Abstract
The successful development of antibody therapeutics depends on the molecules having properties that are suitable for manufacturing, as well as use by patients. Because high solubility is a desirable property for antibodies, screening for solubility has become an essential step during the early candidate selection process. In considering the screening process, we formed a hypothesis that hybridoma antibodies are filtered by nature to possess high solubility and tested this hypothesis using a large number of murine hybridoma-derived antibodies. Using the cross-interaction chromatography (CIC) method, we screened the solubility of 92 murine hybridoma-derived monoclonal antibodies and found that all of these molecules exhibited CIC profiles that are indicative of high solubility (>100mg/mL). Further investigations revealed that variable region N-linked glycosylation or isoelectric parameters are unlikely to contribute to the high solubility of these antibodies. These results support the general hypothesis that hybridoma monoclonal antibodies are highly soluble.
Keywords: cross-interaction chromatography, Glycosylation, hybridoma, immunoglobulin, isoelectric point, monoclonal antibody, solubility
Introduction
Monoclonal antibodies (mAbs) developed as human therapeutics have had substantial success as measured by global sales of marketed pharmaceuticals in recent years because of their exquisite specificity toward a variety of targets and often limited side effects. This success has led to rapid technological advancements that have enabled the discovery and optimization of potent mAb therapeutic candidates, using strategies ranging from transgenic mice to phage and other display selection methods.1 MAbs are complex proteins typically produced in mammalian cell cultures through elaborate manufacturing process designed to yield consistent products. The molecular properties of the mAbs, such as solubility, production yields in certain cell lines, and resistance to chemical modifications, play an essential role in the success of the manufacturing process. As the use of mAbs for chronic disease indications becomes more common, their amenability to convenient delivery methods such as subcutaneous injection has become increasingly important. Subcutaneous delivery requires a small volume (preferred at < 1.5 mL) of a high doses (> 10 mg/kg),2 thus requiring high concentration formulations. As a result, highly soluble mAbs have become a subject of intense research in recent years.3-10
Human therapeutic mAbs are commonly generated using either display methods, including the selection of humanized and affinity-matured murine antibodies, or from transgenic animals. Engineering efforts to improve the solubility of highly potent mAbs have been reported recently.11,12 In these cases, mAbs with poor solubility were derived using phage display selection methods. Our recent experience in screening the solubility of phage-derived mAbs suggests that the solubility screen is a crucial step to eliminate less soluble mAbs early in the process. We began to ask the question whether a solubility screen would be equally crucial for mAbs generated directly from animals. Due to the lack of access to a large number of human mAbs from human or transgenic animal sources, we investigated the solubility properties of murine hybridoma-derived mAbs.
Cross-interaction chromatography (CIC) has emerged as a simple and efficient screening tool to select highly soluble human mAbs.10 This method measures weak protein-protein interactions between a target human mAb in solution phase and human polyclonal antibodies immobilized to a resin matrix, and identifies mAbs that have high solubility (> 100 mg/mL). We have adapted this method to screen the solubility of murine hybridoma-derived antibodies by immobilizing murine polyclonal antibodies to the resin matrix. We evaluated the solubility of 92 murine hybridoma mAbs by the CIC method and found that all antibodies in this study exhibited high solubility profile. These results are consistent with the SEC and DLS measurements. In addition, we investigated the potential contribution from either variable region (V-region) N-linked gylcosylation or isoelectric points (pI) that could have enhanced the solubility at neutral pH. We found that the high solubility profiles of the antibodies unlikely assisted by either V-region N-linked gylcosylation or extreme isoelectric points. These findings support the hypothesis that hybridoma antibodies are more likely to be highly soluble, meeting one of the important requirements for a successful therapeutic mAb development.
Results
Aggregation assessment by size exclusion chromatography (SEC) method
We first evaluated the presence of aggregation in the 92 hybridoma-derived murine antibodies by the SEC-HPLC method. All the antibodies were found to be > 94% monomeric.
Solubility screen by the CIC method
The CIC solubility screen using polyclonal human IgG has been established previously as a high-throughput method to screen for the solubility of human mAbs.10 It was shown that highly soluble antibodies have chromatographic retention factor (k′) values near zero. Antibodies with k’ values > 0.6 are generally significantly less soluble.
