Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is expressed at low levels in skeletal muscle, where it protects against adiposity and insulin resistance via unclear mechanisms. To test the hypothesis that PPARγ directly modulates skeletal muscle metabolism, we created two models that isolate direct PPARγ actions on skeletal myocytes. PPARγ was overexpressed in murine myotubes by adenotransfection and in mouse skeletal muscle by plasmid electroporation. In cultured myotubes, PPARγ action increased fatty acid uptake and incorporation into myocellular lipids, dependent upon a 154 ± 20-fold up-regulation of CD36 expression. PPARγ overexpression more than doubled insulin-stimulated thymoma viral proto-oncogene (AKT) phosphorylation during low lipid availability. Furthermore, in myotubes exposed to palmitate levels that inhibit insulin signaling, PPARγ overexpression increased insulin-stimulated AKT phosphorylation and glycogen synthesis over 3-fold despite simultaneously increasing myocellular palmitate uptake. The insulin signaling enhancement was associated with an increase in activating phosphorylation of phosphoinositide-dependent protein kinase 1 and a normalized expression of palmitate-induced genes that antagonize AKT phosphorylation. In vivo, PPARγ overexpression more than doubled insulin-dependent AKT phosphorylation in lipid-treated mice but did not augment insulin-stimulated glucose uptake. We conclude that direct PPARγ action promotes myocellular storage of energy by increasing fatty acid uptake and esterification while simultaneously enhancing insulin signaling and glycogen formation. However, direct PPARγ action in skeletal muscle is not sufficient to account for the hypoglycemic actions of PPARγ agonists during lipotoxicity.
Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor whose genetic variants result in altered insulin sensitivity and lipid storage (1). The thiazolidinediones (TZD) are PPARγ activators that treat type 2 diabetes by increasing insulin sensitivity, enhancing insulin-dependent glucose disposal. The majority of this glucose disposal improvement occurs in skeletal muscle (2). However, PPARγ is only expressed at low levels in skeletal muscle (3–5), implying that other tissues play an import role in muscle insulin sensitization by TZD (6). Nonetheless, PPARγ expression in skeletal muscle is metabolically important, as best established by muscle-specific PPARγ knockout (MuPPARγKO) mice. Two independent studies found that these mice develop whole-body and hepatic insulin resistance (7, 8). However, skeletal muscle insulin sensitivity of these mice was discrepant between the two studies, with normal or reduced insulin sensitivity reported. These discrepancies leave in question the cell-autonomous actions of skeletal muscle PPARγ on muscle insulin sensitivity (9).
MuPPARγKO studies reveal additional metabolic importance of skeletal muscle PPARγ, in that these mice exhibit elevated serum lipids and excess weight gain (7, 8). A possible mechanism for the hyperlipidemia and adiposity in MuPPARγKO mice is suggested by the finding that fatty acid uptake by skeletal muscle is reduced in MuPPARγKO skeletal muscle (10). This reduced skeletal muscle fatty acid clearance is postulated to be a primary defect and secondarily induce lipid overload in blood and nonmuscle tissues. However, because it is unclear whether the reduced skeletal muscle fatty acid uptake is a primary or secondary defect in MuPPARγKO mice, firm conclusions are not possible. An alternative mechanistic interpretation is that reduced skeletal muscle fatty acid uptake is a secondary phenotype. For example, it is postulated that skeletal muscle PPARγ loss produces skeletal muscle insulin resistance leading secondarily to systemic insulin resistance, hyperlipidemia, and obesity (8), which in turn might reduce skeletal muscle fatty acid uptake (11).
It thus has been suggested that PPARγ in muscle may directly enhance insulin sensitivity and/or fatty acid uptake. However, a perplexing difficulty arises when considering these two postulated direct actions of PPARγ in skeletal muscle, because increases in fatty acid uptake are typically expected to reduce skeletal muscle insulin sensitivity (12, 13). This consideration further brings into question the primary actions of PPARγ on skeletal muscle. It also raises the possibility that the impact of PPARγ on insulin sensitization will be dependent upon fatty acid availability.
Given these unknowns, we have conducted experiments aimed at better defining the direct actions of PPARγ in skeletal muscle. To this end, we created two models that isolate the direct effects of PPARγ on skeletal muscle. The two models overexpress PPARγ above the low endogenous levels in 1) cultured C2C12 skeletal myotubes and 2) in vivo in tibialis anterior (TA) muscle. Using these models, we characterized the cell autonomous effects of PPARγ on both fatty acid metabolism and insulin action. Throughout the manuscript, we will use the terms “insulin signaling” and “insulin sensitivity” to refer to insulin induced intracellular signaling and glucose uptake, respectively. We found that PPARγ increases fatty acid uptake and determined the mechanism involved and the metabolic fate of the fatty acids, because these details influence how the fatty acids might impact insulin signaling. We also found that PPARγ enhanced insulin signaling when lipid availability was low and thus assessed the impact of PPARγ on insulin signaling under abundant lipid conditions that normally inhibit insulin signaling. Surprisingly, PPARγ potentiated insulin signaling under these conditions despite augmenting fatty acid uptake. Thus, cell autonomous PPARγ action in skeletal muscle decouples fatty acid uptake from lipid inhibition of insulin signaling. By contrast to the above strong effects of PPARγ on fatty acid uptake and insulin signaling, the actions of PPARγ on glycolysis, glucose uptake, and fatty acid oxidation were less pronounced and/or negative.
Materials and Methods
Materials
Gene abbreviations, referenced to NCBI gene names, are summarized in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. [9,10-3H]- and [1-14C]-oleic acid were purchased from American Radiolabeled Chemicals (St. Louis, MO); [9,10-3H]-palmitic acid, [γ-32P]-ATP, D-[U-14C]-glucose and 2-[1,2-3H]-deoxy-D-glucose (2DG) from PerkinElmer (Waltham, MA); n-octyl-β-D-glucopyranoside and sn-1,2-diacylglycerol (DAG) kinase from Calbiochem (San Diego, CA); 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) and L-α-phosphatidylinositol from Avanti Polar Lipids (Alabaster, AL); rosiglitazone maleate from Toronto Research Chemicals, Inc. (North York, Ontario, Canada); C2C12 cells from American Type Culture Collection (Manassas, VA); pSV-PPARγ1 from Bruce Spiegelman (Addgene plasmid 8886; Cambridge, MA); pRL-TK from Promega (Madison, WI); and pCMV-βgal from CLONTECH (Palo Alto, CA). pPPREx3-luc (14) was a gift from Xiang Fang (Iowa City, IA). Adenoviral (ad)PPARγ1 (15), a gift from Janardan Reddy (Northwestern University Medical School, Chicago, IL), was prepared by ViraQuest, Inc. (North Liberty, IA) and used at a multiplicity of infection of 250. Albumin was fatty acid free (A8806; Sigma, St. Louis, MO).
