Abstract
Progesterone receptor (PgR) controls the menstrual cycle, pregnancy, embryonic development, and homeostasis, and it plays important roles in breast cancer development and progression. However, the requirement of coregulators for estrogen-induced expression of the PgR gene has not been fully explored. Here we used RNA interference to demonstrate dramatic differences in requirements of 10 different coregulators for estrogen-regulated expression of six different genes, including PgR and the well-studied TFF1 (or pS2) gene in MCF-7 breast cancer cells. Full estrogen-induced expression of TFF1 required all ten coregulators, but PgR induction required only four of the 10 coregulators. Chromatin immunoprecipitation studies demonstrated several mechanisms responsible for the differential coregulator requirements. Actin-binding coregulator Flightless-I, required for TFF1 expression and recruited to that gene by estrogen receptor-α (ERα), is not required for PgR expression and not recruited to that gene. Protein acetyltransferase tat-interactive protein 60 and ATP-dependent chromatin remodeler Brahma Related Gene 1 are recruited to both genes but are required only for TFF1 expression. Histone methyltransferase G9a is recruited to both genes and required for estrogen-induced expression of TFF1 but negatively regulates estrogen-induced expression of PgR. In contrast, histone methyltransferase myeloid/lymphoid or mixed-lineage leukemia 1 (MLL1), pioneer factor Forkhead box A1, and p160 coregulator steroid receptor coactivator-3 are required for expression of and are recruited to both genes. Depletion of MLL1 decreased ERα binding to the PgR and TFF1 genes. In contrast, depletion of G9a enhanced ERα binding to the PgR gene but had no effect on ERα binding to the TFF1 gene. These studies suggest that differential promoter architecture is responsible for promoter-specific mechanisms of gene regulation.
Estrogen regulates growth, differentiation, and other diverse functions in a broad range of target tissues. The canonical mechanism of estrogen receptor (ER)-α action involves binding of estrogen to ERα followed by receptor dimerization and binding to specific estrogen response elements (ERE) located in the promoter or enhancer region of target genes (1). Recently advances in microarray technologies and analysis methods for large-scale genomic expression studies have defined complete sets of estrogen-regulated genes for specific cell and tissue types (2–6). In parallel, global analysis of ERα binding by chromatin immunoprecipitation (ChIP) combined with microarray (ChIP-on-chip) or sequencing (ChIP-seq) has identified the ERα binding sites associated with target genes, providing opportunities for studying the mechanism of transcription complex assembly and the roles of coregulators and pioneer transcription factors in ERα-mediated expression of target genes (5, 7–9). Such studies have suggested that the mechanism by which transcription is activated by ERα can vary on different target genes of ERα.
The progesterone receptor gene (PgR) is an important estrogen-induced gene in normal mammary epithelial and breast cancer cells (10, 11). The PgR gene has two alternate transcription start sites (TSS) that produce two protein isoforms, PgR-A (94 kDa) and PgR-B (120 kDa). Although the transcription of PgR is directly regulated by ERα, there are no consensus ERE motifs for ERα binding near the transcription start sites (10). Early studies suggested that ERα is recruited to the PgR promoter region through interactions with Sp1 or activator protein-1 and a nearby ERE motif half-site (12–14). However, a later genome-wide analysis failed to detect ERα binding near the TSS sites but identified two major ERα binding sites located 168 and 206 kb upstream of the PgR-B TSS (5). A very recent study identified seven ERα binding sites (including the −168 kb and −206 kb sites) located between 48 and 311 kb upstream from the PgR-B TSS; chromatin conformation capture analyses demonstrated that three of these sites (−168 kb, −221 kb, and −311 kb) associate with the TSS region in a 17β-estradiol (E2)-dependent manner, suggesting that these sites are responsible for mediating E2-induced transcription of the PgR gene (15). However, the molecular mechanisms for activation of PgR gene transcription by hormone-activated ERα and its coregulators remain unclear.
Upon hormone treatment, ERα recruits transcriptional coregulators to its target genes. The three steroid receptor coactivator (SRC) or p160 coactivators, which are among the best-characterized nuclear receptor coregulators, function as scaffold proteins to recruit other coregulators (16–18). ATP-dependent chromatin remodeling complexes, histone-modifying enzymes, and many coregulators that contribute to transcription complex assembly through protein-protein interactions have also been implicated in transcriptional activation of various target genes of ERα (19, 20). Several hundred coregulators have been identified so far, and their roles in activating expression of nuclear receptor target genes have been partially characterized for a few coregulators. At the same time, differential requirements for specific coregulators on different target genes of the same nuclear receptor have been observed by several groups (21–25). Selective coregulator requirements are also observed for other types of transcription factors. For example p300, a central coactivator for hypoxic induction of erythropoietin, is required for induction of some target genes of hypoxia-inducible factor 1α, such as VEGF, but not for other target genes, such as PGK and LDHA (26).
