Abstract
The steroid and xenobiotic receptor (SXR) (also known as pregnane X receptor or PXR) is a broad-specificity nuclear hormone receptor that is well known for its role in drug and xenobiotic metabolism. SXR is activated by a wide variety of endobiotics, dietary compounds, pharmaceuticals, and xenobiotic chemicals. SXR is expressed at its highest levels in the liver and intestine yet is found in lower levels in other tissues, where its roles are less understood. We previously demonstrated that SXR−/− mice demonstrate elevated nuclear factor (NF)-κB activity and overexpression of NF-κB target genes and that SXR−/− mice develop lymphoma derived from B-1 lymphocytes in an age-dependent manner. In this work, we show that fetal livers in SXR−/− mice display elevated expression of NF-κB target genes and possess a significantly larger percentage of B-1 progenitor cells in the fetal liver. Furthermore, in utero activation of SXR in wild-type mice reduces the B-1 progenitor populations in the embryonic liver and reduces the size of the B-1 cell compartment in adult animals that were treated in utero. This suggests that activation of SXR during development may permanently alter the immune system of animals exposed in utero, demonstrating a novel role for SXR in the generation of B-1 cell precursors in the fetal liver. These data support our previous findings that SXR functions as a tumor suppressor in B-1 lymphocytes and establish a unique role for SXR as a modulator of developmental hematopoiesis in the liver.
The steroid and xenobiotic receptor (SXR) [also known as pregnane X receptor (PXR), pregnane activated receptor, and nuclear receptor subfamily 1, group I, member 2] is a broad-specificity, low-affinity nuclear hormone receptor. SXR is activated by numerous drugs, hormones, and xenobiotic compounds, including taxol, rifampicin, mifespristone, phenytoin, hyperforin, and clotrimazole. SXR's main role is considered to be the transcriptional control of metabolism enzymes involved in xenobiotic clearance, such as the cytochrome P450 (CYP) genes, conjugating enzymes, and ATP-binding cassette family transporters (1–3).
We and others previously demonstrated that SXR and NF-κB are mutually inhibitory and that SXR knockout (SXR−/−) animals display elevated levels of nuclear factor (NF)-κB target genes (4, 5). Others have confirmed this mutually inhibitory cross talk in the developing liver, describing that ip lipopolysaccharide injection significantly inhibits the expression of both SXR and its target gene CYP3A11, in mouse fetal liver (6, 7). SXR and its target genes CYP3A4 and MDR1 have also been found in human fetal liver using quantitative real-time RT-PCR (qPCR) and immunoblotting (8). Recently, we found that mice lacking SXR develop lymphoproliferations of B-1 cells that develop into multifocal lymphomas with advancing age (9). These data are in accord with published results finding that nfkb1 knockout animals display a diminished peritoneal B-1 cell population (10). Furthermore, we demonstrated that the spleens of SXR−/− animals displayed elevated levels of pro-proliferative NF-κB target gene transcripts and decreased levels of antiapoptotic gene transcripts.
B-1 cells comprise a small fraction of the B cell family and are found primarily in the intestine and peritoneal and pleural cavities, with small numbers found in the spleen (11–14). B-1 cells express low levels of B220 and high levels of IgM and may be further differentiated into B-1a (CD5+, CD11b+) or B-1b (CD5−, CD11b+) B cells (15, 16). Whereas B-2 cells arise from hematopoietic stem cells found in the bone marrow after birth and play a large role in the adaptive immune response, B-1 cell development is thought to occur almost entirely in the fetal liver. B-1 cells generated in the fetal liver migrate to the gut after birth where they continue to self-renew through adulthood and contribute to the innate immune response (16, 17). The fetal liver serves as the major hematopoietic organ during mid to late fetal development. Recent work found that CD19+/B220low cells harvested from mouse fetal liver were able to reconstitute both B-1a and B-1b cells in the peritoneal cavity of recipient severe combined immunodeficient (SCID) mice, whereas no B-2 cells derived from the donor cells were found in the bone marrow or spleen of the recipient animals (18), supporting the hypothesis that a distinct B-1 cell progenitor exists. It has also been proposed that CD19+/B220+ B cell progenitors are the progeny of CD19+/B220low precursors. B220 is up-regulated over time in culture (19), and B-1 progenitor cells generated in the yolk sac and intraembryonic paraaortic splanchnopleura hemogenic endothelium (20) at embryonic d (e)9–e9.5 migrate to the fetal liver and mature into CD19+B220+ cells that ultimately become B-1 lymphocytes (19).
SXR is expressed at its highest levels in the liver (3), B-1 cell progenitors emerge in the embryonic liver (15), and aged SXR−/− animals develop lymphomas derived from B-1 cells (9). Therefore, we sought to investigate the role of SXR in hematopoiesis in the fetal liver and the contribution of SXR to the B-1 cell compartment in the developing embryo. We hypothesized that SXR, or one of its target genes, might play a role in establishing or maintaining the specialized fetal liver microenvironment that imparts B-1 potential, thereby contributing to the selection or maintenance of B-1 cells. Here we show that pro-proliferative and antiapoptotic NF-κB target genes are up-regulated and that checkpoints for the B-1 cell compartment are down-regulated in SXR−/− fetal liver tissue, leading to an increase in CD19+/B220low and CD19+/B220+ B-1 cell precursors. In utero exposure to the mouse SXR agonist pregnenolone-16-α-carbonitrile (PCN) had the opposite effect. PCN exposure in utero reduced the size of the B-1 compartment in adult animals, and the gene expression signature of cells harvested from the peritoneal cavities of these animals revealed a decrease in pro-proliferative transcripts. Together, these results support a role for SXR in developmental modulation of the B-1 cell compartment.
