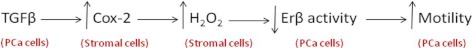Abstract
The tumor microenvironment plays a critical role in supporting cancer cells particularly as they disengage from limitations on their growth and motility imposed by surrounding nonreactive stromal cells. We show here that stromal-derived androgenic precursors are metabolized by DU145 human prostate cancer (PCa) cells to generate ligands for estrogen receptor-β, which act to limit their motility through transcriptional regulation of E-cadherin. Although primary human PCa-associated fibroblasts and the human WPMY-1-reactive prostate stromal cell line maintain this inherent estrogen receptor (ER)β-dependent motility inhibitor activity, they are subverted by TGF-β1 pro-oxidant signals derived from cocultured DU145 PCa cells. Specifically, stromal-produced H2O2, which requires Cox-2, acts as a second paracrine factor to inhibit ERβ activity in adjacent DU145 cells. Chromatin immunoprecipitation analysis reveals that ERβ recruitment to the E-cadherin promoter is inhibited when H2O2 is present. Both neutralization of H2O2 with catalase and prevention of its production by silencing Cox-2 expression in stromal cells restore the motility-suppression activity of stromal-derived ERβ ligand precursors. These data suggest that reactive stromal cells may still have a capacity to limit cancer cell motility through a local endocrine network but must be protected from pro-oxidant signals triggered by cancer cell-derived TGF-β1 to exhibit this cancer-suppressive function.
Since Paget first proposed his seed and soil hypothesis in 1889 (1), increasing attention has been paid to the tumor microenvironment for its role in tumor initiation, development, and progression. Furthermore, tissue recombination experiments with mixed prostate stromal/epithelial cell xenografts first revealed that transformation of epithelial cells is also accompanied by a transdifferentiation of fibroblasts that generates cells [i.e. cancer-associated fibroblasts (CAF) or reactive stroma] that either promote or are permissive to the ultimate formation of a cancerous lesion with metastatic potential (2–4).
One key signaling mediator in the transdifferentiation of fibroblasts into a reactive stromal phenotype is the cytokine TGFβ, most commonly the TGF-β1 isoform. The resulting change in fibroblasts to a myofibroblast phenotype is defined by a coexpression of both fibroblastic and smooth muscle markers, such as vimentin and smooth muscle α-actin, respectively (5). TGFβ's role in tumor development and progression extends beyond its effects on stroma, but its impact on cancer cells is complex. Specifically, TGFβ is tumor suppressive during early phases of cancer development but switches to tumor promoting once the cancer is well established (6, 7). A number of unique components of the TGFβ-signaling pathway have been identified that appear to impact the execution of its diverse actions in cancer progression. Many components of the TGFβ-signaling pathway are altered in cancers, and in some cases these changes are directly correlated with tumor grade, prognosis, or patient outcome (8–12).
In addition to aberrant TGFβ signaling, much attention has recently been paid to the role of chronic inflammation in the development of prostate cancer (13, 14). Several characteristics of chronic inflammation are increased, such as infiltration by inflammatory cells (i.e. macrophages and leukocytes), induction of proinflammatory enzymes such as Cox-2, and production of reactive oxygen species (ROS) and reactive nitrogen species (13). These events are not mutually exclusive, with increased infiltration and induction of Cox-2 leading to some of the increased oxidative stress observed in chronic inflammation. Cox-2 is an inducible isoform of an enzyme responsible for catalyzing the conversion of arachidonic acid to bicyclic peroxides (15). ROS are byproducts of this reaction, and thus Cox-2 can be a significant source of ROS in some cells (16–18). ROS production by cancer cells is necessary for an aggressive phenotype, and highly migratory and invasive prostate cancer (PCa) cell lines produce significantly more H2O2 than their noncancerous or less aggressive counterparts (19).
As with all tissues, the prostate possesses inherent mechanisms for maintenance of homeostasis. In the normal prostate, growth factors such as TGFβ act to limit cell proliferation and maintain normal prostate size (20). Additionally, the prostate is a steroid-dependent organ and relies heavily on a delicate balance of pro- and antiproliferative signals stemming from various steroid hormones. Androgen biosynthesis and signaling play a particularly prominent role in both normal and cancerous prostate biology, but there is increasing recognition for the importance of estrogen action in prostate biology (21). Work done by Risbridger and colleagues (22) highlights the importance of intraprostatic estrogen synthesis by aromatic conversion of androgens, and a series of knockout studies has implicated the estrogen receptor (ER)α subtype in prostatic inflammation and malignancy. ERβ signaling, on the other hand, acts to suppress prostatic growth and PCa cell motility (23). The endogenous ERβ ligands in prostatic tissue are the androgen metabolites 3α-adiol and 3β-adiol, and their importance is underscored by high expression levels of the aldo keto reductase (AKR1C) enzymes, which serve to convert androgenic precursors into the adiols within the prostate tissue (24, 25).
In this study, we sought to examine the basis for PCa cell response to reactive prostate stromal cells in vitro. Our results established a role for cancer cell-derived TGF-β1, acting in a paracrine-signaling network via COX-2-dependent ROS production in neighboring stromal cells, to support aggressive PCa cell motility in vitro. However, we uncovered an inherent motility-suppressive activity present in reactive stromal cells that we identified as an androgenic precursor to an ERβ ligand. Thus, despite their reactive phenotype, these cells are not genetically reprogrammed to fully support cancer progression but rather maintain an intrinsic capacity to limit cancer cell motility. Cancer cells subvert this innate suppression through enhanced production of short-acting mediators, namely ROS, in the surrounding microenvironment.
Results
TGF-β1 signaling in human reactive prostate stromal cells overrides their inherent motility-inhibitory activity toward cocultured PCa cells
We have used a modified scratch assay to examine the impact of stromal cell-derived factors on PCa cell motility in vitro. This assay has been widely used and is well established to represent the classical wound healing migratory response in cells without the focus on chemotaxis seen in Boyden chambers (26). We used this assay to assess motility of the androgen-independent DU145 human PCa cell line when cocultured with human prostate stromal cells. Importantly, in the coculture system that we employ stromal and PCa cells are not in direct contact but share a common growth medium.
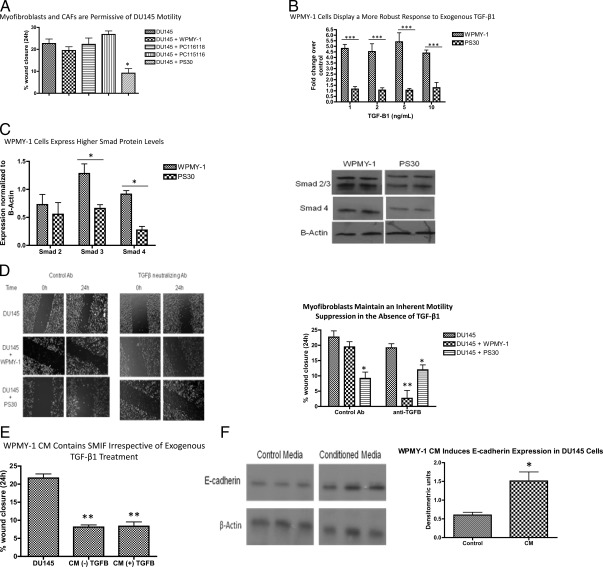
Figure 1A displays the results of a scratch assay with DU145 cells cocultured with either established human stromal cell lines (WPMY-1 and PS30), or with primary stromal cultures isolated from PCa tissue (PC116118 and PC115116). DU145 cells plated at approximately 90% confluence can close a constant diameter wound approximately 22% within 24 h in low-serum media. To exclude the influence of proliferation on wound healing, the doubling time of DU145 cells under the same low-serum conditions was determined to be approximately 40 h. Although the motility of these cells is not significantly affected when cocultured with CAF or WPMY-1 cells, their movement is significantly reduced upon coculture with PS30 cells.
Fig. 1.
