, 41]. In preliminary work, norepinephrine-induced contractions of aortic tissues from our prairie voles were not as stable as those induced by PE. In addition, norepinephrine lacks the adrenergic receptor selectivity of PE and is therefore not as highly recommended as PE for ACh relaxation tests [35]. Norepinephrine can potentially alter endothelial cell function due to stimulation of endothelial alpha-2 and beta adrenergic receptors [51], thereby confounding interpretation of responses to ACh (in terms of their dependence on the endothelium). Finally, all the above aortic rings were rinsed repeatedly once more (with ACh- and PE-free buffer) and then, after precontracting again with PE, treated with multiple graded concentrations of the nitric oxide-donor NP to allow estimation of endothelium-independent relaxation of the underlying smooth muscle (also expressed as % of the PE-induced precontraction). NP is commonly employed for this purpose as a control measure for ACh relaxation tests in large conduit arteries like the aorta [46, 48–50]. In such vessels, the principal relaxing substance released by the endothelium in response to ACh is nitric oxide [52, 53].
2.5 Plasma Analysis
Total cholesterol was determined by a quantitative colorimetric method (EnzyChrom ECCH-100, BioAssay Systems, Hayward, CA), according to the kit instructions [54]. This assay has a lower detection limit of 5 mg/dl. The procedures were first validated to ensure that the values from prairie vole plasma samples fell along the standard curve when diluted with assay buffer according to the kit instructions and read at 340 nm.
2.6 Analysis of Data
2.6.1 Designation of n Values (Sample Sizes)
All data analysis of aortic tissue, cardiac tissue, and plasma was designed to represent individual prairie voles that had been subjected to either social pairing (control condition; n=28 animals total) or social isolation (n=27 animals total). All n values presented in Results, Figures and the Table for tests conducted on these tissues and plasma always refer to the number of individual animals involved in each test.
2.6.2 Exclusion Criteria
Because this is the first study to our knowledge investigating endothelial function in prairie vole vascular tissue, and because endothelium-dependent relaxation of precontracted vascular tissue by ACh (our primary concern) is easily distorted by tissue handling [53, 55–65], we chose to be as conservative as possible in terms of which aortic rings were included in data analysis. A few rings simply failed to contract in response to PE, as encountered before in a small fraction mouse aortic rings [42]. For us, this occurred in a few prairie vole rings following removal of their endothelium as described above. Presumably, that procedure also removed too much smooth muscle. Obviously, we could not calculate either ACh- or NP-induced changes in PE contractions that did not exist.
Data were also disregarded from a number of endothelium-intact aortic tissues that did in fact contract to PE but then completely failed to demonstrate relaxation to ACh, another criterion used before to reject mouse aortic tissues [42]. For us, these tissues only further contracted in response to ACh (at all concentrations tested) but to a similar degree and in a similar number of aortas for both our experimental groups of animals; n=10 from the 28 socially paired animals and n=9 from the 27 socially isolated animals (data not shown). We concluded that the endothelium in such aortas was particularly susceptible to being injured by the amount of stretching they were exposed to during either 1) their difficult dissection from the chest cavity, 2) removal of fat and connective tissue from their outer walls, and/or 3) our need to stretch them to achieve the abovementioned desired resting tension of 1800 mg. That conclusion was based not only on results from our own preliminary efforts to minimize such injury but also on the following previously published findings [53, 55–65]. Excess stretch when deliberately applied is known to impair arterial vascular endothelium-dependent relaxations (including ACh-induced) [53, 56–59], promote release of endothelium contracting factors [53, 55, 59–61, 65], and stimulate endothelial production of reactive oxygen species [53, 62, 63], which in turn contribute to endothelium-dependent contractile response to ACh [64].
In summary, the total number of aortic rings excluded from data analysis because of either lack of contraction in response to PE or, despite intact endothelium, the complete failure of ACh to relax PE contraction was 17 (30%) out of the 56 rings total from the 28 socially paired animals and 16 (30%) out of the 54 rings total from the 27 socially isolated animals. Thus, an equivalent number of rings from each group of animals was considered unresponsive and therefore excluded from data analysis
2.6.3 Statistical Comparisons
Data gathered from all the other aortic tissue rings in this study (70% of the total for each group of animals) were analyzed in the following manner. Responses to multiple graded concentrations of ACh and NP were analyzed separately and after subjecting them to nonlinear regression analysis to compute their respective half-maximally effective concentration (EC50) values, i.e. for all animals whose aortic tissues displayed a sufficiently sigmoid relationship between responses and concentrations for either ACh or NP. These and all other data gathered from the same animals, including K- and PE-induced aortic contractions, plasma analyses, body weight, and heart weight, were then summarized in the form of mean ± standard error of the mean (SEM; per experimental group) and subjected to appropriate forms of analysis-of-variance (ANOVA) plus mean comparison tests to determine statistically significant main factor effects, factor interaction effects and individual cell mean differences. In the absence of repeated measures, independent groups of data were subjected to regular 2-factor ANOVAs to determine these effects. In the presence of repeated measures, data were subjected to mixed-design 2-factor ANOVAs with animal group as an independent factor (paired or isolated) and cumulatively-administered multiple graded concentrations of either ACh or NP as a repeated measures factor. Differences between ANOVA factor levels and/or corresponding individual cell means were considered statistically significant if the standard probability of error (p value) was less than 0.05.