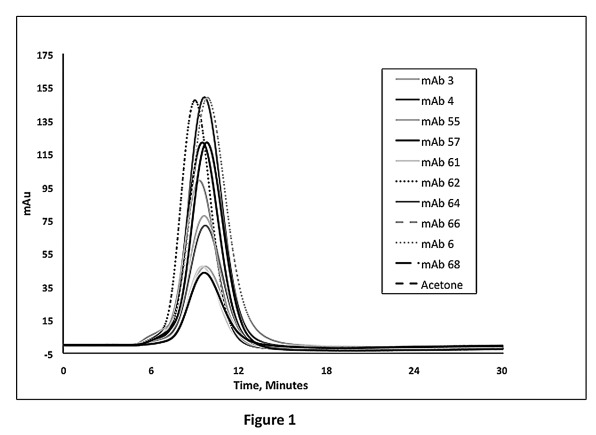
This underlying principle is applicable to antibodies derived from other species. We modified this method to test hybridoma antibodies generated in mice by immobilizing polyclonal murine IgG to a column. Using this method, we evaluated the solubility of 92 hybridoma-derived murine antibodies. These hybridoma antibodies were raised against more than 20 different soluble antigenic proteins. The CIC data showed that all 92 hybridoma-derived mAbs yielded k’ value of < 0.2, suggesting that they are highly likely to reach concentrations above 100 mg/mL at neutral pH (representative data shown in Figure 1; data summarized in Table 1).
Figure 1. Representative murine hybridoma antibody CIC profiles on murine polyclonal IgG coupled column. Peak height differences are due to injection concentration variances (range 0.05–0.20 mg/mL).
Table 1. Retention factor k’ and pI of murine mAb derived from hybridoma1.
|
mAb # |
k'
2
|
k' after deglycosylation |
pI |
| 1 |
0.01 ± 0.02 |
|
7.2 |
| 2 |
0.00 |
0.03 |
7.2 |
| 3 |
-0.02 ± 0.04 |
0.00 |
N.D. |
| 4 |
0.02 |
|
7.1 |
| 5 |
0.01 ± 0.01 |
|
7.1 |
| 6 |
0.00 |
|
7.3 |
|
7 |
0.02 |
|
N.D. |
|
8 |
0.00 |
|
N.D. |
| 9 |
0.00 ± 0.00 |
|
7.7 |
| 10 |
0.00 |
|
7.3 |
| 11 |
0.01 ± 0.03 |
|
N.D. |
| 12 |
0.01 ± 0.01 |
|
7.2 |
| 13 |
0.02 ± 0.04 |
|
5.9 |
| 14 |
0.01 ± 0.02 |
|
7.0 |
| 15 |
0.01 ± 0.01 |
|
N.D. |
| 16 |
0.00 |
|
N.D. |
| 17 |
0.03 ± 0.01 |
|
8.3 |
| 18 |
0.18 |
|
6.5 |
| 19 |
-0.01 ± 0.06 |
|
N.D. |
|
20 |
0.04 |
|
N.D. |
| 21 |
0.01 ± 0.01 |
|
7.2 |
| 22 |
0.11 ± 0.15 |
0.05 |
7.2 |
| 23 |
0.03 ± 0.01 |
|
7.3 |
| 24 |
0.09 |
|
7.0 |
| 25 |
0.02 ± 0.01 |
|
7.4 |
| 26 |
0.16 |
0.13 |
N.D. |
| 27 |
0.01 ± 0.00 |
|
7.0 |
|
28 |
0.09 |
|
N.D. |
| 29 |
0.13 |
0.15 |
6.9 |
| 30 |
0.00 |
|
N.D. |
|
31 |
0.00 |
|
N.D. |
|
32 |
0.00 |
|
N.D. |
| 33 |
0.05 ± 0.01 |
0.06 |
6.9 |
|
34 |
0.09 |
|
N.D. |
| 35 |
0.04 |
0.05 |
N.D. |
| 36 |
0.01 ± 0.03 |
|
7.2 |
| 37 |
0.00 |
|
8.0 |
| 38 |
0.07 |
|
N.D. |
|
39 |
0.00 ± 0.00 |
|
N.D. |
| 40 |
0.02 ± 0.01 |
|
6.9 |
| 41 |