Paracrine signaling modulates cancer cell motility. A, In indirect coculture, DU145 motility is unaffected by the presence of CAF or WPMY-1 cells, but is significantly reduced by PS30 cells. Data represent the mean ± sem from three independent experiments, each repeated in technical triplicate. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 compared with respective DU145 control. B, WPMY-1 and PS30 cells were transiently cotransfected with the 3TP-lux-luciferase (luc) and Renilla-luc reporter plasmids overnight, and then subjected to a 6-h TGF-β1 treatment the following day (0, 1, 2, 5, and 10 ng/ml). Data represent the mean ± sem of three independent experiments, each performed in triplicate. A two-way ANOVA followed by Bonferroni posttest was performed. ***, P < 0.001. C, WPMY-1 cells express higher levels of Smad 3 and Smad 4. A representative blot is shown, and a graphic display of densitometric analysis representing the mean ± sem of three independent experiments is presented. A two-way ANOVA followed by a Bonferroni posttest was performed. *, P < 0.05. D, The left panel is representative images from the modified wound-healing assay; the right panel is a graphic display of all replicates. Data represent the mean ± sem from four independent experiments, each repeated in technical triplicate. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 compared with respective DU145 control. **, P < 0.01 relative to DU145 treated with TGFβ neutralizing antibody. E, Naïve DU145 cells were wounded and media replaced with WPMY-1 CM. Wound closure over 24 h was determined. Data represent the mean ± sem from three independent experiments, each repeated in technical triplicate. A one-way ANOVA followed by Tukey's multiple comparison test was performed. **, P < 0.01 compared with control media. F, DU145 cells were treated for 24 h with either 1% serum-containing media (control) or WPMY-1 CM, followed by Western blot analysis for E-cadherin expression. The left panel shows an image from three independent samples; the right panel is a graphic representation of the normalized densitometry. A t test was performed for pairwise comparison. *, P < 0.05. Ab, Antibody.
WPMY-1 cells exhibit a robust myofibroblast or reactive phenotype in contrast to the fibroblastic phenotype of PS30 cells. We therefore examined TGFβ signaling components in the two cell lines because it is an important contributor to the reactive stromal phenotype in the prostate (27). Transient transfection of both the WPMY-1 and PS30 cell lines with 3TP-lux, a Smad binding element luciferase reporter construct, demonstrated that the WPMY-1 line has a significantly more robust response to exogenous TGF-β1 than PS30 cells (Fig. 1B). Additionally, WPMY-1 cells express significantly higher levels of Smad proteins as indicated by Western blot analysis (Fig. 1C). Thus, unlike PS-30 cells, WPMY-1 cells are TGF-ß1 responsive and therefore susceptible in cocultures to TGF-ß1 produced by DU145 cells (28).
To uncover the role of TGFβ signaling in modulating stromal cell regulation of PCa cell motility, we used an interfering TGF-β1 antibody in coculture assays. As shown in Fig. 1D, inhibition of TGFβ signaling did not affect the motility of DU145 cells but uncovered an inherent motility-inhibitory activity of the WPMY-1 cells. The motility-inhibitory activity of the PS30 cells was not affected by the TGF-β1 neutralizing antibody. These results suggest that although TGF-β1 does not affect DU145 cell movement, it enables cocultured reactive stromal cells to be permissive for cancer cell motility. Additionally, these data suggest that although both reactive and nonreactive prostate stromal cells produce an inherent cancer cell motility-inhibitory factor (hereafter referred to as stromal-derived motility-inhibitory factor, SMIF), reactive stroma respond to TGF-β1 produced by cancer cells to limit either the production or activity of SMIF. As a direct test of this hypothesis, we isolated conditioned media (CM) from WPMY-1 cells grown overnight in 1% serum-containing media with or without exogenous TGF-β1 (5 ng/ml) and added this CM to freshly wounded naïve DU145 cells. Surprisingly, CM from WPMY-1 cells treated with exogenous TGF-β1 significantly inhibited DU145 motility (Fig. 1E). Therefore, either TGF-β1 is necessary but not sufficient to block SMIF activity in WPMY-1 cells, or the effect of TGF-β1 on SMIF is mediated through a short-lived molecule.
Increased motility is a characteristic of epithelial-to-mesenchymal transition (EMT), and one hallmark sign of EMT is loss of the epithelial cell adhesion molecule E-cadherin. To assess whether the effect of CM on DU145 motility could be through induction of E-cadherin expression, we treated naïve DU145 cells with either control or WPMY-1 CM and then subjected the cells to Western blot analysis for E-cadherin. As Fig. 1F shows, CM significantly enhanced expression of E-cadherin.
ROS generated after TGF-β1 signaling overrides the inherent motility-inhibitory activity of WPMY-1 cells
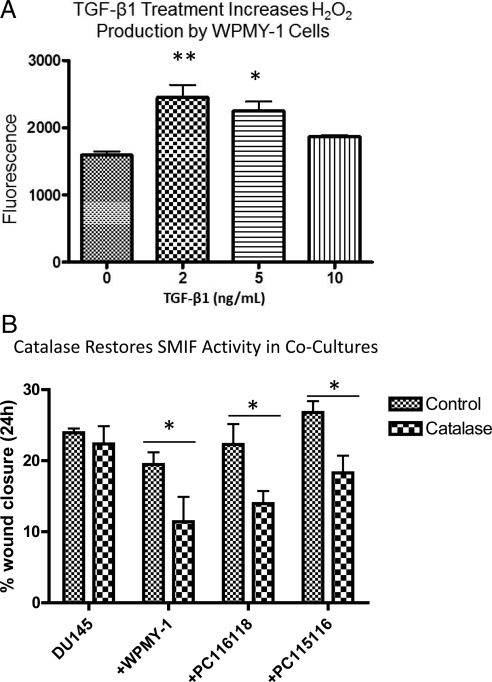
Hydrogen peroxide is one ROS ultimately generated in response to TGF-β1 that can participate in local paracrine signaling. To examine whether TGF-β1 activates an oxidant signaling pathway in WPMY-1 cells, an Amplex Red endpoint assay was performed to measure H2O2 accumulation. As Fig. 2A shows, a 3-h treatment with 2 and 5 ng/ml of TGF-β1 significantly increased production of H2O2. Thus, H2O2 presents a viable candidate for the nontransferrable inhibitor of SMIF. Therefore, wound-healing assays were performed with the addition of catalase (1500 U/ml), a cell-impermeable enzyme that metabolizes H2O2 to H2O and O2. We also assessed whether H2O2 was also responsible for the permissive effect observed in CAF cocultures. As Fig. 2B shows, although addition of catalase did not alter the highly motile phenotype of the DU145 cells, it restored the activity of SMIF when added to DU145/myofibroblast or DU145/CAF cocultures. Therefore, extracellular H2O2 overrides SMIF activity derived from reactive stromal cells leading to efficient motility of cocultured DU145 cells. Importantly, extracellular H2O2 does not regulate the inherent motility of DU145 cells.
Fig. 2.
TGF-β1 treatment triggers H2O2 production in reactive stromal cells, which can limit SMIF activity. A, In response to exogenous TGF-β1, WPMY-1 cells produce increased levels of H2O2 as measured by an endpoint Amplex Red assay. WPMY-1 cells were serum starved for 90 min before addition of TGF-β1 in fresh serum-free media. Cells were incubated with the TGFβ for 3 h, Amplex Red was added to cell cultures, and an endpoint reading was recorded at 1 h. Data represent mean from three biological replicates ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05; **, P < 0.01 relative to untreated WPMY-1 control. B, Addition of catalase to cocultures reverses the permissive role of CAF and WPMY-1 cells on DU145 motility. The modified wound-healing assay was performed with the addition of 1500 U of catalase per 1 ml of media. Data are representative of three independent experiments ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 comparing control coculture with coculture with the addition of catalase.