Materials and Methods
Lymphocyte analysis and flow cytometry
Tissues were gently dissociated using frosted microscope slides and were strained to obtain a single-cell suspension. Cells collected from peritoneal lavages were washed, crypreserved in 10% dimethylsulfoxide (DMSO) in fetal calf serum and were thawed and washed before staining. Cells were washed and resuspended in cold flow cytometry buffer (1% fetal bovine serum, 0.1% sodium azide in PBS). Antibodies were purchased from BD Biosciences (San Diego, CA) [CD19 FITC, CD5 APC, B220 PE Cy5.5, and PerCP-eFluor 710, IgM PE, IgM APC, Lin (Ly-6 C) APC, lymphocyte-specific protein tyrosine kinase (Lck) pY505 AlexaFluor488, and Zeta-chain-associated protein kinase 70 (Zap70) pY319 PE as well as appropriate isotype controls]; Cell Signaling Technology (Danvers, MA) (Bcl-xL AlexaFluor488); Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) [c-myc PE, c-jun PE, and Siglec-10(G)]; and Novus Biologicals (Littleton, CO) (protein tyrosine phosphatase, non-receptor type, PTPN6), and staining was performed as per manufacturer's protocol. Anti-Siglec-G was detected with donkey antigoat PE (Santa Cruz Biotechnology), and anti-PTPN6 was detected with goat antirabbit FITC (eBioscience, San Diego, CA). Staining used to determine progenitor gates (see Fig. 4) is located in Supplemental Fig. 1 (published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Cells were analyzed on FACSCalibur (Becton Dickinson, Mountain View, CA), and data were analyzed using FlowJo (Treestar, Ashland, OR). Each plot is representative of four or more animals.
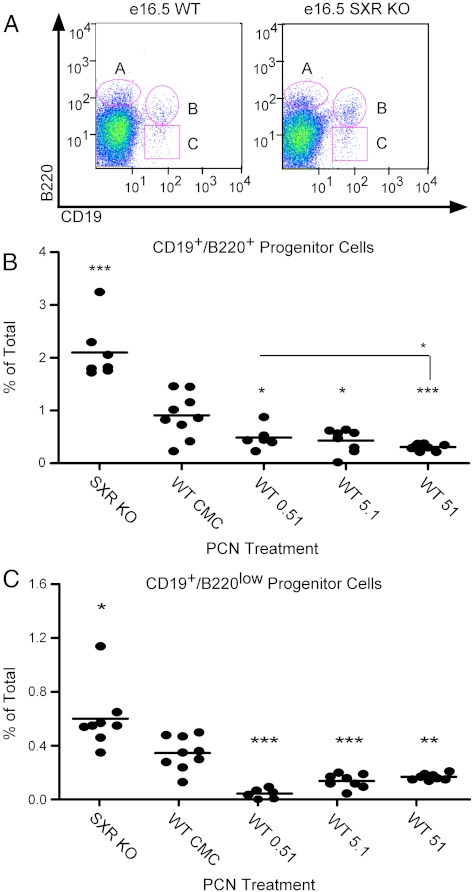
Fig. 4.
Loss of SXR increases B-1 cell progenitors in developing fetal liver, and SXR activation reduces B-1 cell progenitors. Panels A–C, Dams were treated with mouse SXR agonist PCN (at concentration in micromolar indicated) or vehicle control from conception until e16.5. Fetal livers were resected, dissociated, and stained. Cells that stained CD19−/B220+, CD19+/B220+ (gate B), or CD19+/B220low (gate C) are indicated. Panel B, The percentage of total cells residing in gate B (CD19+/B220+) are indicated, and the mean is shown. Statistics are relative to CMC except where indicated. Panel C, The percentage of total cells residing in gate C (CD19+/B220low) is indicated, and the mean is shown. Statistics are relative to CMC. Knockout (KO), n = 8; CMC, n = 9; 0.51 μm, n = 6; 5.1 μm, n = 8; 51 μm, n = 8. *, P < 0.05; **, P < 0.01; ***, P < 0.001. WT, Wild type.
RNA isolation, cDNA preparation, and qPCR
All tissues and cells were flash-frozen in liquid nitrogen and stored at −80 C until RNA preparation. For CD19+ enrichment, resected fetal livers were gently dissociated and strained to obtain a single-cell suspension, and lysis buffer [0.15 m NH4Cl, 10 mm KHCO3, 0.1 mm EDTA (pH 7.3)] was used to remove red blood cells. Cells were washed, counted, and resuspended in 0.5% BSA and 2 mm EDTA in cold PBS. CD19 MicroBeads (MACS Miltenyi Biotec Inc., Auburn, CA) were used to purify CD19+ fractions (∼93% pure) according to the manufacturer's instructions, and cells were flash frozen upon isolation. RNA was isolated using TRIzol Reagent (Invitrogen, Grand Island, NY) according to manufacturer's protocol. cDNA was synthesized using Transcriptor (Roche, Indianapolis, IN). The qPCR was performed using specific primers (Supplemental Table 1) and the SYBR Green QPCR Kit (Roche) in a DNA Engine Opticon Fluorescence Detection System (MJ Research, Applied Biosystems, Foster City, CA). Data were analyzed using the cycle threshold method (normalized to GAPDH) (21).