3. Results
3.1 Vascular Reactivities
Initial contractile tensions induced by high (90 millimolar) K buffer in aortic tissues rings with endothelium intact were 1856 ± 106 mg (n=16) for paired animals and 2009 ± 106 mg (n=18) for socially isolated animals. In aortic rings with endothelium removed they were lower, 681 ± 303 mg (n=9) for paired and 811 ± 133 mg (n=11) for isolated animals. Accordingly, only removal of the endothelium influenced these contractions to a statistically significant extent, not isolation, as determined by 2-factor ANOVA in which factor 1 = group (isolated versus paired animals) and factor 2 = endothelium (intact versus removed) [F(1,50) = 59.88, p<0.0001].
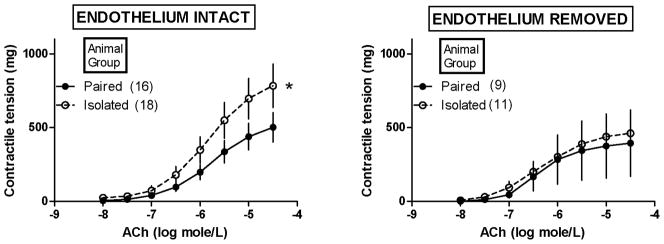
Figure 1 shows the effect of social isolation (versus pairing) of female prairie voles on contractile responses of their aortic tissues to ACh. It is important to note that these tissues were not first precontracted with PE (as illustrated in Figure 2). Most tissues in Figure 1 demonstrated contractile responses to ACh, the mean magnitude of which rose progressively as the concentration of administered ACh was increased. Social isolation significantly enhanced ACh-induced contraction in aortic tissues in which the endothelium was intact [Figure 1, left; significant factor interaction effect from mixed-design 2-factor ANOVA, with factor 1 (independent factor) = isolated versus paired animals; factor 2 (repeated measures factor) = ACh concentrations; F (7,224) = 2.10, p=0.04]. Removal of the endothelium (Figure 1, right) abolished this enhancing effect of social isolation without altering ACh-induced contraction in aortic tissues from paired animals. Accordingly, this enhancing effect was dependent on the presence of the endothelium.
Figure 1.
Effect of isolation versus pairing of female prairie voles on contractile responses of their aortic tissues to acetylcholine (ACh). *Isolation significantly enhanced ACh-induced contractions in aortic tissues with endothelium intact (left), as determined by a significant factor interaction effect from mixed-design 2-factor ANOVA in which factor 1 (independent factor) = isolated or paired animal group and factor 2 (repeated measures factor) = ACh concentrations [F (7,224) = 2.10, p=0.04]. Removal of the endothelium (right) abolished the enhancing effect of isolation without significantly altering ACh contractions in aortic tissues from paired animals. Note: values are shown as mean ± SEM.
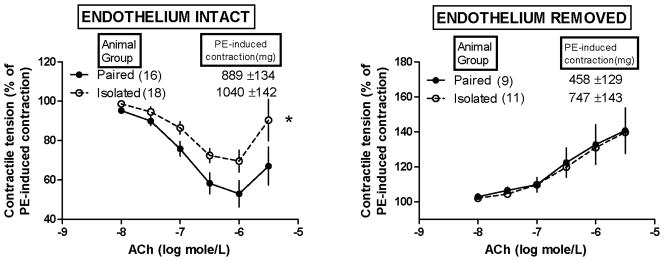
Figure 2.
Effect of isolation versus pairing of female prairie voles on acetylcholine (ACh)-induced changes in their aortic contractile responses to phenylephrine (PE). ACh was not administered until PE-induced contractile tensions stabilized. *Isolation significantly attenuated the ability of ACh to relax PE-induced contractions in aortic tissues with endothelium intact (left), as determined by a significant group effect from mixed-design 2-factor ANOVA in which factor 1 (independent factor) = isolated or paired animal group and factor 2 (repeated measures factor) = ACh concentrations [F (1,32) = 4.92, p=0.03]. Removal of the endothelium (right) not only abolished the effect of isolation but also unmasked an ACh-induced enhancement of PE-induced contractions (similarly in tissues from both groups of animals). Note: values are shown as mean ± SEM.
ACh half-maximally effective concentration (EC50) values for ACh-induced contractions would not compute (via nonlinear regression analysis) for some of the tissues represented in Figure 1. Of those that would compute, the values were not altered to a statistically significant extent by isolation versus pairing of the animals, in tissues with endothelium either intact or removed. Removal of endothelium decreased all EC50 values alike for all animals, as determined by 2-factor ANOVA in which factor 1 = group (isolated versus paired animals) and factor 2 = endothelium (intact versus removed) [F(1,40) = 20.67, p<0.0001]. For endothelium-intact tissues, those values were (expressed in nanomolar ACh concentration units) 2553 ± 456 (n=16) and 2252 ± 319 (n=13) for isolated and paired animals, respectively. For endothelium-removed tissues, they were 480 ± 80 (n=9) and 533 ± 123 (n=6) for isolated and paired animals, respectively.
