Abstract
We have previously reported that the absence of leptin signaling in β-cells enhances glucose-stimulated insulin secretion and improves glucose tolerance in vivo. To investigate the relevance of β-cell leptin signaling in the context of postprandial or therapeutic insulin secretion, we examined the cross talk between leptin and glucagon-like peptide (GLP)-1 and sulfonylurea actions. Single and size-matched islets isolated from control or pancreas-specific leptin receptor knockout (pancreas-ObR-KO) mice were treated either with GLP-1 or with glibenclamide. Leptin suppressed GLP-1-stimulated intracellular Ca2+ concentrations ([Ca2+]i) increase that paralleled the decrease in insulin secretion in controls. In contrast, and as expected, the ObR-KO islets were nonresponsive to leptin, and instead, showed a 2.8-fold greater GLP-1-stimulated [Ca2+]i increase and a 1.7-fold greater insulin secretion. Phosphorylation of cAMP-responsive element binding protein was enhanced, and phosphodiesterase enzymatic activity was suppressed in MIN6 β-cells with ObR knockdown compared with controls. The ObR-KO islets also showed significantly higher glibenclamide-induced insulin secretion compared with control islets, whereas [Ca2+]i was similar to the controls. These data support enhanced insulinotropic effects of glucose, GLP-1, and sulfonylureas in the islets lacking leptin signaling with potential therapeutic implications.
β-Cell growth and function are regulated by nutrients, hormones, and neuronal factors. Among hormones, leptin derived from adipocytes represents an example of interorgan cross talk and influences glucose homeostasis by modulating insulin secretion from pancreatic β-cells independent of its effects on the central nervous system (1, 2). We and others have reported that leptin acts directly on pancreatic β-cells to regulate insulin secretion, β-cell growth, and whole-body glucose homeostasis (3–5). Mice with a pancreas-specific leptin receptor knockout (pancreas-ObR-KO) manifested enhanced glucose-induced insulin secretion both in vivo and in vitro (5) and complemented the previous studies showing inhibitory effects of leptin on insulin gene expression and insulin secretion in human islets (1, 6). In cultured β-cells and isolated rodent islets, leptin also inhibits insulin secretion stimulated by glucagon-like peptide (GLP)-1 and agents that increase intracellular cAMP content, including 3-isobutyl-1-methylxanthine (IBMX) and forskolin (7–10). Activation of phosphatidylinositol 3-kinase and phosphodiesterase (PDE) 3B by leptin and subsequent suppression of intracellular cAMP levels has been suggested to be one of the mechanisms underlying the suppressive effect of leptin on GLP-1-stimulated insulin secretion in β-cells (10).
In vitro studies have also shown that leptin activates ATP-sensitive potassium channel to inhibit insulin secretion, and the effect is completely reversed by application of sulfonylurea agents (11, 12). Because GLP-1 analogs and sulfonylureas are widely used to improve insulin secretion as a form of treatment of type 2 diabetes, we explored the link between leptin, GLP-1, and sulfonylurea signaling using islets from the pancreas-ObR-KO mouse model.
We report that insulin secretion stimulated by GLP-1 or sulfonylureas is enhanced in ObR-KO islets, and in MIN6 β-cells with an ObR gene knockdown using small interfering RNA (siRNA). Together, these data suggest that the induction of leptin resistance in pancreatic β-cells would promote hyperinsulinemia when GLP-1 levels rise in the postprandial state or when sulfonylureas are used to enhance insulin secretion in the treatment of type 2 diabetes.
Materials and Methods
Animals
Pancreas-specific ObR-KO mice were created by crossing mice carrying obr in which exon 1 was flanked with loxP sites (obrlox/lox; ObRlox) (13) with mice expressing cre driven by the Pdx1 promoter (Pdx-Cre) (14) and were maintained on a C57BL/6 background as previously described (5). All animals were housed in specific pathogen-free facilities and maintained on a 12-h light, 12-h dark cycle and fed a standard rodent chow at the Foster Animal Laboratory (Brandeis University, Waltham, MA). All protocols for animal use were approved by the Institutional Animal Care and Use Committee of Joslin Diabetes Center and Brandeis University and were in accordance with National Institutes of Health guidelines. Genotyping was performed on DNA isolated from the tails of 3- to 4-wk-old mice by PCR as previously described (5).
Quantitative real-time PCR
Real-time quantitative PCR of islet or MIN6 β-cell sample was performed as previously described (5, 6). Primers used for real-time PCR were as follows: mouse Obr, 5′-GCTCTTCTGATGTATTTGGAAATC-3′ (forward), and 5′-ACCTGATATTGAAGCGGAAATGG-3′ (reverse); mouse GLP-1 receptor, 5′-AGAACTCTCCTTCACTTCCTTCCA-3′ (forward) and 5′-TCCCAGCATTTCCGAAACTC-3′ (reverse); mouse β-actin, 5′-AGGGCTATGCTCTCCCTCAC-3′ (forward) and 5′-AAGGAAGGCTGGAAAAGAGC-3′ (reverse); mouse Kir6.2, 5′-GTAGGGGACCTCCGAAAGAG-3′ (forward) and 5′-TGGAGTCGATGACGTGGTAG-3′ (reverse); mouse SUR-1, 5′-CCTGGGGGTGCGCTTTCTGC-3′ (forward) and 5′-CCCTGCTGGCTCTGCGTGTCTTT-3′ (reverse). Primers for PDE isoforms are available on request.