Animals and in utero PCN exposure
Animals were identically raised and housed at the University of California, Irvine, and were maintained on a standard diet. For in utero per os treatment studies via drinking water, PCN (Sigma, St. Louis, MO) stocks were dissolved in DMSO, and drinking water was made with 0.5% carboxymethylcellulose (CMC) in autoclaved tap water. CMC control contained the same amounts of CMC and DMSO as experimental groups but no PCN. PCN or CMC was administered from the first day of the pregnancy (e0.5) until either e16.5 for embryo studies or the date of birth of the pups for adult studies, at which point it was replaced with normal drinking water. Dosages were calculated using a 1, 10, or 100 mg/kg treatment, assuming a 20-d treatment, an average pregnancy weight of 30 g, and daily water consumption by dams of 8.5 ml/d (resulting in final water concentrations of 0.5, 5.1, and 51 μm). Timed pregnancies were counted from day of copulation plug (e0.5). Animal care and use was in accordance with applicable standards and approved by the Institutional Animal Care and Use Committees at the University of California, Irvine. SXR−/− mice were descendants of a gift from Ron Evans (Salk Institute, La Jolla, CA); all other animals were purchased from Taconic Farms (Germantown, NY).
Statistics
Differences between groups were analyzed using Student's t Test (parametric) or Mann-Whitney U Test (nonparametric). A P value < 0.05 was considered to be significant. Graphs are presented as the mean ± sem. Analyses were performed with Prism version 4 program (GraphPad Software, San Diego, CA).
Results
SXR is expressed embryonically during fetal development
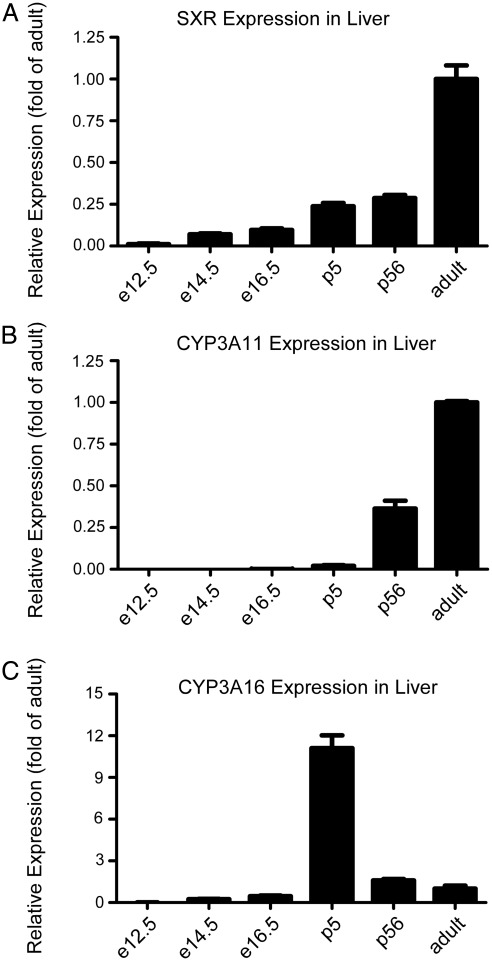
Although it has been reported that SXR is expressed in the developing fetal liver (8) and that its expression is lower than that in the adult liver, little is known about the expression of SXR over developmental time. Therefore, we first characterized SXR expression throughout early development and found that it was detectable as early as e12.5, with moderate expression in fetal liver of increasing age; expression rose after birth (5 d of age) and reached a maximum in healthy adult liver (Fig. 1A). We next sought to correlate SXR expression with CYP3A expression. Although it was detected at all time points, CYP3A11 mRNA was not significantly expressed until after birth (5 d of age), at which point expression was still quite low relative to adult levels (Fig. 1B). Lastly, we sought to characterize the expression of CYP3A16, a neonatal isoform (22), in the developing mouse liver; CYP3A16 mRNA was predominantly expressed in neonatal liver (5 d of age), with expression decreasing with increasing age; the lowest levels were found in fetal tissue (Fig. 1C). This suggests that expression of SXR in the developing fetal liver may have a different function from its known role as a xenobiotic sensor, perhaps as a contributor to regulating proliferation in the B-cell compartment as was suggested by our previous work (9).
Fig. 1.
SXR is expressed embryonically during fetal development. A, SXR mRNA as measured by qPCR. Data are expressed as fold of adult liver expression, and the means are indicated. B, CYP3A11 mRNA; C, CYP3A16 mRNA. Data are expressed as fold of adult liver expression, and means are indicated. e12.5, n = 4; e14.5, n = 7; e16.5, n = 5; postnatal d 5 (p5), n = 4; p56, n = 3; adult, n = 3.
SXR modulates key NF-κB target genes during fetal development
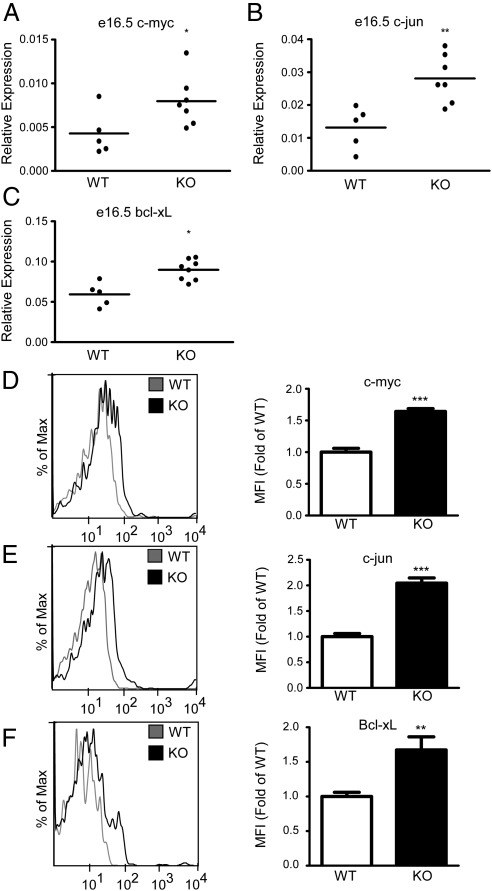
To determine whether pro-proliferation and antiapoptotic markers were up-regulated during development of the fetal liver, we examined embryos harvested from timed pregnancies in wild-type and SXR−/− animals. In fetal liver tissue from e16.5 embryos derived from wild-type and SXR−/− animals, qPCR analysis indicated that the pro-proliferative NF-κB targets c-myc and c-jun were up-regulated approximately 2-fold (Fig. 2, A and B) and the antiapoptotic Bcl-xL was up-regulated approximately 1.3-fold (Fig. 2C). To determine whether these gene expression patterns were found in CD19+ B-1 cell precursors, we analyzed transcripts from magnetically purified cells from e16.5 fetal liver. c-jun and c-myc were significantly up-regulated and Bcl-xL trended toward moderate overexpression (Supplemental Fig. 2). Flow cytometric analysis of intracellular staining of CD19+ B-1 cell precursors in e16.5 fetal liver tissue demonstrated that c-myc (Fig. 2D) and Bcl-xL (Fig. 2F) were up-regulated over 1.5-fold and c-jun (Fig. 2E) was up-regulated approximately 2-fold at the protein level in CD19+ B-1 cell precursors.