Cox-2 is necessary for H2O2 generation in WPMY-1 cells
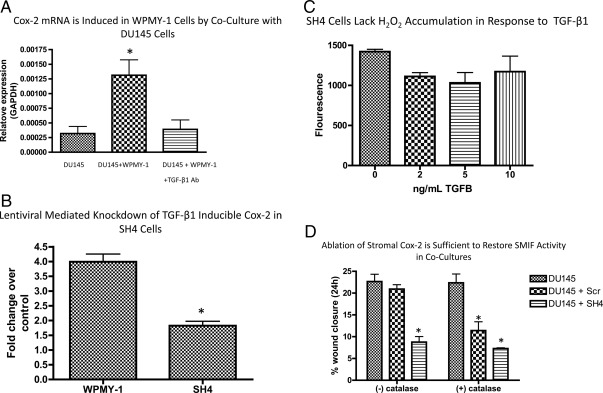
TGF-β1 is an important component of stromal-epithelial cross-talk and is necessary for the transdifferentiation of stromal fibroblasts into a myofibroblastic-reactive stroma phenotype (5, 27). The mobilization of oxidant signaling pathways is used by TGF-β1 to influence paracrine communication. For example, TGF-β1 induces the expression of enzymes such as Cox-2 and some NOX isoforms that generate ROS. In fact, in DU145/WPMY-1 cocultures, Cox-2 mRNA was induced in WPMY-1 cells as revealed by quantitative real-time PCR. TGF-β1's role in this induction was confirmed by inclusion of a TGF-β1 neutralizing antibody, which subsequently abolished the Cox-2 mRNA induction in cocultured WPMY-1 cells (Fig. 3A).
Fig. 3.
TGFβ-inducible Cox-2 in stromal cells generates the H2O2 necessary to limit SMIF activity and permit PCa cell motility in cocultures. A, A transwell insert containing DU145 cells was placed into a chamber containing serum-starved WPMY-1 cells, and the media were replaced with fresh 1% serum-containing RPMI −/+ a TGF-β1 neutralizing antibody. The cells were cocultured for 24 h, and the Cox-2 levels in WPMY-1 cells were determined by qRT-PCR. Data represent the mean ± sem from three independent experiments. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05. B, A lentiviral vector expressing shRNA either scrambled (Scr) or directed against Cox-2 (SH4) was used to stably infect WPMY-1 cells. The resulting lines were subjected to a 24-h treatment with TGF-β1 in serum-free media (5 ng/ml), and Cox-2 mRNA levels were determined by qRT-PCR. Data represent three independent experiments ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05. C, SH4 cells were serum starved for 90 min before addition of TGF-β1 in fresh serum-free media. Cells were incubated with the TGFβ for 3 h, Amplex Red was added to cocultures, and an endpoint reading was recorded at 1 h. Data represent mean from three biological replicates ± sem. D, The modified wound-healing assay was performed with the addition of 1500 U of catalase per 1 ml of media. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 relative to appropriate DU145 control. Ab, Antibody; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To determine to what extent stromal-derived Cox-2 is involved in ROS generation, we first stably expressed a Cox-2 short hairpin RNA (shRNA) (SH4) or scrambled shRNA sequence (Scr) in WPMY-1 cells using recombinant lentiviral vectors. Because basal levels of Cox-2 mRNA are very low in WPMY-1 cells, the efficiency of Cox-2 mRNA knockdown was analyzed via quantitative RT-PCR (qRT-PCR) after treatment of WPMY-1 or SH4 cells with 5 ng/ml TGF-β1. As Fig. 3B shows, TGF-β1 induction of Cox-2 mRNA was reduced 65% in SH4 cells relative to Scr. Because Cox-2 can be a significant source of ROS (17), SH4 cells were subjected to an endpoint Amplex Red assay to measure H2O2 production. As Fig. 3C shows, TGF-β1 is unable to produce a significant increase in H2O2 levels in WPMY-1 cells lacking inducible Cox-2, suggesting that Cox-2 is at least partially responsible for the TGF-β1-induced increase in H2O2 production.
Next, we sought to determine whether ablation of Cox-2 altered the TGF-β1-dependent inhibition of SMIF in WPMY-1 cells. This is of particular relevance because other ROS-generating enzymes are responsive to TGF-β1 in WPMY-1 cells and other fibroblast cells (29). As shown in Fig. 3D, DU145 motility is inhibited when cocultured with SH4 cells but not Scr cells. Importantly, the degree of motility inhibition between DU145/WPMY-1 coculture with catalase and DU145/SH4 coculture is not significantly different, indicating that ablation of Cox-2 is sufficient to reduce H2O2 levels to an extent that restores SMIF activity. To further confirm that stromal Cox-2 is necessary for TGF-β1-inducible H2O2 in WPMY-1 cells, we included catalase (1500 U/ml) in coculture scratch assays. As shown in Fig. 3D, addition of catalase does not further accentuate the inhibitory activity of SH4 cells on DU145 motility in cocultures. Therefore, WPMY-1 cells in which TGF-β1 induction of Cox-2 expression and subsequent H2O2 production is limited (SH4 cells) maintain the ability to limit DU145 motility in coculture. Additionally, stromal Cox-2 is necessary for the H2O2 production that is responsible for limiting the activity of SMIF.
Hydrogen peroxide produced by Cox-2 in WPMY-1 cells modulates the response of DU145 cells to SMIF
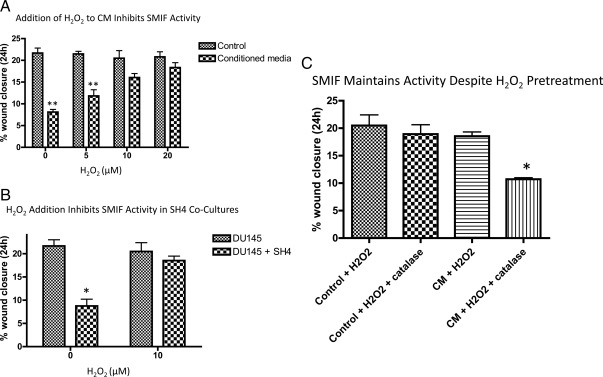
To confirm that H2O2 could alter the activity of SMIF, we added varying physiological concentrations of H2O2 to CM from WPMY-1 cells. Whereas a single bolus of H2O2 (5, 10, and 20 μm) does not significantly influence DU145 motility, H2O2 addition eliminated the motility-inhibitory activity of WPMY-1 CM (Fig. 4A). Furthermore, H2O2 reverses the inhibitory effect of the SH4 cells on DU145 motility in coculture (Fig. 4B). When the major prostatic Cox-2 metabolite PGE2 was added to cocultures, no significant change in motility was observed (data not shown), suggesting that this metabolite of Cox-2 is unlikely to impact SMIF activity.
Fig. 4.
Cox-2-dependent H2O2 generation in WPMY-1 cells inhibits DU145 cell response to SMIF. A, CM loses its inhibitory effect on DU145 motility at concentrations of H2O2 greater than or equal to 10 μm. CM was generated as previously described from WPMY-1 cells. Naïve DU145 cells were wounded, and the media were replaced with CM to which varying amounts of H2O2 had been added. Data represent the mean of six independent experiments ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. **, P < 0.01 relative to appropriate control media. B, Addition of H2O2 (10 μm) to the DU145/SH4 coculture reverses the motility inhibition observed under basal conditions. A modified wound-healing assay as previously described was carried out with and without the addition of H2O2. Data represent the results of four independent experiments ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 relative to appropriate DU145 control. C, CM was incubated with 10 μm H2O2 for 3 h. After this pretreatment, a portion of the media was then treated with catalase (1500 U/ml), and the two different treatment groups were added to wounded naïve DU145 cells. Data represent three independent experiments ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 compared with all other conditions.
Our results suggest that either SMIF is being oxidatively modified by H2O2, or that H2O2 produced by WPMY-1 cells is influencing the response of the DU145 cells to SMIF. To address this issue, CM was pretreated with 10 μm H2O2 for 3 h to allow potential oxidation of SMIF. Before treating wounded naïve DU145 cells with the CM, catalase (1500 U/ml) was added to neutralize the remaining H2O2. As Fig. 4C shows, pretreatment of the WPMY-1 CM with H2O2 did not alter its inhibitory effect on DU145 cell motility. These results imply that SMIF is not being oxidized, but rather that H2O2 is acting directly on DU145 cells to impact their response to SMIF.