TA transfection and insulin action
All rodent studies were approved by the University of Iowa Institutional Animal Care and Use Committee. C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) TA was injected with 12 U of hyaluronidase and 2 h later electroporated (175 v/cm, 20 msec, 10 pulses) with injected plasmid. Mice were studied 1 wk after electroporation, at which time PPARγ mRNA, protein, and activity were enhanced in pSV-PPARγ1 transfected but not contralateral TA (Supplemental Fig. 1, A–C). The TA retained normal morphology without abnormal lipid accumulation despite extensive transfection (Supplemental Fig. 1D). Metabolic studies were performed after overnight fast. The mice were treated with 1 ml of 20% Intralipid ip and 25 U of heparin sc at the start of fasting and again 4 h before TA isolation. Insulin-stimulated thymoma viral proto-oncogene (AKT) phosphorylation in TA was determined 15 min after injection of 5 U of insulin into the inferior vena cava during terminal pentobarbital anesthesia. Insulin-stimulated glucose uptake was determined in other mice during terminal pentobarbital anesthesia. Insulin was infused at 6 mU/kg·min via right jugular catheter after a priming dose of 300 mU/kg. Euglycemia was maintained with variable glucose infusion. During steady state, 0.35 mCi/kg 2DG were administered ip, and tissues were snap frozen 45 min later for [3H]-2DG and [3H]-2DG-6-phosphate determination (16).
PPARγ action in myotubes
C2C12 myoblasts were cultured in high-glucose DMEM, 10% fetal bovine serum, 100 U/ml penicillin, and 50 μg/ml streptomycin at 37 C in a humidified atmosphere containing 5% CO2-95% air. Myotubes were produced by culturing myoblasts at 80% confluence in media containing 2% heat-inactivated horse serum, changed daily for 5 d. Unless noted otherwise, myotubes were adenotransfected for 2 d and exposed to 500 nm rosiglitazone or vehicle for 1 d before harvest. PPARγ mRNA and protein were increased by adPPARγ1, and PPARγ activity was enhanced by adPPARγ plus rosiglitazone (Supplemental Fig. 2, A–C). Rosiglitazone, but not adPPARγ alone, activated a PPARγ-ligand sensing reporter (Supplemental Fig. 2D). Myotube morphology and myocellular marker expression were not affected by treatment (Supplemental Fig. 2, E and F), adipocyte marker expression remained undetectable, and an adipocyte-based TZD target gene was not up-regulated (Supplemental Fig. 2G).
Where indicated, myotubes were treated for 18 h before harvest with 0.75 mm palmitate complexed with 0.3 mm fatty acid-free albumin unless otherwise noted. For insulin stimulation, myotubes were cultured in serum-free media for 3 h followed by addition of 100 nm insulin for 10 min before harvest unless otherwise noted. Expression knockdown was accomplished by stable transfection of myoblasts with short hairpin RNA plasmids from OriGene (Rockville, MD). All created stable transfectant cell lines could be differentiated into myotubes.
Lipid studies
Fatty acid uptake was studied in myotubes that were washed thrice with 2 ml of Krebs-Ringer-HEPES buffer (KRH) [113 mm NaCl, 4.8 mm KCl, 25 mm NaHCO3, 1.4 mm KH2PO4, 2.2 mm CaCl2, 1.4 mm MgSO4, and 5.5 mm glucose (pH 7.4)] plus 0.1% albumin, and once with 2 ml of KRH without albumin. Uptake was initiated with 0.5 μCi/ml [3H]oleate and albumin in 1 ml of KRH at room temperature and stopped by adding ice-cold 0.5% albumin KRH. Cells were lysed in 0.5 ml of 0.1 n NaOH for 30 min before scintillation counting.
Labeled cell lipids were Folch (17) extracted, dissolved in 200 μl of CHCl3:CH3OH (2:1), and separated by thin layer chromatography on LK5D plates (Whatman, Piscataway, NJ) developed in chloroform:methanol:40% methylamine:water (60:36:1.5:1). Migrated radioactivity was quantified by AR-2000 scanner (Bioscan, Inc., Washington, DC) and compared with standards. Phosphatidylinositol and phosphatidylserine were separated on plates pretreated with 2.3% (wt/vol) boric acid in ethanol and developed in chloroform:ethanol:water:triethylamine (30:35:7:35).
Fatty acid oxidation was measured by incubating myotubes in sealed 24-well plates with 0.5 μCi [14C]oleic acid and albumin at 37 C. Oxidation was stopped by adding perchloric acid. CO2 was trapped overnight in 2 m NaOH soaked Whatman no. 3 filter paper and the radioactivity detected by scintillation counting with 5 ml of Budget-Solve (Research Products International, Mt. Prospect, IL). The radioactivity present in the perchloric acid supernatant represented the acid-soluble partial oxidation products.
Ceramide and DAG were quantified (18) in myotubes cultured in 100-mm dishes. Cell lipids were extracted by a modification of the Bligh Dyer method (19), in which 0.04% perchloric acid was added in the phase separation step. The extracted lipid was resuspended in 20 μl of 256 mm n-octyl-β-D-glucopyranoside and 25 mm 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol), 60 μl of reaction buffer (18), 5 μl of water, and 6 μg of DAG kinase. The labeling reaction was initiated with 20 μl of 5 mm and 0.1 μCi/μl [32P]ATP for 30 min at room temperature and stopped by Bligh Dyer extraction. The labeled lipid was separated on silica gel 60 plates (18) and quantified with a Typhoon 9200 phosphorimager (GE Healthcare, Princeton, NJ) and ImageJ (http://rsbweb.nih.gov/ij/) by comparison with codeveloped standards.
Neutral lipid droplets were detected using AdipoRed (40 μl/ml; Lonza, Walkersville, MD), in myotubes cultured on cover slips or skeletal muscle in 10-μm frozen sections cut at −25 C, fixed at 4 C, and photographed using a fluorescein isothiocyanate filter. Cellular triglyceride was measured in myotubes cultured in KRH for 3 h; cell lipids were Folch extracted and reconstituted into isopropanol for triglyceride measurement using GPO-Trinder (Sigma).
Glycogen synthesis
Myotubes were treated with 0 or 100 nm insulin for the final 30 min of serum-free incubation and then for 2 h with 1 μCi/ml [14C]glucose in KRH. Cells were lysed in 300 μl of 1 m KOH, boiled for 10 min, treated with 40 μl of saturated Na2SO4 and 700 μl of acetone, and glycogen precipitated at −70 C. The pellet was dissolved in H2O for scintillation counting.
Other assays
Firefly or Renilla luciferase activity was measured using the relevant Luciferase Assay Reagent (Promega). Chemiluminescence was measured with a Monolight 2001 luminometer (Analytical Luminescence Laboratories, San Diego, CA). Antibodies were against PPARγ (sc-7196; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), CD36 (Cascade Bioscience, Winchester, MA), β-actin (Sigma), phosphotyrosine (clone 4G10; Millipore, Billerica, MA), AKT, phospho-AKT Ser473, Thr308, phosphoinositide-dependent protein kinase 1 (PDK1), phospho-PDK1 Ser241, phospho-glycogen synthase Ser641, phospho-glycogen synthase kinase 3β Ser9, phosphatidylinositol 3 kinase (PI3K)-p85 subunit, phospho-protein kinase C (PKC)θ, PKCθ, and insulin receptor substrate 1 (IRS1) (nos. 9272, 9271, 2965, 3062, 3438, 3891, 9323, 4257, 9377, 2059, and 3015; Cell Signaling Technology, Danvers, MA). CD36 was detected in lysates prepared by Mem-PER Eukaryotic kit (no. 89826; Pierce, Rockford, IL). Immunoprecipitation was performed using protein G (IP-50 kit; Sigma). Band densities were quantified by V750 Pro scanner (Epson, Long Beach, CA) and ImageJ. Real-time PCR was performed on TRIzol (Invitrogen, Carlsbad, CA) extracted RNA using primers designed to span at least one intron. PCR conditions are listed in Supplemental Table 2. PCR progress curves were monitored using SYBR (Stratagene, La Jolla, CA), products were verified by melting curve and agarose gel electrophoresis, and expression quantification was normalized to that of β-actin. PI3K activity in PI3K-p85 immunoprecipitates was determined by thin layer chromatography resolution of [γ-32P]-ATP phosphorylated L-α-phosphatidylinositol (20). Glycolysis in C2C12 myotubes, acutely switched to XF Assay Medium containing 10 mm glutamine and placed in an XF24 Analyzer (Seahorse Bioscience, North Billerica, MA), was assessed from the extracellular acidification rate during serial additions of 10 mm glucose, 100 nm insulin, 2 μm oligomycin, and 100 mm 2-deoxyglucose (21).