Fig. 2.
SXR modulates key NF-κB targets during fetal development. A–C, qPCR analysis of indicated transcripts from fetal livers resected from e16.5 embryos is shown, and the means are indicated. Expression is relative to GAPDH; D–F, flow cytometry of indicated proteins on CD19+-gated cells in e16.5 embryos. Wild type, n = 10; knockout, n = 5. Histograms of representative animals are shown on the left panel, and quantitated protein expression (median fluorescent intensity) relative to wild type is shown on the right panel. Wild type (WT) is shown in the histogram in gray, and knockout (KO) is shown in black. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
SXR modulates key regulators of the B-1 cell compartment during fetal development
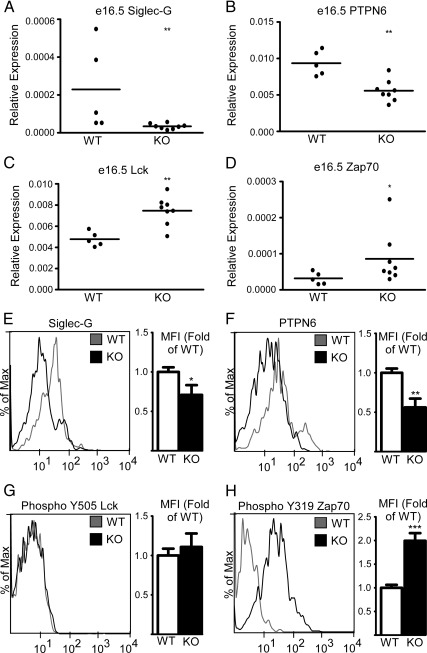
We previously found that Siglec-G, a sialic acid-binding Ig-like lectin, was down-regulated in the spleens of SXR−/− animals (9). Siglec-G knockout mice developed influxes of B-1 cells (23, 24). PTPN6, a target of Siglec-G, with a similar knockout phenotype (25), was also down-regulated in SXR knockout animals, and concordantly, the transcripts of both genes were up-regulated after SXR ligand activation by PCN in wild-type mice (9). We hypothesized that because these transcripts were down-regulated in adult knockout tissues, they might also be down-regulated developmentally, thereby priming the precursor cells to be proliferative later in life. Consistent with our hypothesis, Siglec-G and PTPN6 were found to be down-regulated (Fig. 3A, B) in fetal liver harvested from wild-type and SXR−/− embryos at day e16.5. Next, we hypothesized that down-regulation of Siglec-G and PTPN6 would lead to up-regulation of their pro-proliferative targets in the fetal liver. We found that the PTPN6 target gene Lck (26, 27) was up-regulated in SXR−/− fetal liver at e16.5 (Fig. 3C). Furthermore, the Lck target Zap70, which we previously found to be overexpressed in the spleens of SXR−/− animals (9), was up-regulated in SXR−/− fetal liver at e16.5 (Fig. 4D). In purified CD19+ B-1 precursors, Siglec-G and PTPN6 transcripts were both significantly down-regulated (Supplemental Fig. 3) and Lck and Zap70 trended toward moderate overexpression. Expression of both Siglec-G (Fig. 3E) and PTPN6 (Fig. 3F) was down-regulated at the protein level as measured by flow cytometry in CD19+-gated cells. Lastly, although we did not observe a change in Lck Y505 inactivating phosphorylation (Fig. 3G), Zap70 activating phosphorylation Y319 was up-regulated in SXR knockout CD19+ cells, confirming that Zap70 is in its active state. Intriguingly, Lck is overexpressed in chronic lymphocytic leukemia (28, 29) where it may confer a self-renewing phenotype and its target, Zap70, which is also often over-expressed in chronic lymphocytic leukemia is associated with poor survival and more aggressive disease (30, 31). Our data support a role for SXR in negatively regulating pro-proliferative gene expression during extramedullary hematopoiesis in the developing fetal liver.
Fig. 3.
SXR modulates key regulators of the B-1 cell compartment during fetal development. A–D, qPCR of indicated transcripts from fetal livers resected from e16.5 embryos is shown, and the means are indicated. Expression is relative to GAPDH. E and F, Flow cytometry of indicated proteins on CD19+-gated cells in e16.5 fetal liver. G and H, Phosphorylation of Lck and Zap70 as detected by flow cytometry (PhosFlow) on CD19+-gated cells in e16.5 fetal liver. Wild type, n = 6; knockout, n = 4. Histograms of representative animals are shown on the left panel, and quantitated protein expression (median fluorescent intensity) relative to wild type is shown on the right panel. Wild type (WT) is shown in the histogram in gray, and knockout (KO) is shown in black. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Loss of SXR increases B-1 cell progenitors in developing fetal liver, and SXR activation reduces B-1 cell progenitors
To expand on our findings that SXR modulates expression of transcripts involved in B-1 proliferation in the fetal liver, we examined fetal livers from timed pregnancies to determine whether B cell progenitor cells were affected by the presence or absence of SXR. Wild-type and SXR−/− dams were euthanized at d 16.5 of pregnancy, embryos harvested, and fetal liver tissue collected. Flow cytometric analysis of dissociated fetal liver cells revealed a modest (but not statistically significant) 1.3-fold increase in the population of CD19−/B220+ cells, which are not thought to give rise to B-1 cells (Fig. 4A, upper left gate labeled A). A larger, 2.3-fold increase in CD19+/B220+ B cell progenitors (Fig. 4A, upper right gate labeled B) was observed; knockout tissue displayed 2.1%, as compared with 0.9% in control tissue. Furthermore, over a 1.5-fold increase in CD19+/B220low B cell progenitors (Fig. 4A, lower right gate labeled C) was seen in SXR−/− tissue (an increase from 0.37 to 0.6%). This observation suggests that SXR negatively regulates the growth of B cell progenitors and that loss of SXR leads to an increased population of B cell progenitors in the developing fetal liver, which may ultimately contribute to the malignant phenotype seen in aged animals.
We next asked whether the populations of CD19+/B220+ and CD19+/B220low B-1 cell progenitors in wild-type fetal liver would be affected by chronic oral administration of the mouse SXR agonist PCN. Female mice were treated from mating until e16.5 with PCN or vehicle control. On e16.5, dams were euthanized, embryos were resected, and fetal liver tissue was harvested. SXR activation in the embryos was confirmed by induction of CYP3A11 and CYP3A16 (Supplemental Fig. 4). Flow cytometry revealed that the populations of CD19+/B220+ cells (labeled as gate B in Fig. 4A and quantitatively graphed in Fig. 4B) in fetal liver decreased upon PCN treatment of wild-type animals (representative flow cytometry plots shown in Supplemental Fig. 5). The lowest concentration (0.51 μm) had a mean population of 0.5%, the middle concentration a population of 0.43%, and the highest concentration reduced the percentage of CD19+/B220+ cells down to 0.3%, less than half that of vehicle-treated controls. Analysis of CD19+/B220low cells (labeled as gate C in Fig. 4A and quantitatively graphed in Fig. 4C) indicated that all three concentrations of PCN reduced the percentage of CD19+/B220low cells by more than half as compared with controls. We infer that activation of SXR in utero leads to a reduction of the B-1 cell progenitors in the fetal liver and suggest that SXR plays a crucial role in the embryonic generation of these lymphocyte progenitors.
In utero exposure to murine SXR agonist PCN reduces the size of the B-1 cell compartment
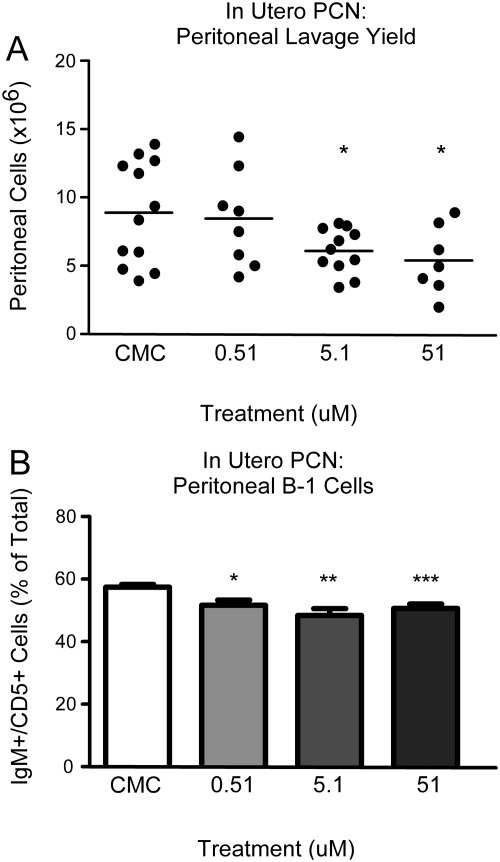
To examine the role of SXR activity in the developing animal, we treated female mice throughout pregnancy with PCN at 0.51, 5.1, or 51 μm or with CMC vehicle alone in their drinking water. In utero PCN exposure served as a tool to elucidate the role of SXR in developmental extramedullary hematopoiesis and in programming of B-1 cell precursors in the fetal tissue. When cells were harvested from 8-wk-old animals via unchallenged peritoneal lavage and counted, the total number of cells recovered varied inversely with PCN concentration in a dose-dependent fashion. The number of cells obtained ranged from approximately 9 × 106 (vehicle alone) and 8.5 × 106 (0.51 μm) to 6 × 106 (5.1 μm) and down to 5.5 × 106 (51 μm) (Fig. 5A). Spleen weight was largely unaffected by the in utero PCN treatment (Supplemental Fig. 6), as were follicular zone and marginal zone B cell populations in the spleen (data not shown). Furthermore, SXR was no longer activated in the 8-wk-old animals that were subjected to in utero exposure to PCN as measured by CYP3A11 expression in the liver (Supplemental Fig. 4C). Based on the observation that treatment with PCN reduced the total number of cells in the peritoneal cavity of the adult animal, we hypothesized that chronic exposure to an SXR agonist in utero leads to a permanent disturbance in the size of the peritoneal B-1 cell population.
