Abstract
The presence or absence of core fucose in the Fc region N-linked glycans of antibodies affects their binding affinity toward FcγRIIIa as well as their antibody-dependent cell-mediated cytotoxicity (ADCC) activity. However, the quantitative nature of this structure-function relationship remains unclear. In this study, the in vitro biological activity of an afucosylated anti-CD20 antibody was fully characterized. Further, the effect of fucose reduction on Fc effector functions was quantitatively evaluated using the afucosylated antibody, its “regular” fucosylated counterpart and a series of mixtures containing varying proportions of “regular” and afucosylated materials. Compared with the “regular” fucosylated antibody, the afucosylated antibody demonstrated similar binding interactions with the target antigen (CD20), C1q and FcγRIa, moderate increases in binding to FcγRIIa and IIb, and substantially increased binding to FcγRIIIa. The afucosylated antibodies also showed comparable complement-dependent cytotoxicity activity but markedly increased ADCC activity. Based on EC50 values derived from dose-response curves, our results indicate that the amount of afucosylated glycan in antibody samples correlate with both FcγRIIIa binding activity and ADCC activity in a linear fashion. Furthermore, the extent of ADCC enhancement due to fucose depletion was not affected by the FcγRIIIa genotype of the effector cells.
Keywords: afucosylated antibody, antibody-dependent cellular cytotoxicity, FcγRIIIA, fucosylation, glycoform variants, Glycosylation, monoclonal antibody
Introduction
The glycans attached to the asparagine at the 297 position (N297) of the Fc region of IgG play a critical role on the effector functions of antibodies.1-3 These N-linked glycans are situated within a cleft formed by the paired heavy chains in the CH2 domain of IgGs such that they may undergo extensive non-covalent interactions with the adjacent protein surface.4-6 There is evidence that interactions between the IgG Fc region and the effector ligands (Fcγ receptors and C1q) are critically dependent on IgG Fc protein–glycan interactions.7,8 Both the conformation and functionality of antibodies can be modulated by manipulation of these oligosaccharides.9,10 Antibodies depleted of N-linked glycans at Asn-297 behave similarly to normal antibodies with respect to antigen binding and Protein A binding capacity. However, they are defective in binding to Fcγ receptors, activating complement and inducing ADCC.4,11-13 Structural and thermodynamic data have shown that the precise structure of the IgG-Fc N-linked glycans helps to determine the binding affinity of the IgG to Fcγ receptors and thus the effector functions of the antibodies.14,15 Specifically, the N-glycans stabilize particular conformations of the CH2 domains and act as spacers, holding the CH2 domains apart to provide an open state of the horseshoe-shaped IgG-Fc fragment, allowing increased accessibility and tighter binding to Fcγ receptors.7,16-18
The majority of human IgG-Fc N-linked glycans are based on a common core structure of biantennary heptapolysaccharide containing GlcNAc and mannose.19,20 Further modification of the core carbohydrate structure through the addition of fucose, as well as bisecting GlcNAc, galactose and sialic acid, substantially increases structural heterogeneity, with more than 30 variant forms possible.21 For both serum-derived endogenous human IgGs and IgG produced from engineered mammalian cell lines, the majority of Fc N-linked glycans carry different degrees of terminal galactosylation resulting in a G0 glycoform, a G1 glycoform and a G2 glycoform. Whereas these glycans are predominantly fucosylated, i.e., contain a fucose attached to the innermost GlcNAc residue in the core structure, small amounts of naturally occurring glycoforms that lack the core fucose have been observed in both human serum-derived and CHO cell produced IgG. It is well-documented that the absence of core fucose in IgG results in higher affinity binding to the FcγRIIIa receptor (both the F158 and V158 allotypes of this receptor) and increased ADCC activity.22-27 In 2002, Shields et al. first reported that recombinant human IgG1 produced from the CHO-Lec13 cell line showed enhanced FcγRIIIa binding and ADCC activity compared with IgGs produced by regular CHO cells. The CHO-Lec13 cell line is deficient in its ability to add fucose to glycans, but produces IgGs with oligosaccharides that are otherwise similar to those found in normal CHO cell lines.22 Similar results were later reported by other groups using afucosylated antibodies produced from engineered CHO cell lines in which α-1,6-fucosyltransferase (FUT8) was either downregulated by RNA interference technology or genetically knocked out.26,27 In fact, enhancement of ADCC activity by fucose removal has been demonstrated with all four subclasses of IgGs, as well as with Fc fusion proteins in both in vitro assays and in vivo animal models.23,25,28-32 Moreover, the antigen density required to induce efficient ADCC activity is lower when the IgG has low, rather than high, fucose content.24 Based on these data, afucosylated forms of therapeutic antibodies are expected to have clinical advantages over the fucosylated forms because of the increased efficiency of ADCC induction and its ability to better compete with endogenous IgGs for binding with FcγRIIIa on effector cells. To date, several antibodies with no or low levels of fucose have been produced from genetically engineered cell lines and are currently in clinical studies as candidate therapeutics for various indications.
ADCC is a critical effector function that has been implicated in the clinical efficacy of a number of therapeutic antibodies.33,34 In ADCC, target cells bound by the Fab regions of antibodies are lysed by activated effector cells following interactions between Fc regions of the antibody and activating Fcγ receptors on effector cells. The potency of ADCC induction by therapeutic antibodies is dependent on binding affinity to both the target ligand and to the activating Fcγ receptors. Therefore, both the efficacy and safety of therapeutic antibodies may be dependent on the ADCC activity, which in turn is closely influenced by the level of afucosylated glycans in the antibody product. To ensure optimal safety and efficacy, the level of afucosylated glycan in therapeutic antibody products should be controlled during the manufacturing process. However, at present, there is no known way to fully regulate the glycosylation activities of CHO cells. Batch-to-batch variations in glycan profiles, including the extent of afucosylated glycans, are commonly observed in CHO cell-derived antibody products. While the impact of afucosylated glycans on FcγRIIIa binding and ADCC is established, the quantitative nature of this structure-function relationship remains unclear. A better understanding of this relationship, specifically its quantitative nature, is crucial for devising an assessment and control strategy to monitor the impact of varying levels of afucosylated glycans (inherent to the production processes) on effector functions of the antibody product.
In this study, we sought to provide a comprehensive in vitro characterization of biological activity of an afucosylated anti-CD20 antibody and to generate quantitative data to measure the affect of fucose reduction on Fc effector functions. We prepared a sample set consisting of an afucosylated AbX produced from a CHO cell line deficient in FUT8, the “regular” fucosylated AbX produced from CHO cells, and a series of mixtures with varying degrees of “regular” and afucosylated AbX. This sample set, hereafter referred to as AbX AF-blends, was quantitatively characterized in a series of in vitro assays including antigen (CD20) binding, Fcγ receptor binding, ADCC, C1q binding, and CDC assays. Results from these studies were used to delineate the relationship between the relative activity of FcγRIIIa binding/ADCC and the level of afucosylated glycans in the antibody samples.
Results
Using capillary electrophoresis to determine fucosylation level
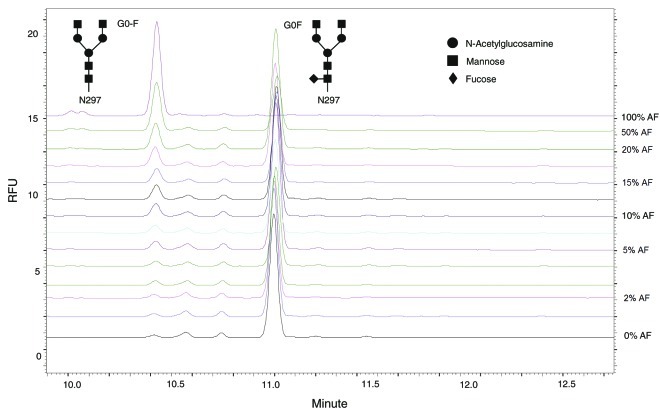
Fluorescent derivatives of glycans were released by PNGase F digestion and quantified by CE-LIF. Peaks were identified using a combination of known standards purchased commercially and enzymatic digests of the glycan structures. In this study, β-galactosidase was used to convert all glycans to the G0 form and the observed amount of G0-F reflected the aggregate of all non-fucosylated species (except the high mannose structures). The AbX AF-blends sample set consisted of 14 samples: the “regular” fucosylated (0% afucosylated AbX); the afucosylated (100% afucosylated AbX); and blended samples containing 1%, 2%, 3%, 4%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, and 50% of the afucosylated AbX, with the “regular” fucosylated AbX added to make up the final content. Samples in the AbX AF-blends were identified by the content of the afucosylated AbX, including the starting material. Therefore “regular” fucosylated AbX is referred as 0% AF, whereas the afucosylated AbX is referred as 100% AF. An overlay of glycan analysis data from the various blends is shown in Figure 1. The increase in the level of G0-F is apparent, together with a corresponding decrease in G0F, as increasing amounts of afucosylated material are added to the blend.
