Abstract
Adipocytes are highly specialized cells that play a central role in lipid homeostasis and the maintenance of energy balance. Obesity, an excessive accumulation of adipose tissue, is a major risk factor for the development of Type 2 diabetes mellitus (T2DM), cardiovascular disease, and hypertension. A variety of studies suggest that obesity and T2DM can be linked to a breakdown in the regulatory mechanisms that control the expression and transcriptional activity of PPARγ. PPARγ is a nuclear hormone receptor that functions as a master switch in controlling adipocyte differentiation and development. Also important in controlling glucose homeostasis and insulin sensitivity, PPARγ is a ligand-dependent transcription factor that is the functional receptor for the anti-diabetic thiazolidinediones (TZDs). In the last fifteen years, a variety of covalent modifications of PPARγ activity have been identified and studied. These covalent modifications include phosphorylation, ubiquitylation, O-GlcNAcylation and SUMOylation. Covalent modifications of PPARγ represent key regulatory mechanisms that control both PPARγ protein stability and transcriptional activity. A variety of PPARγ transgenic models, including mice heterozygous for PPARγ, have demonstrated the importance of PPARγ expression in glucose homeostasis and insulin resistance. In the following review, we have highlighted the regulation of PPARγ by covalent modifications, the interplay between these interactions and how these post-translational modifications impact metabolic disease states.
Keywords: Adipocyte, PPARγ, Adipogenesis, Ubiquitylation, SUMOylation
1.1. Role of PPARγ in disease
Our understanding of adipose tissue biology and the role of adipocytes in obesity-related diseases such as type 2 diabetes (T2DM) has benefited greatly from the discovery that the lipid-activated peroxisome proliferator-activated receptor gamma (PPARγ, NR1C3) nuclear receptor is essential for adipocyte development [1–3]. Subsequent to its discovery as a critical adipogenic transcription factor, PPARγ was identified as the pharmacological target of the insulin-sensitizing thiazolidinediones (TZD) that have been widely used to treat insulin resistance associated with T2DM [4, 5]. Since that time, various genetic studies using animal models of tissue-specific PPARγ deletions have shown that activation of PPARγ in adipose tissue is central to the insulin-sensitizing effects of the TZDs. Mice lacking white adipose tissue [6] are not responsive to TZDs and deletion of PPARγ in adipose tissue causes insulin resistance in adipose and liver, but not in skeletal muscle [7]. Inhibiting PPARγ activity in mature adipocytes leads to insulin resistance associated with decreased expression of key genes required for insulin signaling in adipocytes, reduced uptake of free fatty acids by adipocytes and increased lipolysis [8, 9]. Gain-of-function experiments show that adipocyte-specific constitutive activation of PPARγ in mature adipocytes can regulate whole body insulin sensitivity [10] without stimulating adipogenesis. Collectively, these observations indicate that PPARγ activation is as important to lipid and glucose metabolism in fully formed adipocytes as it is for adipocyte development.
1.2. Regulation of PPARγ activity by protein-protein interactions and ligand binding
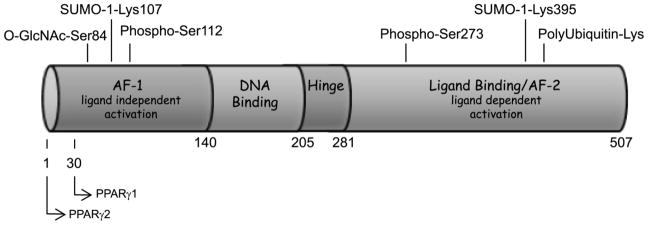
PPARγ is a ligand-activated transcription factor that is expressed in adipocytes as PPARγ2 and PPARγ1; the two forms differing by a thirty amino acid N-terminal extension found in PPARγ2 (refer to Figure 1). While PPARγ1 is expressed in multiple tissues [11], PPARγ2 is expressed primarily in adipocytes [12]. PPARγ2 is the more adipogenic PPARγ isoform in vitro [13] and it is also the isoform transcriptionally regulated by nutrition [11]. PPARγ has an overall domain structure typical of nuclear hormone receptors. This includes an N-terminal activation function-1 (AF-1) domain that functions as a ligand-independent activation domain and confers specificity of target gene activation [14]. PPARγ also contains a DNA binding domain followed by a hinge domain and a large ligand binding domain that contains a short region at the C-terminus called the activation function 2 (AF-2) domain that is responsible for ligand-dependent activation [12] (refer to Figure 1). PPARγ transcriptional activity is regulated at multiple levels. PPARγ forms a heterodimeric complex with RXRα that is mediated by the PPARγ ligand binding domain. Interaction with RXRα is required for PPARγ to bind the PPARγ responsive element of its target gene promoters. Once bound to DNA, PPARγ activation can be modulated by a large number of coregulators classified as corepressors and coactivators whose association with PPARγ is determined by ligand binding to PPARγ. Corepressor proteins that regulate PPARγ activity include the nuclear receptor corepressor NCoR-1 and its homolog SMRT (silencing mediator for retinoid and thyroid receptors) [15, 16] and the receptor interacting protein 140 (RIP 140) [17]. In recent studies of transgenic mice lacking NCoR in adipocytes, it was shown that deletion of this corepressor leads to increased adipogenesis, reduced inflammation, and enhanced systemic insulin sensitivity [18]. These observations are reminiscent of a TZD treatment and support other studies that show transcriptionally active PPARγ promotes insulin sensitivity. In the absence of ligand, PPARγ can be bound to target gene promoters while associated with the nuclear corepressors that recruit chromatin-modifying enzymes to regulate access to the DNA as has been shown in the glycerol kinase promoter [19]. Of note, the most active cis-regulatory elements bound by PPARγ differ between mouse and man [20]. Also, studies in adipocytes and macrophages have demonstrated that PPARγ DNA binding is predominantly cell type specific [21]. Upon ligand binding, the corepressor proteins are replaced by coactivating proteins that promote PPARγ transcriptional activity. A major category of the coactivators is the p160 family of proteins that includes the cAMP response element binding protein (CBP)/p300 and Steroid Receptor Coactivators (SRC)-1,-2,-3, which recruit histone modifiers to the chromatin structure (reviewed in [22]). A second category of coactivators includes subunits of the mediator complex such as the PPAR–binding protein (PBP)/thyroid hormone receptor-associated protein (TRAP) 220/vitamin D receptor-associated protein (DRIP) 205 [23–25]. These coactivators interact with the general transcriptional machinery to control assembly of the transcription preinitiator complex [23]. TRAP220/DRIP205, originally cloned as a coactivator of the vitamin D receptor [24], interacts directly with PPARγ[25]. An additional coactivator, PRIP (peroxisome proliferators-activated receptor gamma interacting protein) serves to link TRAP220/DRIP205 bound PPARγ to the CBP/p300 coactivator [26]. These coregulators orchestrate the selective binding of PPARγ to an enormous array of gene promoters.
Figure 1.
Schematic of PPARγ2 covalent modifications
Our understanding of the genes regulated by PPARγ binding has greatly expanded from the set of genes initially identified as direct targets of this nuclear receptor. Earlier studies established that PPARγ controls the expression of genes that are required for lipid synthesis, transport, and storage [1, 27–29] in adipocytes. Recent studies that take advantage of chromatin immunoprecipitation and genome-wide sequencing technology demonstrate the PPARγ/RXRα heterodimer binds to more than 5,000 sites, directly activating the expression of a substantial number of genes involved in lipid and glucose metabolism during adipogenesis and in mature adipocytes [30–32]. In a majority of the binding sites, C/EBPα is also present [30, 32], in agreement with the accumulated evidence that PPARγ and C/EBPα activities coordinate to form a fully functional insulin sensitive adipocyte. The overlap between PPARγ and C/EBPα binding sites persists in mature adipocytes [32].
1.3. Regulation of PPARγ by covalent modifications
As indicated above, PPARγ can be associated with a large number of proteins. These important protein-protein interactions are non covalent, but these interactions control PPARγ activity and can be significantly regulated by covalent modifications of PPARγ. PPARγ is known to be modified by phosphorylation, SUMOylation, O-GlcNAcylation, and ubiquitylation (refer to Figure 1). The multiple covalent modifications of PPARγ increase the levels of control of both PPARγ expression and transcriptional activity that contribute to its ability to modulate adipocyte development and insulin sensitivity.