0.04 |
|
N.D. |
| 42 |
0.06 ± 0.03 |
|
7.0 |
| 43 |
0.00 ± 0.03 |
|
N.D. |
| 44 |
0.05 |
|
N.D. |
|
45 |
0.00 |
|
7.4 |
| 46 |
0.00 ± 0.00 |
|
7.4 |
| 47 |
0.12 ± 0.02 |
|
7.7 |
| 48 |
0.00 |
|
7.2 |
| 49 |
0.09 ± 0.01 |
|
7.3 |
|
50 |
0.04 |
|
N.D. |
| 51 |
0.04 ± 0.01 |
|
7.6 |
| 52 |
0.04 ± 0.00 |
|
6.9 |
| 53 |
0.03 ± 0.01 |
0.00 |
N.D. |
| 54 |
0.16 |
|
N.D. |
| 55 |
0.00 ± 0.04 |
|
7.1 |
| 56 |
0.04 |
|
N.D. |
| 57 |
0.04 ± 0.01 |
|
7.4 |
| 58 |
0.03 |
|
N.D. |
|
59 |
0.01 |
|
7.3 |
| 60 |
0.01 ± 0.01 |
|
N.D. |
| 61 |
0.06 ± 0.01 |
|
N.D. |
| 62 |
0.06 ± 0.01 |
|
7.6 |
|
63 |
0.07 ± 0.10 |
|
7.6 |
|
64 |
0.06 |
|
N.D. |
| 65 |
0.00 |
|
7.7 |
| 66 |
0.04 ± 0.06 |
|
6.9 |
| 67 |
0.03 ± 0.04 |
0.11 |
5.8 |
| 68 |
0.02 ± 0.03 |
|
7.0 |
|
69 |
0.04 |
|
N.D. |
|
70 |
0.08 |
|
N.D. |
| 71 |
0.02 ± 0.03 |
0.05 |
7.8 |
|
72 |
0.12 |
|
N.D. |
| 73 |
0.03 |
|
N.D. |
|
74 |
0.03 |
|
N.D. |
| 75 |
0.01 |
|
7.0 |
| 76 |
0.03 ± 0.04 |
|
8.3 |
|
77 |
0.07 |
|
N.D. |
|
78 |
0.13 |
|
N.D. |
|
79 |
0.03 |
|
7.2 |
| 80 |
0.04 |
|
6.9 |
|
81 |
0.07 |
|
N.D. |
| 82 |
0.02 ± 0.02 |
|
N.D. |
|
83 |
0.05 |
|
N.D. |
|
84 |
0.04 |
|
N.D. |
| 85 |
0.03 |
|
6.6 |
|
86 |
0.04 |
|
N.D. |
| 87 |
0.05 ± 0.04 |
0.00 |
N.D. |
|
88 |
-0.02 |
|
N.D. |
|
89 |
0.09 |
|
6.9 |
|
90 |
0.01 |
|
5.5 |
|
91 |
-0.01 |
|
6.2 |
| 92 | -0.03 | 5.5 |
1Samples with italic numbers were not subject to MALDI-TOF-MS analysis; Samples 89–92 have V-region sequences available and do not contain consensus N-linked glycosylation site; for the antibodies identified to contain V-region glycosylation, the retention factors (k’) upon deglycosylation are listed. 2The errors are calculated from n = 2 chromatographic experiments
Aggregation assessment by dynamic light scattering (DLS) method
To further assess the solution property of these mAbs, we performed DLS experiments on a randomly selected set of 14 mAbs. All these mAbs were found to have hydrodynamic radii and polydispersity that indicate predominantly monomer (Table 2). The DLS results are consistent with the above SEC and CIC results.