Figure 1. Overlay of electropherograms from CE-LIF with blended AbX samples. Glycan analysis was performed following β-galactosidase treatment of samples to convert all glycans to either G0F or G0-F. The top line corresponds to 100% afucosylated AbX, with a peak at G0-F but a flat line at G0F, indicating that the AbX produced by that cell line is not fucosylated (100% G0-F, 0% G0F); the bottom line is the “regular” AbX which contains mostly G0F. Intermediate lines show data from different blends of normal AbX and afucosylated AbX, showing the expected mixtures of G0-F and G0F peaks. The amount of afucosylated material in AbX AF-Blends whose electropherograms were stacked from top to bottom are 100%, 50%, 20%, 17.5%, 15%, 12.5%, 10%, 7.5%, 5%, 4%, 3%, 2%, 1%, 0%. RFU = relative fluorescence unit.
Since the “regular” AbX material contained approximately 1.3% afucosylated glycans, this value was used to estimate the total theoretical G0-F content in various AF-blends. As shown in Table 1, the CE measurement of G0-F in the samples was consistent with the expected values, but often under-recovered G0-F by about 15% when measured within the 5–20% G0-F range (e.g., the observed level of 7.4% G0-F may reflect an underlying value of 8.4%). The reason for this slight discrepancy between theoretical and actual CE measured values for G0-F is not clear, but could be attributed to variations in the efficiency of PNGase F digestion or the quantum efficiency of the fluorophore used for CE, which was conjugated in close proximity to the fucose.
Table 1. Levels of afucosylated glycans in AbX AF-blends.
|
Afucosylated Material |
Theoretical G0-F (T) |
Observed |
Difference |
|
G0-F (O) |
(O-T)/T |
||
| 100% |
100% |
98% |
-2.2% |
| 50% |
51% |
46% |
-8.6% |
| 20% |
21% |
18% |
-15% |
| 17.5% |
19% |
16% |
-16% |
| 15% |
16% |
14% |
-16% |
| 12.5% |
14% |
11% |
-16% |
| 10% |
11% |
9.5% |
-15% |
| 7.5% |
8.7% |
7.4% |
-15% |
| 5% |
6.2% |
5.4% |
-13% |
| 4% |
5.2% |
4.8% |
-8.2% |
| 3% |
4.3% |
4.1% |
-4.0% |
| 2% |
3.3% |
3.2% |
-3.8% |
| 1% |
2.3% |
2.3% |
0.1% |
| 0% | 0% | 1.3% | 1.3% |
Theoretical G0-F was calculated assuming that the “regular” fucosylated material contains 1.3% afucosylated glycans and the afucosylated material contains 100% of afucosylated glycans; for example, the theoretical G0-F value of AbX AF-blend containing 10% afucosylated material is calculated as 0.1 x100% + 0.9 x 1.3% = 11%. Observed G0-F values are obtained from the CE-based glycan analysis.
Antigen Binding Activity
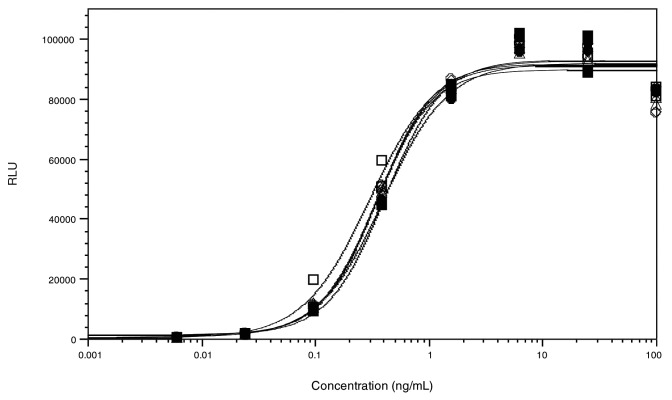
Binding of selected AbX AF-blends to target cells was measured with a cell-based assay using WIL2-S cells, which express a high level of target antigen (CD20) on the cell surface. Test antibodies bound to WIL2-S cells were detected with an electrochemiluminescent method using the MSD technology. Since there is no glycosylation site in the CDR of AbX, changes in the fucosylation level of this monoclonal antibody should not affect its binding to cell surface target antigens. Representative dose-response binding curves of AbX AF-blends to WIL2-S cells are presented in Figure 2. Samples tested included AF-blends containing 0%, 5%, 10%, 20%, 50%, and 100% afucosylated AbX. As expected, there were no discernible differences in CD20 binding among the AF-blends tested (Fig. 2).
Figure 2. Antigen binding activity of select AbX AF-blends measured in a cell-based binding assay. Human B lymphoma cell line WIL2-S were coated on plates and incubated with various concentrations of AbX AF-blends. Antibodies bound to CD20 on WIL2-S were detected with goat anti-human IgG polyclonal antibodies. Results for AbX AF-blends containing 0% (○), 5% (□), 10% (△), 20% (◇), 50% (●), and 100% (■) of the afucosylated material are shown. RLU = Relative luminescence unit
C1q Binding and CDC Activities
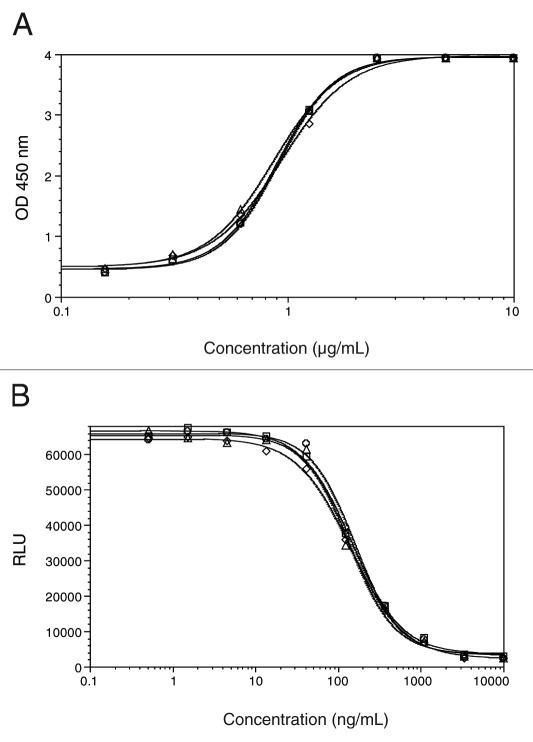
The affect of fucose reduction on C1q binding was assessed by an ELISA using purified human C1q. Test antibodies included the “regular” fucosylated AbX (0%AF), the afucosylated AbX (100%AF) and 2 blended samples containing 10% and 50% afucosylated AbX (10%AF and 50%AF) respectively. As shown in Figure 3A, all four samples showed comparable C1q binding activity as demonstrated by overlapping dose-response curves. Further, the CDC activity of these samples was assessed in a cell-based assay using WIL2-S as target cells and complement derived from normal human serum. As shown in Figure 3B, comparable CDC activities were observed for all 4 AbX samples with varying degrees of afucosylated glycans. This finding is consistent with published literature, which reports that removal of core fucose in N-glycans of antibodies showed no affect on CDC activity of the antibodies.25
Figure 3. (A) C1q binding and (B) CDC activities of select AbX AF-blends. C1q binding was assessed using purified human C1q by ELISA. CDC against human B lymphoma cell line WIL2-S cells was induced by the human serum complement in the presence of the test antibodies. Extent of cell lysis was measured by CellTiter-Glo which detects ATP from live cells. Samples tested included those containing 0% (○), 10% (□), 50% (△), and 100% (◇) of the afucosylated material.
Fcγ Receptor Binding Activity
The Fcγ receptor binding activity of AbX AF-blends was measured by ELISA-based methods using a panel of recombinant human Fcγ receptors (Ia, IIa-R131, IIb, IIIa-F158, and IIIa-V158). Antibody samples in monomeric form were tested for binding with the high-affinity receptor FcγRI (CD64) and in multimeric form (cross-linked with a F(ab’)2 fragment of goat anti-human kappa chain) for binding with the low-affinity receptors, i.e., FcγRII (CD32) and FcγRIII (CD16). Twelve AbX AF-blends were tested for binding with FcγRII and FcγRIII, including those containing 0%, 1%, 2%, 3%, 4%, 5%, 7.5%, 10%, 15%, 20%, 50%, and 100% of afucosylated AbX. Only six samples were tested for binding with FcγRI; the amount of afucosylated AbX in these samples were 0%, 5%, 10%, 20%, 50%, 100% .