1.3.1. Phosphorylation of PPARγ
The most well described of these modifications is phosphorylation of PPARγ. Growth factor activation of the mitogen activated protein kinases (MAPK) results in phosphorylation of PPARγ2 in the N-terminal AF-1 domain at serine112 (Ser82 in PPARγ1) [33–37], the single MAPK consensus recognition site in PPARγ. The first studies on PPARγ phosphorylation were prompted by observations of the MAPK-inducing growth factors including epidermal growth factor (EGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) inhibited adipogenesis. Mutation of serine112 to alanine abolishes both the growth factor stimulated phosphorylation of PPARγ at serine112 and the inhibitory effect of PDGF and EGF on PPARγ transcriptional activity [35]. In vitro studies have demonstrated that the non-phosphorylated Ser112Ala mutant form of PPARγ is more active than wild-type PPARγ in the presence of ligand [33, 36, 37]. The MAPK-mediated phosphorylation of PPARγ at serine112 in the AF-1 domain functions to inhibit PPARγ activity by regulating ligand binding in the C-terminal ligand binding domain of PPARγ [38]. The physiological relevance of PPARγ phosphorylation at serine112 was demonstrated in mice homozygous for the Ser112Ala mutation of PPARγ2. Although diet-induced obesity is associated with insulin resistance, elimination of the phosphorylation site protected the Ser112Ala mice from diet-induced insulin resistance even though the mice were obese [39]. Preservation of insulin sensitivity in the obese Ser112Ala mice correlated with higher expression of PPARγ target genes and increased serum levels of adiponectin, an insulin-sensitizing factor secreted exclusively by adipocytes. An additional PPARγ phosphorylation site at serine273 in the PPARγ ligand-binding domain that is regulated by Cdk5 [40, 41] may have important implications for the treatment of T2DM. Cdk5-dependent phosphorylation is known to play a role in neurobiology and neurodegenerative diseases [42, 43], but this kinase is also expressed in non-neuronal tissue, including the insulin secreting pancreatic beta cells [44], and adipocytes [40]. Cdk5 activity can be regulated by TNF-α [45], a pro-inflammatory cytokine that is up-regulated in adipose tissue in conditions of obesity and insulin resistance [46]. The Cdk5-mediated phosphorylation of PPARγ in adipose tissue has been linked to obesity [40]. Importantly, treatment with thiazolidinediones, the insulin sensitizing drugs that act as high affinity PPARγ ligands, increases PPARγ transcriptional activity and decreases cdk5-dependent phosphorylation of PPARγ2 at serine273. However, Cdk5 phosphorylation at serine273 of PPARγ2 does not regulate PPARγ transcriptional activity in general, but does alter the expression of metabolically important target genes in adipocytes such as adiponectin. Moreover, PPARγ ligands that are less potent agonists than the thiazolidinediones, but have insulin-sensitizing effects, also inhibit Cdk5-mediated phosphorylation in PPARγ2. Transgenic mice lacking the corepressor NCoR in adipocytes exhibit improvements in adipogenesis, reduced inflammation, and enhanced systemic insulin sensitivity and a notable decrease in CDK5-mediated PPARγ ser273 phosphorylation [18]. These studies suggest that NCoR can function as an adaptor protein to enhance the ability of CDK5 to associate with and phosphorylate PPARγ. Collectively, these studies suggest that phosphorylation of PPARγ2 at serine273 by Cdk5 is a key determinant of whole body insulin sensitivity and that new classes of anti-diabetic drugs may emerge that are based on regulating Cdk5-mediated phosphorylation of the PPARγ ligand binding domain. One compound that fits these criteria has recently been described [41].
In addition to modulating PPARγ transcriptional activity, MAPK signaling also affects PPARγ by regulating the subcellular location of PPARγ. These data demonstrate an interaction of MEK1 with the C-terminal AF2 domain of PPARγ, yet this interaction does not directly alter phosphorylation of PPARγ [47, 48]. However, there is evidence that cytoplasmic-nuclear shuttling of PPARγ may be regulated by phosphorylation of PPARγ in the AF-1 domain at serines 46 and 51 of PPARγ2 (Ser 16 and Ser 21 of PPARγ1) [49]. In this instance, phosphorylation of PPARγ is mediated by casein kinase II (CK-II) and leads to cytoplasmic localization of PPARγ and decreased PPARγ transcriptional activity. These studies were largely performed in HEK 293 cells and in adipocytes the overall majority of PPARγ is found in the nucleus. Hence, the physiological relevance of these observations is still unclear.