Figure 2 shows the effect of social isolation (versus pairing) of female prairie voles on ACh-induced changes in their aortic contractile responses to PE. ACh was not administered until these PE-induced contractions (i.e. precontractions) were fully stabilized (at the mg tension values shown in Figure 2). As with K-induced contractions described above, only removal of the endothelium influenced these contractions to a statistically significant extent, as determined by 2-factor ANOVA in which factor 1 = group (isolated versus paired animals) and factor 2 = endothelium (intact versus removed) [F(1,50) = 5.99, p = 0.018]. Accordingly, removal of the endothelium significantly lowered PE-induced contractions (p<0.05) while isolation tended to increase them although this tendency did not achieve statistical significance and there was no statistically significant factor interaction detected by this ANOVA.
In aortic tissues with endothelium intact (Figure 2, left) the PE-induced contractions appeared to be progressively relaxed with increasing concentration of ACh but only up to 10−6 molar (i.e. 1000 nanomolar). Above 10−6 molar, such ACh-induced relaxation of PE contraction appeared to be partially reversed. Thus, maximum ACh-induced relaxation of PE contraction was observed at 10−6 molar. That maximum relaxation was notably less in aortic tissues from socially isolated versus paired animals (30% versus 47%) and the mixed-design 2-factor ANOVA revealed a statistically significant attenuating effect of social isolation (versus pairing) on the overall relaxant responsiveness to ACh across the entire range of ACh concentrations [Figure 2 left; significant animal group effect from mixed-design 2-factor ANOVA in which factor 1 (independent factor) = isolated or paired animals and factor 2 (repeated measures factor) =ACh concentrations; F (1,32) = 4.92, p=0.03]. The deliberate removal of the endothelium from an additional set of aortic tissues abolished all this relaxant responsiveness to ACh including the social isolated-related attenuation of it, and unmasked in its place an ACh-induced enhancement of PE-induced contraction (similarly so in tissues from both groups of animals).
ACh EC50 values for ACh-induced changes in PE-induced contractions would not compute (via nonlinear regression analysis) for some of the tissues represented in Figure 2. For the ACh-induced relaxations of PE contractions in a few of the endothelium-intact tissues (Figure 2, left), the ACh EC50 values would only compute when the individual responses to ACh (employed in the nonlinear regression analysis) were limited to those observed with all but the highest ACh test concentration administered (i.e. the one above 10−6 molar). Of those EC50 values that would compute, they were not altered to a statistically significant extent by isolation versus pairing of the animals, in tissues with endothelium either intact or removed.
Removal of the endothelium increased all EC50 values alike for all animals, as determined by 2-factor ANOVA in which factor 1 = group (isolated versus paired animals) and factor 2 = endothelium (intact versus removed) [F(1,47) = 28.84, p<0.0001]. For endothelium-intact tissues, these values were (expressed in nanomolar ACh concentration units) 183 ± 47 (n=18) and 137 ± 31 (n=16) for isolated versus paired animals, respectively. For endothelium-removed tissues, they were 454 ± 89 (n=8) and 444 ± 51 (n=9) for isolated versus paired animals, respectively.
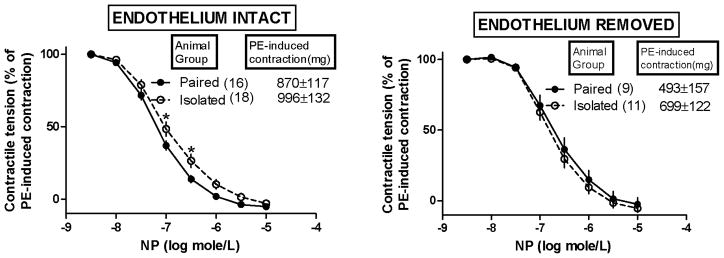
Figure 3 shows the effects of social isolation (versus pairing) of female prairie voles on NP-induced changes in their aortic contractile responses to PE. NP was not administered until these PE-induced contractions (i.e. precontractions) were fully stabilized (at the mg tension values shown in Figure 3). Statistical analysis of these PE-induced contractions with ANOVA yielded results identical to those described above for the PE-induced contractions shown in Figure 2.
Figure 3.
Effect of isolation versus pairing of female prairie voles on nitroprusside (NP)-induced changes in their aortic contractile responses to phenylephrine (PE). NP was not administered until PE-induced contractile tensions stabilized. *Isolation slightly but significantly attenuated the ability of submaximally-effective (though not maximally-effective) concentrations of NP to relax PE-induced contractions in aortic tissues with endothelium intact (left), as determined by both a significant group effect and a significant interaction effect from mixed-design 2-factor ANOVA in which factor 1 (independent factor) = isolated or paired animal group and factor 2 (repeated measures factor) = NP concentrations [F(1,32)=4.34, p=0.04; interaction [F (7,224)=2.45, p=0.02]. Removal of the endothelium (right) abolished this effect of isolation. Additional effects of endothelial removal on half-maximally effective concentration (EC50) values for NP are described in the text. Note: values are shown as mean ± SEM.
In all aortic tissues illustrated in Figure 3, all of the PE-induced contractions were inhibited progressively with increasing concentrations of NP, up to 10−5 molar at which level all PE-induced contractions were completely eliminated (i.e. ≥ 100% inhibition). Social isolation did not influence this maximal NP-induced elimination of PE-induced contractions. However, in aortic tissues with endothelium intact, social isolation did slightly attenuate the ability of submaximally-effective levels of NP to relax PE-induced contractions [Figure 3, left; as determined by both a significant main animal group effect and a significant interaction effect from mixed-design 2-factor ANOVA in which factor 1 (independent factor) = isolated or paired animal group and factor 2 (repeated measures factor) = NP concentrations; main effect of animal group: F(1,32)=4.34, p=0.04; interaction: F (7,224)=2.45, p=0.02]. This attenuating effect was dependent on the presence of the endothelium (i.e. it was abolished by removal of the endothelium; Figure 3, right).