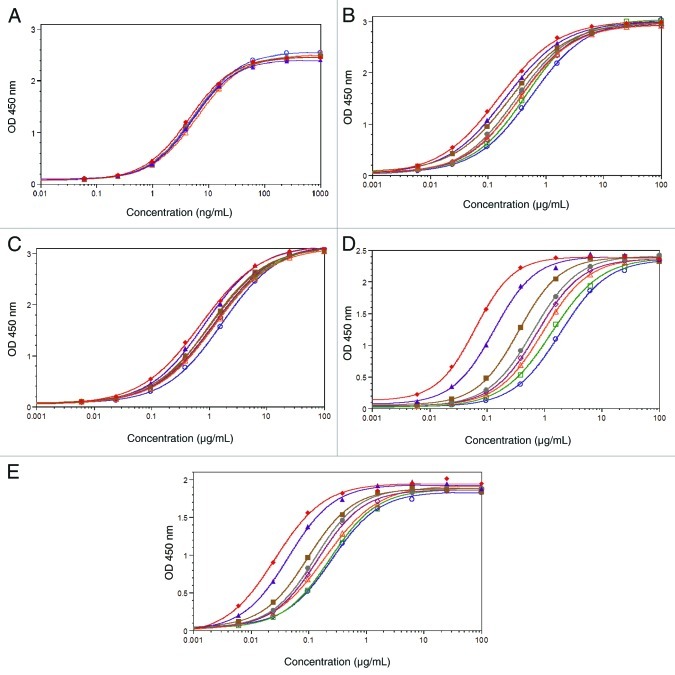
Representative binding curves are shown in Figure 4. Overlapping dose-response curves were observed for the binding of AbX AF-blends to Fcγ receptor Ia (Fig. 4A). The binding curves from samples containing increasing amounts of afucosylated glycans showed a small but apparent leftward shift for Fcγ receptors IIa (Fig. 4B) as well as IIb (Fig. 4C) and a much more profound leftward shift for both allotypes of FcγRIIIa (Figs. 4D and 4E). Overall, the extent of leftward shifts in binding curves among test samples appeared to be proportional to the respective content of afucosylated AbX. The leftward shift results in reduced EC50 value and is indicative of increased binding affinity. Overall, it appeared that removal of core fucose had a minimal affect on the binding of AbX with FcγRIa, moderate impact on the binding with Fcγ receptors IIa and IIb, and marked affect on the binding with IIIa. The assays were performed in multiple runs and the relative binding activity was calculated by inversely normalizing the EC50 values of test samples with those of the “regular” fucosylated AbX (0% afucosylated AbX). This exercise provided better quantification of the effect of afucosylation in different samples and allowed direct comparison of receptors with varying binding affinities. Of note, given that minimal differences were observed at upper asymptote of binding curves, it was justified to use EC50 values to calculate relative binding activity. The mean relative binding activity values are summarized in Table 2. Compared with the “regular” fucosylated AbX that had a relative binding activity value of 1, the afucosylated AbX showed a respective relative binding activity of 35 and 9 for F158 and V158; 3.6 and 2.4 for FcγRIIa and IIb, and 1.1 for FcγRIa.
Figure 4. Binding responses of AbX AF-blends to human Fcγ receptors. Dose-response binding curves of AbX AF-blends to various human Fcγ receptors were generated from ELISA-based binding assays using soluble recombinant Fcγ receptors. The test antibodies were assayed in monomeric form for (A) Fcγ Receptor Ia, and in multimeric forms for (B)IIa-R131, (C)IIb, (D)IIIa-F158, (E)IIIa-V158. All data points were collected in duplicate and the mean absorbance values from duplicate wells at OD 450nm were plotted against the antibody concentration. Binding curves for 0% (blue ○), 2% (green □), 5% (orange △), 7.5% (magenta ◇), 10% (gray ●), 20% (brown ■), 50% (purple ▲), 100% (red ◆) of the afucosylated material are shown. The results shown were obtained from one representative data set out of at least three in vitro binding experiments.
Table 2. Summary of mean relative Fcγ receptor binding activity of AbX AF-Blends.
|
Percent |
FcγRIIIa-F158 |
FcγRIIIa-V158 |
FcγRIIa-R131 |
FcγRIIb |
FcγRIa |
|||||
|
Afucosylated Material |
Mean (n = 6) |
SD |
Mean (n = 6) |
SD |
Mean (n = 4) |
SD |
Mean (n = 4) |
SD |
Mean (n = 3) |
SD |
| 0% |
1.00 |
NA |
1.00 |
NA |
1.00 |
NA |
1.00 |
NA |
1.00 |
NA |
| 1% |
1.34 |
0.11 |
1.10 |
0.26 |
1.16 |
0.09 |
1.13 |
0.01 |
ND |
NA |
| 2% |
1.49 |
0.16 |
1.16 |
0.17 |
1.37 |
0.18 |
1.31 |
0.03 |
ND |
NA |
| 3% |
1.78 |
0.14 |
1.24 |
0.28 |
1.34 |
0.10 |
1.26 |
0.10 |
ND |
NA |
| 4% |
1.96 |
0.24 |
1.32 |
0.21 |
1.35 |
0.04 |
1.27 |
0.00 |
ND |
NA |
| 5% |
2.31 |
0.22 |
1.55 |
0.44 |
1.30 |
0.08 |
1.20 |
0.05 |
0.88 |
0.06 |
| 7.5% |
2.81 |
0.31 |
1.81 |
0.50 |
1.62 |
0.02 |
1.60 |
0.09 |
ND |
NA |
| 10% |
3.34 |
0.12 |
2.05 |
0.50 |
1.64 |
0.16 |
1.38 |
0.02 |
1.00 |
0.04 |
| 15% |
4.35 |
0.36 |
2.53 |
0.47 |
1.72 |
0.01 |
1.63 |
0.03 |
ND |
NA |
| 20% |
6.00 |
0.46 |
2.98 |
0.76 |
1.66 |
0.11 |
1.50 |
0.22 |
0.94 |
0.03 |
| 50% |
15.7 |
1.23 |
6.12 |
0.75 |
2.29 |
0.08 |
1.70 |
0.15 |
0.98 |
0.04 |
| 100% | 34.9 | 6.25 | 9.05 | 0.80 | 3.62 | 0.42 | 2.40 | 0.13 | 1.13 | 0.11 |
Relative Fcγ receptor binding activity = [EC50 0% Afucosylated material/ EC50 Sample]. SD = Standard deviation; NA = Not applicable; ND = Not done.
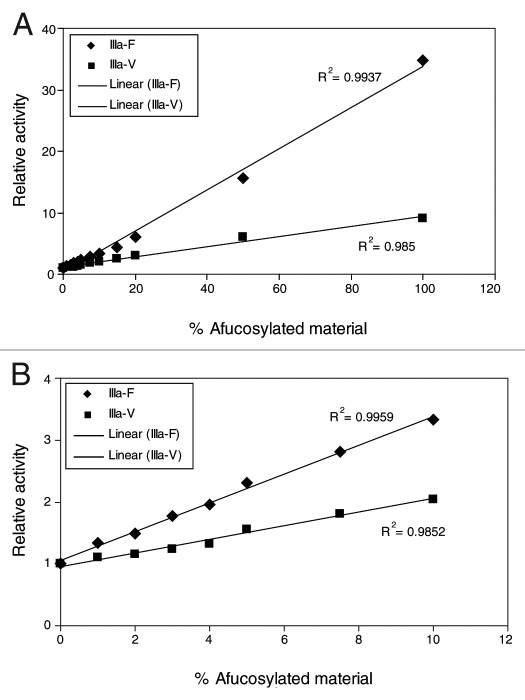
The relative binding activity was plotted against the level of afucosylation and the data were fitted with a linear model to further examine the nature of the relationship between FcγRIIIa binding activity and afucosylation. Proportional increases in relative FcγRIIIa binding were observed for AF-blends with increasing amount of afucosylated AbX (Fig. 5A); the R-squared values for the linear fit with F158 and V158 allotypes are 0.994 and 0.985, respectively. Given that levels of afucosylated glycans in CHO cell-derived IgG are usually less than 10%, further analysis was performed using data generated from samples containing afucosylated glycan levels ranging from 0–10%. As shown in Figure 5B, a positive linear correlation between relative binding activity and the amount of afucosylated AbX in AF-blends (0%, 1%, 2%, 3%, 4%, 5%, 7.5%, and 10%) was clearly demonstrated; the R-squared values for the linear fit with the F158 and V158 allotypes are 0.996 and 0.985, respectively. It is worth noting that this linear relationship was also observed with relative binding activity expressed as a function of the total content of afucosylated glycans represented by the theoretical G0-F and observed G0-F values (Table 1).