Table 2. DLS measurements of a selective set of murine hybridoma mAb.
|
mAb # |
Radius (nm) |
% Polydispersity |
% Monomer by mass |
| 62 |
5.5 |
17 |
100 |
| 63 |
5.2 |
14 |
100 |
| 65 |
5.1 |
13 |
100 |
| 66 |
5.3 |
13 |
100 |
| 67 |
5.4 |
11 |
99 |
| 68 |
5.3 |
12 |
100 |
| 71 |
5.5 |
19 |
100 |
| 75 |
4.7 |
7 |
98 |
| 76 |
4.7 |
10 |
97 |
| 79 |
4.8 |
10 |
100 |
| 80 |
5.1 |
16 |
100 |
| 85 |
5.0 |
13 |
100 |
| 89 |
4.7 |
20 |
100 |
| 90 | 4.7 | 7 | 100 |
The potential effect of variable region N-linked glycosylation
Although all the mAbs used in this study showed highly soluble CIC profiles, it is possible that some of these antibodies rely on additional N-linked glycosylation moieties in the variable region to maintain high solubility. Our laboratory, as well as others, has experienced situations in which antibodies with low solubility became highly soluble upon the addition of an N-linked glycosylation moiety in the variable domain.11,12
The consensus N-linked glycosylation site consists of a three-residue motif Asn-X-Ser /Thr, where X can be any amino acid residue except Pro.13,14 Five of the 92 murine hybridoma antibodes in this study have sequences available that do not contain any consensus N-linked glycosylation site in their V-region, while the remaining 87 mAbs were not sequenced. To investigate whether a significant number of these 87 antibodies have additional N-linked glycosylation moiety that may enhance their solubility, we performed enzymatic deglycosylation experiments on 63 of the 87 hybridoma antibodies and assessed their molecular weight changes by MALDI-TOF-MS. The MALDI-TOF-MS method has been successfully used to monitor enzymatic deglycosylation of antibodies.15
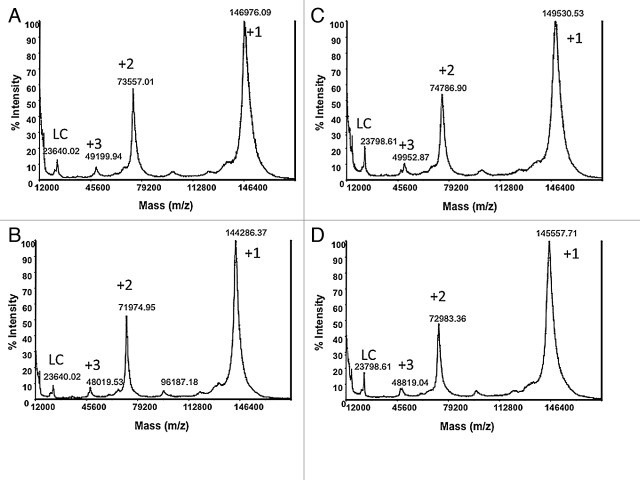
The mAbs showing intact molecular weight higher than 148 kDa typically contain additional glycosylation in the V-region, while those under ~148 kDa contain only Fc glycosylation.15 Representative MALDI-TOF-MS spectra of two murine antibodies (mAb 3 and mAb 17), before and after treatment with PNGase F, are shown in Figure 2. The majority of the 63 mAbs had molecular weights in the vicinity of 148 kDa and were found to be glycosylated in the Fc region only, where a ~2.5–3 kDa decrease in molecular weight (m/z of +1 ion) was observed upon deglycosylation (Fig. 2A and 2B). Eleven antibodies were identified as potentially having N-linked oligosaccharide in the variable domain, exhibiting intact mass significantly above 148 kDa (e.g., Figure 2C) and a mass loss of more than ~3 kDa upon the enzymatic treatment (e.g., Figure 2D). mAb 3 (in Figure 2C and 2D) seems to contain a mixture of IgGs with 3 or 4 N-linked glycans of which a large majority of molecules seems to contain at least 3 glycans. The CIC profiles of the 11 antibodies before and after the non-denaturing PNGase F treatment were compared. The profiles and the k’ values before and after the treatment are similar (Table 1), suggesting that no significant changes in solubility occurred upon the removal of the N-linked oligosaccharides in the V-region.
Figure 2. Comparison of MALDI-TOF-MS spectra before and after deglycosylation. (A) intact mAb 17 and (B) deglycosylated mAb 17; (C) intact mAb 3 and (D) deglycosylated mAb 3. +1 is singly charged molecular ion, +2 is doubly charged molecular ion, +3 is triply charged molecular ion and LC is free light chain.