Fig. 5.
In utero exposure to murine SXR agonist PCN reduces the size of the B-1 cell compartment. A, Cells collected from unchallenged peritoneal lavage from 8-wk-old male animals chronically treated in utero with PCN were counted, the absolute numbers of cells are indicated, and the means are shown. B, Cells collected from unchallenged peritoneal lavage from the above animals were analyzed for the expression of IgM and CD5, the percentage of cells that stained positive as determined by flow cytometry was quantitated, and the mean is indicated. CMC, n = 12; 0.51 μm, n = 8; 5.1 μm, n = 11; 51 μm, n = 7. A minimum of two litters per condition are represented. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To address whether chronic in utero PCN treatment reduced the percentage of B-1 lymphocytes in the peritoneal cavity, cells harvested via unchallenged peritoneal lavage were stained for B cell markers, analyzed via flow cytometry (representative flow cytometry plots shown in Supplemental Fig. 7), and the percentage of IgM+/CD5+ cells in the peritoneal cavity of in utero-treated 8-wk-old animals was quantitated. Vehicle-treated animals had a mean IgM+/CD5+ population of 57.46% of total cells (for a total B-1 cell yield of approximately 5.2 × 106), whereas the 0.51 μm-treated animals were found to have 51.65% (4.4 × 106 B-1 cells), the 5.1 μm-treated animals had 48.4% (2.9 × 106 B-1 cells), and the 51 μm-treated animals displayed a mean of 50.61% (approximately 2.8 × 106 B-1 cells) (Fig. 5). These data support the conclusion that in utero exposure to murine SXR agonist PCN decreases the total number of cells in the peritoneal cavity that were recovered by unchallenged peritoneal lavage. Moreover, SXR activation reduces the percentage of B-1 cells in the peritoneal cavity and consequently the total number of B-1 cells.
In utero exposure to SXR agonist PCN dampens proliferative signals in peritoneal cells harvested from 8-wk-old animals
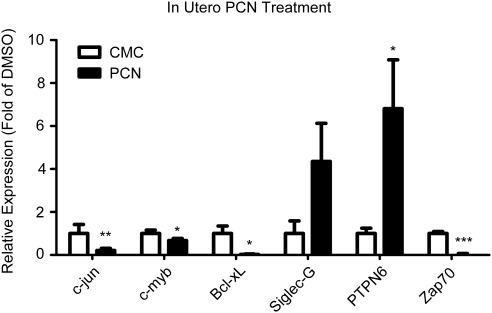
We harvested cells from unchallenged peritoneal lavage of 8-wk-old animals treated in utero with PCN to determine whether chronic in utero activation of SXR blunts expression of transcripts known to modulate the B-1 cell compartment. The qPCR analysis of mRNA transcript levels revealed that pro-proliferative c-jun and c-myb and antiapoptotic Bad were down-regulated as compared with vehicle-treated animals (Fig. 6). The B-1 cell checkpoints Siglec-G and PTPN6 were up-regulated in animals that were chronically exposed to PCN in utero, and expression of pro-proliferative Zap70 was significantly reduced (Fig. 6). Together, these results support the conclusion that activation of SXR by PCN during fetal development leads to decreased pro-proliferative signals, negative regulation of B-1 cell growth, and a diminished peritoneal B-1 cell population in the adult animal.
Fig. 6.
In utero exposure to SXR agonist PCN dampens proliferative signals in peritoneal cells harvested from 8-wk-old animals. mRNA of specific transcripts is measured by qPCR. Data are expressed as fold of CMC (vehicle) expression, and means are indicated. CMC, n = 12; 5.1 μm, n = 11. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
The results presented above lead us to conclude that SXR plays a role in the generation and maintenance of the B-1 cell compartment in the mouse fetal liver. SXR is expressed at high levels in the fetal liver, and pro-proliferative transcripts are up-regulated in SXR−/− fetal liver, whereas genes that regulate apoptosis and checkpoint control are down-regulated in SXR−/− fetal liver. We interpret these data to indicate that loss of SXR increases the pool of B-1 cell precursors in the fetal liver and that activation of SXR in utero reduces the population of B-1 cell progenitors. Furthermore, we found that activation of SXR during embryonic development reduced both the total number of peritoneal B-1 B cells and the percentage of B-1 cells in the peritoneal cavity. Therefore, SXR may be a crucial regulator of negative feedback in B-1 lymphocytes in the embryonic liver and in extramedullary hematopoiesis in the developing embryo. Loss of SXR may disturb steady-state conditions before birth, contributing to deregulated cell proliferation or progression past normal checkpoints. We note that although the pro-proliferative gene expression profile of B-1 cell progenitors is similar to that of whole fetal liver, the trend in B-1 cell progenitors is less robust, suggesting that SXR and NF-κB signaling in fetal hepatocytes or other cells may influence the development of B-1 cell progenitors in the tissue. Hepatocyte nuclear factor 4α (HNF-4α) is known to regulate the expression of a variety of genes in the fetal and adult liver and is required for expression of SXR in the fetal liver (32). Kamiya et al. (33) found that HNF-4α is crucial for the regulation of SXR and its xenobiotic metabolism target genes and showed that HNF-4α was required for transcriptional activation of SXR in fetal hepatocytes. The role of fetal hepatocytes in the development of B-1 cell progenitors is not well understood. It is clear that additional research is needed to uncover the role of SXR, as well as other transcription factors such as NF-κB and HNF-4α, in both fetal hepatocytes and in developmental hematopoiesis as well as in the intersection of the two in the fetal liver.