Statistical analysis
Results are presented as means ± sem. The significance of differences between groups was assessed by one-way ANOVA using Tukey's honestly significant difference test for post hoc determination of pairwise significance using R.
Results
PPARγ stimulates myocellular uptake of fatty acid
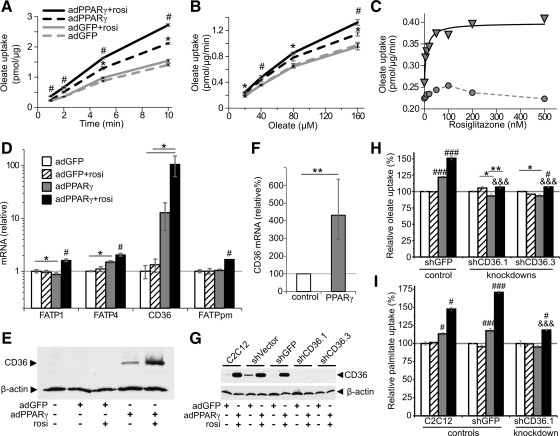
Myocellular uptake of the fatty acid oleate was increased by adPPARγ and further so by adPPARγ plus rosiglitazone (Fig. 1, A and B). The increase was detected within 1 min after the addition of oleate, suggesting enhancement in the early step(s) of cellular fatty acid accumulation, and it occurred across a range of oleate concentrations. Rosiglitazone exhibited an ED50 of 9.7 nm for stimulating oleate uptake in adPPARγ-transfected myotubes (Fig. 1C). By contrast, rosiglitazone had no effect on oleate uptake in ad green fluorescent protein (GFP)-transfected myotubes (Fig. 1, A–C). The ability of PPARγ to increase fatty acid uptake was dependent upon the differentiation of myoblasts to striated myotubes (Supplemental Table 3).
Fig. 1.
PPARγ increases fatty acid uptake and incorporation into myotubes mediated by induction of CD36 expression. Oleate was complexed with 40 μm albumin. A and B, Uptake of [9,10-3H]oleate during variable (A) incubation length (80 μm oleate) or (B) oleate concentration (5-min incubation). Cells were transfected with adGFP (gray lines) or adPPARγ (black lines) and treated with vehicle (dashed lines) or rosiglitazone (rosi) (solid lines). A and B, Results are representative of two or more independent determinations. *, P < 0.05; **, P < 0.005 vs. adGFP-transfected groups; #, P < 0.05; ###, P < 0.0005 vs. all other groups; n = 3. C, Rosiglitazone dose-response for uptake of 80 μm [9,10-3H]oleate assayed after a 30-min incubation in myotubes transfected with adGFP (circles) or adPPARγ (triangles). The solid line represents the best fit of ED50 = 9.7 ± 2.3 nm. D, Expression in myotubes of FATP, CD36, and FABPpm. Bar graph groups: adGFP + vehicle (white), adGFP + rosiglitazone (hatched), adPPARγ + vehicle (gray), or adPPARγ + rosiglitazone (black). E, CD36 protein in myotubes. F, CD36 mRNA in TA transfected with 25 μg pSV-PPARγ1 (gray) or pCMV-βgal (white, control) (n = 10 leg pairs). G, Knockdown of CD36 protein in myotubes stably transfected with short-hairpin constructs directed against CD36 (shCD36.1 and shCD36.3) but not control transfectants (shVector and shGFP). H and I, Uptake of 80 μm [9,10-3H]-oleate (H) or [9,10-3H]-palmitate (I) complexed with 40 μm albumin over 30 min in the indicated cell lines. D–I, n = 3 unless otherwise indicated. *, P < 0.05; **, P < 0.005 for the indicated comparison; #, P < 0.05; ###, P < 0.0005 vs. all others groups; &&&, P < 0.00001 for impaired PPARγ + rosiglitazone response in indicated shCD36 vs. control shGFP myotubes.
Up-regulation of CD36 is required for PPARγ-increased fatty acid uptake
We examined the effect of PPARγ on the expression of gene products known to enhance plasma membrane fatty acid uptake. PPARγ action in myocytes modestly increased the expression of CD36 proteins (FATP) 1 and 4 (FATP1/4) and fatty acid binding protein plasma membrane (FABPpm), but it increased CD36 (also known as fatty acid translocase) expression over 100-fold (Fig. 1D). Protein levels of CD36 were likewise increased by PPARγ action (Fig. 1E). We also developed a model of PPARγ overexpression in vivo as described above, in which muscle CD36 expression was likewise increased (Fig. 1F). To test whether CD36 induction was responsible for increased fatty acid uptake, we created stable C2C12 transfectant cells with near total knockdown of CD36 (shCD36) (Fig. 1G). Stable transfectant myotubes with CD36 knockdown had a greatly diminished oleate uptake response to PPARγ action as compared with control transfectant myotubes (Fig. 1H). Likewise, palmitate uptake was increased in control transfectant and normal C2C12 myotubes in response to PPARγ action, whereas lack of CD36 greatly impaired this response (Fig. 1I).