The effect of isoelectric point (pI) on solubility
The isoelectric point (pI) of a protein is defined as the pH where a protein is charge-neutral and intramolecular electrostatic repulsion is at its lowest. Therefore, proteins are typically least soluble at their pI.16 As the solubility of proteins can be significantly improved in pH ranges far from their pI, we investigated the possibility that our antibodies are highly soluble in the PBS buffer because they carry a large amount of charges at neutral pH. The pI values for 50 hybridoma mAbs randomly selected from the panel of 92 mAbs were determined by isoelectric focusing (listed in Table 1). We found that for all mAbs measured, the pIs fell in the range of 5.5–8.3, with the majority of them (~90%) within 6–8. Therefore, the charges of the mAbs under investigation are unlikely to aid the high solubility profiles at neutral pH.
Discussions
Development of antibody therapeutics is a complex process that involves the expression and purification of the antibodies from mammalian cell cultures and formulation in stabilizing buffers that minimize aggregation and chemical degradation over a long period of time. The molecular properties of the antibodies profoundly affect the efficiency of the process. For high concentration formulations, the solubility of the antibodies is one of the most important properties that determine the success.
We have found that screening for solubility is a very important step in the selection of phage-derived therapeutic antibodies. Hybridoma antibodies have been hypothesized as being filtered by nature and thus more likely to be highly soluble compared with antibodies that are selected using display selection methods. We examined this hypothesis by evaluating the solubility of a large number of murine hybridoma antibodies using a modified CIC method. The CIC method, with human polyclonal IgG coupled to the column matrix, has been previously established as a high-throughput solubility screening method for human therapeutic mAbs.10 The retention factor k’ is used to describe the interaction between the antibodies in the stationary phase and the antibody in the solution phase. We have replaced human polyclonal IgG with murine polyconal IgG on the column to screen for the solubility of murine hybridoma mAbs. Our evaluation of 92 murine hybridoma-derived mAbs showed that all of them exhibited high solubility profiles with k’ < 0.2. The SEC data on all the mAbs and DLS data on a selected set of mAbs indicate the lack of significant aggregation in these mAb solutions.
We further explored alternative reasons to account for the results. Low solubility, or the tendency to aggregate at relatively low concentrations, is thought to be due to the presence of specific hydrophobic sequence motifs that are prone to participating in intermolecular interactions.17 While nature may have selected antibody sequences that are devoid of such aggregation-prone hydrophobic motifs, it may have employed other means to make antibodies highly soluble. One of such possibilities is having additional glycosylation in the V-region to shield the hydrophobic motifs. Such glycosylation are likely N-linked glycan since O-linked glycosylation is rare for antibodies.18 All antibodies generated from mammalian sources have N-linked oligosaccharides attached to Asn297 in the Fc region, which are somewhat buried and affect the conformation of the CH2 domain along with the effector functions.18-20 A significant percentage of natural antibodies is known to have glycosylation in the variable region. Approximately 18% of the heavy chain variable region sequences in the Kabat database contain a consensus N-linked glycosylation site. Experimentally, about 15–25% of the Fab fragments and 15% of the light chains isolated from human myeloma proteins were found to contain N-linked oligosaccharides.21 The additional N-linked glycosylation may play an important role in solubility in addition to affinity, specificity, half-life, and immunogenicity.22-24 We found in a previous study that the addition of an N-linked oligosaccharide in the V-region dramatically altered the solubility of an antibody.11 A similar improvement in solubility was reported by Pepinsky et al. when new N-linked glycosylation sites were introduced to the CH1 domain of an antibody with poor solubility.12
In this case, we found that ~17% of the antibodies studied by MALDI-TOF-MS have additional N-linked glycosylation in the V-region, consistent with the previously reported percentage. The CIC profiles of these mAbs did not change upon deglycosylation, which suggests that the presence of the glycans do not affect their solubility. Based on this data, we conclude that the antibodies in our study unlikely rely on additional N-linked glycosylation to possess high solubility.
Antibody solubility is highly dependent on solution conditions such as pH, salt, and other excipients. The hybridoma antibodies could have extremely low or high pIs to render them highly charged and thus highly soluble due to charge repulsion in the functional pH range. However, we found that ~90% of the mAbs that we measured have pIs in a relatively narrow range of 6–8, while all the mAbs that we measured have pIs between 5.5 and 8.3. This data makes charge repulsion an unlikely contributor to the high solubility. This observed pI range is in agreement with previous reports on the pI of hybridoma mAbs against a variety of antigens (e.g., ref. 25,26).