Measurements of intracellular Ca2+ concentrations ([Ca2+]i) and insulin secretion in islets
Mouse islets were isolated as previously described (5), and single, size-matched islets were incubated in different concentrations of glucose with or without mouse recombinant leptin (Sigma Chemical Co., St. Louis, MO), GLP-1 (7–36) amide (Sigma), or glibenclamide (Sigma) as indicated and assayed for insulin secretion and [Ca2+]i as described previously (5, 15, 16). Briefly, after being isolated from mice, a single pancreatic islet was perfused in microfluidic chamber with different concentrations of glucose with or without leptin, GLP-1, or glibenclamide as indicated. The [Ca2+]i measurements in single islet were performed by ratiometric fluorescence using fura-2 as a Ca2+ indicator dye. Basal levels were determined by taking the average [Ca2+]i from measurements at 3 mm glucose for the 3 min before a step change to 8 mm and assigning the value 100% basal. Increased flux from islet stimulation is presented as a % increase over this level. These calculations did not involve total [Ca2+]i content.
Measurement of insulin content in mouse islets
Islet insulin content was measured by acid ethanol extraction followed by RIA (17, 18). Insulin content was measured in 10 size-matched islets in both genotypes and expressed as picograms of insulin per islet.
Cell culture and transfection of siRNA
MIN6 β-cells were incubated at 37 C and 5% CO2 in DMEM supplemented with 15% fetal bovine serum, and penicillin and streptomycin. Predesigned siRNA for mouse leptin receptor (siObr) (Ambion, Inc., Austin, TX) was transfected in MIN6 β-cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) as described previously (5). Serum starved cells were treated 48 h after transfection with mouse leptin and/or exendin-4 (Sigma) for 15 min as indicated. Insulin release from MIN6 β-cells stimulated by 1 μm glibenclamide was measured after 1-h preincubation with Krebs-Ringer buffer (KRB) with 3.3 mm glucose after siRNA transfection (19). Insulin released in KRB for 30 min was normalized by protein content in each well, and data were expressed as picograms insulin per μg protein per minute.
Western blotting
Protein samples extracted from isolated islets or MIN6 cell lysates were subjected to SDS-PAGE and immunoblotting (5). Antibodies to phospho-cAMP response element-binding protein (CREB) (Ser133), CREB, phospho-Akt (Ser473) and Akt were from Cell Signaling Technology (Danvers, MA). Anti-α-tubulin antibody was from Abcam, Inc. (Cambridge, MA). Anti-PDE3B, Kir6.2, and SUR-1 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase -conjugated goat antirabbit IgG, goat antimouse IgG, and mouse antigoat IgG were from Santa Cruz Biotechnology.
PDE activity assay
Two million MIN6 cells were seeded in six-well plates and 24 h later, the cells were transfected with either scramble control or siObR as described above. Media were changed 48 h after transfection to either 5.5 or 25 mm glucose for 16 h. Cells were then lysed with 300 μl per well of radioimmune precipitation assay buffer (20). PDE activities were determined using the cyclic nucleotide PDE assay kit (Enzo Life Sciences, Inc., Farmingdale, NY). Cell lysates were first applied to gel filtration column provided by the assay kit to remove excess phosphates and nucleotides before being used for PDE activity determination according to the manufacturer's protocol.
Insulin release from mouse islets
C57BL/6J mouse islets were isolated and cultured overnight in RPMI media containing 2.8 mm glucose and 1% BSA. Next day, the islets were incubated for 1 h in KRB with 2.8 mm glucose, 1% BSA and followed by 1 h of incubation in KRB containing leptin in the presence or absence of cilostamide (Sigma). Insulin released in the incubation media was measured by RIA and normalized by DNA content in each islet sample as reported previously (20).
Statistics
All data are presented as mean ± sem and were analyzed using an unpaired two-tailed Student's t test or ANOVA as appropriate. P < 0.05 was considered significant.
Results
Pancreatic islets lacking ObR exhibit enhanced insulinotropic effects of GLP-1 stimulation
To evaluate whether leptin signaling affects GLP-1 action on glucose-stimulated insulin secretion, we treated single, size-matched islets isolated from control or pancreas-ObR-KO mice with or without 10 nm GLP-1(7–36) amide and measured [Ca2+]i by ratiometric fluorescence and insulin secretion by serial immunoassay on a microfluidic chip. GLP-1 enhanced glucose-stimulated [Ca2+]i increase by 32.9% (peak [Ca2+]i, vehicle vs. GLP-1, 155.5 ± 16.2% vs. 206.6 ± 11.4% basal; P < 0.05; n = 5), and this effect was suppressed by 22.7% with simultaneous treatment with leptin in ObRlox control islets (peak [Ca2+]i, 159.8 ± 10.9% basal; P < 0.05 vs. GLP-1 without leptin; n = 5) (Fig. 1, A and C).
Fig. 1.