The motility-inhibitory activity of WPMY-1 cells acts via ERβ in DU145 cells
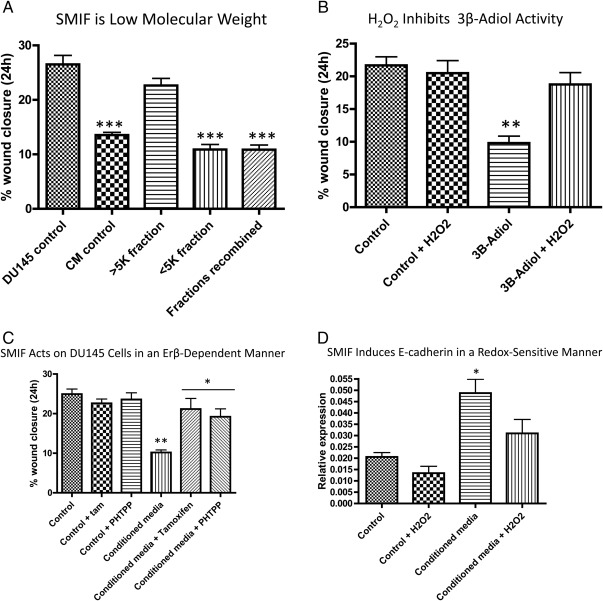
For an initial assessment of the identity of SMIF produced by WPMY-1 cells, we used a column fractionation technique to separate the components in the CM into high (>5 kDa) and low (<5 kDa) molecular mass fractions. These separate fractions were then applied to naïve DU145 cells and their motility measured. As Fig. 5A shows, the low-molecular mass fraction retains the inhibitory activity present in intact WPMY-1 cell CM on DU145 cell motility. The high-molecular mass fraction is permissive for DU145 motility, and readdition of the low-molecular mass fraction again restores the motility suppression observed with complete CM.
Fig. 5.
SMIF from WPMY-1 CM blocks DU145 motility in an ERβ-dependent manner. A, WPMY-1 CM was fractionated into high- and low-molecular mass fractions, and each fraction was tested independently for biological activity. The low molecular mass fraction (pore size <5 kDa) retains inhibitory effects on DU145 motility. Data are representative of four independent biological replicates ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed; ***, P < 0.001 compared with both DU145 control and the high-molecular mass fraction. B, Addition of exogenous 3β-adiol (10−6 m) is able to significantly inhibit DU145 motility. This is reversed when 10 μm H2O2 is added to the media. Data are representative of four independent biological replicates ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. **, P < 0.01 compared with all other conditions. C, Naïve DU145 cells were wounded and treated with either control or WPMY-1 CM with 4-hydroxytamoxifen (10−7 m) or PHTPP (0.1 μm). Results are representative of four independent biological replicates ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. ***, P < 0.001 compared with conditions containing control media; *, P < 0.05 compared with CM. D, Naïve DU145 cells were treated for 18 h with either control or WPMY-1 CM, −/+ 10 μm H2O2. The cells were harvested in Trizol and subjected to qRT-PCR for E-cadherin transcript levels. Results are indicative of three independent samples and are displayed ± sem. A one-way ANOVA followed by Tukey's multiple comparison test was performed. *, P < 0.05 relative to all other conditions.
In lieu of further purification of the CM 5 kDa and below fraction, we assessed candidate low molecular mass inhibitors of cancer cell motility. Two androgen derivatives, 5α-androstane-3α,17β-adiol (3α-adiol) and 5α-androstane-3β,17β-adiol (3β-adiol) have been found to increase cancer cell adhesion and decrease cell motility in an ERβ-dependent manner (30, 31). These androgen metabolites do not bind androgen receptor but are potent ligands for ERβ (31). DU145 cells express the ERβ isoform but no detectable ERα (30–32). Therefore, to determine whether this pathway was responsible for oxidant-dependent inhibition of motility in coculture, we examined the impact of H2O2 on the motility inhibitory activity of exogenous 3β-adiol (10−6 m).
Consistent with previously published reports, 3β-adiol suppressed DU145 cell motility (30, 31). However, this inhibitory effect is reversed by H2O2 (Fig. 5B). To further establish the role of ERβ in regulating the motility response of DU145 cells to WPMY-1-generated factors, we added tamoxifen, which acts as an ERβ antagonist, and the selective ERβ antagonist 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) to the wound-healing assays. As Fig. 5C shows, addition of both tamoxifen and PHTPP reverses the motility inhibitory effect of WPMY-1 CM. Thus, the WPMY-1-produced SMIF acts on ERβ in DU145 cells to limit their motility.
Previous work has shown E-cadherin to be a downstream target of ERβ signaling that is responsible for the decrease in motility seen with 3β-adiol treatment (31). We had previously shown that WPMY-1 cell CM induces E-cadherin expression in DU145 cells so we therefore sought to determine whether the addition of H2O2 to CM would reverse this effect. DU145 cells were treated with either control or CM −/+ H2O2 for 18 h, and qRT-PCR analysis was performed for E-cadherin mRNA expression. As Fig. 5D shows, WPMY-1 cell CM significantly increased E-cadherin expression, which is lost when H2O2 (10 μm) is present.
SMIF-induced activity of ERβ is oxidation sensitive in DU145 cells
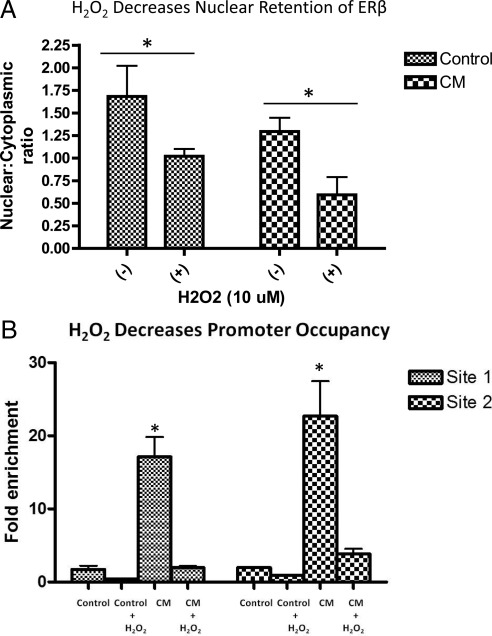
H2O2 could interfere with ERβ action at multiple levels to limit the induction of E-cadherin brought about by WMPY-1 CM. For example, when DU145 cells are treated with H2O2, nuclear retention of ERβ is decreased (Fig. 6A). Although this is observed in both control and CM conditions, the effect of H2O2 is only seen when the ERβ signaling pathway is activated by some component of CM.
Fig. 6.
Oxidized ERβ exhibits less DNA binding at the E-cadherin promoter.A, Nuclear and cytoplasmic lysates were prepared from DU145 cells and subjected to Western blot analysis for ERβ protein. Results are displayed as densitometric analysis from three independent blots. A two-way ANOVA followed by a Bonferroni posttest was performed. *, P < 0.05. B, DU145 cells were grown in either control or CM −/+ H2O2 (10 μm) for 18 h. ChIP using ERβ antibodies was performed, and qRT-PCR analysis for previously defined ERβ-binding sites within the E-cadherin promoter proximal region (Table 2) revealed a significant increase in promoter occupancy in the presence of CM. This increase was reversed when H2O2 was present in CM. Results are representative of three independent experiments. A one-way ANOVA followed by Tukey multiple comparison test was performed. *, P < 0.05 compared with all other conditions at that promoter site.
To determine whether this decreased nuclear retention of ERβ in the presence of H2O2 was associated with a concomitant decrease in transcriptional activity, we next performed chromatin immunoprecipitation (ChIP). Specifically, ERβ occupancy was assessed on E-cadherin promoter proximal binding sites (Table 1) identified in ChIP assays using MCF-7 breast cancer cells (Charn, T.H., Z. Madak-Erdogan, and B.S. Katzenellenbogen, unpublished information) As Fig. 6B shows, under control conditions there is minimal promoter occupancy, and this remains unaffected by the presence of H2O2. When CM containing active SMIF is added to DU145 cells, a significant increase in promoter occupancy is seen at two distinct ERβ-binding sites within the E-cadherin promoter proximal region. Importantly, this increased ERß promoter occupancy is reversed when H2O2 is added to the CM. Therefore, a paracrine ROS signaling network initiated within reactive stromal cells by DU145 cell-derived TGFß1 acts in the cancer cells to limit ERß genomic action.