Figure 5. Correlation between relative FcγRIIIa binding activity and amount of afucosylated materials in AbX AF-blends. Relative binding activity was calculated by inverse normalization of EC50 values of samples with EC50 of the sample containing 0% of the afucosylated material (100% “regular” fucosylated material). Mean relative binding activity values (Table 2) were plotted against levels of afucosylated material in the samples and the data points were fitted to a linear model using Excel.
ADCC Assay
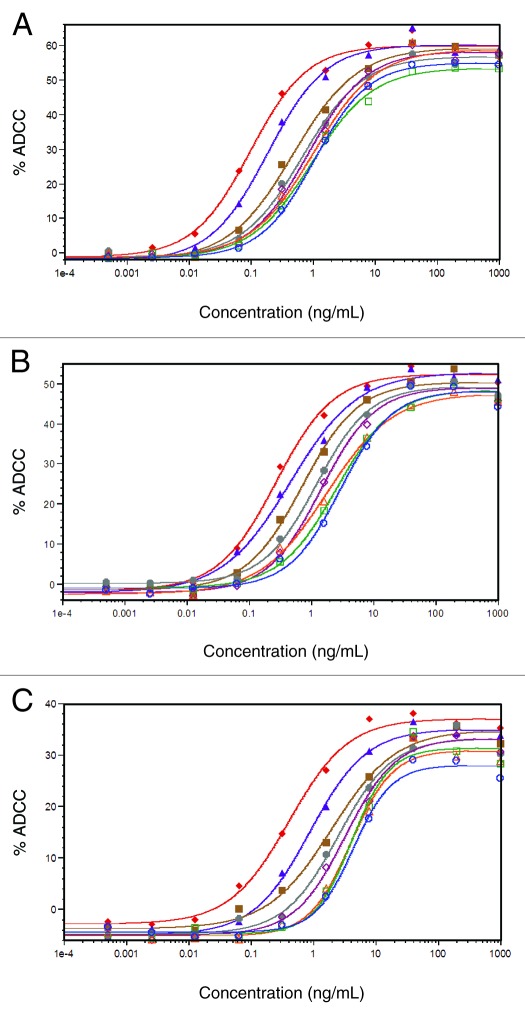
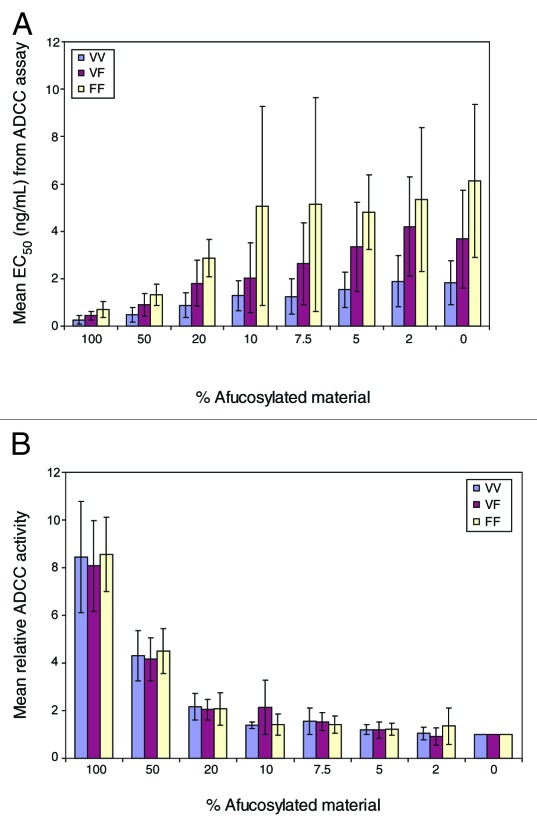
The ADCC activity of selected AbX AF-blends was assessed using a human B lymphoma cell line, WIL2-S, as target cells and PBMC from healthy donors with pre-determined FcγRIIIa genotype as effector cells. Due to constraints on the volume of fresh blood readily available, we tested 8 samples in this study: the “regular” fucosylated (0% AF-blend), the afucosylated (100% AF-blend), plus blended samples containing 2%, 5%, 7.5%, 10%, 20%, and 50% of the afucosylated AbX. The study involved 16 donors: 5 were homozygous for FcγRIIIa-F158 (FF), 5 were homozygous for FcγRIIIa-V158 (VV), and 6 were heterozygous (VF). Dose-response curves from representative experiments using effector cells carrying each of the 3 different FcγRIIIa genotypes (VV, VF, and FF) are presented in Figure 6A, B and C. Consistent with published results,23,35 samples with higher levels of afucosylated material showed enhanced ADCC activity with effector cells from donors of all three FcγRIIIa genotypes as evidenced by leftward shifts, indicating reduced EC50, and higher upper asymptote, indicating increased maximal ADCC level. Of note, the differences in leftward shifts among samples appeared to be more profound than differences at the upper asymptote. Nevertheless, both changes appeared to correlate with the amount of afucosylated glycans in the sample. Figure 7A shows mean EC50 values calculated from dose-response curves fitted with a 4 Parameter Logistic nonlinear regression model. As evidenced by the large error bars, the variability for most of the mean values were high even though the data were calculated from donors carrying the same FcγRIIIa genotype. This wide range of variability is typical of ADCC assays using PBMC as effector cells and is largely due to donor-to-donor variability. Additionally, we observed a notable rank order of EC50 values from donors with different FcγRIIIa genotypes (Fig. 7A). The lowest mean EC50 values came from effector cells carrying the VV genotype; the highest EC50 value came from FF effector cells; an intermediate EC50 value was obtained from effector cells from VF donors. This indicates that effector cells carrying VV genotype exert the highest level of ADCC activity, followed by those carrying VF, and the effector cells carrying the FF genotype have the lowest ADCC activity.
Figure 6. Activity of AbX samples consisting of varying mixtures of fucosylated and afucosylated materials in ADCC assays with effector cells carrying different FcγRIIIa genotypes. All experiments were performed using human B lymphoma WIL2-S cells as target cells and human peripheral blood mononuclear cells (PBMC) as effector cells at an Effector cell/Target cell ratio of 25:1. The extent of cell lysis was measured by LDH activity assay. (A) Representative ADCC curves from assays using PBMC expressing homozygous FcγRIIIa-V158 (VV); (B) Representative ADCC curves from assays using PBMC expressing both FcγRIIIa-V158 and -F158 (VF); (C) Representative ADCC curves from assays using PBMC expressing homozygous FcγRIIIa-F158 (FF). Data are expressed as percent cell lysis by ADCC and concentration of the antibodies. Samples tested included those containing 0% (blue ○), 2% (green □), 5% (orange △), 7.5% (magenta ◇), 10% (gray ●), 20% (brown ■), 50% (purple ▲), 100% (red ◆) of afucosylated material. The results shown were obtained from one representative data set out of at least five in vitro ADCC assays.
Figure 7. (A) Mean EC50 value and(B) Mean Relative ADCC activity of select AbX AF-blend samples with effector cells carrying different FcγRIIIa genotypes (VV: homozygous V158; VF: heterozygous with both V158 and F158; FF: homozygous F158). Relative ADCC activity was calculated by inverse normalization of EC50 values of samples with EC50 value of the sample containing 0% afucosylated material. Data presented are mean values from at least five in vitro ADCC experiments using different donors; error bars indicate corresponding standard deviations.