PPARγ action increases fatty acid incorporation into myocellular lipids with little effect on oxidation
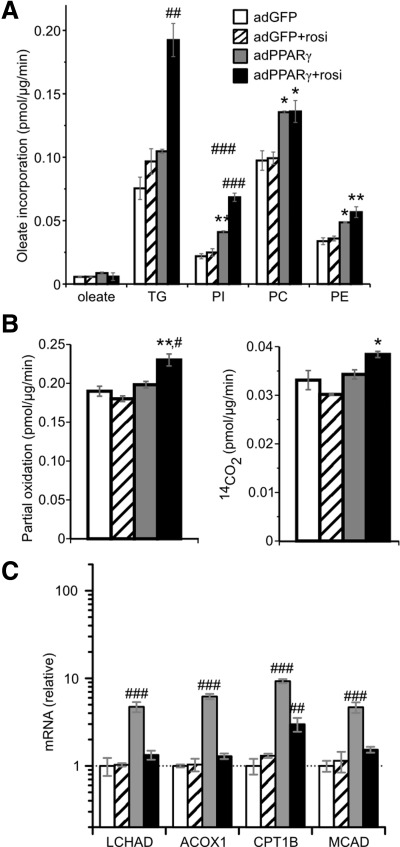
Increased fatty acid uptake might produce a variety of biological responses depending upon the metabolic fate of the fatty acids. Thus, we examined the incorporation of oleate into various lipid classes in myocytes. Unexpectedly, myocellular levels of nonesterified oleate were unchanged by PPARγ and/or rosiglitazone (Fig. 2A). Rather, adPPARγ increased oleate incorporation into myocellular lipids, including triglycerides and the phospholipids phosphatidylinositol, phosphatidylcholine, and phosphatidylethanolamine, with further increases produced by PPARγ plus rosiglitazone. Despite the increase in oleate incorporation into triglyceride, microscopically visible lipid droplets were not increased by adPPARγ plus rosiglitazone in cells cultured under routine conditions (Supplemental Fig. 3A, upper panels). However, myocellular lipid droplets accumulated when myotubes were cultured with supplemental oleate, with further droplet increases induced by adPPARγ (Supplemental Fig. 3A, lower panels). These effects on visible lipid droplets were congruent with total cellular triglyceride levels (Supplemental Fig. 3B). We also examined complete and partial oxidation of oleate, which were only modestly stimulated by PPARγ plus rosiglitazone, whereas adPPARγ alone had no effect (Fig. 2B). By contrast, larger increases in myotube fatty acid oxidation were produced by a 30-min preincubation with 2,4-dinitrophenol (Supplemental Fig. 3C). To further investigate the effect of PPARγ on fatty acid oxidation, we examined the expression of key fatty acid oxidation genes. Although PPARγ increased expression of these genes, activation of PPARγ with rosiglitazone reduced their expression nearly to baseline (Fig. 2C), contrasting the monotonic increases of FATP (Fig. 1D) with PPARγ activity (Supplemental Fig. 2C). These findings, coupled with the weak increases of fatty acid oxidation induced by PPARγ, to a degree less than the increases in fatty acid uptake, suggest that PPARγ does not directly promote fatty acid oxidation in muscle. This contrasts the actions of PPARα and PPARβ/δ, which stimulate fatty acid oxidation in skeletal muscle and myotubes (22–26).
Fig. 2.
Myocellular incorporation and metabolism of fatty acids. A, Incorporation of 80 μm [9,10-3H]oleate complexed with 40 μm albumin into cellular unesterified oleate, triglycerides (TG), phosphatidylinositol (PI), phosphatidylcholine (PC), and phosphatidylethanolamine (PE) over 30 min. B, Oxidation of 80 μm [1-14C]oleate complexed with 40 μm albumin over 2 h to acid soluble metabolites (partial oxidation, left panel) and 14CO2 (right panel). Results are representative of two of more independent determinations. C, Expression in myotubes of genes involved in fatty acid oxidation. Groups: adGFP + vehicle (white bars), adGFP + rosiglitazone (rosi) (hatched), adPPARγ + vehicle (gray), or adPPARγ + rosiglitazone (black). *, P < 0.05; **, P < 0.005 vs. adGFP-transfected groups; #, P < 0.05; ##, P < 0.005; ###, P < 0.0005 vs. all other groups, n = 3.
Impact of myocellular PPARγ on insulin signaling during low lipid availability
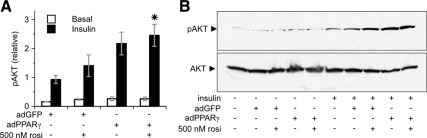
We assessed the impact of PPARγ on insulin-stimulated AKT phosphorylation in routine cultures, which have no added lipid or fatty acid in the media. Under these conditions, adPPARγ and/or rosiglitazone enhanced insulin-stimulated AKT phosphorylation (Fig. 3, A and B). Basal AKT phosphorylation remained low in the absence of insulin, indicating that PPARγ enhanced the sensitivity of AKT phosphorylation to insulin stimulation. Rosiglitazone enhanced insulin-stimulated AKT phosphorylation at concentrations as low as 5 nm, both in myotubes transfected with adGFP or adPPARγ (Supplemental Fig. 4, A and B).
Fig. 3.
PPARγ and/or rosiglitazone (rosi) augment insulin signaling in myotubes cultured without added lipid. A and B, PPARγ increases phosphorylation of AKT (pAKT) (Ser473) in response to insulin in C2C12 myotubes. A, white bars, Basal conditions (n = 2–4); black bars, 100 nm insulin for 10 min (n = 6–8). *, P < 0.01 vs. adGFP + insulin. B, Representative blot.
PPARγ restores lipid-inhibited insulin signaling
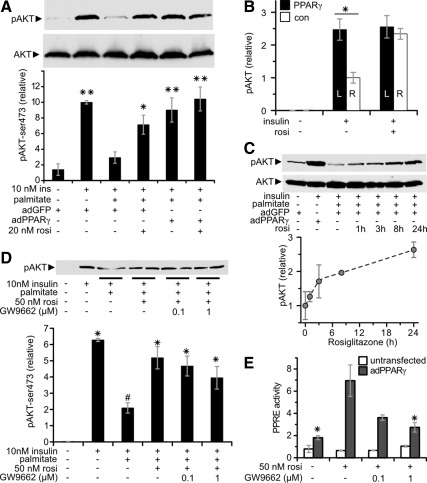
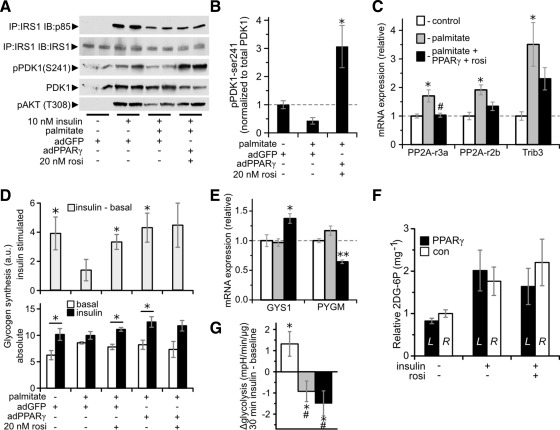
Insulin sensitivity in muscle is typically diminished by increased fatty acid exposure and uptake. Thus, extrapolation from routine cell culture experiments to the in vivo situation is problematic because of the low lipid availability conditions typically employed in culture. This is of further concern, because CD36 overexpression and fatty acid exposure are reported to synergistically induce insulin resistance in myotubes (27). It could be envisioned that PPARγ's effect to enhance insulin signaling would be abolished or even reversed under lipid replete conditions. Thus, to delineate the relationship between PPARγ-stimulated lipid uptake and insulin signaling, we assessed myotubes cultured in media containing palmitate. As expected, 0.75 mm palmitate reduced insulin-stimulated AKT phosphorylation (Fig. 4A, lane 2 vs. 3). However, even though fatty acid uptake was increased, rosiglitazone and/or adPPARγ enhanced insulin-stimulated AKT phosphorylation 2.4 ± 0.4- to 3.6 ± 0.5-fold (Fig. 4A). The combination of adPPARγ plus rosiglitazone fully restored AKT phosphorylation to levels observed in the absence of palmitate. The restoration of lipid-inhibited AKT insulin signaling by PPARγ action occurred in both myoblasts and myotubes (Supplemental Fig. 5A) and in CD36 knockdown myotubes (Supplemental Fig. 5B).
Fig. 4.