Islets lacking leptin receptor exhibit greater GLP-1 effects on glucose-stimulated [Ca2+]i increase and insulin release. A, B, D, and E, Representative traces of intracellular Ca2+ flux (A and B) and insulin release (D and E) measured in primary size-matched islets isolated from 6-month-old male ObRlox (A and D) and ObR-KO (B and E) mice in different glucose concentrations with or without 10 nm GLP-1(7–36) amide or 10 nm leptin. C, Percent changes in [Ca2+]i from basal glucose (3 mm) in response to 8 mm glucose treated as indicated (mean ± sem; *, P < 0.05; **, P < 0.01; n = 5). F, Percent changes in insulin secretion in response to glucose (8 mm) from basal glucose (3 mm) treated as indicated (mean ± sem; *, P < 0.05; **, P < 0.01; n = 5). G, Islet insulin content was measured in acid-ethanol extracts from 5-month-old ObRlox and ObR-KO mice by RIA. Values are expressed as means ± sem in picograms/islet (n = 3; P = 0.222). H, Expression of GLP-1 receptor gene in size-matched ObRlox and ObR-KO islets assessed by quantitative real-time PCR (P = 0.766; n = 3). Data are obtained from islet samples from 6-month-old mice and are shown as mean ± sem.
In ObR-KO islets, GLP-1 enhanced glucose-stimulated [Ca2+]i increase, which was significantly greater than in size-matched control islets (peak [Ca2+]i; ObRlox vs. KO; 206.6 ± 11.4% vs. 579.1 ± 94.1% basal; P < 0.01; n = 5; Fig. 1C), and this effect was not suppressed by leptin, consistent with the absence of functional leptin receptors in the islets (Fig. 1, B and C). There was virtually no difference in basal [Ca2+]i levels at 3 mm glucose between genotypes (ObRlox, 111.7 ± 17.3; ObR-KO, 129.3 ± 34.8 nm; P = 0.052; n = 20). In agreement with the alterations in [Ca2+]i, GLP-1 also enhanced glucose-stimulated insulin secretion (GSIS) (peak insulin; vehicle, 238.3 ± 20.9 vs. GLP-1; 413.5 ± 39.3% basal; P < 0.01; n = 5) and failed to enhance GSIS in the presence of leptin in ObRlox islets (239.9 ± 18.8% basal; P < 0.01 vs. GLP-1 without leptin; n = 5) (Fig. 1, D and F). In ObR-KO islets which already exhibited greater GSIS than ObRlox islets (385.9 ± 42.7% basal; P < 0.05 vs. ObRlox without GLP-1; n = 5), insulin secretion was augmented even further by GLP-1 (650.2 ± 74.0% basal; P < 0.05 vs. ObRlox with GLP-1; n = 5; Fig. 1F) and was virtually unaffected by leptin treatment (499.6 ± 67.3% basal; P = 0.170 vs. GLP-1 without leptin) (Fig. 1, D and F). The GLP-1 effects on [Ca2+]i and insulin release in the presence of 8 mm glucose were 7.2-fold and 1.5-fold greater, respectively, in ObR-KO islets than controls (% δ increases; [Ca2+]i, 51.1 and 366.8%; insulin release, 175.2 and 264.4% in ObRlox and ObR-KO, respectively. Fig. 1, C and F, red columns), indicating that the enhanced GLP-1-stimulated insulin secretion in ObR-KO islets is independent of background glucose effects.
Insulin content in size-matched islets was similar between genotypes (Fig. 1G), indicating the stimulatory effects of GLP-1 are unlikely due to differences in the amount of insulin in β-cells. Expression of GLP-1 receptor (GLP-1R) levels in size-matched islets, evaluated by real-time PCR, was also similar between the two groups (Fig. 1H). These results indicate that GLP-1 has significantly greater effects on Ca2+ flux and insulin secretion in islets lacking leptin signaling and that these effects are independent of differences in β-cell mass and GLP-1R expression but secondary to alterations in GLP-1-induced intracellular signaling in islet β-cells.
Because the ObR-KO mice exhibit impaired GSIS after high-fat diet feeding (5), we further explored how islets lacking leptin signaling are affected by lipotoxic signals. Chronic treatment with palmitate (48 h) before glucose stimulation decreased glucose-induced [Ca2+]i response, indicating lipotoxic effects by palmitate, to a similar extent in islets from both genotypes (control, 38.5; KO, 35.9% decrease; n = 5; P = 0.518) (Supplemental Fig. 1, A–C, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). However, whereas chronic palmitate treatment impaired GSIS by 21.0% in control islets (Supplemental Fig. 1, D and F), the KO islets showed an even greater impairment in GSIS (47.9% decrease; n = 5, P < 0.05 vs. control) (Supplemental Fig. 1, E and F). These data indicate the ObR-KO islets are more susceptible to lipotoxic effects of palmitate than controls by mechanisms independent of the KATP channel-Ca2+ pathway.