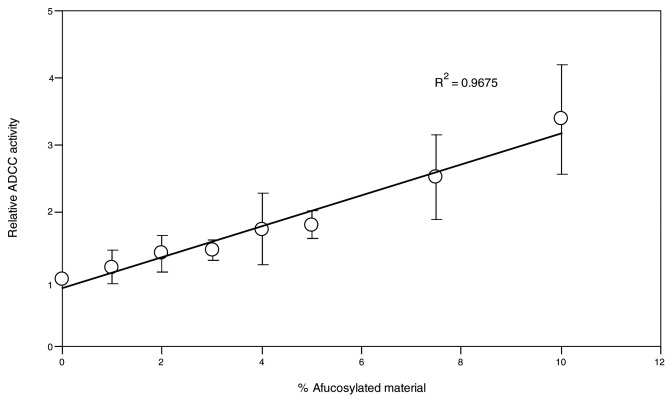
Relative ADCC activity was calculated by inversely normalizing the EC50 value of each sample to the EC50 value from the “regular” AbX (0% AF-blend) to better quantify the effect of fucose reduction on ADCC activity. The resulting mean relative ADCC activity values are summarized in Figure 7B. As evidenced by the reduced size of error bars compared with those in Figure 7A, normalization within a data set generated from a single donor significantly reduces the variability of the mean value; this confirms that the high variability seen in the raw data are primarily caused by inter-donor variability. Whereas EC50 values appear to differ according to the FcγRIIIa genotype of the effector cells (Fig. 7A), the relative ADCC activity in relation to the extent of afucosylated glycans remains similar despite the difference in FcγRIIIa genotypes (Fig. 7B). This result clearly demonstrates that the effect of fucose reduction on ADCC activity is independent of the FcγRIIIa genotype of the effector cells. The relationship between the amount of afucosylated glycan and ADCC activity was further analyzed in a scatter graph (data not shown). A positive trend was observed where increasing amounts of afucosylated material generally led to higher levels of ADCC activity. Fitting the data to a linear model yielded R-squared values ranging from 0.94 to 0.97 depending on the FcγRIIIa genotype of the effector cells (data not shown). However, due to the wide range of afucosylated materials tested and the relatively small number of samples, specifically, those carrying low level (< 10%) of afucosylated AbX, the resulting R-squared values from the linear fit might be skewed by samples carrying high levels of afucosylated material. Further, given the high variability of the PBMC-based ADCC assay, it is technically challenging to accurately detect small differences in ADCC activity associated with samples that contain a low level of afucosylated material. Engineered NK cell lines have been shown to behave similarly to purified NK cells and to offer improved performance in ADCC assays.36 To confirm the linear relationship between levels of afucosylated glycan and relative ADCC activity, additional ADCC studies were performed using an engineered NK cell line that expresses a high level of stably transfected human FcγRIIIa-F158 as effector cells. In this study, AbX AF-blends containing 0%, 1%, 2%, 3%, 4%, 5%, 7.5%, and 10% of afucosylated material were tested in the NK cell line based ADCC assays. As shown in Figure 8, a linear relationship between relative ADCC activity and the amount of afucosylated glycan in the AF-blends was clearly demonstrated; the R-squared value for the linear fit was 0.967.
Figure 8. Activity of AbX samples consisting of varying mixtures of fucosylated and afucosylated materials in ADCC assays using an engineered NK cell line as effector cells. The engineered NK cell line expresses stably transfected human FcγRIIIa-F158 and was used in conjunction with the human B lymphoma WIL2-S as target cells at an effector cell/target cell ratio of 5:1. The extent of cell lysis was measured by LDH activity assay. Samples tested included those containing 0%, 1%, 2%, 3%, 4%, 5%, 7.5%, and 10% of afucosylated material. Data presented are mean values from three in vitro ADCC experiments; error bars indicate corresponding standard deviations. Relative ADCC activity was calculated by inverse normalization of EC50 values of samples with EC50 value of the sample containing 0% afucosylated material. The data was fitted to a linear model using Excel.
Effect of Afucosylated Glycans in AbX Production Batches on FcγRIIIa Binding and ADCC activities
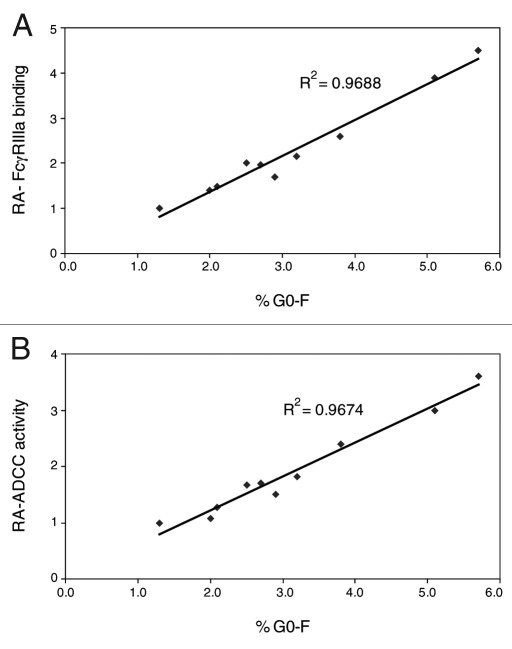
Ten samples of AbX from different production runs were evaluated to verify the effects of afucosylated glycans on effector functions of AbX samples produced from wild type CHO cells. The relative FcγRIIIa-F158 binding and relative ADCC activity were plotted against the level of afucosylated glycans (% G0-F) measured by glycan analysis. As shown in Figure 9A and 9B, linear relationships between the amount of afucosylated glycan and either the relative FcγRIIIa-F158 binding activity or the level of relative ADCC activity based on EC50 values were clearly demonstrated. The R-squared value for the FcγRIIIa binding data are 0.9688; the R-squared value for the relative ADCC data are 0.9674.
Figure 9. Relationship between amount of afucosylated glycans and (A) relative FcγRIIIa-F158 binding activity (B) relative ADCC activity of selected AbX samples. Samples were tested in an ELISA-based FcγRIIIa-F158 binding assay and engineered NK cells-based ADCC assays. Relative activity (RA) of FcγRIIIa-F158 binding and ADCC activity based on EC50 values were calculated by inverse normalization with the corresponding EC50 value of the “regular” fucosylated AbX, which is one of the samples tested. %G0-F is the level of afucosylated glycans in the sample measured by the glycan analysis.
Discussion
Removal of core fucose from IgG-Fc N-glycans has been shown to enhance FcγRIIIa binding and ADCC activity of antibodies. Structural models indicate that the core fucose in Fc N-glycan may present steric hindrance for the FcγRIII glycan at N162, thereby preventing optimal interactions between the antibody and the receptor.37 For therapeutic antibodies produced from mammalian cell lines, some variation in the level of afucosylated glycans is routinely observed in different production batches. Although these differences are usually small, given the affect of afucosylated glycan on FcγRIIIa binding and ADCC, it is important to monitor the content of afucosylated glycan in the antibody product during production processes and assess the affect on biological activities with appropriate assays. In this study, we sought to quantitatively characterize effects of fucose reduction on in vitro biological activities of a humanized antibody. The results of our study confirm that the content of afucosylated glycans in antibodies does not affect target binding, C1q binding and CDC activity. More importantly, our data show a strong linear correlation between levels of afucosylated glycans and both the FcγRIIIa binding and ADCC activity of the antibody.
Fcγ receptors play a vital role in the in vivo mechanisms by which antibodies mediate their activity. Activating Fcγ receptors such as FcγRIIa and IIIa are involved in activation of cytotoxic activity of effector cells such as NK cells, monocytes, macrophages and neutrophils. On the other hand, the inhibitory FcγRIIb has been shown to modulate B cell activity, humoral tolerance and plasma-cell survival.38 Since many cell types co-express activating and inhibitory Fcγ receptors, the effector functions of therapeutic antibodies are counterbalanced by differential interactions with both the activating FcγRIa/IIa/IIIa and the inhibitory FcγRIIb.38 The outcome of these interactions may influence both the efficacy and safety of the therapeutic antibodies. Using ELISA-based binding assays, we showed that increasing the afucosylated glycan content of AbX led to markedly increased binding activity toward FcγRIIIa, moderate increases in IIa and IIb binding, but no affect on binding with Ia. While it is difficult to ascertain the biological significance of the moderate increase in binding with FcγRIIa and IIb by afucosylated antibodies, the increase in binding activity toward FcγRIIIa appeared to result in proportional increase in ADCC activity. Additionally, the changes in afucosylated glycan content and relative ADCC potency derived from normalized EC50 values appeared to correlate in a linear fashion. Given that this linear relationship was also observed among samples carrying low (< 10%) levels of afucosylated glycans, it is clear that small differences in levels of afucosylated glycans routinely observed in production batches of CHO cell produced antibody products can result in considerable changes in biological activity. Due to the lack of clinical data and the complexity of molecular mechanisms underlying immune effector functions, the significance of these differences relative to the in vivo functions of AbX remains unclear. However, it does not minimize the importance of monitoring levels of afucosylated glycans in antibody products. Currently, there are several engineered antibodies with improved effector functions under clinical development; the availability of data from these clinical studies should further our understanding on the correlation between in vitro effector functions and in vivo efficacy of a therapeutic antibody.