PPARγ and/or rosiglitazone (rosi) restore palmitate-inhibited insulin signaling to AKT phosphorylation. A, Insulin-stimulated AKT phosphorylation (pAKT) (Ser473) in C2C12 myotubes. n = 4–7; *, P < 0.01; **, P < 0.001 vs. no insulin. Results are representative of multiple independent determinations. B, Insulin-stimulated pAKT in TA transfected for 1 wk with 15 μg of pSV-PPARγ1 (black bars; left) or pCMV-βgal [white bars; R, Right control (con)]. All mice were treated with Intralipid and where indicated 0.125 U/g of insulin iv for 15 min and/or 10 mg/kg·d rosiglitazone by gavage for 3 d (*, P < 0.005 for the indicated comparison by paired t test; n = 6–11). C, Time course required for rosiglitazone to restore insulin signaling in adGFP-transfected myotubes treated with 0.75 mm palmitate for 18 h; n = 2. Right panel shows representative blot. D, AKT phosphorylation in C2C12 myotubes receiving the indicated treatments. *, P < 0.05 vs. control (lane 1); #, P < 0.05 vs. insulin (lane 2); n = 4, except leftmost two conditions n = 2. D, PPAR reporter element activity (PPRE) measured and normalized as in Supplemental Fig. 2C. *, P < 0.05 vs. rosiglitazone + PPARγ treated; n = 3, except in nontransfected myotubes n = 2.
In vivo, the same considerations regarding fatty acid uptake and insulin sensitivity also apply. Similar to the situation in myotubes, CD36 expression in vivo in skeletal muscle promotes insulin resistance (12, 13). Hence, the marked increase in CD36 expression in skeletal muscle transfected with PPARγ could be envisioned to reduce insulin signaling. Thus, to fully delineate the relationship in vivo between PPARγ action and insulin signaling, we assessed the impact of PPARγ overexpression in lipid-treated mice. Under these conditions, PPARγ overexpression in skeletal muscle enhanced insulin-stimulated AKT phosphorylation 2.5 ± 0.3-fold in the TA muscle, with AKT phosphorylation remaining virtually undetected in the basal state (Fig. 4B). As expected, rosiglitazone enhanced insulin-stimulated AKT phosphorylation in the control TA but had no additional effect in the PPARγ-treated TA.
In myotubes, the effect of rosiglitazone to increase insulin-stimulated AKT phosphorylation progressed over 24 h of treatment, with improvement evident within 1–3 h (Fig. 4C). Interestingly, rosiglitazone restoration of palmitate-inhibited AKT phosphorylation occurs under conditions where rosiglitazone has minimal effect on PPAR activity (see Supplemental Fig. 2C). To further address whether PPARγ is required for the ability of rosiglitazone to restore palmitate-inhibited insulin signaling, we treated myotubes with the covalently-acting irreversible PPARγ antagonist GW9662, which has an IC50 of under 10 nm (28). GW9662 only weakly reduced the ability of rosiglitazone to restore AKT phosphorylation (Fig. 4D), including at concentrations that antagonized the majority of rosiglitazone-induced PPAR activity at PPAR response element in PPARγ-transfected myotubes (Fig. 4E).
Responsiveness of the PDK1 AKT signaling nexus to insulin is enhanced by PPARγ
To determine the aspects of the insulin signaling pathway modulated by PPARγ, we assessed insulin signaling events downstream and upstream of AKT phosphorylation in palmitate-treated myotubes. Far upstream of AKT, neither palmitate nor PPARγ altered insulin-stimulated IRS1 tyrosine phosphorylation (Supplemental Fig. 6A). By contrast, palmitate inhibited the insulin-stimulated association of IRS1 with PI3K subunit p85, but PPARγ action failed to restore this signaling event (Fig. 5A). Likewise, PPARγ action did not increase insulin-stimulated IRS1-associated PI3K activity (Supplemental Fig. 6B). Palmitate reduced the activation state of PDK1, as assessed by Ser241 phosphorylation (29), whereas PPARγ enhanced PDK1 activation to above baseline levels (Fig. 5, A and B). Consistent with its effect on AKT Ser473 phosphorylation, PPARγ action also restored AKT phosphorylation at Thr308 (Fig. 5A). Palmitate increased the expression of several genes known to antagonize AKT activation, including regulatory subunits of protein phosphatase 2A (30) and tribbles 3 (31) (Fig. 5C). PPARγ action reversed these increases, suggesting additional mechanisms by which PPARγ may enhance AKT signaling.
Fig. 5.
Impact of PPARγ and/or rosiglitazone (rosi) on insulin signaling and glucose metabolism. A, Insulin signaling events measured using the indicated immunoprecipitations (IP) and immunoblots (IB). B, Quantitation of the ratio of phospho-Ser241- to total-PDK1 in myotubes treated with 10 nm insulin. *, P < 0.05 vs. all other groups; n = 4. C, mRNA expression in C2C12 myotubes. *, P < 0.05 vs. control; #, P < 0.05 vs. palmitate-treated cells; n = 4. D, Glycogen synthesis in arbitrary units (a.u.) over 30 min in C2C12 myotubes treated with 0 (basal, white bars) or 100 nm insulin (black bars). Top panel shows the difference between the insulin-stimulated and basal states (gray bars). n = 3; *, P < 0.05 for change induced by insulin. E, mRNA expression in C2C12 myotubes. *, P < 0.05; **, P < 0.005 vs. all other groups; n = 4. F, Glucose transport, measured as 2-[3H]-deoxyglucose-6-phosphate (2DG-6P) content normalized to tissue weight, in TA transfected for 1 wk with 15 μg of pSV-PPARγ1 (black bars, [L]eft) or pCMV-βgal (white bars, [R]ight control) of mice rapidly infused with 2DG. All mice were Intralipid treated and where indicated received euglycemic infusion of 6 mU/kg·min insulin (see Supplemental Fig. 7A) and/or 10 mg/kg·d rosiglitazone by gavage for 3 d. There were no significant differences between sides (n = 5–7) by paired t tests. G, Insulin-induced change in C2C12 myotube glycolysis as determined from the average extracellular acidification rate (see Supplemental Fig. 7B) in the 30 min after vs. before addition of insulin and normalized to the change observed in the absence of insulin. *, P < 0.05 for nonzero change by z test; #, P < 0.01 vs. control by t test, n = 9. C, E, and G, white bar, Control; gray, 0.75 mm palmitate treated; black, palmitate + adPPARγ + 20 nm rosiglitazone.
Impact of PPARγ on myocellular glucose metabolism
We also examined the impact of PPARγ on glucose metabolism As expected, insulin-stimulated glycogen synthesis was inhibited by palmitate (Fig. 5D). However, both adPPARγ and 20 nm rosiglitazone restored insulin-stimulated glycogen synthesis (Fig. 5D). This effect may have been related to PPARγ action induced increases in glycogen synthase and decreases in glycogen phosphorylase expression (Fig. 5E). By contrast, PPARγ action did not enhance insulin signaling to glycogen synthase kinase and glycogen synthase (Supplemental Fig. 6, C and D). CD36 was not required for adPPARγ plus rosiglitazone to prevent palmitate inhibition of insulin-stimulated glycogen synthesis (Supplemental Fig. 6E).
Despite these increases in insulin signaling, PPARγ overexpression failed to enhance insulin-stimulated glucose-tracer uptake in vivo in TA muscle, because there were no differences in glucose uptake between PPARγ- and control-transfected muscles in the basal, insulin-stimulated, or rosiglitazone plus insulin states (Fig. 5F). Likewise, PPARγ action did not restore palmitate-inhibited insulin-dependent glycolysis (Fig. 5G). These findings may be explained by reductions in hexokinase, 6-phosphofructo-2-kinase, and glucose transporter type 4 induced by PPARγ action in myotubes (Supplemental Fig. 7C).