Knockdown of leptin receptor promotes GLP-1-induced intracellular signaling in MIN6 β-cells
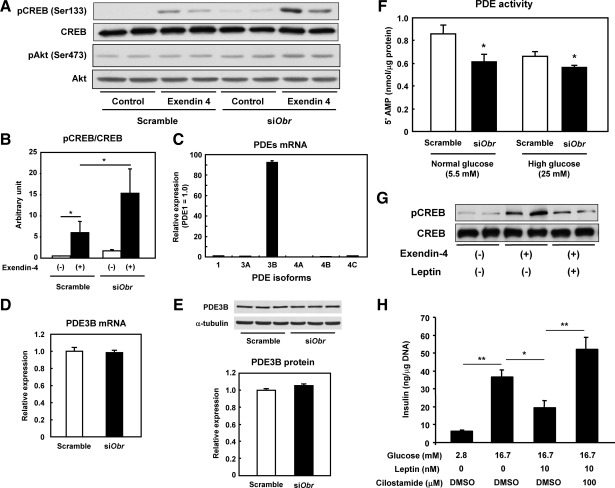
To examine the mechanisms underlying the enhanced effects of GLP-1 on glucose-stimulated [Ca2+]i increase and insulin secretion in ObR-KO islets, we used an in vitro approach by knocking down the ObR gene using siRNA in MIN6 β-cells. Forty eight hours after transfection of siRNA for mouse ObR (siObr), the ObR gene expression was reduced by approximately 80% as reported previously (5). Acute treatment with exendin-4, a GLP-1 analog, promoted phosphorylation of CREB at Ser133 in control β-cells, and the effect was significantly enhanced in cells treated with siObr (Fig. 2, A and B), indicating an up-regulation of the intracellular GLP-1 receptor signaling pathway in β-cells with attenuated leptin signaling. Exendin-4 treatment also enhanced activation of the survival kinase Akt, a central component of the insulin/IGF-I signaling pathway, in cells treated with siObr compared with control cells (Fig. 2A). Next, we examined the expression levels of PDE, which degrade intracellular cAMP and are known to be important regulators of the cAMP/protein kinase A (PKA) signal transduction system (21). Among several isoforms that could be detected, we observed that PDE3B was the most abundant in MIN6 β-cells (Fig. 2C), consistent with studies in rat islets and a hamster β-cell line (10). We next examined the effects of reduced leptin signaling on PDE expression and activity in MIN6 β-cells. A transient Obr knockdown did not significantly alter mRNA (Fig. 2D) or protein expression levels (Fig. 2E) of PDE3B; however, the PDE activity was significantly blunted in MIN6 β-cells treated with siObr compared with control cells at both normal (5.5 mm) and high (25 mm) glucose concentrations (Fig. 2F). These results indicate that the reduced leptin signaling by Obr knockdown decreases PDE activity and consequently enhances the GLP-1/CREB signaling pathway in β-cells. We also observed that leptin treatment reduces the CREB phosphorylation in response to exendin-4 in control MIN6 cells (Fig. 2G) and that the inhibitory effects of leptin on GSIS are blocked by cilostamide, a PDE3 inhibitor, in control mouse islets (Fig. 2H), confirming that the leptin effects on β-cell function are mediated via activation of PDE3B.
Fig. 2.
Enhanced GLP-1-induced intracellular signaling in MIN6 β-cells with ObR knockdown. A, MIN6 β-cells were transfected with scramble control or siObr for 48 h and treated with or without 10 nm exendin-4 for 15 min after overnight serum starvation at 5.5 mm glucose. Total-cell lysates were extracted and subjected to Western blot analyses for phospho-CREB (Ser133) and total CREB. B, The relative expression of phospho-CREB normalized to total CREB is shown as mean ± sem in the graph (*, P < 0.05; n = 3). C, mRNA expression for PDE isoforms in MIN6 β-cells was analyzed by real-time PCR. mRNA level normalized by β-actin was expressed as relative to PDE1 mRNA (mean ± sem; n = 6). Expression level of PDE3B mRNA (D) or protein (E) in MIN6 β-cells transfected with scramble control or siObr was analyzed by real-time PCR or Western blotting, respectively. mRNA level was normalized by β-actin and expressed as mean ± sem (P = 0.938; n = 6). Protein expression level was quantified by densitometry, normalized by α-tubulin, and expressed as mean ± sem (P = 0.393, n = 4), F, MIN6 β-cells were transfected with scramble control or siObR for 48 h, and the PDE enzymatic activity of the cells was measured at 5.5 or 25 mm glucose (mean ± sem; *, P < 0.05 vs. scramble control; n = 4) G, MIN6 β-cells were treated with control or 10 nm exendin-4, with or without 10 nm leptin, for 15 min after 1-h preincubation with or without leptin. Total-cell lysate was subjected to Western blot analyses for phospho-CREB (Ser133) and total CREB. H, C57BL/6J mouse islets were treated with glucose, leptin, and/or cilostamide, respectively, as indicated for 1 h after 1-h precincubation at 2.8 mm glucose. Insulin released in culture media was measured by RIA. Values are expressed as mean ± sem (ng insulin per μg DNA; *, P < 0.05; **, P < 0.01; n = 6).