Table 1.
Primer sequences for real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| Cox-2 | 5′-ATCACAGGCTTCCATTGACC-3′ | 5′-CAGGATAGAGCTCCACAGCA-3′ |
| E-cadherin | 5′-TGAAGGTGACAGAGCCTCTGGAT-3′ | 5′-TGGGTGAATTCGGGCTTGTT-3′ |
| GAPDH | 5′-TTGCCATCAATGACCCCTTCA-3′ | 5′-CGCCCCACTTGATTTTGGA-3′ |
SMIF is an androgenic precursor to an ERβ ligand
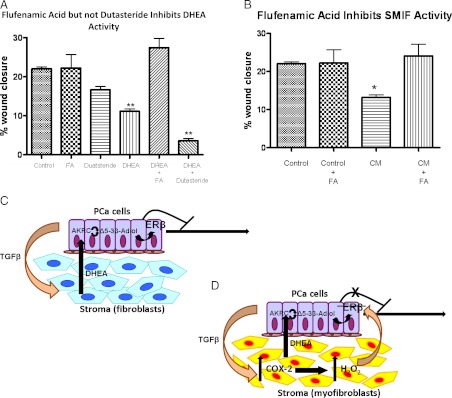
3β-Adiol can be synthesized from a number of androgenic precursors through the enzymatic pathway involving 17β-hydroxysteroid dehydrogenases, also known as aldo-keto reductases (AKR1C) (33). DU145 cells express AKR1C enzymes and are capable of catalyzing redox reactions at the C17 position of steroid hormones (34, 35). Previous work has shown that the androgenic precursor dehydroepiandrosterone (DHEA) can be metabolized to ERβ ligands through the activity of these AKR1C enzymes; specifically, the AKR1C3 subtype can convert DHEA directly to the highly potent androstene Δ5-3β-adiol, whereas additional enzymatic steps involving the activity of 5α-reductase can lead to the production of the androstane, 3β-adiol (36). Thus, we hypothesize that SMIF is an androgenic precursor that is metabolized in DU145 cells to an ERβ ligand. To test this hypothesis, we performed DU145 cell motility assays in the presence of the known AKR1C1–3 inhibitor flufenamic acid (FA) as well as the 5α-reductase inhibitor dutasteride (37). As Fig. 7A shows, although DHEA inhibits DU145 motility, this inhibition is lost when FA, but not when dutasteride, is added. Therefore the androstene Δ5-3β-adiol, rather than the androstane 3β-adiol, is responsible for the motility-suppressive effects of DHEA because dutasteride failed to reverse the suppressive effect of DHEA on DU145 motility. Furthermore, because DU145 cells are AR negative, DHEA is likely exerting its effects via a 3β-adiol metabolite acting on ERβ. Similarly, FA reverses the inhibitory activity of CM, suggesting that an androgenic precursor in SMIF (e.g. DHEA) is being metabolized to Δ5-3β-adiol through an AKR1C-dependent pathway (Fig. 7B).
Fig. 7.
SMIF is an androgenic precursor metabolized by the DU145 cells to an ERβ ligand. A, FA, but not dutasteride, blocks the inhibitory activity of DHEA. Wound-healing assays were performed on DU145 cells treated with either and DHEA (100 nm), FA (50 μm), or dutasteride (1 μm) where indicated. Results are representative of four independent experiments, each done in technical quadruplicate. A one-way ANOVA followed by Dunnett's multiple comparison test using untreated DU145 cells as the control column was performed. **, P < 0.01. B, FA inhibits SMIF activity in CM. Wound-healing assays were performed on DU145 cells treated with CM ± FA (50 μm). Results are representative of four independent experiments, each done in technical quadruplicate. A one-way AONVA followed by Tukey's multiple comparison test was performed. *, P < 0.05. C, In a normal fibroblast, DHEA is constitutively produced and secreted. It is metabolized by 17βHSD enzymes in the DU145 cells into potent ERβ ligands, which then act to limit motility. D, In reactive myofibroblasts, locally produced TGF-β1 stimulates the production of ROS, which is secreted in the form of H2O2. The H2O2 acts as a second paracrine factor to limit the transcriptional activity of ERβ, thus alleviating its inhibitory effect on DU145 motility.
Given these data, we propose a working model to describe the bidirectional communication between the stromal and epithelial compartments with respect to the microenvironment's role in cancer cell motility. Figure 7, C and D, indicate the major points of interest in the stromal/epithelial communication cascade: first, irrespective of their state of reactivity, prostate stromal cells have the capacity to produce a precursor to an ERβ ligand (e.g. DHEA) that is a potent inhibitor of PCa cell motility. However, locally produced TGF-β1 by PCa cells induces a proinflammatory and pro-oxidant milieu in TGF-β1 hyperresponsive stromal cells, leading to up-regulation of Cox-2. H2O2 produced in a Cox-2-dependent manner in stromal cells blocks the motility-inhibitory effect of an ERβ ligand produced in cancer cells from stromal cell-derived precursors.
Discussion
The development of PCa can be considered a coevolution of both the epithelial and stromal cells; indeed, the latter develop their own unique gene signature during cancer progression that has potential predictive value in determining a patient's outcome (10). Among some of the key features of a reactive stroma are heterogeneity in TGFβ signaling, acquisition of a myofibroblastic phenotype similar to that seen in normal wound healing, and an increase in oxidative stress as shown by elevated production of ROS (5, 19, 38). The work presented herein, however, suggest that despite these alterations even reactive, cancer-associated stromal cells possess the inherent capacity to limit PCa cell motility through the production of a precursor to an ERβ ligand. The loss of inhibition over cancer cell motility is determined not by an irreversible adaptation preventing production of this precursor by the stromal cell, but rather an alteration in the redox status of the surrounding milieu resulting from increased TGF-β1-dependent ROS production. By providing TGF-β1, cancer cells not only drive stromal cell transdifferentiation but subvert a local endocrine-signaling network using stromal cell steroid precursors that would otherwise limit their ability to migrate and invade surrounding tissue. Our results are consistent with clinical studies that establish a role for ERβ in preventing EMT and maintaining a lower grade of PCa. Tissue staining from PCa specimens demonstrates that high-grade PCa shows a concurrent loss of both ERβ and E-cadherin (24). Furthermore, an increased risk of PCa has been correlated with genetic mutations within the AKR1C family of enzymes responsible for metabolizing DHEA and other androgenic precursors into potent ERβ ligands (39, 40).
Importantly, we have demonstrated that the stromal cells, irrespective of their reactive phenotype, maintain the ability to produce a precursor to an ERβ ligand that acts to limit cancer cell motility. This is seen in both a myofibroblastic cell line (WPMY-1) as well as primary cultures of CAF isolated from prostatectomy specimens (PC115116 and PC116118). The disruption of PCa ERβ signaling that we observe is not due to a genetic or epigenetic alteration, but rather is the result of increased H2O2 production by surrounding stromal cells that ultimately inhibits ERβ activity in adjacent PCa cells. This decrease in responsiveness is seen at both the biological and molecular level, as measured by a loss of inhibition in wound-healing assays in which H2O2 is present (either exogenous or endogenously produced) and a failure of ERβ-dependent E-cadherin induction in DU145 cells when H2O2 is added concomitantly with the myofibroblast cell CM. ERβ has been shown previously to be sensitive to oxidation, which occurs primarily from modifications in redox-sensitive cysteine motifs in the second zinc finger. The resulting conformational change destabilizes ERβ and ultimately prevents its DNA binding (41, 42). We observed a defect in nuclear retention of ERβ and a subsequent loss of induction of E-cadherin in DU145 cells exposed to H2O2 contained in stromal cell CM. These effects on ERβ signaling that impact the response to stromal-derived ERβ ligands (or ligand precursors) could be due to direct oxidation of the receptor and/or other components of its signaling pathway.