NP EC50 values for the NP-induced relaxations of PE-induced contractions shown in Figure 3 were increased significantly by removal of the endothelium but only in aortic tissues from the paired animals, as determined by a significant factor interaction effect from a 2-factor ANOVA in which factor 1 = group (isolated versus paired animals) and factor 2 = endothelium (intact versus removed) [F(1,50) = 9.689, p=0.003]. Accordingly, in tissues from socially paired animals, NP EC50 values (expressed in nanomolar NP concentration units) were increased over 4-fold by removal of the endothelium, i.e. from 75 ± 8 (n=16 endothelium-intact tissues) to 325 ± 65 (n=9 endothelium-removed tissues). In tissues from socially isolated animals, NP EC50 values were only increased insignificantly by removal of the endothelium, i.e. from 162 ± 24 (n=18 endothelium-intact tissues) to 222 ± 28 (n=11 endothelium-removed tissues). Taken together with all data illustrated in Figure 3, these results indicated 1) that aortic endothelium in the normal (paired) female prairie voles sensitized the underlying smooth muscle to relaxant effects of intermediate (submaximally-effective) concentrations of NP (near its EC50 concentration) and 2) that this sensitizing action was impaired in the aortic endothelium of the socially isolated female prairie voles.
3.2 Heart Weights, Body Weights, and Plasma Analysis
Table 1 displays absolute heart and body weights, heart-to-body weight ratios, and total cholesterol in the paired and isolated groups. Social isolation did not affect body weight (p>0.05), but it was associated with a significant increase in heart weight [t(32) = 3.19, p<0.002]. This led to a significant increase in heart-to-body weight ratio in the isolated group, relative to paired conditions [t(32) = 2.49, p<0.009]. Total cholesterol levels did not differ between paired and isolated groups (p>0.05).
Table 1.
Effect of social isolation versus pairing on body weight, heart weight, and total cholesterol.
| Absolute Body Weight (g) | Absolute Heart Weight (g) | Heart Weight-Body Weight | Total Cholesterol (mg/dl) | |
|---|---|---|---|---|
| Paired | 32.3 ± 1.2 | 0.14 ± 0.007 | 0.0043 ± 0.0002 | 44.7 ± 9.5 |
| Isolated | 34.3 ± 1.0 | 0.17 ± 0.005* | 0.0050 ± 0.0002* | 46.3 ± 7.4 |
Note: values are shown as mean ± SEM for n=16 paired and n=18 isolated animals (except for cholesterol, which includes a subgroup of n=8 paired and n=8 isolated animals).
P < 0.05 vs. respective paired value.
4. Discussion
The present study was undertaken to investigate endothelial-dependent vascular function in the socially isolated prairie vole. This animal model has considerable utility for the investigation of interactions among negative social experiences, mood, and cardiac function [29, 43]. The most important new finding from the present study was that chronic social isolation of the adult female prairie vole significantly attenuated endothelium-dependent relaxation responses to ACh in freshly-isolated adrenergically-precontracted arterial tissue. The arterial tissue studied was aortic but this finding is nonetheless remarkably similar to the results recently observed with the small arteries biopsied from adult depressed patients; most of whom were also female [40, 41]. This finding is also similar to the results with ACh recently observed with thoracic aortas removed from male mice subjected to unpredictable chronic mild stressors [42]. This study is the first to describe vascular function in the prairie vole. The present findings therefore provide a foundation for using the prairie vole model for additional investigations of vascular dysfunction associated with negative social experiences and depression.
Another important new finding from the present study was that chronic social isolation of the adult female prairie vole slightly but significantly attenuated the ability of submaximally-effective (though not maximally-effective) concentrations of the nitric oxide-donor NP to relax adrenergically-precontracted aortic tissues; an effect requiring the presence of the endothelium. Also, the NP-induced relaxations of aortic tissues from the socially paired control animals exhibited a novel response to removal of the endothelium not seen before in other species. Removing the endothelium notably decreased their smooth muscle sensitivity to submaximal NP, as evidenced by a 4-fold greater mean EC50 concentration value for NP’s relaxation of the endothelium-removed versus the endothelium-intact tissues. This impact of endothelial removal did not occur in tissues from the socially isolated animals. One explanation for this phenomenon is that normal prairie vole aortic endothelium spontaneously releases a substance that increases their aortic smooth muscle sensitivity to the relaxant effects of nitric oxide and nitric oxide- donors in general and that social isolation impairs that release. It is not likely that this phenomenon relates to an effect of basally-released endothelial nitric oxide itself, because that is generally considered to do just the opposite, i.e. not sensitize but rather desensitize smooth muscle to relaxant effects of exogenous sources of nitric oxide like NP [66–68]. Thus, relaxant responses to NP in normal aortic tissues from other species (e.g. rat and rabbit) are sometimes reported to be increased (not decreased) by removal of the endothelium [66–70]. Accordingly, some have suggested that NP-induced relaxations are not entirely independent of the endothelium [69, 70]. Our results with prairie vole aortic tissue would essentially suggest the same but only regarding relaxations induced by submaximally-effective, not maximally-effective, concentrations of NP. But our results would also suggest that the prairie vole aorta may afford an opportunity to further explore and identify a vasoactive endothelial-derived substance not yet detected in vascular tissues from other species (thus up to now escaping recognition).