Sulfonylureas enhance glucose-induced insulin secretion independent of Ca2+ concentrations in ObR-KO islets
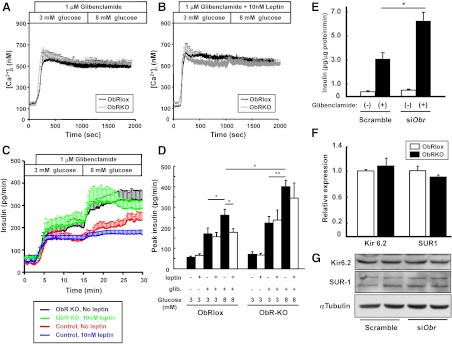
Next, to examine whether the absence of leptin receptors in islets affects insulin secretion stimulated by sulfonylureas, we perfused single, size-matched islets isolated from control or ObR-KO mice with 1 μm glibenclamide in the presence of 3 or 8 mm glucose. Glibenclamide treatment raised [Ca2+]i by approximately 5-fold in both ObRlox and ObR-KO islets (Fig. 3A). Further, islets of both genotypes failed to show significant differences in [Ca2+]i in response to glucose (Fig. 3A), and neither responded to leptin in the presence of glibenclamide treatment (compare Fig. 3, A and B). These findings suggest a complete closure of KATP channels with saturation of [Ca2+]i in the presence of glibenclamide. In the presence of glibenclamide, we did not observe a decrease in [Ca2+]i that is associated with increased sarcoplasmic endoplasmic reticulum Ca2+-ATPase activity as reported elsewhere (22). Further investigation of the decrease in [Ca2+]i suggests this is detectable in both control and ObR-KO islets; however, its observation when glucose is stepped up from 3 to 8 mm glucose appears to be dependent on islet handling and conditions that are influenced by technical variables including perfusion time (data not shown).
Fig. 3.
Islets lacking leptin receptor exhibit greater insulin secretion stimulated by sulfonylureas. A and B, Representative traces of [Ca2+]i concentrations measured in primary size-matched islets isolated from 6-month-old male ObRlox and ObR-KO mice in different glucose concentrations with 1 μm glibenclamide with (B) or without (A) 10 nm leptin. C, Insulin release was measured in size-matched islets perfused as in panels A and B. Peak insulin release from islets in response to glibenclamide and/or glucose was expressed as mean ± sem(pg/min) in the graph (D) (*, P < 0.05; **, P < 0.01; n = 7 ∼ 8). E, MIN6 β-cells were transfected with scramble control or siObr for 48 h and treated with 0.1% DMSO or 1 μm glibenclamide for 30 min after preincubation with KRB at 3.3 mm glucose for 30 min. Insulin released in culture media was measured by RIA. Values are expressed as mean ± sem in picograms insulin per μg protein per min (*, P < 0.01; n = 6). F, Expression of Kir 6.2 and SUR-1 in size-matched ObRlox and ObR-KO islets assessed by quantitative real-time PCR (P = 0.562 for Kir6.2; P = 0.317 for SUR-1; n = 3). Data are obtained from islet samples from 6-month-old mice and are shown as mean ± sem. G, Western blot analysis of total cell lysates for Kir6.2 and SUR-1 in MIN6 β-cells transfected with scramble control or siObr for 48 h.
Leptin treatment did not alter glibenclamide-stimulated islet insulin release at basal 3 mm glucose (Fig. 3, C and D), in either group, and this was compatible with the similar [Ca2+]i in both genotypes. However, ObR-KO islets released 23.5% more insulin in response to glibenclamide than controls at 3 mm glucose (peak insulin levels before and after glibenclamide; ObRlox, 55.6 ± 6.0 to 170.6 ± 28.5; ObR-KO, 70.3 ± 13.1 to 222.9 ± 31.7 pg/min; n = 7 or 8) (Fig. 3, C and D), suggesting that ObR-KO islets secrete significantly more insulin by mechanisms that are independent of the KATP channel. Interestingly, in the presence of glibenclamide, insulin secretion in ObRlox control islets was increased by 53.2% upon glucose stimulation (peak insulin at 3 and 8 mm glucose, 170.6 ± 28.5 and 261.4 ± 28.5 pg/min, respectively. P < 0.05 between 3 and 8 mm glucose, n = 8), and this effect was significantly suppressed by leptin (170.1 ± 17.5 pg/min; P < 0.05 vs. 8 mm glucose without leptin). The glucose-induced effect on insulin secretion upon glibenclamide treatment was even greater in the ObR-KO group than controls (400.4 ± 31.1 pg/min; P < 0.05 vs. ObRlox), and those islets exhibited virtually no response to leptin (344.0 ± 74.0 pg/min; P = 0.562 vs. ObR-KO at 8 mm glucose without leptin, n = 7) (Fig. 3, C and D). These results suggest that, during glibenclamide treatment when KATP channels are fully inactivated, glucose stimulates insulin secretion independent of the KATP channel-Ca2+ pathway. The data also suggest that leptin suppresses a potential KATP channel-independent pathway by glucose, and the absence of ObR allows activation of this pathway in mouse islets. We confirmed the suppressive effect of leptin on [Ca2+]i increase and GSIS in ObRlox islets and enhanced [Ca2+]i increase and GSIS in ObR-KO islets in the presence of 0.1% dimethyl sulfoxide (DMSO) as a vehicle (data not shown), indicating that DMSO itself has no effect on islet function in these experiments.