1.3.2. SUMOylation of PPARγ
PPARγ is reversibly modified by SUMO-1 (small ubiquitin-related modifier) at lysine107 (lysine77 in PPARγ1) and lysine395 (lysine365 in PPARγ1) [50–53]. First identified in 1995 [54] as a suppressor of a centromere-associated protein, SUMO-1 is an approximately 10 kD protein that regulates protein activity, stability, or cellular location. Although found in the cytoplasm and nucleus, SUMO-1 is best described as regulating a wide range of nuclear functions, including replication, transcription, DNA repair, and cytoplasmic-nuclear protein transport [55]. SUMOylation of PPARγ in the N-terminal AF-1 domain (lysine107) strongly represses PPARγ transcriptional activity, suggesting SUMO-1 modification of PPARγ at lysine107 accounts for the repressive effect of amino acids 100–138 [56]. The potential for “crosstalk” between SUMO-1 modification at lysine107 and phosphorylation at serine112 within this region is supported by at least one study showing that SUMOylation of PPARγ is impaired in the phosphorylation deficient form of PPARγ2 (Ser112Ala) [52], consistent with the repressive effect of serine112 phosphorylation.
SUMOylation-mediated repression of PPARγ transcriptional activity occurs even though only a small percentage of PPARγ protein is modified by SUMO-1 at steady-state. This disproportionate effect of SUMOylation on PPARγ activity is consistent with the effect of SUMOylation on other transcription factors and may be due to SUMO-dependent recruitment of “downstream” cofactors that regulate PPARγ activity. SUMOylation of PPARγ in the AF-1 domain occurs within a sequence specific context found in many nuclear receptors that is termed a “synergy control” motif [57, 58]. At promoters containing multiple response elements, complex protein-protein interactions with transcription factors give rise to synergistic interactions that control gene expression patterns [59, 60]. The repressive activity of the synergy control motif is determined by SUMO-1 (or SUMO-2) modification of a lysine within the synergy control region and the SUMO-1 modification provides a surface for interactions with transcriptional repressors such as p300 [61] and the histone deacetylases [62]. Evidence of the effect of SUMOylation on the PPARγ AF-1 domain synergy control motif awaits additional experiments to examine the interactions of PPARγ when modified at lysine107 by SUMO-1. However, ligand-dependent SUMOylation of PPARγ1 at lysine365 (lysine395 in PPARγ2) has been shown to promote the interaction of PPARγ with nuclear receptor corepressor (NCoR)-histone deacetylase-3 (HDAC3) complexes in macrophages [53]. This SUMO-1 dependent interaction prevents removal of the NCoR-HDAC3 complexes by proteasomal degradation and maintains repression of PPARγ activity. The physiological relevance of PPARγ SUMOylation has been observed in FGF21 null mice that have alterations in PPARγ signaling. Mice lacking FGF21 are lipodystropic and have less body fat and decreased expression of PPARγ target genes [63]. These transgenic mice also have increased PPARγ SUMOylation and FGF21 is capable of inhibiting PPAR SUMOylation at lysine107. Collectively, these observations suggest that hormones including FGF21 modulate PPARγ transcriptional activity by regulating SUMOylation at lysine107 and contribute to whole body insulin sensitivity. To date, no studies have examined the SUMOylation of the PPARγ2 ligand-binding domain in adipocytes. Hopefully, future studies will determine if this modification occurs in adipocytes and if there is any “cross-talk” between SUMO-1 modification at lysine395 and Cdk5-dependent phosphorylation at serine273.
1.3.3. Ubiquitylation of PPARγ
SUMO-1 is structurally related to ubiquitin, an 8.5 kD polypeptide that regulates myriad cellular functions ranging from receptor-mediated endocytosis to regulation of transcription when covalently attached to targeted proteins [64]. Proteins modified by multiple ubiquitin polypeptides can be targeted to a number of possible fates, including proteasomal degradation, the best described endpoint for a polyubiquitylated protein. Covalent modification of proteins by ubiquitin occurs via a highly regulated cascade of enzymes that catalyze the activation of ubiquitin followed by the transfer of ubiquitin to the targeted protein. The process of activating and transferring ubiquitin to a substrate is repeated multiple times to form the polyubiquitin chains that are recognized by the proteasome.