One possible, though unlikely, explanation for the attenuated relaxation responses to ACh demonstrated here is that it was dependent on the magnitude of PE-induced (adrenergically-mediated) precontraction of the underlying smooth muscle of the aortic tissue. That precontraction tended to be somewhat greater for the socially isolated animals (i.e. 1040 ± 142 versus 889 ± 134 mg of contractile tension for isolated versus paired animals, respectively; as shown in Figure 2, left). Although this tendency did not achieve statistical significance it was nonetheless notable. However, this explanation is highly unlikely for three reasons. First, the relaxant effects of ACh are expressed as relative (percent) not absolute changes in the PE-induced precontractions. This is common practice in all vascular contraction/relaxation experiments involving precontractions [35, 46, 48–50, 71–74] and is designed to correct for anticipated individual tissue differences in the absolute magnitude of the precontractile tensions. Second, the statistically significant attenuating effect of chronic social isolation on ACh-induced relaxation in the endothelium-intact tissues in question (Figure 2, left) is most noticeable at the maximum level of that relaxation (which occurred at the same ACh concentration, 10−6 molar, in tissues from both paired and isolated animals). If that were due to a non-specific difference in the underlying smooth muscle’s maximum capacity to respond to vasorelaxant agents in general then it should have occurred with maximum NP-induced relaxation as well. However, there was no difference at all in the maximum NP-induced relaxation of PE precontractions in the same tissues (Figure 3, left) even though these tissues exhibited nearly the same statistically insignificant difference in the PE-induced precontractile tensions (i.e. 996 ± 132 versus 870 ± 117 mg of contractile tensions for isolated versus paired animals, respectively; as shown in Figure 3, left). Third, removal of the endothelium clearly abolished the statistically significant attenuating effect of social isolation on ACh-induced relaxations of the PE-induced precontractions without abolishing the statistically insignificant difference in those precontractions themselves (Figure 2, right).
A more likely explanation for the attenuating effect of social isolation on ACh-induced relaxation is that it specifically impaired endothelial cell function, including normal endothelial cell responses to ACh, independent of any non-specific changes in smooth muscle cell function. As in depressed human subjects [40, 41] and the chronic mild stress model of depression in mice [42], mechanisms responsible for such impairment in the socially isolated prairie voles of the present study remain to be fully elucidated. In humans and other animal models, it is widely known that diabetes, hypercholesterolemia and systemic hypertension are three major risk factors for endothelial dysfunction and subsequent atherosclerosis, and the effects of all three have been demonstrated repeatedly in the form of impaired endothelial release of protective relaxing substances in response to ACh [35]. We did not measure blood glucose in the socially isolated prairie voles of this study, however water intake measured previously in similar protocols [22, 31, 75] showed no signs of diabetes (i.e. polydipsea). Circulating cholesterol levels were similar in paired and isolated animals, however heart-body weight ratios were significantly increased in isolated animals, as observed in a previous study with identical groups of animals [22]. This would be consistent with a sustained elevation in systemic arterial pressures over the four-week period of social isolation, similar to previous findings in human and other animal models of social isolation [12, 27]. The chamber of the heart that contributes most to heart weight (i.e. left ventricle) typically enlarges after long-term (chronic) systemic hypertension [76–80]. Also consistent with this theory, social isolation caused a statistically significant enhancement of ACh-induced contraction, observed specifically in aortic tissues with endothelium intact and not first precontracted with PE (Figure 1, left). That enhancement suggests abnormally excessive release of contracting substances from the endothelium. Of the three major risk factors mentioned above, hypertension is the one most often reported to induce excessive release of contracting substances from the endothelium [81–86], i.e. in addition to impairing its release of relaxing substances. This has been demonstrated repeatedly in the form of endothelium-dependent contractile responses to ACh (in hypertensive versus normotensive arteries neither of which are first precontracted in any way) [87–89]. Typically, such contractile responses are most notable at concentrations of ACh higher than those required to stimulate release of relaxing factors from the endothelium [81–83, 90]. This is remarkably similar to what we observed with the aortic tissues obtained from the socially isolated prairie voles of the present study. This then suggests elevated blood pressure as a function of social isolation in the present study.