To confirm that the effects observed in islets are specific to β-cells, we examined glibenclamide-induced insulin secretion in static incubation experiments using MIN6 β-cells with Obr knockdown. Glibenclamide stimulated insulin secretion by 8.4-fold in scramble control cells, whereas its insulinotropic effect was approximately 2-times greater in cells with Obr knockdown (siObr; 6.5 ± 0.8 vs. scramble; 3.2 ± 0.6 pg/μg protein/min; P < 0.01; n = 6) (Fig. 3E). These results confirm that inhibiting leptin signaling in β-cells allows for an enhanced glibenclamide-induced insulin secretion. We observed no difference in Kir6.2 or SUR-1 gene expression levels between islets of either genotype (Fig. 3F), and no difference was observed in the expression levels of Kir6.2 or SUR-1 protein in MIN6 β-cells treated with scramble or Obr knockdown (Fig. 3G).
Discussion
We have previously reported a direct role for leptin signaling in the secretory function of pancreatic β-cells by creating a pancreas-specific ObR-KO mouse model (5). In the same study we observed elevated plasma insulin concentrations in vivo in both fasting and fed states with a greater difference between groups in the fed state. Indeed, plasma glucose tended to be lower in the fed state in the mutants whereas fasting blood glucose levels were similar between groups. Those findings indicated that the inhibitory effects of leptin on insulin secretion are more pronounced during the postprandial state when GLP-1, one of the incretin hormones that is secreted by intestinal L-cells after enteral nutrient ingestion (23), is released. Previous reports have suggested that leptin inhibits glucose-induced insulin secretion that is potentiated by agents that increase intracellular cAMP content such as GLP-1 and IBMX in rodent β-cell lines and rat perfused pancreas (7–10). However, direct evidence for interactions between leptin and GLP-1 signaling in the β-cell has, to our knowledge, not been reported.
In this study we have further explored the cross talk between leptin and GLP-1 signaling pathways and report that leptin suppresses GLP-1-potentiated insulin secretion in control islets. GLP-1-induced [Ca2+]i increase was also suppressed by leptin, indicating that the effects of GLP-1 to increase GSIS, by affecting β-cell pathways before Ca2+ flux, such as KATP channel activity, mitochondrial function, and/or glucose metabolism, is potentially inhibited by leptin. The notion that the inhibitory effects of leptin are more pronounced in the presence of GLP-1-stimulation is supported by our observation that islets lacking leptin receptors exhibit significantly greater [Ca2+]i increase and insulin secretion when treated with GLP-1 compared with control islets. The greater GLP-1 effects on [Ca2+]i and insulin release that occur independent of the prevailing levels of glucose in ObR-KO islets, and in the absence of significant differences in islet insulin content and GLP-1 receptor expression levels between size-matched control and ObR-KO islets, clearly suggest that GLP-1-induced intracellular signaling pathways are altered in β-cells lacking leptin receptors. On average, the first phase of [Ca2+]i was stronger than the first phase of insulin secretion. Whereas first-phase [Ca2+]i was consistently observed, we detected some variability in insulin secretion. Typical [Ca2+]i traces with first-phase insulin secretion are presented in Supplemental Fig. 2. One possible explanation for these observations is the day-to-day variability in islet preparations that differentially impact [Ca2+]i and insulin secretion measurements. We also cannot completely rule out an unpredictable disruption in the flow path of the microfluidic system used for insulin secretion measurements that influences the detection of a sharp first phase in some experiments (e.g. small clogs can alter flow and/or reduce temporal resolution). Nevertheless, careful testing of the chips used for these experiments did not reveal defects that could have directly contributed to the observed effects.
The ObR-KO islets exhibited an impaired GSIS to a greater extent than control islets after chronic exposure to palmitate, a saturated fatty acid. This finding is compatible with our in vivo data showing a pronounced β-cell dysfunction after high-fat diet feeding in ObR-KO mice (5) and suggests that in the normal situation leptin signaling protects islet β-cells from lipotoxic signals. Because GLP-1 has also been shown to protect pancreatic β-cells against lipotoxic β-cell dysfunction caused by chronic exposure to free fatty acid via the reduction of β-cell endoplasmic reticulum stress (24), it is tempting to speculate that leptin coordinates with GLP-1 for protecting β-cell function in lipotoxic states such as obesity and diabetes.
GLP-1 and GLP-1 receptor agonists mediate insulinotropic effects on β-cells by promoting CREB phosphorylation at Ser133 and regulating CREB activity via PKA-dependent pathways, especially in the presence of elevated plasma glucose levels (23). The elevated CREB phosphorylation in response to treatment with the GLP-1 analog, exendin-4, along with reduced PDE enzymatic activity in MIN6 β-cells with ObR knockdown suggests an up-regulation of the GLP-1R-cAMP production-PKA cascade in our studies. Indeed, leptin activates phosphatidylinositol 3-kinase and PDE3 and consequently reduces cytosolic cAMP in cultured rodent β-cells (10, 25, 26). Our data showing that leptin reduces phospho-CREB response to exendin-4 in MIN6 cells and leptin inhibition of GSIS is blocked by a PDE3 inhibitor in mouse islets confirms leptin inhibition of β-cell function via activation of PDE3. GLP-1-induced CREB activation leads to insulin receptor substrate 2 activation, a component of insulin signaling, and promotes islet cell survival and function (27). In agreement with cross talk between both signaling pathways, phosphorylation of Akt, a protein downstream of insulin receptor substrate 2 that is induced by exendin-4, was also up-regulated in β-cells with ObR knockdown. Thus, the absence of stimulatory effects of leptin on PDE activity and the consequent up-regulation of cellular cAMP/PKA signaling coupled with activation of proteins in the insulin/growth factor signaling pathway are potential explanations for enhanced GLP-1 action in β-cells lacking leptin receptors.