PPARγ proteins have a short half-life in adipocytes [65] and the turnover rate of PPARγ proteins is regulated by the ubiquitin-proteasome system under ligand-independent and ligand-dependent conditions [66, 67]. Ligand binding increases ubiquitin modification of PPARγ and proteasomal degradation of PPARγ and in vitro assays indicate PPARγ is modified by ubiquitin in the ligand binding domain, although the exact site of ubiquitylation has not been identified [66, 68]. Although ubiquitylation occurs in the absence of the AF2 domain, the AF2 domain is required for maximal ubiquitin modification of the PPARγ ligand binding domain [68]. This suggests ubiquitylation of PPARγ may precede coactivator binding of the AF-2 domain and that ubiquitin modification of PPARγ functions as an integral part of PPARγ transcriptional activation. The link between ubiquitin modification of PPARγ and activation of PPARγ is further supported by the observations that inhibiting proteasome activity increases PPARγ activity and ubiquitylation in general is required for PPARγ activity in adipocytes [50, 68].
The simplest interpretation of the studies on PPARγ ubiquitylation is that ubiquitin modification of PPARγ regulates PPARγ activity by controlling the abundance of PPARγ proteins. Regulation of PPARγ protein steady state levels by promoting ubiquitin modification of PPARγ may represent an important aspect of the action of the insulin sensitizing thiazolidinediones. Genetic studies using mouse models of insulin resistance show decreased PPARγ gene expression is as effective as thiazolidinedione activation of PPARγ in improving insulin sensitivity [69–71]. The decreased PPARγ gene expression is associated with lower steady state levels of PPARγ protein in adipose tissue [72], lending substantial support to the possibility that modification of PPARγ by the ubiquitin-proteasome in adipocytes is an important regulation of systemic insulin sensitivity that can be exploited in the treatment of type 2 diabetes.
1.3.4. O-GlcNAcylation of PPARγ
A type of glycosylation that occurs on both cytosol and the nuclear proteins is the β-O-linked N-acetylglucosamine (O-GlcNAc) post-translational modification. Recent mutational analysis and mass spectrometry studies have revealed that the threonine54 in the AF-1 domain of PPARγ1 (threonine84 in PPARγ2) is a major O-GlcNAc site [73]. The transcriptional activity of wild type PPARγ was inhibited in the presence of an inhibitor of this modification, whereas the threonine mutant (Thr54Ala) was unresponsive to inhibitor treatment. Although these studies were conducted in vitro in cultured 3T3-L1 adipocytes, the results clearly show that O-GlcNAcylation of PPARγ functions to reduce PPARγ transcriptional activity.
1.3.5. Potential interplay of PPARγ post-translational modifications
The phosphorylation and SUMOylation sites in the AF-1 domain of PPARγ are contained within a highly conserved motif (ψ KxExxSP, where ψ is a hydrophobic residue; in PPARγ the sequence is IK107 VEPAS112P) that is considered to be a phosphorylation-dependent SUMOylation motif. Many of the proteins containing this motif are transcription factors, including the nuclear receptors [74]. In PPARγ, phosphorylation and SUMOylation in the AF-1 domain function to repress transcriptional activity. Although phosphorylation within the “phospho-SUMO switch” in other nuclear receptors acts to block SUMOylation of the AF-1 domain [75], the evidence that SUMOylation is impaired in the phosphorylation-deficient form of PPARγ [52] supports the possibility that phosphorylation of serine112 regulates SUMOylation of lysine 107 to repress PPARγ activity. In addition, phosphorylation of serine112 may affect ubiquitylation of PPARγ since inhibition of MAPK signaling alters the stability of PPARγ and the level of PPARγ ubiquitylation [66, 67]. This may also be the case for SUMOylation. While SUMOylation of lysine107 can occur in the absence of ubiquitylation, inhibiting SUMO-1 modification of lysine107 increases proteasomal degradation of PPARγ [50], suggesting SUMOylation regulates ubiquitin modification of the PPARγ ligand-binding domain. Stabilization of PPARγ protein via SUMO-1 modification is consistent with studies that indicate SUMOylation and ubiquitylation have opposing functions, but the interplay between SUMOylation and ubiquitylation in PPARγ may be more complex and involve cooperative as well as antagonistic functions [76].
Phosphorylation, SUMOylation, and ubiquitylation work in concert with acetylation in many transcription factors, including several of the nuclear receptors [77]. PPARγ contains twenty potential acetylation sites [78], although no sites in PPARγ have been definitively identified as modified by acetylation. However, the possibility of acetylation accompanying phosphorylation, SUMOylation, and ubiquitylation in regulating PPARγ activity is intriguing given that four of the potential sites for acetylation overlap or are in close proximity to SUMOylation and phosphorylation sites in the AF-1 (at lysines 107 and 119 of PPARγ2) and ligand binding domain (at lysine 268, 272 and 382). Although speculative, it seems highly likely that acetylation will participate with other covalent modifications in regulating the transcriptional activity of PPARγ.