The endothelial dysfunction demonstrated here in isolated prairie voles may be due to sustained autonomic imbalance [51, 91]. For instance, a high level of sympathetic activity is a risk factor for cardiovascular pathophysiology, whereas a high level of parasympathetic activity can antagonize sympathetic actions and has protective effects on the cardiovascular system [92, 93]. Previous findings indicate reduced vagal and elevated sympathetic nerve activities to the heart following 4 weeks of social isolation in this species [22]. If social isolation were to also elevate sympathetic nerve activity to the vasculature, it could cause hypertension (as described above) and thus, the endothelial dysfunction observed here. Clearly this hypothesis requires further investigation with direct blood pressure measurements. However, autonomic imbalance (with increased sympathetic nerve activity) may link psychosocial risk factors (including depression) to endothelial dysfunction by way of other mechanisms [51]. These include sympathetically-mediated changes in endothelial cell structure, its immunoreactivity, and its uptake of low-density lipoprotein (LDL) cholesterol [51]. In the prairie vole, altered autonomic regulation of the heart, including both elevated sympathetic activity and withdrawal of parasympathetic activity, chronically increases resting heart rate, reduces heart rate variability, and increases the vulnerability to cardiac arrhythmias [22, 32]. High resting heart rate has emerged as another independent hemodynamic risk factor for atherosclerosis in humans and is thought to damage vascular endothelium by the proinflammatory effects of its exposure to more oscillatory shear stress [94].
Finally, there are reports of unusual responses to ACh in arteries from animals exposed to other social stressors. For example, exposing male rats or mice to social stress in the form of chronic crowding [72–74] or complex housing conditions [71] has been shown more often to enhance than to impair relaxant responses to ACh in precontracted arteries (including aorta but also femoral arteries). To our knowledge, there are no reports of enhanced endothelium-dependent relaxations in humans with depression or other affective disorders. Thus, the relevance of these unusual contrasting findings in rats and mice exposed to social crowding remains to be determined. Yet mechanisms responsible for them are worthy of investigation if for no other reason than to serve as control measures for studies with social isolation and to determine possible species differences in the responses to social stressors. Collectively, our present results plus all these previous diverse findings from other animals provide a very broad foundation from which to focus further studies of the vascular endothelium in different social settings. Further studies focused on social experiences, behavior, and vascular function using animal models will improve our understanding of behavioral and neurobiological mechanisms underlying depression and cardiovascular disease.
Highlights.
We investigated vascular endothelial dysfunction in response to social isolation
Aortic tissue was studied in female prairie voles following social isolation
Relaxation of precontracted aortic tissue was reduced by social isolation vs. pairing
The findings increase knowledge of social experiences and endothelial function
Acknowledgments
The investigators would like to thank Meagan LaRocca and Kristin Preihs for assistance. This research was supported by the National Institutes of Health grant MH077581 and by internal university funds (Midwestern University and Northern Illinois University).
Role of the Funding Source
This research was supported by the National Institutes of Health grant MH077581 and by internal university funds (Midwestern University and Northern Illinois University). The funding sources had no involvement in the study design; collection analysis or interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacob D. Peuler, Email: jpeule@midwestern.edu.
Melissa-Ann L. Scotti, Email: melissa.scotti@gmail.com.
Laura E. Phelps, Email: lphelp@midwestern.edu.
Neal McNeal, Email: nealmcneal@gmail.com.
Angela J. Grippo, Email: angelagrippo@niu.edu.
References
- 1.Glassman A, Shapiro P. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155:4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Penninx B, Beekman A, Honig A, Deeg D, Schoevers R, van Eijk J, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–7. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Freedland K, Rich M, Skala J, Carney R, Davila-Roman V, Jaffe A. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 4.Carney R, Freedland K. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–7. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 5.Van der Kooy K, Van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 6.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–10. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–36. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- 8.Do DP, Dowd JB, Ranjit N, House JS, Kaplan GA. Hopelessness, depression, and early markers of endothelial dysfunction in US adults. Psychosom Med. 2010;72:613–9. doi: 10.1097/PSY.0b013e3181e2cca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev. 2011;19:130–42. doi: 10.1097/CRD.0b013e31820e8106. [DOI] [PubMed] [Google Scholar]
- 10.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 11.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46:S39–52. [PubMed] [Google Scholar]
- 12.Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, et al. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–17. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: A prospective study of French employees of Electricity of France-Gas of France: the GAZEL cohort. Am J Epidemiol. 2004;159:167–74. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosom Med. 2009;71:836–42. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom Med. 2008;70:282–7. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, et al. Social networks are associated with lower mortality rates among women with suspected coronary disease: The National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2004;66:882–8. doi: 10.1097/01.psy.0000145819.94041.52. [DOI] [PubMed] [Google Scholar]