The absence of leptin's inhibitory effects on [Ca2+]i in the presence of sulfonylureas in ObRlox control islets is compatible with previous studies reporting that leptin induces KATP channel activation and membrane hyperpolarization by generating phosphatidylinositol (3,4,5)-trisphosphate and actin depolymerization in cultured β-cells and mouse islets (11, 25, 26, 28). Glibenclamide-induced increase in [Ca2+]i was neither suppressed by leptin nor enhanced further by glucose, indicating that KATP channels are closed and voltage-dependent Ca2+ channels are open in the presence of sulfonylureas. The ObR-KO islets failed to exhibit any differences in [Ca2+]i compared with controls when perfused with glibenclamide, possibly because the effects of ObR KO are minimal when KATP channels are already inactivated by sulfonylureas.
Despite the lack of a significant change in [Ca2+]i, insulin release was augmented at stimulatory glucose (8 mm) in the presence of glibenclamide in both control and ObR-KO islets. Because the β-cell plasma membrane is fully depolarized by glibenclamide, the increment in insulin release by glucose reflects KATP channel-independent mechanisms (29–31). Our data also suggest that inhibitory effects of leptin on KATP channel-independent effects of glucose-stimulated insulin release are evident in control islets and are further enhanced in the ObR-KO group. Because islets in both groups contain similar insulin content, the KATP channel-independent effects of glucose, which are enhanced in the ObR-KO islets, suggest the cross talk between leptin signaling and glucose-induced KATP channel-independent signaling primarily impacts secretory function. The increase in glibenclamide-induced insulin release at basal glucose (3 mm) in ObR-KO islets without significant differences in [Ca2+]i, islet insulin content, or the expression of KATP channel between genotypes, indicates that the insulinotropic effect of sulfonylureas is augmented in islets lacking leptin signaling. Similar effects in MIN6 β-cells with ObR knockdown confirm that the interaction between glibenclamide and leptin occurs directly at the level of the β-cell.
Our observations in the leptin receptor KO group provide direct evidence for a specific inhibitory effect of leptin on GLP-1-stimulated glucose secretion (summarized in Fig. 4) and is consistent with earlier in vitro studies (10, 25, 26). For example, leptin reduces the GLP-1-mediated increase in insulin release and cytosolic Ca2+ concentration in perfused rat pancreas (8). Leptin also inhibits insulin secretion stimulated by GLP-1, IBMX, forskolin, or dibutyryl-cAMP in rat islets (9) and in the INS-1 β-cell line (7). The enhanced intracellular GLP-1 signaling effects in β-cells, when functional leptin receptors are absent, could underlie, in part, the hyperinsulinemia in the fed compared with the fasting state in pancreas-ObR-KO mice (5). Future studies aimed at restoring ObRb expression specifically in pancreatic β-cells to study the consequences on long-term regulation of insulin-secretory function in the db/db mouse model will provide insights into the importance of leptin signaling in β-cells for the maintenance of glucose homeostasis.
Fig. 4.
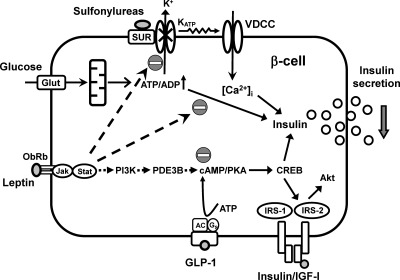
Schematic depicting the potential signaling cross talk between leptin, glucose, GLP-1, and sulfonylureas in the regulation of β-cell biology. AC, Adenylyl cyclase; Jak, Janus family of tyrosine kinases; PI3K, phosohatidylinositol 3-kinase; Stat, signal transducer and activator of transcription.
In summary, the enhanced insulinotropic actions of sulfonylureas (e.g. glibenclamide) and incretin hormones (e.g. GLP-1) in models with attenuated leptin signaling in the β-cells have several implications. First, it will be worth investigating whether the hyperinsulinemia observed in leptin-resistant obese diabetic patients occurs as a consequence of the absence of leptin's inhibitory effects on β-cell secretion. Second, these observations suggest potential regulatory feedback effects between β-cells and adipose tissue in the overall regulation of leptin secretion. Finally, it would be useful to consider leptin therapy in obese individuals that exhibit severe hyperinsulinemia especially in response to treatment with GLP-1 analogs and/or sulfonylureas.
Supplementary Material
Acknowledgments
We thank Liz Morgan and Kellianne Parlee (Joslin Diabetes Center, Boston, MA) for excellent assistance with formatting the manuscript and G.G. Holz Ph.D. (New York University, New York, NY) for discussions.
This work was supported by an American Diabetes Association Research Award (7–07-RA-84) (to R.N.K.) and in part by National Institutes of Health Grant RO1 DK67536. T.M. was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Young Scientists B 21790878) and The Osaka Medical Research Foundation for Incurable Diseases. C.W.L. is supported by National Institutes of Health Grant 1-K99 DK090210-01.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- [Ca2+]i
- Intracellular Ca2+ concentration
- CREB
- cAMP response element-binding protein
- DMSO
- dimethyl sulfoxide
- GLP
- glucagon-like peptide
- GSIS
- glucose-stimulated insulin secretion
- IBMX
- 3-isobutyl-1-methylxanthine
- KO
- knockout
- KRB
- Krebs-Ringer buffer
- PDE
- phosphodiesterase
- PKA
- protein kinase A
- siRNA
- small interfering RNA.