1.4. The role of PPARγ post-translational modifications in metabolic disease
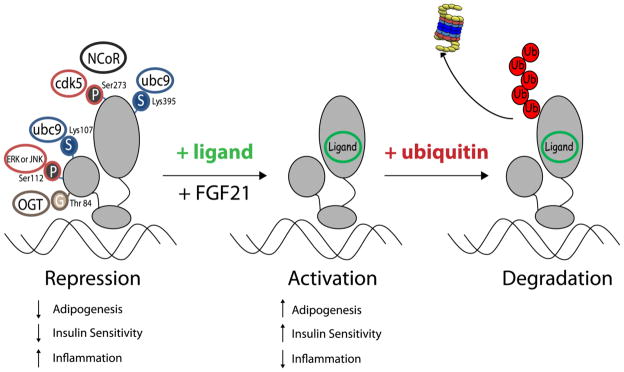
It appears that all of the PPARγ covalent modifications identified to date including phosphorylation, SUMOylation, O-GlcNAcylation and ubiquitylation largely function to decrease PPARγ transcriptional activity and/or expression levels (see Figure 2). For over a decade, we have known that PPARγ has been implicated in the regulation of systemic insulin sensitivity. This was first demonstrated when PPARγ was shown to be the functional receptor for the synthetic anti-diabetic thiazolidinediones (5). Moreover, direct evidence from genetic studies in humans has revealed that mutations in the ligand-binding domain of PPARγ are associated with severe insulin resistance and T2DM [79]. To date, none of the naturally occurring genetic mutations in humans have been shown to occur at the exact residues in PPARγ where covalent modifications are known to occur. Nonetheless, these post-translational modifications have important effects on PPARγ activity and expression levels. However, a well documented missense mutation in the PPARγ2 genes results in the conversion of proline to glutamine at position 115 and some studies indicate this polymorphism is associated with obesity [80, 81]. Ectopic expression of this mutant PPARγ results in decreased serine phosphorylation and increased PPARγ transcriptional activity [80]. Collectively, these studies revealed that the Pro115Gln mutation in PPAR2 enhanced the differentiation of adipocytes and this phenotype was likely a result of decreased serine phosphorylation.
Figure 2.
Covalent Modifications of PPARγ are associated with transcriptional repression.
As reviewed above and summarized in Table 1, there are a large number of studies demonstrating how covalent modification of PPARγ plays a role in modulating its activity and expression levels. In the last year, two new PPARγ covalent modifications have been discovered including O-GlcNAcylation and CDK5 mediated serine phosphorylation. Also, FGF21 has recently been identified as a potent modulator of PPARγ SUMOylation [63]. It will be interesting to learn whether other insulin sensitizers act by modulating PPARγ covalent modifications. Transgenic models of PPARγ modulation have shown that the activity and expression of PPARγ plays a role in both adipocyte development and insulin sensitivity. Yet, few studies on covalent modification of PPARγ have been performed in the whole animal setting. To date, the best evidence to support that covalent modifications of PPARγ may contribute to metabolic disease states comes from the Lazar laboratory. In these studies, mice containing a PPARγ2 gene that cannot be phosphorylated on serine112 developed obesity following high fat feeding but were partially protected from diet induced insulin resistance [34]. Hopefully, future studies will reveal equally important roles for other post translational modification of PPARγ under physiological and/or pathological conditions. The ability to modulate PPARγ activity and improve insulin sensitivity by specifically inhibiting a covalent modification of PPARγ may represent a viable therapeutic option in the treatment of insulin resistance and T2DM.
Table I.
Covalent Modifications of PPARγ2
| Modification of PPARγ2 | Site | Function |
|---|---|---|
| Phosphorylation | Serine 112 | General decrease in transcriptional activity |
| Serine 273 | Decrease in activation of specific target genes | |
| SUMOylation | Lysine 107 | Inhibits transcriptional activity |
| Lysine 395 | Prevents NCoR removal to maintain repression of activity | |
| Ubiquitylation | Ligand Binding Domain | Targets PPARγ for degradation to reduce protein expression |
| O-GlcNAcylation | Threonine 84 | Inhibits ligand-independent transcriptional activity |
Highlights.