Impact of PPARγ on lipotoxic metabolism and signaling
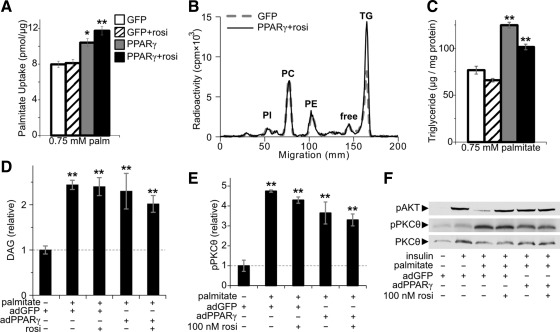
There are various possible mechanisms by which PPARγ could protect against palmitate induced insulin resistance, including 1) decreased palmitate uptake, 2) enhanced clearance of palmitate-derived cellular lipids, 3) decreased lipotoxic lipid metabolites DAG or ceramide, or 4) abrogation of signaling through PKCθ, a DAG-dependent kinase that induces insulin resistance (32). By contrast, we found that PPARγ action increased palmitate uptake in myocytes even under lipotoxic conditions (Fig. 6A). Furthermore, PPARγ action increased palmitate incorporation into triglyceride and increased total myocellular triglyceride (Fig. 6, B and C). Additionally, PPARγ activity did not alter clearance of palmitate-labeled cellular triglyceride or phospholipids, and visible neutral lipid droplets remained sparse in all palmitate-treated conditions (Supplemental Fig. 8, A and B). As expected, palmitate exposure increased myocellular DAG and led to phosphorylation of PKCθ (Fig. 6, D–F). PPARγ activity partly restored DAG and phosphorylated PKCθ to baseline levels. A similar pattern was observed in myocellular levels of ceramide in response to palmitate and PPARγ action (Supplemental Fig. 8C). These findings show that direct PPARγ action in myocytes is sufficient to enhance selected aspects of insulin signaling even though it does not channel lipids away from muscle and only partly abrogates lipotoxic metabolism and signaling.
Fig. 6.
Impact of PPARγ on lipotoxic lipid handling and signaling. A, Uptake of palmitate (palm) over 30 min in myotubes cultured with 0.75 mm palmitate + 0.3 mm albumin; n = 3. Bar graph groups: adGFP + vehicle (white), adGFP + rosiglitazone (rosi) (hatched), adPPARγ + vehicle (gray), or adPPARγ + rosiglitazone (black). B, Incorporation of 0.75 mm 0.5 μCi/ml [9,10-3H]palmitate + 0.3 mm albumin into myocellular lipids over 24 h, showing the distribution of lipid radioactivity in thin layer chromatograms from myotubes treated with adGFP and vehicle (dotted gray line) or adPPARγ and 500 nm rosiglitazone (solid line), identifying peaks corresponding to phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidylethanolamine (PE), unesterified palmitate (free), and triglycerides (TG). C, Total triglyceride was measured in myotubes incubated for 18 h with media containing 0.75 mm palmitate + 0.3 mm albumin (n = 3); groups as above. Myotube levels of (D) DAG (n = 6–8 per group) and (E) phosphorylated (Thr538) PKCθ (n = 3). F, Representative immunoblot of phospho (p)AKT, pPKCθ, and total PKCθ. *, P < 0.05; **, P < 0.01 vs. adGFP, no palmitate control. Concomitant pAKT is shown to allow comparison with the degree of insulin signaling restoration.
Discussion
The role of PPARγ in skeletal muscle has been elusive, in part because of difficulty in separating primary from secondary phenomena in muscle-specific loss-of-function studies (i.e. MuPPARγKO mice). The overexpression studies presented here confirm that direct PPARγ action in skeletal muscle modulates energy metabolism and insulin signaling, as predicted by loss-of-function studies. However, the overexpression studies further show that PPARγ modulates the manner in which lipid metabolism and insulin signaling interact. These PPARγ effects counter a portion of the dysmetabolism and impaired signaling that characterize skeletal muscle in type 2 diabetes. The results also suggest that an overarching and hitherto unrecognized role of PPARγ in skeletal muscle is to promote storage of energy by increasing uptake and esterification of fatty acids and by increasing insulin sensitivity in a manner that promotes glycogen formation.
Fatty acid uptake capability in skeletal muscle is reduced in type 2 diabetes, although this is offset by the increased circulating fatty acid concentrations (33, 34). This reduced fatty acid uptake by muscle may be pathologic and is postulated to contribute to adiposity and insulin resistance in other tissues (11). Thus, the increase in fatty acid uptake induced by direct PPARγ action in skeletal muscle that we observed could be anticipated to produce metabolic benefits by reducing lipid loading in other tissues. The effect of PPARγ to enhance fatty acid uptake was potent, with the increased uptake approaching the degree induced by direct overexpression of CD36 or FATP (27, 35). We find that PPARγ up-regulates a variety of fatty acid transporters in skeletal muscle, including CD36, FATP, and FABPpm, similar to previous work using PPARγ agonists (36–38). However, we demonstrate that up-regulation of CD36 accounts for the majority of the increase in fatty acid uptake induced by PPARγ direct action in myocytes. These findings are consistent with reduced fatty acid uptake and a tendency for reduced CD36 expression in MuPPARγKO muscle (10).
Whether direct PPARγ action improves insulin sensitivity in skeletal muscle has been unclear. This uncertainty is exemplified by loss-of-function studies using MuPPARγKO mice (7, 8), in which conclusions about direct PPARγ muscle action on insulin signaling are complicated by systemic dysmetabolism. One approach to study direct PPARγ action free of confounders is to use a myotube model. Our studies show direct PPARγ action in skeletal myotubes improves insulin signaling, similar to previous myotube-based experiments (39). Because lipids are ubiquitously available in vivo, the artificially low fatty acid availability in routine cell culture might have produced insulin signaling enhancements that would not be observed in vivo. We thus also performed studies under lipid-rich conditions that normally inhibit insulin signaling. Under these conditions, we find that PPARγ directly ameliorates the lipotoxic inhibition of insulin signaling to AKT in vivo and in cultured myotubes despite increasing lipid uptake. Mechanistically, we find that PPARγ improves the PDK1-AKT insulin signaling nexus through a coordinate approach of restored PDK1 activity and reduced expression of genes that antagonize AKT phosphorylation. Palmitate inhibits PDK1 activity via activation of PKCθ (32). Whether the modest reduction of DAG levels and PKCθ activity induced by PPARγ action in skeletal muscle is sufficient to account for the improved PDK1 activity remains to be determined. PPARγ action also improved insulin-stimulated glycogen formation, again mediated by an apparent coordinate mechanism involving up-regulation of the glycogen synthase and down-regulation of glycogen phosphorylase isoforms expressed in skeletal muscle. Down-regulation of protein phosphatase 2A, a mediator of palmitate inhibition of insulin signaling to AKT phosphorylation and glycogen synthesis (40), may also be important to these improvements in insulin signaling by PPARγ. Alternatively, PPARγ action to increased insulin-stimulated glycogen formation may be related to PPARγ-increased fatty acid uptake, because increased fatty acid metabolism is associated with preferential shunting of intracellular glucose to glycogen rather than to glycolysis (41).