None of the 92 randomly selected murine hybridoma antibodies in this study exhibited a low solubility CIC profile. This is in sharp contrast to our experiences with screening a large number of human antibodies selected from phage display experiments, where we have observed a higher incidence of mAbs with low solubility as indicated by their CIC profiles (unpublished data). For some targets, we found that more than 30% of the mAbs selected from phage libraries exhibited low solubility CIC profile. A direct comparison was not attempted in this report because the antibodies were against different antigens. A comparison of antibodies derived from phage display selection method and hybridoma method against the same target is the subject of future investigations.
The results from this study provide an anecdotal support to the hypothesis that hybridoma antibodies are more likely to be highly soluble. Our experiences are that, with the additional scrutiny for solubility in place, all antibody generation methods can lead to highly potent, as well as highly soluble, antibodies for therapeutic applications. It is also important to note that high solubility is just one of the desirable properties of antibodies, and it alone does not guarantee the success of the complex development process involving expression, purification, and formulation of mAbs.27,28
Materials and Methods
Source of murine hybridoma antibodies
The hybridoma antibodies used in this study were obtained by immunizing Balb/c mice with > 20 different soluble proteins. Mice that demonstrated positive serum titers for the antigens were selected for hybridoma fusion. Splenocytes, which contain antibody-producing B cells from titer positive mice, were fused with an immortal cell line and the resulting hydridoma cells were cultured under selection conditions that allowed only hybridoma cells to grow. Growth-positive hybridomas secreting antigen-specific antibodies were selected for limited dilution subcloning. Binding assays were utilized to select antibodies that bound to the respective antigens specifically. While the majority of the 92 murine antibodies are in subclass IgG1, the rest are divided in subclasses IgG2a and IgG2b.
For most of the antibodies, a two-step pH elution purification process with protein G was used. The first step used was with 250 mM citrate buffer pH 4.5 while the second step employed was with 100 mM glycine buffer at pH 2.5. Most of the mAbs were eluted at pH 2.5 step. Some of the antibodies used in this study were purified using either MabSelect with one step elution using 0.1M sodium citrate pH 3.0, or MabSuRe with one step elution using 0.1M sodium acetate pH 3.5. Regardless of the capturing method, the eluted fractions were neutralized with 10% the elution volume of 1M TRIS-HCl pH 8.0 and dialyzed into PBS buffer. The purity of the antibodies was assessed by SDS-PAGE gel electrophoresis to be > 95%.
Size exclusion chromatography
The quality of the antibodies was further assessed using SEC-HPLC chromatography. Approximately 20ul of each sample (1–2 mg/mL) was loaded onto a Tosoh TSKgel G3000Wx1 column and eluted for 20 min in PBS buffer (Gibco Catalog #: 14190–136) with measurements taken at 215 nm and 280 nm wavelengths.
Cross-interaction chromatography (CIC) method
Murine polyclonal antibodies purified from pooled mouse serum were purchased from Sigma-Aldrich (I5381). Murine polyclonal antibodies were coupled to the resin matrix at ~30 mg/mL using the protocol described by Jacobs et al.10 Purified murine hybridoma mAbs in PBS buffer were injected to the murine IgG-coupled column and the control column, respectively, with concentrations range from 0.05 to 0.20 mg/mL. The retention times were used to calculate the retention factor k’ values reported in Table 1:
(Ref. 29)
Vr represents the elution volume of the sample on the protein coupled column, Vo the elution volume from a control column, Tr the retention time on the protein coupled column, and Tm the retention time on the control column. A number of samples were run twice on the same column.
In search of an antibody that can serve as a negative control, we tested a low solubility human antibody CNTO60711 on the murine IgG-coupled column. We found that it showed delayed elution time although not as pronounced as it did on a human IgG-coupled column (k’ = 0.3–0.4 on murine IgG column; k’ = 1.4 on human IgG column), Due to the absence of a low solubility murine antibody, we included this human mAb in our chromatography experiments as a negative control (data not shown).