PPARγ is important in fat cell development
PPARγ plays a role in glucose homeotstasis and an an anti-diabetic drug target.
The activity of of PPARγ is largely effected by covalent modifications..
The activity of PPARγ is highy regulated by phosphorylation.
The activity of PPARγ is highy regulated by SUMOylation
The activity of PPARγ is highy regulated by ubiquitin modification.
Acknowledgments
American Diabetes Association Basic Science Award and National Institutes of Health (NIH R56DK089020) (to Z.E.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 2.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 4.Harris PK, Kletzien RF. Localization of a pioglitazone response element in the adipocyte fatty acid-binding protein gene. Mol Pharmacol. 1994;45:439–445. [PubMed] [Google Scholar]
- 5.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 6.Chao L, Marcus-Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E, Gavrilova O, Reitman ML. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest. 2000;106:1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O’Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 9.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 10.Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD, Hong SH, Castro GL, Yin YQ, Nelson MC, Hsiao G, Greaves DR, Downes M, Yu RT, Olefsky JM, Evans RM. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci U S A. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 13.Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev. 2002;16:27–32. doi: 10.1101/gad.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummasti S, Tontonoz P. The peroxisome proliferator-activated receptor N-terminal domain controls isotype-selective gene expression and adipogenesis. Mol Endocrinol. 2006;20:1261–1275. doi: 10.1210/me.2006-0025. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RN. Nuclear receptor corepressors and PPARgamma. Nucl Recept Signal. 2006;4:e003. doi: 10.1621/nrs.04003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J Biol Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 17.Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M, White R. Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor alpha. Molecular endocrinology. 2007;21:1581–1592. doi: 10.1210/me.2007-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, Bandyopadhyay G, Scadeng M, Ofrecio JM, Nalbandian S, Olefsky JM. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefterova MI, Steger DJ, Zhuo D, Qatanani M, Mullican SE, Tuteja G, Manduchi E, Grant GR, Lazar MA. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Molecular and cellular biology. 2010;30:2078–2089. doi: 10.1128/MCB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 23.Rachez C, Freedman LP. Mediator complexes and transcription. Curr Opin Cell Biol. 2001;13:274–280. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 24.Fondell JD, Ge H, Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci U S A. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge K, Guermah M, Yuan CX, Ito M, Wallberg AE, Spiegelman BM, Roeder RG. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 26.Qi C, Surapureddi S, Zhu YJ, Yu S, Kashireddy P, Rao MS, Reddy JK. Transcriptional coactivator PRIP, the peroxisome proliferator-activated receptor gamma (PPARgamma)-interacting protein, is required for PPARgamma-mediated adipogenesis. J Biol Chem. 2003;278:25281–25284. doi: 10.1074/jbc.C300175200. [DOI] [PubMed] [Google Scholar]
- 27.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. The Journal of biological chemistry. 1999;274:3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- 28.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. Embo J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 29.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, Chiu KP, Pettersson S, Wei CL, Ruan Y, Liu ET. De-novo identification of PPARgamma/RXR binding sites and direct targets during adipogenesis. PLoS One. 2009;4:e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Berger J, Zhou G, Elbrecht A, Biswas S, White-Carrington S, Szalkowski D, Moller DE. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 1996;271:31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 35.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 36.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 37.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. The Journal of biological chemistry. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 38.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 39.Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, Kaestner KH, Lazar MA. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 40.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schurer SC, Vidovic D, Shulman GI, Spiegelman BM, Griffin PR. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su SC, Tsai LH. Cyclin-Dependent Kinases in Brain Development and Disease. Annu Rev Cell Dev Biol. 2010 doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- 43.Dhavan R, Tsai LH. A decade of CDK5, Nature reviews. Molecular cell biology. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 44.Lilja L, Yang SN, Webb DL, Juntti-Berggren L, Berggren PO, Bark C. Cyclin-dependent kinase 5 promotes insulin exocytosis. The Journal of biological chemistry. 2001;276:34199–34205. doi: 10.1074/jbc.M103776200. [DOI] [PubMed] [Google Scholar]