B-1a cells secrete large amounts of IgM, termed natural antibody, which impart protection against select encapsulated bacteria (including Streptococcus pneumoniae). B-1a cells help protect against mucosal pathogens and assist in tissue maintenance (34); published findings have shown that the natural antibodies to the influenza virus are almost entirely produced by B-1 cells (35). Natural IgM secretion by B-1 cells is required for maximal protection against deaths due to influenza virus. Enhanced mortality was observed after infection of mice that lacked B-1 cell-derived IgM but had normal levels of B-2 cell-derived IgM (35, 36). Protection against infection was partially restored after IgM transfer from wild-type, uninfected animals. Similarly, Blimp-1-deficient B-1 cells produce less natural antibody than wild-type B-1 cells and are also defective in providing early protection against influenza infection (37). These data suggest that B-1 cells assist in the frontline innate response against select antigens and contribute to the layered and intricate adaptive immune response. Moreover, deficiencies in the B-1 cell compartment may have detrimental effects on health and immune response, particularly before the generation of the full B-2 cell repertoire. We infer that SXR activation in utero reduces the number of B-1 cells in the offspring of treated dams and that mice and presumably humans exposed in utero to SXR agonists would generate an insufficient immune response against influenza infection.
Natural IgM secreted by B-1 cells can also bind to oxidized low-density lipoproteins, which can subsequently decrease the progress of atherosclerosis (38, 39). This suggests that a decrease in B-1 cells and, consequently, in natural antibody could lead to an increased progression of atherosclerosis, implicating SXR activation in not only immune disorders but also in additional aspects of human health. This is in accord with the observations that loss of SXR (which produces more B-1 cells) decreases atherosclerosis in apoE-deficient mice (40) and that activation of SXR (which decreases the number of B-1 cells) accelerates atherosclerosis in apoE-deficient mice (41). B-1 cell-generated antibody may also be specific for oxidized lipids (38) and antigens found on apoptotic cells (42). Together, these findings suggest that loss of B-1 cells due to inappropriate SXR activation may contribute to atherosclerosis and its sequelae.
B-1 cells also contribute to the neonatal immune response (15); thus, our findings suggest that taking drugs, herbal supplements, or xenobiotics that activate SXR during pregnancy may reduce the number of B-1 cells found neonatally, thereby reducing the immune capacity of the newborn. It is plausible that in utero exposure to SXR antagonists could subsequently increase the number of circulating B cells after birth, contributing to autoimmune diseases and other B cell-derived disorders. Published work demonstrates that B-1 cells found in the peritoneal cavity turn over relatively slowly (43), suggesting that alterations to the signals controlling their growth and proliferation may have a sizable impact on their population pool. It is possible that in utero priming of B cell progenitors during development may reduce the amount of circulating natural antibody, reducing the neonatal response to certain bacterial antigens. Ongoing work aims to determine the full extent of in utero SXR agonist exposure on various lymphoid components and immune responses to select immunological challenges and pathogens.
In conclusion, it is likely that SXR and its target genes contribute to the microenvironment that influences the fate and function of developing lymphocytes in the fetal liver. Inactivation or loss of the receptor may encourage proliferation of the B-1 cell compartment, whereas activation of the receptor may attenuate the production of B-1 cells. Importantly, chronic exposure to an SXR agonist during pregnancy is likely to disturb the developing B-1 progenitor cell compartment that will later populate the peritoneal cavity and gut after birth. It will be of interest to examine the role of drugs and nutraceuticals that activate or antagonize SXR during pregnancy on subsequent immune function.
Supplementary Material
Acknowledgments
We thank Gina M. Turco and Jhyme K. L. Laude for technical assistance, Edward L. Nelson for helpful comments on the manuscript, and Raquel Chamorro Garcia, Jose J. Limon, and David A. Fruman for experimental advice. We thank the Fruman lab for use of the Miltenyi MACS cell purification equipment and the UC Irvine Flow Cytometry Core Facility for use of the BD FACSCalibur.
We acknowledge support from National Cancer Institute Grant T32 CA9054 (to S.C.C.) and National Institutes of Health Grant ES015849 (to B.B.).
S.C.C. conceived the study, designed and performed experiments, analyzed data, and wrote the paper. B.B. supervised data collection and analysis and wrote the paper.
Disclosure Summary: S.C.C. has nothing to declare. B.B. is a named inventor on U.S. patents US 6,756,491, US 6,809,178, US 6,984,773, and US 7,214,482 related to SXR.
NURSA Molecule Pages†:
Nuclear Receptors: PXR.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- CMC
- Carboxymethylcellulose
- CYP
- cytochrome P450
- DMSO
- dimethylsulfoxide
- e
- embryonic d
- HNF-4α
- hepatocyte nuclear factor 4α
- Lck
- lymphocyte-specific protein tyrosine kinase
- NF
- nuclear factor
- PCN
- pregnenolone-16-α-carbonitrile
- PTPN6
- protein tyrosine phosphatase, non-receptor type 6
- qPCR
- quantitative real-time RT-PCR
- Siglec-G
- Sialic acide-binding Ig-like lectin G
- SXR
- steroid and xenobiotic receptor
- Zap70
- Zeta-chain-associated protein kinase 70.