Dynamic light scattering experiments
Particle sizes and distributions of samples were determined on a DynaPro Plate Reader DLS instrument (Wyatt Technologies Corporation) at 20°C and 1 mg/mL. Measurements were made using a Corning® 384-well black plate with clear flat bottom polystyrene (CLS3540) and 40μL sample in each well. For every measurement, 20 runs were performed. The refractive index of 1.333 at 589 nm for PBS buffer at 20°C was used (a standard value embedded in the software by the manufacturer). The method of cumulants was used to analyze the data. When a distribution of size is present, the effective radius is obtained by averaging the mass-weighted radii.
Degylcosylation experiments
The deglycosylation of the mAbs under non-denaturing condition was accomplished by treating the mAbs with PNGase F (New England BioLabs, Cat# P0705L) in 20 mM TRIS-HCl buffer, pH 7.0 at 37°C for 24 h. Mabs resulting from this treatment were used in the CIC analysis and the MALDI-TOF-MS analysis.
MALDI-TOF-MS analysis
MALDI-TOF-MS data of the glycosylated and deglycosylated mAb samples were acquired using a Voyager DE instrument from Applied BioSystems (Foster City, CA). The instrument was externally calibrated with a protein calibration kit (Sigma).
Isoelectric focusing
Isoelectric-focusing (IEF) gel electrophoresis was performed using Novex Pre-Cast Vertical pH 3–10 IEF Gels (Invitrogen). Antibodies were loaded at 5 μg each. The detailed procedures were conducted according to the manufacturer’s protocol. The pI of each antibody listed is the mid-point of the multiple bands observed on the gel due to charge heterogeneity introduced by the variability of the N-linked glycosylation.
Acknowledgments
The authors are grateful to Kimberly Mellon, Adrienne Clements-Egan, Mike Rycyzyn, Jessica Saggers, Jennifer Yohrling, Natalie Fursov and Erin Kennedy for providing murine hybridoma antibodies.
Glossary
Abbreviations:
- mAb
monoclonal antibody
- SEC
size exclusion chromatography
- CIC
cross-interaction chromatography
- DLS
dynamic light scattering
- IgG
immunoglobulin G
- Fc
constant domain
- Fv
variable domain
- Fab
antigen binding fragment
- V-region
variable region
- MALDI-TOF-MS
matrix assisted laser desorption ionization-time of flight-mass spectrometry
- IEF
isoelectric focusing
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/19869
References
- 1.Nieri P, Donadio E, Rossi S, Adinolfi B, Podestà A. Antibodies for therapeutic uses and the evolution of biotechniques. Curr Med Chem. 2009;16:753–79. doi: 10.2174/092986709787458380. [DOI] [PubMed] [Google Scholar]
- 2.Dani B, Platz R, Tzannis ST. High concentration formulation feasibility of human immunoglubulin G for subcutaneous administration. J Pharm Sci. 2007;96:1504–17. doi: 10.1002/jps.20508. [DOI] [PubMed] [Google Scholar]
- 3.Jespers L, Schon O, Famm K, Winter G. Aggregation-resistant domain antibodies selected on phage by heat denaturation. Nat Biotechnol. 2004;22:1161–5. doi: 10.1038/nbt1000. [DOI] [PubMed] [Google Scholar]
- 4.Famm K, Winter G. Engineering aggregation-resistant proteins by directed evolution. Protein Eng Des Sel. 2006;19:479–81. doi: 10.1093/protein/gzl032. [DOI] [PubMed] [Google Scholar]
- 5.Lienqueo ME, Mahn A, Navarro G, Salgado JC, Perez-Acle T, Rapaport I, et al. New approaches for predicting protein retention time in hydrophobic interaction chromatography. J Mol Recognit. 2006;19:260–9. doi: 10.1002/jmr.776. [DOI] [PubMed] [Google Scholar]
- 6.Demeule B, Lawrence MJ, Drake AF, Gurny R, Arvinte T. Characterization of protein aggregation: the case of a therapeutic immunoglobulin. Biochim Biophys Acta 2007; 1774:146–53. [DOI] [PubMed]
- 7.Harn N, Allan C, Oliver C, Middaugh CR. Highly concentrated monoclonal antibody solutions: direct analysis of physical structure and thermal stability. J Pharm Sci. 2007;96:532–46. doi: 10.1002/jps.20753. [DOI] [PubMed] [Google Scholar]