- 45.Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor Necrosis Factor-α Regulates Cyclin-dependent Kinase 5 Activity during Pain Signaling through Transcriptional Activation of p35. Journal of Biological Chemistry. 2009;284:2275–2284. doi: 10.1074/jbc.M805052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2007;27:803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle. 2007;6:1539–1548. doi: 10.4161/cc.6.13.4453. [DOI] [PubMed] [Google Scholar]
- 49.von Knethen A, Tzieply N, Jennewein C, Brune B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J Cell Sci. 2010;123:192–201. doi: 10.1242/jcs.055475. [DOI] [PubMed] [Google Scholar]
- 50.Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes Res. 2004;12:921–928. doi: 10.1038/oby.2004.112. [DOI] [PubMed] [Google Scholar]
- 51.Ohshima T, Koga H, Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem. 2004;279:29551–29557. doi: 10.1074/jbc.M403866200. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes to cells : devoted to molecular & cellular mechanisms. 2004;9:1017–1029. doi: 10.1111/j.1365-2443.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 53.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meluh PB, Koshland D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Molecular biology of the cell. 1995;6:793–807. doi: 10.1091/mbc.6.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 56.Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and -2 isoforms and influence of insulin. J Biol Chem. 1997;272:20230–20235. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- 57.Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc Natl Acad Sci U S A. 2003;100:15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iniguez-Lluhi JA, Pearce D. A common motif within the negative regulatory regions of multiple factors inhibits their transcriptional synergy. Mol Cell Biol. 2000;20:6040–6050. doi: 10.1128/mcb.20.16.6040-6050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 60.Ellwood K, Huang W, Johnson R, Carey M. Multiple layers of cooperativity regulate enhanceosome-responsive RNA polymerase II transcription complex assembly. Mol Cell Biol. 1999;19:2613–2623. doi: 10.1128/mcb.19.4.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, Garcia-Wilson E, Perkins ND, Hay RT. P300 transcriptional repression is mediated by SUMO modification. Mol Cell. 2003;11:1043–1054. doi: 10.1016/s1097-2765(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 62.Yang SH, Sharrocks AD. SUMO promotes HDAC-mediated transcriptional repression. Mol Cell. 2004;13:611–617. doi: 10.1016/s1097-2765(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 63.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast Growth Factor-21 Regulates PPARgamma Activity and the Antidiabetic Actions of Thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 65.Waite KJ, Floyd ZE, Arbour-Reily P, Stephens JM. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J Biol Chem. 2001;276:7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 66.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 67.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 68.Kilroy GE, Zhang X, Floyd ZE. PPAR-gamma AF-2 Domain Functions as a Component of a Ubiquitin-dependent Degradation Signal. Obesity (Silver Spring) 2009;17:665–673. doi: 10.1038/oby.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 70.Miles PD, Barak Y, Evans RM, Olefsky JM. Effect of heterozygous PPARgamma deficiency and TZD treatment on insulin resistance associated with age and high-fat feeding. Am J Physiol Endocrinol Metab. 2003;284:E618–626. doi: 10.1152/ajpendo.00312.2002. [DOI] [PubMed] [Google Scholar]
- 71.Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anghel SI, Bedu E, Delucinge Vivier C, Descombes P, Desvergne B, Wahli W. Adipose tissue integrity as a prerequisite for systemic energy balance : A critical role for PPARgamma. J Biol Chem. 2007 doi: 10.1074/jbc.M702490200. [DOI] [PubMed] [Google Scholar]
- 73.Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochemical and Biophysical Research Communications. 2012;417:1158–1163. doi: 10.1016/j.bbrc.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 74.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-Dependent Antagonism of Sumoylation Derepresses Progesterone Receptor Action in Breast Cancer Cells. Molecular Endocrinology. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 76.Helle DU. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends in Cell Biology. 2005;15:525–532. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Tian L, Popov VM, Pestell RG. Acetylation and nuclear receptor action. J Steroid Biochem Mol Biol. 2011;123:91–100. doi: 10.1016/j.jsbmb.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li A, Xue Y, Jin C, Wang M, Yao X. Prediction of Nepsilon-acetylation on internal lysines implemented in Bayesian Discriminant Method. Biochemical and biophysical research communications. 2006;350:818–824. doi: 10.1016/j.bbrc.2006.08.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 80.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 81.Clement K, Hercberg S, Passinge B, Galan P, Varroud-Vial M, Shuldiner AR, Beamer BA, Charpentier G, Guy-Grand B, Froguel P, Vaisse C. The Pro115Gln and Pro12Ala PPAR gamma gene mutations in obesity and type 2 diabetes. Int J Obes Relat Metab Disord. 2000;24:391–393. doi: 10.1038/sj.ijo.0801191. [DOI] [PubMed] [Google Scholar]