References
- 1. Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. 1998. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12:3195–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. 1998. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou C, Verma S, Blumberg B. 2009. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal 7:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, Blumberg B. 2006. Mutual repression between steroid and xenobiotic receptor and NF-κB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. 2006. Role of NF-κB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. JBiol Chem 281:17882–17889 [DOI] [PubMed] [Google Scholar]
- 6. Xu DX, Chen YH, Wang JP, Sun MF, Wang H, Wei LZ, Wei W. 2005. Perinatal lipopolysaccharide exposure downregulates pregnane X receptor and Cyp3a11 expression in fetal mouse liver. Toxicol Sci 87:38–45 [DOI] [PubMed] [Google Scholar]
- 7. Li XY, Zhang C, Wang H, Ji YL, Wang SF, Zhao L, Chen X, Xu DX. 2008. Tumor necrosis factor α partially contributes to lipopolysaccharide-induced downregulation of CYP3A in fetal liver: its repression by a low dose LPS pretreatment. Toxicol Lett 179:71–77 [DOI] [PubMed] [Google Scholar]
- 8. Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H. 2005. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol 231:75–85 [DOI] [PubMed] [Google Scholar]
- 9. Casey SC, Nelson EL, Turco GM, Janes MR, Fruman DA, Blumberg B. 2011. B-1 Cell Lymphoma in Mice Lacking the Steroid and Xenobiotic Receptor, SXR. Mol Endocrinol 25:933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pohl T, Gugasyan R, Grumont RJ, Strasser A, Metcalf D, Tarlinton D, Sha W, Baltimore D, Gerondakis S. 2002. The combined absence of NF-κ B1 and c-Rel reveals that overlapping roles for these transcription factors in the B cell lineage are restricted to the activation and function of mature cells. Proc Natl Acad Sci USA 99:4514–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med 157:202–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kroese FG, Ammerlaan WA, Deenen GJ. 1992. Location and function of B-cell lineages. Ann NY Acad Sci 651:44–58 [DOI] [PubMed] [Google Scholar]
- 13. Kantor AB, Herzenberg LA. 1993. Origin of murine B cell lineages. Annu Rev Immunol 11:501–538 [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. 1985. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161:1554–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorshkind K, Montecino-Rodriguez E. 2007. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nature reviews 7:213–219 [DOI] [PubMed] [Google Scholar]
- 16. Montecino-Rodriguez E, Dorshkind K. 2006. New perspectives in B-1 B cell development and function. Trends Immunol 27:428–433 [DOI] [PubMed] [Google Scholar]
- 17. Hardy RR. 2006. B-1 B cell development. J Immunol 177:2749–2754 [DOI] [PubMed] [Google Scholar]
- 18. Montecino-Rodriguez E, Leathers H, Dorshkind K. 2006. Identification of a B-1 B cell-specified progenitor. Nat Immunol 7:293–301 [DOI] [PubMed] [Google Scholar]
- 19. Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. 2011. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA 108:1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. 1993. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 364:67–70 [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 22. Itoh S, Satoh M, Abe Y, Hashimoto H, Yanagimoto T, Kamataki T. 1994. A novel form of mouse cytochrome P450 3A (Cyp3a-16). Its cDNA cloning and expression in fetal liver. Eur J Biochem 226:877–882 [DOI] [PubMed] [Google Scholar]
- 23. Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P, Liu Y. 2007. Siglecg limits the size of B1a B cell lineage by down-regulating NFκB activation. PLoS ONE 2:e997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. 2007. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nature immunology 8:695–704 [DOI] [PubMed] [Google Scholar]
- 25. Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. 2007. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27:35–48 [DOI] [PubMed] [Google Scholar]
- 26. Chiang GG, Sefton BM. 2001. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J Biol Chem 276:23173–23178 [DOI] [PubMed] [Google Scholar]
- 27. Cuevas B, Lu Y, Watt S, Kumar R, Zhang J, Siminovitch KA, Mills GB. 1999. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem 274:27583–27589 [DOI] [PubMed] [Google Scholar]
- 28. Majolini MB, D'Elios MM, Galieni P, Boncristiano M, Lauria F, Del Prete G, Telford JL, Baldari CT. 1998. Expression of the T-cell-specific tyrosine kinase Lck in normal B-1 cells and in chronic lymphocytic leukemia B cells. Blood 91:3390–3396 [PubMed] [Google Scholar]
- 29. Abts H, Jücker M, Diehl V, Tesch H. 1991. Human chronic lymphocytic leukemia cells regularly express mRNAs of the protooncogenes lck and c-fgr. Leuk Res 15:987–997 [DOI] [PubMed] [Google Scholar]
- 30. Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ. 2004. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med 351:893–901 [DOI] [PubMed] [Google Scholar]
- 31. Schroers R, Griesinger F, Trümper L, Haase D, Kulle B, Klein-Hitpass L, Sellmann L, Dührsen U, Dürig J. 2005. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia 19:750–758 [DOI] [PubMed] [Google Scholar]
- 32. Li J, Ning G, Duncan SA. 2000. Mammalian hepatocyte differentiation requires the transcription factor HNF-4α. Genes Dev 14:464–474 [PMC free article] [PubMed] [Google Scholar]
- 33. Kamiya A, Inoue Y, Gonzalez FJ. 2003. Role of the hepatocyte nuclear factor 4α in control of the pregnane X receptor during fetal liver development. Hepatology 37:1375–1384 [DOI] [PubMed] [Google Scholar]
- 34. Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev 11:34–46 [DOI] [PubMed] [Google Scholar]
- 35. Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 192:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA 96:2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savitsky D, Calame K. 2006. B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med 203:2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. 2009. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 119:1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 105:1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sui Y, Xu J, Rios-Pilier J, Zhou C. 2011. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J Lipid Res 52:1652–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou C, King N, Chen KY, Breslow JL. 2009. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J Lipid Res 50:2004–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. 2009. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol 182:5363–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deenen GJ, Kroese FG. 1992. Murine peritoneal Ly-1 B cells do not turn over rapidly. Ann NY Acad Sci 651:70–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.