We have identified stromal Cox-2 as a necessary component in the generation of H2O2 responsible for loss of ERβ signaling in the DU145 cells and, as such, a driving factor in the loss of motility inhibition by a myofibroblastic stroma. This is in accordance with previous work showing that Cox-2 is overexpressed in primary PCa with metastatic potential. Of particular relevance is the work done by Rao et al. (43) showing an inverse relationship between Cox-2 and E-cadherin expression in PCa tissue, which coincides with our model which describes an induction of stromal Cox-2 ultimately leading to decreased E-cadherin expression in adjacent PCa cells. Our model, describing a bidirectional paracrine communication network, further underscores the importance of considering the biological impact of Cox-2 in cells that comprise the tumor microenvironment and not just cancer cells themselves. In fact, a recent study demonstrated that overexpression of Cox-2 in the stroma of laryngeal squamous cell carcinoma specimens correlated with a worse tumor grade, suggesting an important role for Cox-2 in the cancer microenvironment (44). Cox-2 is also overexpressed in the surrounding stroma in neoplastic and cancerous prostate tissue (45). Finally, in vivo experiments with lung carcinoma cells showed that Cox-2-expressing tumor cells were unable to grow in a Cox-2 −/− host, highlighting the importance of stromal Cox-2 in cancer growth and progression (46). Our results add to this growing interest in stromal-derived ROS signaling by identifying the consequences of a Cox-2-dependent signaling mediator (H2O2) on cancer cell response to a steroid metabolite produced within their microenvironment that would normally have the capacity to limit cancer cell motility.
Activation of ERβ by this adiol induces E-cadherin expression and subsequently increases cell adhesion, ultimately leading to a decrease in motility (31). The loss of motility inhibition in DU145 cells when H2O2 is present in either shared or CM suggests that the sensitivity of this cellular response is due to a redox sensitivity of this endocrine communication network. Because addition of H2O2 can inhibit exogenous adiol action, it is unlikely that H2O2 influences metabolism of androgenic precursors in PCa cells, but rather that it acts directly on ERβ, resulting in the subsequent loss of adiol action. Blocking the production or action of the second paracrine factor, H2O2, at any point in the cascade, restores the cancer cells' ability to respond to locally produced Δ5-3β-adiol in an ERβ-dependent manner. A simple schematic describing the interrelation of TGFβ, oxidative stress, and motility in bidirectional communication between PCa and stromal cells is presented below.
In summary, we have highlighted the importance of oxidative stress within the local milieu with respect to permitting DU145 cancer cell motility despite constitutive production of androgenic precursors by reactive stromal cells. Interestingly, DU145 cells do not intrinsically respond to either TGF-β1 or H2O2, but use both molecules to favorably alter their microenvironment to allow their movement and spread to proceed unimpaired. Elucidation of this complex paracrine/local endocrine signaling interaction between cancer cells and their surrounding stroma provides multiple points for therapeutic intervention and offers evidence to support the rational design of a new treatment paradigm for advanced aggressive prostate cancer. For example, Food and Drug Administration-approved Cox-2 inhibitors could be combined with additional antioxidants to ensure a reduction in local ROS production, thus restoring the activity of ERβ in the cancer cells to respond to locally produced ligands.
Materials and Methods
Chemicals and reagents
Recombinant human TGF-β1 and TGF-β1 neutralizing antibody were purchased from R&D Systems (Minneapolis. MN) and were reconstituted according to the manufacturer's protocol. PGE2, FA, and catalase were purchased from Sigma-Aldrich (St. Louis, MO) and were reconstituted according to the manufacturer's instructions. Amicon Ultra-4 centrifugal filter devices for fractionating conditioned media were purchased from Millipore Corp. (Billerica, MA). 4-Hydroxytamoxifen and PHTPP were purchased from Tocris Bioscience (Ellisville, MO). DHEA was purchased from Steraloids (Newport, RI). 3β-Adiol was purchased from Sigma-Aldrich. Dutasteride was kindly provided by Zhou Wang of the University of Pittsburgh Cancer Institute. Antibodies against E-cadherin, Smad 2/3, and Smad 4 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against ERβ were purchased from Millipore Corp. and obtained from the laboratory of Benita Katzenellenbogen. Antibody against β-actin and secondary horseradish peroxidase (HRP)-conjugated antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture
WPMY-1, PS30, PC3, and DU145 cell lines are commercially available and were purchased from American Type Culture Collection (Manassas, VA). SH4 cells were generated as described below. Primary cultures PC116118 and PC115116 were obtained from radical prostatectomy specimens through UPCI from the laboratory of William LaFramboise. The cells were maintained in monolayer at 37 C in a 5% CO2 incubator in RPMI-1640 supplemented with 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were routinely passaged at a confluence of approximately 90%.
Transient transfection
Cells were transfected using Lipofectamine LTX with Plus Reagent (Invitrogen, Carlsbad, CA). WPMY-1 and PS30 cells were plated at approximately 70% confluency in a 12-well plate and grown overnight in antibiotic-free RPMI-1640 media with 5% FBS. The following day, per well amounts of 0.5 μg 3TP-lux, 0.1 μg Renilla-luc, and 0.5 μl Plus reagent were incubated in opti-mem for 5 min. LTX reagent (1.5 μl) was added, and the complex was incubated at room temperature for 30 min. The mixture was then added to the cells dropwise and incubated overnight. The following day cells were serum starved for approximately 2 h followed by treatment with TGF-β1 (0, 1, 2, 5, and 10 ng/ml). Cells were lysed in passive lysis buffer (Promega Corp., Madison, WI), and a dual-injector luminometer was used to record both firefly and Renilla luciferase relative light unit values. Firefly luciferase values were normalized to Renilla.
Western blotting
Whole-cell lysates were prepared by lysing WPMY-1 cells in radioimmune precipitation assay buffer. Total protein (15 μg) was run on a 10% acrylamide gel and transferred to a nitrocellulose membrane using a Transblot SD Semi-Dry transfer apparatus (Bio-Rad Laboratories, Inc., Hercules, CA). Membranes were blocked for 1 h at room temperature in 5% nonfat dry milk in PBS containing 0.1% Tween (PBS-T). The indicated antibody was added to a solution of 5% BSA/PBS-T in a concentration of 1:1000 and incubated overnight at 4 C with gentle rocking. Membranes were washed for 5 min three times in PBS-T and then incubated with HRP-conjugated secondary antibody in a concentration of 1:3000 for 30 min and room temperature. Membranes were washed in PBS-T an additional three times, and enhanced chemiluminescence reagents were used to detect the HRP signal.
Nuclear and cytoplasmic fractionation was performed following the publicly available Lammond Laboratory protocol (http://www.lamondlab.com/pdf/CellFractionation.pdf). The nuclear and cytoplasmic fractions were then treated as previously detailed for the whole-cell lysate protocol.
Knockdown assays
A lentivirus set containing five unique shRNA sequences specific for Cox-2 (Homo sapiens prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) was purchased from UPCI Lentiviral Core Facility (Pittsburgh, PA). WPMY-1 cells were seeded at 50% confluency in Optimem and grown overnight. The following day the media was replaced with fresh Optimem containing lentivirus and a final concentration of 8 μg/ml polybrene. Cells were incubated approximately 18 h at 37 C, and the following day the media were replaced with fresh RPMI-1640 containing 10% FBS. On d 3 after the infection, cells were passaged through a selection media containing 2 μg/ml puromycin for 5 d. Viable cells were expanded, and a quantitative real-time PCR experiment was performed to confirm knockdown of Cox-2 mRNA. The cell population demonstrating the greatest viability and knockdown was named SH4.
Indirect coculture wound-healing assay
Prostate stromal cells were plated in six-well dishes at a density of 3 × 105 cells per well and grown overnight. Prostate epithelial tumor cells DU145 were seeded on coverslips in six-well plates at a density of 2.5 × 105 cells per well and grown overnight. The following day, the stromal cells were placed in serum-free media (RPMI-1640 + 1% pen/strep) for approximately 2 h. The epithelial cells were scratched using a 200-μl pipette tip, and the coverslip was then transferred cell-side up to the stromal cell-containing well. The media were immediately replaced with RPMI-1640 + 1% FBS + 1% pen/strep and, when indicated, catalase was used at a final amount of 1500 U/ml. The wound was imaged at time zero. The coculture system was incubated, and the same areas were then imaged at time 24 h. The denuded zone was measured at time zero and again at time 24 h, and the percent wound closure was calculated by subtracting the 0-h wound size from the 24 h wound size and multiplying by 100. TGF-β1 neutralizing antibody was used at a final concentration of 10 μg/ml.