Our results have implications regarding mechanisms of TZD action, indicating that muscle PPARγ plays a direct role in TZD-induced insulin sensitization in skeletal muscle, although with important limitations. It is possible that muscle PPARγ activation by TZD is required but not sufficient for the improvement in muscle insulin sensitivity. Specifically, our data would fit with a model by which direct TZD action in skeletal muscle improves insulin signaling at the PDK1-AKT signaling nexus, but indirect TZD action is required to produce full enhancement of insulin signaling especially as pertains to glucose uptake.
The strengths and weaknesses of the PPARγ overexpression models described here are complementary with those of previous knockout (MuPPARγKO) studies (7, 8, 10). Major strengths of our models include the isolation of the direct effects of PPARγ on skeletal muscle, avoiding potent effects of PPARγ activation in other tissues (3, 6, 42), and avoidance of a systemic phenotype. Another strength of this work is avoidance of high TZD concentrations (>10−6 m), which have PPARγ-independent effects (43–45) and avoidance of constructs that couple PPARγ to exogenous transcriptional activation domains. Nonetheless, interpretation of our results must heed that overexpressed PPARγ could enter into modes of action that do not occur at endogenous levels. Tempering this caution is the high degree to which these results fit with previous loss-of-function studies. Additionally, our in vivo studies demonstrates that only very modest PPARγ overexpression, less than endogenous levels in brown adipose tissue, is needed to greatly enhance insulin signaling at the level of AKT phosphorylation.
It is informative to compare PPARγ action in muscle with its action in liver, where it is also expressed at low levels. Similar to our findings in muscle, PPARγ action in liver promotes lipid accumulation (15). However, contrasting our muscle findings, PPARγ action in liver does not promote insulin sensitivity (46) and actually worsens hepatic insulin resistance in ob/ob mice (47). The direct action of PPARγ in skeletal muscle to improve proximal insulin signaling differs from that of PPARα, a related transcription factor, and PPARγ coactivator-1α, a related transcriptional coactivator. PPARα contributes to insulin resistance in muscle (22), an effect thought to be related to increased fatty acid oxidation (48). PPARγ coactivator-1α overexpression in skeletal muscle induces insulin resistance, an effect thought mediated by increased CD36 expression and lipid uptake (49). By contrast, fatty acid oxidation does not appear to have a major role in PPARγ action, and the PPARγ-induced increase in CD36 and fatty acid uptake does not cause insulin resistance in skeletal muscle. It is possible that simultaneous activation of PPARγ plus PPARα and/or PPARβ/δ could have beneficial effects on fatty acid uptake, oxidation, and insulin sensitivity.
Surprisingly, rosiglitazone did not require demonstrable increases in PPAR activity to induce improvements in lipid-inhibited insulin signaling. Furthermore, an irreversible antagonist of PPARγ did not prevent rosiglitazone from improving insulin signaling. These results suggest that rosiglitazone may restore insulin signaling in a manner independent of PPARγ. These results, although provocative, do not allow definitive conclusions about rosiglitazone's mechanisms of actions. Although there is extensive precedent for PPARγ-independent actions of rosiglitazone, these invariably involve high concentrations (e.g. 5–100 μm), whereas we observe rosiglitazone actions at concentrations as low as 5–20 nm. Nanomolar-range nongenomic rosiglitazone actions have been reported (50) but, in contrast to our findings, occur rapidly and are inhibited by GW9662 and thus presumably require PPARγ in a nongenomic role.
Endogenous expression of PPARγ in human skeletal muscle varies considerably between individuals (3–5). Our results predict that individuals with higher PPARγ expression in skeletal muscle will have enhanced insulin signaling, fatty acid disposal, and sensitivity to TZD. This prediction is supported by the fact that skeletal muscle PPARγ message levels are lower in obese subjects with insulin resistance compared with those with intact insulin sensitivity (51).
We conclude that in skeletal muscle, direct PPARγ action induces a program of increased myocellular fatty acid uptake while remarkably preserving insulin-induced AKT phosphorylation and glycogen formation. Interventions to selectively raise PPARγ expression or activity in skeletal muscle may have beneficial effects on metabolic health.
Supplementary Material
Acknowledgments
We acknowledge the kind gifts of adPPARγ from Dr. Janardan Reddy (Department of Pathology, Northwestern University Medical School, Chicago, IL) and pPPREx3-luc from Dr. Xiang Fang (Department of Biochemistry, University of Iowa). We also thank Terry Kaduce, and Dr. Chris Adams, Dr. Ken Volk (University of Iowa), and Dr. Kohjiro Ueki (University of Tokyo, Tokyo, Japan) for advice, and Brian Fink (University of Iowa) for experimental assistance and advice.
This work was supported by National Institutes of Health Grants R01-DK081548 and K08-DK064906 (to A.W.N.) and by Veterans Affairs Medical Research Funds (W.I.S.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: PPAR-γ;
Ligands: Rosiglitazone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ad
- Adenoviral
- AKT
- thymoma viral proto-oncogene
- DAG
- diacylglycerol
- 2DG
- 2-[1,2-3H]-deoxy-D-glucose
- FABPpm
- fatty acid binding protein plasma membrane
- FATP
- fatty acid transport protein
- GFP
- green fluorescent protein
- IRS1
- insulin receptor substrate 1
- KRH
- Krebs-Ringer-HEPES buffer
- MuPPARγKO
- muscle-specific PPARγ knockout
- PDK1
- phosphoinositide-dependent protein kinase 1
- PI3K
- phosphatidylinositol 3 kinase
- PKC
- protein kinase C
- PPARγ
- peroxisome proliferator-activated receptor γ
- TA
- tibialis anterior
- TZD
- thiazolidinedione.