The in vitro ADCC activities of the AbX AF-blends were assessed using PBMC from donors with pre-determined FcγRIIIa genotypes. Based on EC50 values derived from complete dose-response curves, we were able to quantify the increase in ADCC activity in relation to varying levels of afucosylated glycans in the AbX AF-blends. Our data clearly indicate that increasing levels of afucosylated glycans lead to increased ADCC activity and that the extent of increase correlates linearly with the amount of afucosylated glycans. Furthermore, the extent of increase in ADCC activity appeared to be minimally affected by FcγRIIIa genotypes because AbX AF-blends showed comparable relative ADCC activity regardless of the FcγRIIIa genotype of the effector cells. Overall, our data indicate an 8–10 fold increase in ADCC activity with afucosylated AbX over the regular fucosylated AbX. Some previous studies reported substantially higher (> 100 fold) increases in ADCC activity due to fucose depletion.23,39 However, the quantification in those studies was largely based on estimated values extrapolated from incomplete dose-response curves. In contrast, our data was based on multiple samples with varying degrees of reduction in afucosylated glycans and relative ADCC activity quantified with EC50 values derived from complete dose-response curves. In addition, the approximately 10-fold increase in ADCC activity found in afucosylated AbX was also observed in assays using either fresh NK cells or engineered NK cell lines as target cells (unpublished data). It is worth noting that whereas proportional shifts were observed toward higher maximal levels of ADCC at saturating concentrations, reflecting improvements in the relative efficacy of the variants, the differences were typically within a 2-fold range. This limited range of the upper asymptote, which represents maximum ADCC activity, justifies the use of EC50 values as an expression of relative ADCC activity. However, this approach to quantification might under-estimate the effects by failing to account for changes in the upper asymptote of the dose-response curves. Nevertheless, given that movements in the upper asymptote were also proportional to levels of afucosylated glycans in the samples, the omission of this parameter should not compromise the validity of the observed linear relationship between the afucosylated glycan level and the ADCC activity. Of note, both the ADCC and the FcγR binding assays used in this study are non-validated characterization assays in which the degree of similarity (parallelism) between dose response curves was not assessed. The observation of significant shifts toward higher maximal levels of ADCC activity, but not of FcgRIIIa binding activity, at saturating concentrations reflects intrinsic limitations of different in vitro effector function assays. For product release testing, the most relevant biological activity (potency) of the product is typically measured by quantitative comparison of dose-dependent response of reference and sample preparations in a precise and accurate manner to ensure manufacture consistency. A more precise and accurate measurement of ADCC activity of antibody product that takes into account the differences in the upper asymptote may be achieved by employing a parallel-logistics assay where parallelism assessment is part of the system suitability criteria and relative ADCC potency is measured with a constrained 4- or 5-parameter parallel curve model.
The ability of antibodies to induce ADCC depends on the binding affinities to both the antigen on target cells and to the Fcγ receptors on effector cells. One of the factors that influences binding affinity between IgG and FcγRIIIa is the genetic polymorphism in FcγRIIIa that results in the expression of valine (V) or phenylalanine (F) at amino acid 158. Structural and functional studies have shown that FcγRIIIa residue 158 is directly involved in hydrophobic contact with IgG-Fc and that the FcγRIIIa-V158 allotype binds to IgG1 with higher affinity than the FcγRIIIa-F158 allotype. Additionally, immune effector cells bearing the FcγRIIIa-V158 allele have been shown to mediate more potent ADCC activity than those bearing the F allele in in vitro ADCC assays.40 Further, clinical studies have shown that non-Hodgkin lymphoma patients carrying the FcγRIIIa-V158 allotype responded better to rituximab treatment than those carrying the FcγRIIIa-F158 allotype.41,42 Similar findings were also reported for breast cancer patients treated with trastuzumab.43,44 Consistent with these published reports, we observed a clear and notable rank order of EC50 values from donors with different FcγRIIIA genotypes. The lowest EC50 values (highest ADCC activity) came from effector cells carrying VV genotypes; the highest EC50 value came from FF effector cells; an intermediate EC50 value was obtained with effector cells from the VF donors. This result confirms that immune effector cells bearing the FcγRIIIa-V158 allele are generally more potent in inducing ADCC activity than those bearing the F158 allele. In spite of such differences in ADCC potency, effector cells carrying different FcγRIIIa genotypes showed similar degrees of increase in relative ADCC activity by AbX AF-blends in our assays, indicating that the effect of N-glycan fucose reduction on ADCC activity of the antibody is independent of the FcγRIIIa genotype of the effector cells.
Afucosylated glycan content, in contrast, had a more profound affect on binding activity toward FcγRIIIa-F158 in the ELISA based binding assays. Specifically, compared with the “regular” fucosylated AbX, there were 9- and 35-fold increases in the afucosylated AbX for binding with FcγRIIIa-V158 and F158, respectively. This discrepancy could be due to differences in assay systems and the sample preparation involved in the ELISA method. Due to the low binding affinity, binding between monomeric IgG and FcγRII and III could not be detected in ELISA-based assays, which required multiple washing cycles between each incubation step. To overcome this obstacle, the samples of antibodies were routinely tested in multimeric forms via cross-linking with anti-kappa F(ab’)2. It is conceivable that cross-linked samples with high afucosylated glycan content might exert avidity approaching the limit of the dynamic range of the assay, resulting in under-quantification of the binding activity with the V158 allotype. Further, additional binding studies using a surface plasmon resonance-based immunoassay showed comparable changes of 20-fold in binding affinity between “regular” and afucosylated AbX for both FcγRIIIA allotypes (unpublished data). Together, the present observation of the differential affect of afucosylated glycans on the binding with FcγRIIIa allotypes is likely due to inherent limitation of the ELISA-based assay system.
During clinical development and manufacturing, different assay strategies appropriate to different types of antibody products should be devised to assess effector functions. Selection of effector function assays is typically based on the effector function potential of the product and depends on its IgG subclass, glycoform, and mechanism of action, as well as the biological property of the target molecule.3 For antibody products where Fc effector function is a part of the mechanism of action, effector function testing should be tightly coordinated with other analytical assessments of product quality attributes. The characterization of IgG1 Fc glycosylation is of particular importance because of the established correlation between fucose reduction and effector functions.
In both serum-derived IgG and IgG produced from CHO cells with conventional processes, there are typically less than 10% afucosylated glycans. An antibody could carry an afucosylated N-glycan in each of the two heavy chains. However, given the small amount of the afucosylated glycans present and the fact that glycan biosynthesis is intrinsically random as well as heterogeneous, it is more likely that most naturally occurring afucosylated glycans exist in antibodies that are half-afucosylated, that is, where one heavy chain carries an afucosylated glycan and the other heavy chain in the same molecule carries a fucosylated glycan. Crystal structures show that the Fcγ receptor binding site on IgG-Fc is localized to the hinge proximal region of the CH2 domain and includes the lower hinge region.4-7 More importantly, the binding site is asymmetrical with both heavy chains making contact with a single FcγRIII molecule, which dictates that one receptor can only bind to one IgG-Fc at a time.4-7 This 1:1 stoichiometry of the IgG-Fc/FcγRIII complex is critical for normal immune functions because it prevents constant stimulation of the immune system by monomeric immunoglobulins that are present at high concentrations in the serum. The structural model of IgG-Fc/FcγRIII complex also predicts that only one of the two Fc-fucose residues needs to be absent for increased binding affinity toward FcγRIII.37 It implies that a homogenously afucosylated antibody should behave similarly to a half-afucosylated antibody in terms of FcγRIII binding and ADCC activities. Most of the data in this study were generated from antibody samples carrying mixtures of homogenously fucosylated or homogenously afucosylated glycans and not from half-afucosylated antibodies. To ensure that our findings are generally applicable to “regular” antibody samples, a panel of 10 AbX samples produced from wild-type CHO cells were evaluated for the FcγRIIIa binding and ADCC activities. Linear relationships between the amount of afucosylated glycans and either the FcγRIIIa binding activity or the ADCC activity were clearly demonstrated, suggesting that the quantitative data generated by blended samples can be extended to batch samples that presumably contain mostly half-afucosylated antibodies. The observation of over 3-fold increase in ADCC activity with product batches containing varying amount of afucosylated glycans demonstrates that glycoform can profoundly influence effector functions of the product and underscores the importance to better control the production process to provide adequate manufacturing consistency. However, controlling glycoform fidelity remains a huge challenge to pharmaceutical industry to date. Manipulation of culture medium and culture conditions for mammalian cells can have substantial influence on the glycoform profiles of products and may allow for manipulation of the glycoform profiles over the time of a production run.45 Other control strategies include cell engineering, glycoengineering, and development of alternative production vehicles.
Our observation of linear relationships between the afucosylated glycan content of IgGs and both FcγRIIIa binding and ADCC activity provides direct evidence that helps to define the quantitative nature of this structure-activity relationship. A well-defined structure-activity relationship is invaluable for the manufacture and control of therapeutic antibody products. It allows cross-validation of analytical and activity assays for the control system and provides meaningful biological information to the product development process where function assays are not routinely performed. Nevertheless, given the complexity of molecular interactions between Fcγ receptors and IgG glycoforms, and the fact that the present study involves a humanized IgG1 with a native Fc sequence, the observed linear relationship between afucosylated glycan content and Fc effector function should not be overly generalized. It is possible that alternative relationships exist for other antibody molecules with different isotypes/subclasses or alternative Fc sequences.