Conditioned media
Stromal cells were plated at the same density as the indirect coculture assay and grown overnight. The following day they were serum starved for approximately 2 h after which the media were replaced with fresh media containing 1% charcoal-stripped phenol-red free serum. Where indicated, exogenous TGF-β1 was added when indicated at a final concentration of 5 ng/ml. The cells were incubated overnight, and the media were collected the following day, centrifuged at 1500 × g for 3 min, and stored at −20 C. Before column fractionation, media were syringe filtered. The ultrafiltrate was diluted into fresh serum-free media, and the lower filtrate was used without any additional dilutions. CM was thawed and placed on freshly wounded naïve DU145 cells, and the wounds were imaged at time zero and time 24 h. Wound closure was calculated as described above.
RNA isolation, reverse transcription, and real-time PCR
WPMY-1 prostate stromal cells were plated in six-well plates at a density of 2.5 × 105 cells per well and were grown overnight. The following day they were placed in serum-free media and were serum starved for approximately 2 h. TGF-β1 (0, 2, 5, and 10 ng/ml) was then added, and the cells were incubated overnight. WPMY-1 cells from coculture were grown in the lower chamber of a transwell system and cocultured in 1% FBS RPMI-1640 media for 24 h with a DU145-containing insert. The following day the media were removed, the cells were washed in sterile 1× PBS, and harvested in 500 μl cold Trizol. RNA was extracted using the RNeasy kit from QIAGEN (Valencia, CA) and was quantified on the Nanodrop ND-1000. cDNA was synthesized using the iScript kit from Bio-Rad Laboratories, Inc., according to the kit protocol, and the final product was diluted to a total volume of 100 μl using nuclease-free water. Quantitative real-time PCR was performed using the iTaq Sybr green kit from Bio-Rad. Table 1 shows the primer sequences that were used. Samples were run through an initial denaturation step of 95 C for 10 min followed by 40 cycles of 95 C for 30 sec, 55 C for 1 min, and 72 C for 1 min. Relative expression was determined using the comparative Ct method.
Measurement of ROS production
WPMY-1 cells were plated at a density of 3000 cells per well in black-walled clear bottom 96-well tissue culture plates (Greiner Bio-One, Radnor, PA) in phenol-red-free RPMI-1640 containing 5% FBS. The following day, cells were serum starved for approximately 90 min, and TGF-β1 was added in fresh serum-free media. The cells were incubated for 3 h and H2O2 production was measured using an Amplex Red Enzyme Assay (Invitrogen).
Chromatin immunoprecipitation (ChIP)
DU145 cells were grown to approximately 80% confluence on 15-cm plates and treated for 18 h with either control or WPMY-1 CM −/+ H2O2 (10 μm). The following day, media were replaced with fresh complete growth medium (5% FBS) containing 1% formaldehyde for cross-linking. Cells were incubated at 37 C for 20 min, followed by addition of glycine and an additional 10 min incubation at room temperature. Cells were washed once in 1× PBS, and then collected in ice-cold 1× PBS containing protease inhibitors. Cells were pelleted at 2000 rpm for 10 min at 4 C. PBS was discarded, and cells were resuspended in lysis buffer containing protease inhibitors. After a 15-min incubation on ice, cells were sonicated at maximum setting using a bioruptor with 30 sec on/off pulses for 10 min. Cells were incubated on ice 5 min, and an additional 5 min of sonication was performed. A DNA gel was run to ensure adequate shearing of the chromatin to approximately 1-kb fragments. Sheared chromatin was diluted in ChIP dilution buffer containing protease inhibitors to a final concentration of 250 μg/ml. Antibodies were linked to antimouse IgG magnetic beads in low-salt immune complex buffer for 6 h at 4 C. Anti-ERβ antibodies were used at a final concentration of 2 μg/ml. During antibody linking, chromatin samples were precleared using fresh magnetic beads, and a portion of this precleared sample was used as input sample. The remaining precleared chromatin was then incubated overnight at 4 C with the antibody-linked beads. The following day, beads were washed for 15 min once each with low-salt immune complex buffer, high-salt immune complex buffer, LiCl-immune complex buffer, and 1× Tris-EDTA buffer. After the final wash, beads were resuspended in 150 μl Tris-EDTA for a final rinse before addition of 400 μl elution buffer. Proteinase K (5 μl) was added to each sample and they were incubated overnight at 65 C. The following day DNA was extracted using phenol-chloroform-isoamyl alcohol (25:24:1). Glycogen (2 μl) and sodium acetate (35 μl) were added, and samples were vortexed, followed by addition of 800 μl ice-cold ethanol. Samples were incubated at −20 C for 1–2 h and then centrifuged at 13,000 rpm for 60 min at 4 C. Pellet was washed once with 70% EtOH and respun, after which the pellet was allowed to air dry on ice for 2 h. Pellet was resuspended in 30 μl RNase-free water, and the DNA was then subjected to quantitative real-time PCR analysis (described above). Table 2 below lists the primers used for two different ERβ-binding sites within the E-cadherin promoter. RT-PCR results were calculated using the ΔΔCt method and are presented as assay site immunoprecipitation fold enrichment.
Table 2.
Primer sequences for ChIP assay
| Forward | Reverse | |
|---|---|---|
| E-cadherin site 1 (coordinates 67326210-67326900 on human chromosome 16) | 5′-GACCTGAGACCTTTGGCCCCTA-3′ | 5′-TATCTCCTCTTGGCGAACTTGG-3′ |
| E-cadherin site 2 (coordinates 67328620-67329310 on human chromosome 16) | 5′-CAATCAGCGGTACGGGGGGCGG-3′ | 5′-GGTTCTTTCCAGCATTTATCCT-3′ |
Statistical analysis
Two-sample comparisons were performed using Student's t test. Multiple comparisons were performed using a one-way ANOVA followed by the Tukey test or a two-way ANOVA followed by a Bonferroni posttest. All data are represented ± sem and are representative of more than or equal to three independent biological replicates. P values <0.05 are considered significant.
Acknowledgments
ChIP assays were performed with the help of Teresa Liu at the University of Pittsburgh. ERβ antibody was kindly provided by Benita Katzenellenbogen from the University of Illinois. Finally, intellectual insights were provided by Guillermo Romero, Patrick Pagano. and Roberto Di Maio of the University of Pittsburgh.
This work was supported by Department of Defense award 09-1-0497 and National Institutes of Health grant R01 DK078394.
Disclosure Summary: The authors have no conflicts of interest to disclose in the conduction of the work herein or during preparation of the manuscript.
NURSA Molecule Pages†:
Nuclear Receptors: ER-β.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- AKR
- Aldo keto reductase
- CAF
- cancer-associated fibroblasts
- ChIP
- chromatin immunoprecipitation
- CM
- conditioned media
- DHEA
- dehydroepiandrosterone
- EMT
- epithelial-to-mesenchymal transition
- ER
- estrogen receptor
- FA
- flufenamic acid
- FBS
- fetal bovine serum
- HRP
- horseradish peroxidase
- PCa
- prostate cancer
- PBS-T
- PBS containing 0.1% Tween
- PHTPP
- 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- qRT-PCR
- quantitative RT-PCR
- ROS
- reactive oxygen species
- Scr
- scrambled shRNA sequence
- SH4
- Cox-2 shRNA
- shRNA
- short hairpin RNA.