References
- 1. Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JP. 2010. The association between the peroxisome proliferator-activated receptor-γ2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 171:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersen KF, Krssak M, Inzucchi S, Cline GW, Dufour S, Shulman GI. 2000. Mechanism of troglitazone action in type 2 diabetes. Diabetes 49:827–831 [DOI] [PubMed] [Google Scholar]
- 3. Loviscach M, Rehman N, Carter L, Mudaliar S, Mohadeen P, Ciaraldi TP, Veerkamp JH, Henry RR. 2000. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia 43:304–311 [DOI] [PubMed] [Google Scholar]
- 4. Kruszynska YT, Mukherjee R, Jow L, Dana S, Paterniti JR, Olefsky JM. 1998. Skeletal muscle peroxisome proliferator- activated receptor-γ expression in obesity and non- insulin-dependent diabetes mellitus. J Clin Invest 101:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapsys NM, Kriketos AD, Lim-Fraser M, Poynten AM, Lowy A, Furler SM, Chisholm DJ, Cooney GJ. 2000. Expression of genes involved in lipid metabolism correlate with peroxisome proliferator-activated receptor γ expression in human skeletal muscle. J Clin Endocrinol Metab 85:4293–4297 [DOI] [PubMed] [Google Scholar]
- 6. Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. 2007. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 117:1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. 2003. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 112:608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. 2003. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 9:1491–1497 [DOI] [PubMed] [Google Scholar]
- 9. Tontonoz P, Spiegelman BM. 2008. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- 10. Norris AW, Hirshman MF, Yao J, Jessen N, Musi N, Chen L, Sivitz WI, Goodyear LJ, Kahn CR. 2008. Endogenous peroxisome proliferator-activated receptor-γ augments fatty acid uptake in oxidative muscle. Endocrinology 149:5374–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaak EE. 2004. Basic disturbances in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc 63:323–330 [DOI] [PubMed] [Google Scholar]
- 12. Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. 1999. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 274:26761–26766 [DOI] [PubMed] [Google Scholar]
- 13. Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA, Romijn JA, Havekes LM, Voshol PJ. 2003. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res 44:2270–2277 [DOI] [PubMed] [Google Scholar]
- 14. Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. 2005. Activation of peroxisome proliferator-activated receptor α by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther 314:260–270 [DOI] [PubMed] [Google Scholar]
- 15. Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. 2003. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J Biol Chem 278:498–505 [DOI] [PubMed] [Google Scholar]
- 16. Mauvais-Jarvis F, Virkamaki A, Michael MD, Winnay JN, Zisman A, Kulkarni RN, Kahn CR. 2000. A model to explore the interaction between muscle insulin resistance and β-cell dysfunction in the development of type 2 diabetes. Diabetes 49:2126–2134 [DOI] [PubMed] [Google Scholar]
- 17. Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- 18. Signorelli P, Hannun YA. 2002. Analysis and quantitation of ceramide. Methods Enzymol 345:275–294 [DOI] [PubMed] [Google Scholar]
- 19. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 20. Ueki K, Yamauchi T, Tamemoto H, Tobe K, Yamamoto-Honda R, Kaburagi Y, Akanuma Y, Yazaki Y, Aizawa S, Nagai R, Kadowaki T. 2000. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J Clin Invest 105:1437–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. 2007. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292:C125–C136 [DOI] [PubMed] [Google Scholar]
- 22. Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. 2005. A potential link between muscle peroxisome proliferator- activated receptor-α signaling and obesity-related diabetes. Cell Metab 1:133–144 [DOI] [PubMed] [Google Scholar]
- 23. Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. 2003. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 100:15924–15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coll T, Alvarez-Guardia D, Barroso E, Gómez-Foix AM, Palomer X, Laguna JC, Vázquez-Carrera M. 2010. Activation of peroxisome proliferator-activated receptor- δ by GW501516 prevents fatty acid-induced nuclear factor-κb activation and insulin resistance in skeletal muscle cells. Endocrinology 151:1560–1569 [DOI] [PubMed] [Google Scholar]
- 25. Sauerberg P, Olsen GS, Jeppesen L, Mogensen JP, Pettersson I, Jeppesen CB, Daugaard JR, Galsgaard ED, Ynddal L, Fleckner J, Panajotova V, Polivka Z, Pihera P, Havranek M, Wulff EM. 2007. Identification and synthesis of a novel selective partial PPARδ agonist with full efficacy on lipid metabolism in vitro and in vivo. J Med Chem 50:1495–1503 [DOI] [PubMed] [Google Scholar]
- 26. Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. 2002. Peroxisome proliferator-activated receptor-α regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51:901–909 [DOI] [PubMed] [Google Scholar]
- 27. Bastie CC, Hajri T, Drover VA, Grimaldi PA, Abumrad NA. 2004. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes 53:2209–2216 [DOI] [PubMed] [Google Scholar]
- 28. Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. 2002. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41:6640–6650 [DOI] [PubMed] [Google Scholar]
- 29. Casamayor A, Morrice NA, Alessi DR. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J 342(Pt 2):287–292 [PMC free article] [PubMed] [Google Scholar]
- 30. Sablina AA, Hector M, Colpaert N, Hahn WC. 2010. Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res 70:10474–10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du K, Herzig S, Kulkarni RN, Montminy M. 2003. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300:1574–1577 [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Liu M, Riojas RA, Xin X, Gao Z, Zeng R, Wu J, Dong LQ, Liu F. 2009. Protein kinase Cθ (PKCθ)-dependent phosphorylation of PDK1 at Ser504 and Ser532 contributes to palmitate-induced insulin resistance. J Biol Chem 284:2038–2044 [DOI] [PubMed] [Google Scholar]
- 33. Labbé SM, Croteau E, Grenier-Larouche T, Frisch F, Ouellet R, Langlois R, Guérin B, Turcotte EE, Carpentier AC. 2011. Normal postprandial nonesterified fatty acid uptake in muscles despite increased circulating fatty acids in type 2 diabetes. Diabetes 60:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelley DE, Simoneau JA. 1994. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Digel M, Staffer S, Ehehalt F, Stremmel W, Ehehalt R, Füllekrug J. 2011. FATP4 contributes as an enzyme to the basal and insulin mediated fatty acid uptake of C2C12 muscle cells. Am J Physiol Endocrinol Metab 301:E785–E796 [DOI] [PubMed] [Google Scholar]
- 36. Sato O, Kuriki C, Fukui Y, Motojima K. 2002. Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor α and γ ligands. J Biol Chem 277:15703–15711 [DOI] [PubMed] [Google Scholar]
- 37. Frohnert BI, Hui TY, Bernlohr DA. 1999. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem 274:3970–3977 [DOI] [PubMed] [Google Scholar]
- 38. Benton CR, Koonen DP, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heikkila JJ, Bonen A. 2006. Differential effects of contraction and PPAR agonists on the expression of fatty acid transporters in rat skeletal muscle. J Physiol 573:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verma NK, Singh J, Dey CS. 2004. PPAR-gamma expression modulates insulin sensitivity in C2C12 skeletal muscle cells. Br J Pharmacol 143:1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. 2001. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Cζ, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes 50:2210–2218 [DOI] [PubMed] [Google Scholar]
- 41. Hue L, Taegtmeyer H. 2009. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE. 2004. Selective disruption of PPARγ 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA 101:10703–10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J, Hsueh WA, Beavo JA, Coleman RA, Bornfeldt KE. 2007. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-γ-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakajima T, Iwasawa K, Oonuma H, Imuta H, Hazama H, Asano M, Morita T, Nakamura F, Suzuki J, Suzuki S, Kawakami Y, Omata M, Okuda Y. 1999. Troglitazone inhibits voltage-dependent calcium currents in guinea pig cardiac myocytes. Circulation 99:2942–2950 [DOI] [PubMed] [Google Scholar]
- 45. Brunmair B, Gras F, Neschen S, Roden M, Wagner L, Waldhäusl W, Fürnsinn C. 2001. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-γ-mediated changes in gene expression. Diabetes 50:2309–2315 [DOI] [PubMed] [Google Scholar]
- 46. Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. 2003. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278:34268–34276 [DOI] [PubMed] [Google Scholar]
- 47. Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr, Reitman ML, Gonzalez FJ. 2003. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 111:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56 [DOI] [PubMed] [Google Scholar]
- 49. Benton CR, Holloway GP, Han XX, Yoshida Y, Snook LA, Lally J, Glatz JF, Luiken JJ, Chabowski A, Bonen A. 2010. Increased levels of peroxisome proliferator-activated receptor γ, coactivator 1α (PGC-1α) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia 53:2008–2019 [DOI] [PubMed] [Google Scholar]
- 50. Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, Shirai A, Nakamura M, Iso-O N, Li Y, Hara M, Tsukamoto K, Moriyama N, Kudo A, Kawakami H, Yamauchi T, Kubota N, Kadowaki T, Kume H, Enomoto Y, Homma Y, Seki G, Fujita T. 2011. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPAR-dependent nongenomic signaling. Cell Metab 13:550–561 [DOI] [PubMed] [Google Scholar]
- 51. Macias-Gonzalez M, Moreno-Santos I, García-Almeida JM, Tinahones FJ, Garcia-Fuentes E. 2009. PPARγ2 protects against obesity by means of a mechanism that mediates insulin resistance. Eur J Clin Invest 39:972–979 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.