Materials and Methods
Preparation of AbX AF-blends
AbX is a humanized anti-CD20 antibody based on IgG1 heavy chains and kappa light chains. For this study, greater than 98% fucosylated AbX was produced from the CHO DP12 cell line. Completely afucosylated AbX was produced from a CHO cell line deficient in FUT8. Both materials showed comparable physicochemical properties including purity, solubility and low level of high mannose glycoform or aggregation (data not shown). Blends of afucosylated variants were generated by mixing the “regular” fucosylated antibodies with the afucosylated materials. The resulting AbX AF-blends consisted of 14 samples: the “regular” fucosylated, the afucosylated, plus blended samples containing 1%, 2%, 3%, 4%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, and 50% of the afucosylated AbX. “Regular” fucosylated AbX was added to make up the final content. Of note, samples in the AbX AF-blends were identified by the content of the afucosylated AbX, including the starting material. Thus, the “regular” fucosylated sample is referred as 0% AF, whereas the afucosylated AbX is referred as 100% AF.
Using CE-LIF to determine the fucose content of antibody glycans
The fucose content of Fc glycans was determined by CE using a LIF method as the detection mode.46 Briefly, the samples (300 μg) were transferred to pH 7.5 buffer containing 20 mM sodium phosphate, 50 mM EDTA and 0.02% NaN3; then digested with 10 milliunits of PNGase F (New England Biolabs, Ipswich, MA) for 15 h at 37°C. The antibody was precipitated by heating the solution at 95°C for 5 min and the precipitate was removed by centrifugation. The supernatant solutions were further treated with 10 milliunits of β-galactosidase (Prozyme, Hayward, CA) for 2 h at 37°C. The solutions were then dried in a centrifugal vacuum evaporator and the released glycans were used to generate derivatives with APTS (Beckman Coulter, Indianapolis, IN) in a 15% acetic acid solution containing sodium cyanoborohydride. The derivative formation reaction was conducted for 2 h at 55°C. A Beckman PA-800 system was used for the CE analysis in a running buffer of 40 mM ε-amino caproic acid/acetic acid (pH 4.5) and 0.2% HPMC. This system was equipped with a N-CHO coated capillary (50 μm i.d. x 60.2 cm, Beckman Coulter) and a LIF detection module using an argon-ion laser (488 nM excitation, 520 nM emission). A water plug was injected into the capillary at 0.2 psi for 5 sec prior to sample loading. Samples were then injected into the capillary at 0.5 psi for 10 sec. The separation of the oligosaccharide derivatives was performed at 500V/cm (30 kV) with the capillary temperature maintained at 20°C.
CD20 Binding Assay
Binding of AbX to CD20 antigen was measured using a cell-based electrochemiluminescent assay as described by Lu et al.47 Briefly, WIL2-S cells, a human B lymphoma cell line expressing a high level of CD20, were washed with phosphate buffered saline (PBS) and seeded at 25,000 cells/well in 25 μL PBS on 96-well MULTI-ARRAY High Bind plates (MSD, Gaithersburg, Maryland). WIL2 cells were incubated for one hour at room temperature (RT) to allow cell attachment to the carbon surface. The plates were then blocked with 15% FBS in PBS for 30 min at RT with mild agitation and incubated with serial dilutions of antibodies (2.74–2000 ng/mL) in assay buffer (PBS containing 10% FBS). After 1 h incubation at RT with mild agitation, the plates were washed and bound antibodies were detected by adding ruthenium-labeled F(ab′)2 goat anti-human IgG Fc in assay buffer in the presence of Tris-based Read Buffer T (MSD). Upon electrochemical stimulation, luminescent signals were quantified with a Sector Imager 6000 reader (MSD). Dose-response binding curves were generated by plotting the mean luminescent signals from duplicates of sample dilutions against the sample concentrations and fitting the data points to a four-parameter model using SoftMax Pro (Molecular Devices, Sunnyvale, CA).
Fcγ Receptor Binding by ELISA
The binding activities of test antibodies toward various human Fcγ receptors were assessed by ELISA-based ligand binding assays.48 The human Fcγ receptors were expressed as fusion proteins containing the extracellular domain of the Fcγ receptor linked to a Gly/6xHis/glutathione S-transferase (GST) polypeptide tag at the C-terminus. For the low-affinity receptors (Fcγ RIIa [CD32A], Fcγ RIIb [CD32B] and Fcγ RIIIa [CD16]), antibodies were tested as multimers by cross-linking with F(ab’)2 fragments of goat anti-human kappa chain antibodies (MP Biomedicals, Solon, OH) at an approximate molar ratio of 1:3. For the high-affinity receptor (FcγRIa), the antibodies were assayed as monomers without cross-linking. Sample and reagent dilutions were prepared in an assay buffer containing PBS, 0.5% BSA and 0.05% Tween-20. Plates were washed with PBS containing 0.05% Tween-20 using an ELx405™ plate washer (Biotek Instruments, Winooski, VT) after each incubation step.
Plates were coated with an anti-GST antibody (Genentech) in a 0.05 M sodium carbonate buffer (pH 9.6) overnight at 4°C. After blocking with the capture antibody, the plates were incubated with Fcγ receptors for 1 h at RT. Serially diluted test antibodies were added either as monomers (for binding with FcγRIA) or as multimeric complexes (for binding with FcγRIIa, IIb and IIIa) and the plates were incubated for 2 h at RT. Antibodies bound to the Fcγ receptor were linked to HRP conjugated F(ab’)2 fragment of goat anti-human F(ab’)2 (Jackson ImmunoResearch Laboratories, West Grove, PA) and substrate TMB (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added. The plates were incubated for 5−20 min at RT, depending on the Fcγ receptors tested, to allow color development. The reaction was terminated with 1 M H3PO4 and absorbance determined at 450nm. Dose-response binding curves were generated by plotting the mean absorbance values from duplicates of sample dilutions against the sample concentrations and fitting the data points to a four-parameter model using SoftMax Pro (Molecular Devices) to obtain the EC50 values. For comparison, relative activity was calculated for each sample by inversely normalizing its EC50 value with that of a reference molecule using the following formula:
Relative Activity = EC50 Reference/EC50 Sample
ADCC Assay
ADCC assays were performed using PBMC from healthy donors as effector cells and WIL2-S, a human B lymphoma cell line, as target cells. Briefly, PBMC were isolated from fresh blood of healthy human donors by Ficoll-Paque (GE Healthcare, Milwaukee, WI) density gradient centrifugation. Target cells (4 x 104) prepared in assay medium (RPMI 1640 with 1% BSA and 100 units/mL penicillin and streptomycin) were added to each well in round-bottom, 96-well tissue culture plates. Serial dilutions of antibodies (10,000 to 0.0038 ng/mL following 4-fold dilutions in series) were added to the plates containing the target cells (50 μL/well) and incubated for 30 min at 37°C with 5% CO2 to allow opsonization. After incubation, PBMC (1.0 x 106) were added to each well, in assay medium, for a 25:1 effector:target cell ratio and the plates were incubated further for 4 h. The plates were then centrifuged and the supernatants were assayed for lactate dehydrogenase activity using a Cytotoxicity Detection Kit (Roche Diagnostics Corporation, Indianapolis, IN). Cell lysis was quantified by measuring absorbance at 490 nm using a microplate reader SpectraMax®190 (Molecular Devices). Absorbance of wells containing only the target cells served as the control for background (Low Control), whereas wells containing target cells lysed with Triton-X100 provided the maximum available signal (High Control). AICC was measured in wells containing target and effector cells without the antibody.
The extent of specific ADCC was calculated as follows:
% ADCC = [A490nm(sample) – A490nm(AICC)]/[A490nm(High Control) – A490nm(Low Control)]
Dose-response curves were generated by plotting the mean ADCC values from duplicates of antibody sample dilutions against the antibody concentration. The EC50 values were calculated by fitting the data points to a four-parameter equation using SoftMax Pro. For comparison, relative activity was calculated for each sample by inversely normalizing its EC50 value with that of a reference molecule using the following formula:
Relative Activity = EC50 Reference/EC50 Sample
In select experiments, an engineered NK cell line expressing stably transfected human Fcγ RIIIa-F158 was used as effector cells,36 but the effector:target cell ratio was changed to 5:1 and the incubation time was reduced to 3 h. The rest of the procedures were the same as described above.