References
- 1. Paget S. 1989. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101 [PubMed] [Google Scholar]
- 2. Barclay WW, Woodruff RD, Hall MC, Cramer SD. 2005. A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer. Endocrinology 146:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cunha GR. 1994. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 74:1030–1044 [DOI] [PubMed] [Google Scholar]
- 4. Cunha GR, Hayward SW, Wang YZ. 2002. Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485 [DOI] [PubMed] [Google Scholar]
- 5. Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. 2002. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923 [PubMed] [Google Scholar]
- 6. Akhurst RJ, Derynck R. 2001. TGF-β signaling in cancer–a double-edged sword. Trends Cell Biol 11:S44–S51 [DOI] [PubMed] [Google Scholar]
- 7. Barrack ER. 1997. TGF β in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate 31:61–70 [DOI] [PubMed] [Google Scholar]
- 8. Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. 2007. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-β1 expression in colon carcinoma: a retrospective study. BMC Cancer 7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lahn M, Kloeker S, Berry BS. 2005. TGF-β inhibitors for the treatment of cancer. Expert Opin Investig Drugs 14:629–643 [DOI] [PubMed] [Google Scholar]
- 10. Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. 2003. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res 9:4792–4801 [PubMed] [Google Scholar]
- 11. Reis ST, Pontes-Júnior J, Antunes AA, Sousa-Canavez JM, Abe DK, Cruz JA, Dall'oglio MF, Crippa A, Passerotti CC, Ribeiro-Filho LA, Viana NI, Srougi M, Leite KR. 2011. Tgf-β1 expression as a biomarker of poor prognosis in prostate cancer. Clinics (Sao Paulo) 66:1143–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy L, Hill CS. 2006. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17:41–58 [DOI] [PubMed] [Google Scholar]
- 13. Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature 420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. 2005. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis 26:1170–1181 [DOI] [PubMed] [Google Scholar]
- 15. Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. 1999. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem 274:22903–22906 [DOI] [PubMed] [Google Scholar]
- 16. Giannoni E, Bianchini F, Calorini L, Chiarugi P. 2011. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal 14:2361–2371 [DOI] [PubMed] [Google Scholar]
- 17. Im JY, Kim D, Paik SG, Han PL. 2006. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med 41:960–972 [DOI] [PubMed] [Google Scholar]
- 18. Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. 2009. Oxidative stress in prostate cancer. Cancer Lett 282:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. 2008. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68:1777–1785 [DOI] [PubMed] [Google Scholar]
- 20. Trinh BQ, Barengo N, Naora H. 2011. Homeodomain protein DLX4 counteracts key transcriptional control mechanisms of the TGF-β cytostatic program and blocks the antiproliferative effect of TGF-β. Oncogene 30:2718–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad N, Kumar R. 2011. Steroid hormone receptors in cancer development: a target for cancer therapeutics. Cancer Lett 300:1–9 [DOI] [PubMed] [Google Scholar]
- 22. Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. 2004. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab 89:2434–2441 [DOI] [PubMed] [Google Scholar]
- 23. Ellem SJ, Risbridger GP. 2009. The dual, opposing roles of estrogen in the prostate. Ann NY Acad Sci 1155:174–186 [DOI] [PubMed] [Google Scholar]
- 24. Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. 2010. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas C, Gustafsson JÅ. 2011. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 11:597–608 [DOI] [PubMed] [Google Scholar]
- 26. Jones JI, Doerr ME, Clemmons DR. 1995. Cell migration: interactions among integrins, IGFs and IGFBPs. Prog Growth Factor Res 6:319–327 [DOI] [PubMed] [Google Scholar]
- 27. Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. 2005. Profiling molecular targets of TGF-β1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech Ageing Dev 126:59–69 [DOI] [PubMed] [Google Scholar]
- 28. Wilding G, Zugmeier G, Knabbe C, Flanders K, Gelmann E. 1989. Differential effects of transforming growth factor β on human prostate cancer cells in vitro. Mol Cell Endocrinol 62:79–87 [DOI] [PubMed] [Google Scholar]
- 29. Sampson N, Koziel R, Zenzmaier C, Bubendorf L, Plas E, Jansen-Dürr P, Berger P. 2011. ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol Endocrinol 25:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dondi D, Piccolella M, Biserni A, Della Torre S, Ramachandran B, Locatelli A, Rusmini P, Sau D, Caruso D, Maggi A, Ciana P, Poletti A. 2010. Estrogen receptor β and the progression of prostate cancer: role of 5α-androstane-3β,17β-diol. Endocr Relat Cancer 17:731–742 [DOI] [PubMed] [Google Scholar]
- 31. Guerini V, Sau D, Scaccianoce E, Rusmini P, Ciana P, Maggi A, Martini PG, Katzenellenbogen BS, Martini L, Motta M, Poletti A. 2005. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor β subtype. Cancer Res 65:5445–5453 [DOI] [PubMed] [Google Scholar]
- 32. Linja MJ, Savinainen KJ, Tammela TL, Isola JJ, Visakorpi T. 2003. Expression of ERα and ERβ in prostate cancer. Prostate 55:180–186 [DOI] [PubMed] [Google Scholar]
- 33. Knudsen KE, Penning TM. 2010. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 21:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carruba G, Adamski J, Calabrò M, Miceli MD, Cataliotti A, Bellavia V, Lo Bue A, Polito L, Castagnetta LA. 1997. Molecular expression of 17β hydroxysteroid dehydrogenase types in relation to their activity in intact human prostate cancer cells. Mol Cell Endocrinol 131:51–57 [DOI] [PubMed] [Google Scholar]
- 35. Castagnetta LA, Carruba G, Traina A, Granata OM, Markus M, Pavone-Macaluso M, Blomquist CH, Adamski J. 1997. Expression of different 17β-hydroxysteroid dehydrogenase types and their activities in human prostate cancer cells. Endocrinology 138:4876–4882 [DOI] [PubMed] [Google Scholar]
- 36. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. 2011. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bauman DR, Rudnick SI, Szewczuk LM, Jin Y, Gopishetty S, Penning TM. 2005. Development of nonsteroidal anti-inflammatory drug analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: potential antineoplastic agents that work independently of cyclooxygenase isozymes. Mol Pharmacol 67:60–68 [DOI] [PubMed] [Google Scholar]
- 38. Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, II, Revelo MP, Bhowmick NA, Hayward SW. 2011. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res 71:1272–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang BL, Zheng SL, Hawkins GA, Isaacs SD, Wiley KE, Turner A, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. 2002. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res 62:1784–1789 [PubMed] [Google Scholar]
- 40. Park JY, Tanner JP, Sellers TA, Huang Y, Stevens CK, Dossett N, Shankar RA, Zachariah B, Heysek R, Pow-Sang J. 2007. Association between polymorphisms in HSD3B1 and UGT2B17 and prostate cancer risk. Urology 70:374–379 [DOI] [PubMed] [Google Scholar]
- 41. Atsriku C, Scott GK, Benz CC, Baldwin MA. 2005. Reactivity of zinc finger cysteines: chemical modifications within labile zinc fingers in estrogen receptor. J Am Soc Mass Spectrom 16:2017–2026 [DOI] [PubMed] [Google Scholar]
- 42. Whittal RM, Benz CC, Scott G, Semyonov J, Burlingame AL, Baldwin MA. 2000. Preferential oxidation of zinc finger 2 in estrogen receptor DNA-binding domain prevents dimerization and, hence, DNA binding. Biochemistry 39:8406–8417 [DOI] [PubMed] [Google Scholar]
- 43. Rao DS, Gui D, Koski ME, Popoviciu LM, Wang H, Reiter RE, Said JW. 2006. An inverse relation between COX-2 and E-cadherin expression correlates with aggressive histologic features in prostate cancer. Appl Immunohistochem Mol Morphol 14:375–383 [DOI] [PubMed] [Google Scholar]
- 44. Kourelis K, Vandoros G, Kourelis T, Papadas T, Goumas P, Sotiropoulou-Bonikou G. 2009. Low COX2 in tumor and upregulation in stroma mark laryngeal squamous cell carcinoma progression. Laryngoscope 119:1723–1729 [DOI] [PubMed] [Google Scholar]
- 45. Banerjee AG, Liu J, Yuan Y, Gopalakrishnan VK, Johansson SL, Dinda AK, Gupta NP, Trevino L, Vishwanatha JK. 2003. Expression of biomarkers modulating prostate cancer angiogenesis: differential expression of annexin II in prostate carcinomas from India and USA. Mol Cancer 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. 2000. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest 105:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]