- 17.Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, et al. Social network and coronary artery calcification in asymptomatic individuals. Psychosom Med. 2005;67:343–52. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. Am J Epidemiol. 1988;128:370–80. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- 19.Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155:700–9. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, et al. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Ann Epidemiol. 2008;18:476–83. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bosch O, Nair H, Ahern T, Neumann I, Young L. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–15. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62:1162–70. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–44. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 25.Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, et al. Oxytocin Mediates Social Neuroprotection After Cerebral Ischemia. Stroke. 2011 doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, DeVries AC. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosom Med. 2010;72:519–26. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 27.Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci USA. 2010;107:16342–7. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter C, Devries A, Getz L. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–14. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 29.Grippo AJ. The utility of animal models in understanding links between psychosocial processes and cardiovascular health. Soc Personal Psychol Compass. 2011;5:164–79. doi: 10.1111/j.1751-9004.2011.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grippo A, Cushing B, Carter C. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grippo A, Sgoifo A, Mastorci F, McNeal N, Trahanas D. Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Auton Neurosci Basic Clin. 2010;156:44–50. doi: 10.1016/j.autneu.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertension. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lüscher TF, Vanhoutte PM. The endothelium: modulator of cardiovascular function. Boca Raton: CRC Press. 1990:1–6. 10, 116–130. [Google Scholar]
- 36.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–8. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 37.Broadley A, Korszun A, Jones C, Frenneaux M. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–4. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–9. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Yiu KH, Tse HF. Relationships between vascular dysfunction, circulating endothelial progenitor cells, and psychological status in healthy subjects. Depress Anxiety. 2011;28:719–27. doi: 10.1002/da.20839. [DOI] [PubMed] [Google Scholar]
- 40.Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–9. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, et al. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–40. doi: 10.1161/HYPERTENSIONAHA.110.152801. [DOI] [PubMed] [Google Scholar]
- 42.Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O’Donnell J, et al. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav. 2011;103:540–6. doi: 10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Grippo AJ. Mechanisms underlying altered mood and cardiovascular dysfunction: The value of neurobiological and behavioral research with animal models. Neurosci Biobehav Rev. 2009;33:171–80. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phelps LE, Peuler JD. Evidence of direct smooth muscle relaxant effects of the fibrate gemfibrozil. J Smooth Muscle Res. 2010;46:125–42. doi: 10.1540/jsmr.46.125. [DOI] [PubMed] [Google Scholar]
- 46.Peuler J, Johnson B, Schiebinger R, Zemel M, Iannucci A. Effects of oral calcium and potassium on endothelium-dependent relaxation in hypertensive rats. Am J Physiol. 1994;267:H540–7. doi: 10.1152/ajpheart.1994.267.2.H540. [DOI] [PubMed] [Google Scholar]
- 47.Peuler JD, Lee JHM, Smith JM. 4-Aminopyridine antagonizes the acute relaxant action of metformin on adrenergic contraction in the ventral tail artery of the rat. Life Sci. 1999;65:PL287–93. doi: 10.1016/s0024-3205(99)00522-6. [DOI] [PubMed] [Google Scholar]
- 48.Zemse SM, Hilgers RHP, Simkins GB, Rudic RD, Webb RC. Restoration of endothelin-1-induced impairment in endothelium-dependent relaxation by interleukin-10 in murine aortic rings. Can J Physiol Pharmacol. 2008;86:557–65. doi: 10.1139/Y08-049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayasi R, Akamine EH, Davel AP, Rodrigues MAM, Carvalho CRO, Rossoni LV. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J Hypertension. 2010;28:2111–9. doi: 10.1097/HJH.0b013e32833ca68c. [DOI] [PubMed] [Google Scholar]
- 50.d’Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol. 2010;108:1041–51. doi: 10.1152/japplphysiol.01440.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–64. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 52.Woodman OL, Wongsawatkul O, Sobey CG. Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries. Clin Exp Pharmacol Physiol. 2000;27:34–40. doi: 10.1046/j.1440-1681.2000.03199.x. [DOI] [PubMed] [Google Scholar]
- 53.Bernatova I, Conde M, Kopincova J, González MC, Puzserova A, Arribas SM. Endothelial dysfunction in spontaneously hypertensive rats: focus on methodological aspects. J Hypertension. 2009;27:S27–31. doi: 10.1097/01.hjh.0000358834.18311.fc. [DOI] [PubMed] [Google Scholar]
- 54.Henein HY, Younan SM, Rashed LA, Fakhry A. Effect of adrenomedullin gene delivery on insulin resistance in type 2 diabetic rats. J Adv Res. 2011;2:57–64. [Google Scholar]
- 55.Rinaldi G, Bohr D. Endothelium-mediated spontaneous response in aortic rings of deoxycorticosterone acetate-hypertensive rats. Hypertension. 1989;13:256–61. doi: 10.1161/01.hyp.13.3.256. [DOI] [PubMed] [Google Scholar]
- 56.Sipkema P, van der Linden PJW, Westerhof N, Yin FCP. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J Biomech. 2003;36:653–9. doi: 10.1016/s0021-9290(02)00443-8. [DOI] [PubMed] [Google Scholar]
- 57.Dainty I, McGrath J, Spedding M, Templeton A. The influence of the initial stretch and the agonist-induced tone on the effect of basal and stimulated release of EDRF. Br J Pharmacol. 1990;100:767–73. doi: 10.1111/j.1476-5381.1990.tb14090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy S, Wadsworth RM, Wainwright CL. Effect of antiproliferative agents on vascular function in normal and in vitro balloon-injured porcine coronary arteries. Eur J Pharm. 2003;481:101–7. doi: 10.1016/j.ejphar.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Sekiguchi F, Adachi T, Matsubara H, Matsuda K, Kita K, Shimamura K, et al. Spontaneous and agonist-induced contractions and endothelium-dependent relaxation in aortae from SHRSP and WKY rats under various levels of passive force. Clin Exp Pharmacol Physiol. 1996;23:483–9. doi: 10.1111/j.1440-1681.1996.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 60.Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contraction to stretch in canine basilar arteries. Am J Physiol. 1987;252:H671–3. doi: 10.1152/ajpheart.1987.252.3.H671. [DOI] [PubMed] [Google Scholar]