C1q Binding Assay
Human C1q binding to AbX-AF blends was measured by ELISA.49 Antibodies were serially diluted and coated on high binding microtiter plate wells at 2–8°C with overnight incubation. The plates were sequentially incubated with 2 mg/mL human C1q (Quidel Corporation, Santa Clara, CA) for 2 h at RT, goat anti-human C1q (Accurate Chemical, Westbury, NY) for 1 h at RT, and donkey anti-goat IgG-HRP (Millipore, Billerica, MA) for 1 h at RT after blocking with 3% BSA/PBS (1 h at RT). Plates were washed with PBS containing 0.05% Tween-20 using an ELx405™ plate washer (Biotek Instruments) after each incubation step. Peroxidase activity was detected with TMB substrate (Kirkegaard and Perry Laboratories) and the plates incubated at RT for 15 min to allow color development. The reaction was terminated with 1 M H3PO4 and absorbance measured at 450 nm (the background measured at 650 nm was subtracted for each well) using a microplate reader SpectraMax®190 (Molecular Devices). Dose-response binding curves were generated by plotting the mean absorbance values from duplicates of sample dilutions against the sample concentrations and then fitting the data points to a four parameter logistic model using SoftMax Pro. In a separate experiment, an anti-human Fab-HRP conjugate (Jackson ImmunoResearch Laboratories) was used to determine equivalent coating of antibodies to the wells of the microtiter plate.
CDC Assay
CDC activities of test antibodies were assessed by a cell-based assay using WIL2-S cells as target cells. AbX-AF blends were serially diluted in assay medium (RPMI 1640 medium supplemented with 1% FBS) and distributed into a 96-well opaque-walled microtiter plate (Costar Corning Inc., Lowell, MA). The plate was incubated with 5% CO2 for 2 h at 37°C after WIL2-S cells (5x104 cells/well) and normal human serum complement (Quidel Corporation) were added. After the incubation, the CellTiter-Glo reagent (Promega Corp., Madison, WI), which assays for ATP in metabolically active cells, was added and the plate was incubated for 10 min at RT with constant shaking. The extent of cell lysis was quantified by measuring intensity of luminescence with a plate reader SpectraMax M5 (Molecular Devices). Dose-response binding curves were generated by plotting the mean luminescence signal from duplicates of sample dilutions against the sample concentrations and then fitting the data points to a four-parameter model using SoftMax Pro (Molecular Devices).
Disclosure of Potential Conflicts of Interest
All the authors are employees of Genentech, Inc., which supported the study financially.
Acknowledgments
The authors would like to thank Patty Siguenza for support on the project, Kyra Cowan and Bob Kelley for helpful comments on the manuscript, and colleagues at the research blood program, the sample transportation program, and the CritRS group for providing critical reagents.
Glossary
Abbreviations:
- AbX
a humanized anti-CD20 IgG1 antibody
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AICC
antibody-independent cellular cytotoxicity
- APTS
8-aminopyrene-1,3,6-trisulfonic acid
- BSA
bovine serum albumin
- CDC
complement-dependent cytotoxicity
- CDR
complementarity determining region
- CE-LIF
capillary electrophoresis-laser induced fluorescence
- CV
coefficient of variation
- EC50
the concentration of test antibody at which 50% of its maximal binding activity is observed
- EDTA
ethylene-diamine-tetra-acetic acid
- ELISA
enzyme linked immunosorbent assay
- Fab
fragment of antigen-binding
- FBS
fetal bovine serum
- Fc
fragment, crystalizable
- GlcNAc
N-acetylglucosamine
- G0 glycoform
no terminal galactose on either arm of the oligosaccharide chain
- G1 glycoform
terminal galactose on one arm of the oligosaccharide chain
- G2 glycoform
terminal galactose on both arms of the oligosaccharide chain
- G0-F
afucosylated G0 glycoform
- GST
glutathione S-transferase
- HPMC
hydroxypropylmethyl-cellulose
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- MSD
meso scale discovery
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PNGase F, Peptide
N-Glycosidase F
- RFU
Relative Fluorescence Unit
- RLU
Relative Luminescence Unit
- RT
room temperature
- TMB
3,3′,5,5′-Tetramethylbenzidine
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/19941
References
- 1.Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30:356–62. doi: 10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–8. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10:101–11. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 4.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–73. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 5.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 6.Sondermann P, Kaiser J, Jacob U. Molecular basis for immune complex recognition: a comparison of Fc-receptor structures. J Mol Biol. 2001;309:737–49. doi: 10.1006/jmbi.2001.4670. [DOI] [PubMed] [Google Scholar]
- 7.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/S0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 8.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–89. doi: 10.1016/S0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 9.Raju TS, Briggs JB, Borge SM, Jones AJ. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology. 2000;10:477–86. doi: 10.1093/glycob/10.5.477. [DOI] [PubMed] [Google Scholar]
- 10.Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, et al. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–47. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- 11.Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983;80:6632–6. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989;143:2595–601. [PubMed] [Google Scholar]
- 13.Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989;259:347–53. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leatherbarrow RJ, Rademacher TW, Dwek RA, Woof JM, Clark A, Burton DR, et al. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985;22:407–15. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara C, Brünker P, Suter T, Moser S, Püntener U, Umaña P. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng. 2006;93:851–61. doi: 10.1002/bit.20777. [DOI] [PubMed] [Google Scholar]
- 16.Lund J, Takahashi N, Pound JD, Goodall M, Nakagawa H, Jefferis R. Oligosaccharide-protein interactions in IgG can modulate recognition by Fc gamma receptors. FASEB J. 1995;9:115–9. doi: 10.1096/fasebj.9.1.7821750. [DOI] [PubMed] [Google Scholar]
- 17.Radaev S, Sun PD. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J Biol Chem. 2001;276:16478–83. doi: 10.1074/jbc.M100351200. [DOI] [PubMed] [Google Scholar]
- 18.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36:1370–80. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 19.Saba JA, Kunkel JP, Jan DC, Ens WE, Standing KG, Butler M, et al. A study of immunoglobulin G glycosylation in monoclonal and polyclonal species by electrospray and matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem. 2002;305:16–31. doi: 10.1006/abio.2002.5651. [DOI] [PubMed] [Google Scholar]
- 20.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–7. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 21.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–64. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 22.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 23.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–55. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 24.Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, et al. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11:2327–36. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- 25.Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–60. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, et al. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng. 2004;88:901–8. doi: 10.1002/bit.20326. [DOI] [PubMed] [Google Scholar]
- 27.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–22. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 28.Scallon B, McCarthy S, Radewonuk J, Cai A, Naso M, Raju TS, et al. Quantitative in vivo comparisons of the Fc gamma receptor-dependent agonist activities of different fucosylation variants of an immunoglobulin G antibody. Int Immunopharmacol. 2007;7:761–72. doi: 10.1016/j.intimp.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, Ishida T, Utsunomiya A, Sato F, Mori F, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R gamma(null) mice in vivo. J Immunol. 2009;183:4782–91. doi: 10.4049/jimmunol.0900699. [DOI] [PubMed] [Google Scholar]
- 30.Shoji-Hosaka E, Kobayashi Y, Wakitani M, Uchida K, Niwa R, Nakamura K, et al. Enhanced Fc-dependent cellular cytotoxicity of Fc fusion proteins derived from TNF receptor II and LFA-3 by fucose removal from Asn-linked oligosaccharides. J Biochem. 2006;140:777–83. doi: 10.1093/jb/mvj207. [DOI] [PubMed] [Google Scholar]
- 31.Iida S, Misaka H, Inoue M, Shibata M, Nakano R, Yamane-Ohnuki N, et al. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIa. Clin Cancer Res. 2006;12:2879–87. doi: 10.1158/1078-0432.CCR-05-2619. [DOI] [PubMed] [Google Scholar]
- 32.Natsume A, Wakitani M, Yamane-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, et al. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded antibody comprising a single-chain antibody linked the antibody constant region. J Immunol Methods. 2005;306:93–103. doi: 10.1016/j.jim.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Griggs J, Zinkewich-Peotti K. The state of the art: immune-mediated mechanisms of monoclonal antibodies in cancer therapy. Br J Cancer. 2009;101:1807–12. doi: 10.1038/sj.bjc.6605349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 35.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 36.Schnueriger A, Grau R, Sondermann P, Schreitmueller T, Marti S, Zocher M. Development of a quantitative, cell-line based assay to measure ADCC activity mediated by therapeutic antibodies. Mol Immunol. 2011;48:1512–7. doi: 10.1016/j.molimm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–6. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 39.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–18. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 40.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–10. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 42.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 44.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 45.Werner RG, Kopp K, Schlueter M. Glycosylation of therapeutic proteins in different production systems. Acta Paediatr Suppl. 2007;96:17–22. doi: 10.1111/j.1651-2227.2007.00199.x. [DOI] [PubMed] [Google Scholar]
- 46.Ma S, Nashabeh W. Carbohydrate analysis of a chimeric recombinant monoclonal antibody by capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1999;71:5185–92. doi: 10.1021/ac990376z. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Wong WL, Meng YG. A high throughput electrochemiluminescent cell-binding assay for therapeutic anti-CD20 antibody selection. J Immunol Methods. 2006;314:74–9. doi: 10.1016/j.jim.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 49.Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–84. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]