References
- 1. Kieffer TJ, Habener JF. 2000. The adipoinsular axis: effects of leptin on pancreatic β-cells. Am J Physiol Endocrinol Metab 278:E1–E14 [DOI] [PubMed] [Google Scholar]
- 2. Myers MG., Jr 2004. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59:287–304 [DOI] [PubMed] [Google Scholar]
- 3. Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ. 2006. The pancreatic β cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 4:291–302 [DOI] [PubMed] [Google Scholar]
- 4. Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. 2005. Mechanisms of compensatory β-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54:2294–2304 [DOI] [PubMed] [Google Scholar]
- 5. Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. 2007. Disruption of leptin receptor expression in the pancreas directly affects β cell growth and function in mice. J Clin Invest 117:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. 1997. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100:2729–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahrén B, Havel PJ. 1999. Leptin inhibits insulin secretion induced by cellular cAMP in a pancreatic B cell line (INS-1 cells). Am J Physiol 277:R959–R966 [DOI] [PubMed] [Google Scholar]
- 8. Fehmann HC, Bode HP, Ebert T, Karl A, Göke B. 1997. Interaction of GLP-I and leptin at rat pancreatic B-cells: effects on insulin secretion and signal transduction. Horm Metab Res 29:572–576 [DOI] [PubMed] [Google Scholar]
- 9. Poitout V, Rouault C, Guerre-Millo M, Briaud I, Reach G. 1998. Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans. Endocrinology 139:822–826 [DOI] [PubMed] [Google Scholar]
- 10. Zhao AZ, Bornfeldt KE, Beavo JA. 1998. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest 102:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. 1997. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J Physiol 504:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ning K, Miller LC, Laidlaw HA, Watterson KR, Gallagher J, Sutherland C, Ashford ML. 2009. Leptin-dependent phosphorylation of PTEN mediates actin restructuring and activation of ATP-sensitive K+ channels. J Biol Chem 284:9331–9340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. 2001. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. 2003. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 13:1070–1074 [DOI] [PubMed] [Google Scholar]
- 15. Dishinger JF, Kennedy RT. 2007. Serial immunoassays in parallel on a microfluidic chip for monitoring hormone secretion from living cells. Anal Chem 79:947–954 [DOI] [PubMed] [Google Scholar]
- 16. Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN. 2006. Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- 17. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. 1999. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339 [DOI] [PubMed] [Google Scholar]
- 18. Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. 2007. Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR. 2005. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122:337–349 [DOI] [PubMed] [Google Scholar]
- 20. Liew CW, Bochenski J, Kawamori D, Hu J, Leech CA, Wanic K, Malecki M, Warram JH, Qi L, Krolewski AS, Kulkarni RN. 2010. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse β cells. J Clin Invest 120:2876–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holz GG, Heart E, Leech CA. 2008. Synchronizing Ca2+ and cAMP oscillations in pancreatic β-cells: a role for glucose metabolism and GLP-1 receptors? Focus on “regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach”. Am J Physiol Cell Physiol 294:C4–C6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mourad NI, Nenquin M, Henquin JC. 2011. Metabolic amplification of insulin secretion by glucose is independent of β-cell microtubules. Am J Physiol Cell Physiol 300:C697–C706 [DOI] [PubMed] [Google Scholar]
- 23. Drucker DJ. 2006. The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- 24. Cunha DA, Ladrière L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. 2009. Glucagon-like peptide-1 agonists protect pancreatic β-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes 58:2851–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford ML. 2000. Essential role of phosphoinositide 3-kinase in leptin-induced K(ATP) channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem 275:4660–4669 [DOI] [PubMed] [Google Scholar]
- 26. Ning K, Miller LC, Laidlaw HA, Burgess LA, Perera NM, Downes CP, Leslie NR, Ashford ML. 2006. A novel leptin signalling pathway via PTEN inhibition in hypothalamic cell lines and pancreatic β-cells. EMBO J 25:2377–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. 2003. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev 17:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harvey J, Hardy SC, Irving AJ, Ashford ML. 2000. Leptin activation of ATP-sensitive K+ (KATP) channels in rat CRI-G1 insulinoma cells involves disruption of the actin cytoskeleton. J Physiol 527:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henquin JC. 2000. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49:1751–1760 [DOI] [PubMed] [Google Scholar]
- 30. Liu YJ, Cheng H, Drought H, MacDonald MJ, Sharp GW, Straub SG. 2003. Activation of the KATP channel-independent signaling pathway by the nonhydrolyzable analog of leucine, BCH. Am J Physiol Endocrinol Metab 285:E380–E389 [DOI] [PubMed] [Google Scholar]
- 31. McClenaghan NH, Flatt PR, Ball AJ. 2006. Actions of glucagon-like peptide-1 on KATP channel-dependent and -independent effects of glucose, sulphonylureas and nateglinide. J Endocrinol 190:889–896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.