- 61.Harder DR. Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res. 1987;60:102–7. doi: 10.1161/01.res.60.1.102. [DOI] [PubMed] [Google Scholar]
- 62.Hishikawa K, Lüscher TF. Pulsatile stretch stimulates superoxide production in human aortic endothelial cells. Circulation. 1997;96:3610–6. doi: 10.1161/01.cir.96.10.3610. [DOI] [PubMed] [Google Scholar]
- 63.Howard AB, Alexander R, Nerem RM, Griendling K, Taylor W. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol Cell Physiol. 1997;272:C421–7. doi: 10.1152/ajpcell.1997.272.2.C421. [DOI] [PubMed] [Google Scholar]
- 64.Yang D, Félétou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–10. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanhoutte P. Endothelium-dependent contractions in arteries and veins. J Vasc Res. 1987;24:141–4. doi: 10.1159/000158688. [DOI] [PubMed] [Google Scholar]
- 66.Moncada S, Rees D, Schulz R, Palmer R. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci USA. 1991;88:2166–70. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pohl U, Busse R. Endothelium-derived relaxant factor inhibits effects of nitrocompounds in isolated arteries. Am J Physiol. 1987;252:H307–13. doi: 10.1152/ajpheart.1987.252.2.H307. [DOI] [PubMed] [Google Scholar]
- 68.Ralevic V, Mathie RT, Alexander B, Burnstock G. NG-nitro-L-arginine methyl ester attenuates vasodilator responses to acetylcholine but enhances those to sodium nitroprusside. J Pharm Pharmacol. 1991;43:871–4. doi: 10.1111/j.2042-7158.1991.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 69.Shirasaki Y, Su C, Lee T, Kolm P, Cline W, Nickols G. Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharmacol Exp Ther. 1986;239:861–6. [PubMed] [Google Scholar]
- 70.Shirasaki Y, Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharm. 1985;114:93–6. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- 71.Webb RC, Vander AJ, Henry JP. Increased vasodilator responses to acetylcholine in psychosocial hypertensive mice. Hypertension. 1987;9:268–76. doi: 10.1161/01.hyp.9.3.268. [DOI] [PubMed] [Google Scholar]
- 72.Puzserova A, Bernatova I. Chronic social stress increases nitric oxide-dependent vasorelaxation in normotensive rats. Interdiscip Toxicol. 2010;3:109–17. doi: 10.2478/v10102-010-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernatova I, Csizmadiova Z, Kopincova J, Puzserova A. Vascular function and nitric oxide production in chronic social-stress-exposed rats with various family history of hypertension. J Physiol Pharmacol. 2007;58:487–501. [PubMed] [Google Scholar]
- 74.Bernatova I, Csizmadiova Z. Effect of chronic social stress on nitric oxide synthesis and vascular function in rats with family history of hypertension. Life Sci. 2006;78:1726–32. doi: 10.1016/j.lfs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, et al. 24-Hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosom Med. 2011;73:59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickinson C, Thomson A. High blood-pressure and stroke. Necropsy study of heart-weight and left ventricular hypertrophy. Lancet. 1960;2:342–5. doi: 10.1016/s0140-6736(60)91483-5. [DOI] [PubMed] [Google Scholar]
- 77.Beznák M. Changes in heart weight and blood pressure following aortic constriction in rats. Can J Biochem Physiol. 1955;33:995–1002. [PubMed] [Google Scholar]
- 78.Raaschou F. Liver function and hypertension: blood pressure and heart weight in chronic hepatitis. Circulation. 1954;10:511–6. doi: 10.1161/01.cir.10.4.511. [DOI] [PubMed] [Google Scholar]
- 79.Kubo M, Ochiai T, Kato J, Ishida R. Pharmacological studies on TA-6366, a new ACE inhibitor: II. Effect of long-term administration from the pre-hypertensive stage on blood pressure, relative heart weight and ACE activity of various tissues in spontaneously hypertensive rats (SHRs) Jpn J Pharmacol. 1991;57:517–26. doi: 10.1254/jjp.57.517. [DOI] [PubMed] [Google Scholar]
- 80.Simpson LO. A mouse model of spontaneous renal hypertension. Blood pressure, heart weight, kidney weight and proteinuria relationships in NZB x OUW F1 hybrid female mice. Pathology. 1980;12:347–57. doi: 10.3109/00313028009077096. [DOI] [PubMed] [Google Scholar]
- 81.Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–8. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- 82.Vanhoutte P, Boulanger C. Endothelium-dependent responses in hypertension. Hypertens Res. 1995;18:87–98. doi: 10.1291/hypres.18.87. [DOI] [PubMed] [Google Scholar]
- 83.Vanhoutte PM, Félétou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–58. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanhoutte PM, Tang EHC. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586:5295–304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflugers Arch. 2010;459:1015–23. doi: 10.1007/s00424-009-0783-7. [DOI] [PubMed] [Google Scholar]
- 86.Vanhoutte P. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57:526–31. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- 87.Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Félétou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–45. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang D, Gluais P, Zhang JN, Vanhoutte PM, Félétou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundamen Clin Pharmacol. 2004;18:321–6. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 89.Wong MSK, Man RYK, Vanhoutte PM. Calcium-independent phospholipase A2 plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am J Physiol. 2010;298:H1260–6. doi: 10.1152/ajpheart.01068.2009. [DOI] [PubMed] [Google Scholar]
- 90.Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–8. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- 91.Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–7. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 92.Levy M. Cardiac sympathetic-parasympathetic interactions. Fed Proc. 1984;43:2598–602. [PubMed] [Google Scholar]
- 93.Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45:643–52. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]