- 8.Trevino SR, Scholtz JM, Pace CN. Measuring and increasing protein solubility. J Pharm Sci. 2008;97:4155–66. doi: 10.1002/jps.21327. [DOI] [PubMed] [Google Scholar]
- 9.Tartaglia GG, Pawar AP, Campioni S, Dobson CM, Chiti F, Vendruscolo M. Prediction of aggregation-prone regions in structured proteins. J Mol Biol. 2008;380:425–36. doi: 10.1016/j.jmb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SA, Wu S-J, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27:65–71. doi: 10.1007/s11095-009-0007-z. [DOI] [PubMed] [Google Scholar]
- 11.Wu S-J, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–51. doi: 10.1093/protein/gzq037. [DOI] [PubMed] [Google Scholar]
- 12.Pepinsky RB, Silvian L, Berkowitz SA, Farrington G, Lugovskoy A, Walus L, et al. Improving the solubility of anti-LINGO-1 monoclonal antibody Li33 by isotype switching and targeted mutagenesis. Protein Sci. 2010;19:954–66. doi: 10.1002/pro.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall RD. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;40:17–26. [PubMed] [Google Scholar]
- 14.Bause E, Hettkamp H. Primary structural requirements for N-glycosylation of peptides in rat liver. FEBS Lett. 1979;108:341–4. doi: 10.1016/0014-5793(79)80559-1. [DOI] [PubMed] [Google Scholar]
- 15.Raju TS, Scallon BJ. Glycosylation in the Fc domain of IgG increases resistance to proteolytic cleavage by papain. Biochem Biophys Res Commun. 2006;341:797–803. doi: 10.1016/j.bbrc.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Tanford C. Physical Chemistry ofMacromolecules. New York: John Wiley, 1961. [Google Scholar]
- 17.Agrawal NJ, Kumar S, Wang X, Helk B, Singh SK, Trout BL. Aggregation in protein-based biotherapeutics: computational studies and tools to identify aggregation-prone regions. J Pharm Sci. 2011;100:5081–95. doi: 10.1002/jps.22705. [DOI] [PubMed] [Google Scholar]
- 18.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/S0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 20.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–8. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelberg HL, Abel CA, Fishkin BG, Grey HM. Localization of the carbohydrate within the variable region of light and heavy chains of human gamma g myeloma proteins. Biochemistry. 1970;9:4217–23. doi: 10.1021/bi00823a025. [DOI] [PubMed] [Google Scholar]
- 22.Wright A, Tao MH, Kabat EA, Morrison SL. Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO J. 1991;10:2717–23. doi: 10.1002/j.1460-2075.1991.tb07819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coloma MJ, Trinh RK, Martinez AR, Morrison SL. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1-->6) dextran antibody. J Immunol. 1999;162:2162–70. [PubMed] [Google Scholar]
- 24.Rudd PM, Wormald MR, Harvey DJ, Devasahayam M, McAlister MS, Brown MH, et al. Oligosaccharide analysis and molecular modeling of soluble forms of glycoproteins belonging to the Ly-6, scavenger receptor, and immunoglobulin superfamilies expressed in Chinese hamster ovary cells. Glycobiology. 1999;9:443–58. doi: 10.1093/glycob/9.5.443. [DOI] [PubMed] [Google Scholar]
- 25.Mayers GL, Bankert RB. Immunochemistry of monoclonal antibodies. Transplant Proc. 1980;12:413–6. [PubMed] [Google Scholar]
- 26.Tron F, Jacob L, Bach J-F. Murine monoclonal anti-DNA antibodies with an absolute specificity for DNA have a large amount of idiotypic diversity. Proc Natl Acad Sci U S A. 1983;80:6024–7. doi: 10.1073/pnas.80.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Adv Drug Deliv Rev. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Kozlowski S, Swann P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev. 2006;58:707–22. doi: 10.1016/j.addr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Tessier PM, Sandler SI, Lenhoff AM. Direct measurement of protein osmotic second virial cross coefficients by cross-interaction chromatography. Protein Sci. 2004;13:1379–90. doi: 10.1110/ps.03419204. [DOI] [PMC free article] [PubMed] [Google Scholar]