Most recently integrative mass spectrometry-based analysis identified extensive interaction networks derived from the human endogenous coregulator complexome and revealed that many coregulators assemble into multisubunit protein complexes (27). It is noteworthy that not all coregulators form biochemically stable protein complexes but instead participate in many transient protein-protein interactions on target genes to facilitate transcriptional activation after recruitment by nuclear receptors (28). For instance, SRC-3 makes many transient interactions with nuclear receptors and a diverse array of other coregulators in a cell state-dependent manner (29). Thus, it appears that most coregulators participate in multiple transient interactions with other coregulators, possibly to form gene-specific coregulator complexes, which are required for efficient recruitment to or activation of specific target genes, depending on the specific regulatory context and chromatin architecture of each promoter or enhancer element.
In the current study, we conducted a systematic comparison of the requirements for 10 different coregulators on the estrogen-induced expression of six different genes in MCF-7 breast cancer cells, including the TFF1 (or pS2) and PgR genes. The dramatically different coregulator requirements of these two genes were then used to explore the different mechanisms of transcription complex assembly on these two genes and the specific contributions of selected coregulators to the transcription process. ChIP was used to explore whether different coregulator requirements involved differential recruitment of coregulators to the two genes by ERα or differences in the chromatin architecture or regulatory context of the TFF1 and PgR promoters. We also demonstrated divergent effects of some coregulators on the stable association of ERα with ERE associated with the TFF1 and PgR promoters.
Results
Induction of PgR gene expression in breast cancer cells treated with E2
To establish the time frame for coregulator action, we first examined PgR gene expression in breast cancer cells as a function of time after addition of E2. The increase in PgR mRNA level was observed as early as 3 h after E2 addition and remained elevated for at least 24 h after E2 addition (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Because steady-state mRNA levels are dependent on not only transcription rate but also RNA processing and degradation rate, we also examined the PgR pre-mRNA level, which is a better indicator of the instantaneous rate of transcription (Supplemental Fig. 1B). The PCR primers used detect pre-mRNA for both PgR-A and PgR-B. E2 treatment increased PgR pre-mRNA levels within 3 h, and a further elevation was observed at 24 h.
To confirm the importance of ERα for E2 induction of PgR gene expression, MCF-7 cells were treated with ERα antagonist ICI 182.780 (ICI) before and during E2 treatment of MCF-7 cells. ICI treatment almost completely inhibited E2 induction of PgR gene expression (Supplemental Fig. 1C). In addition MDA-MB231 breast cancer cells, which lack ERα, did not exhibit increased levels of PgR mRNA after E2 treatment (Supplemental Fig. 1D), supporting the ERα dependency of PgR gene expression.
Differential requirement of nuclear receptor coactivators for E2-regulated expression of six different genes
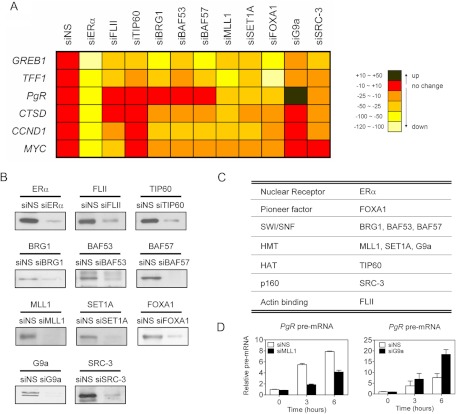
To explore the phenomenon of selective coregulator requirement more comprehensively, we selected 10 coregulators with previously established roles in ERα-mediated transcription and examined their requirement for E2-induced expression of six different target genes of ERα (GREB1, TFF1, PgR, CTSD, CCND1, and MYC) (Fig. 1A). MCF-7 cells were transfected with nonspecific siRNA (siNS) or small interfering RNA (siRNA) specific for each coregulator, and depletion of each coregulator was monitored by immunoblotting (Fig. 1B). Similar levels of depletion were obtained in multiple experiments (Supplemental Fig. 2). Forkhead box A1 (FOXA1) is a pioneer factor required for efficient binding of ERα to ERE in a chromatin environment (8, 9, 30). Brahma Related Gene 1 (BRG1, a core ATPase), BAF53 (BRG1 associated factor 53), and BAF57), are components of the SWI/SNF (SWItch/Surcrose NonFermentable) chromatin-remodeling complex which is one of the first coregulators to occupy a number of ERα target genes upon E2 treatment and is required for E2-induced expression of several genes (24, 31, 32). Myeloid lymphoid or mixed-lineage leukemia (MLL1) and SET domain containing 1A (SET1A) are histone H3 Lys-4 specific methyltransferases required for E2-induced expression of and ERα binding to several target genes (24). Tat-interactive protein 60 (TIP60) is a histone acetyltransferase required for E2-induced expression of and acetylation of H2A on multiple target genes (24). Flightless-I (also called FLII), an actin binding protein, is required for E2-induced recruitment of the SWI/SNF complex to the TFF1 promoter region (31). G9a, a histone H3 Lys-9 (H3K9) methyltransferase with corepressor and coactivator activities, and p160 coactivator SRC-3 are differentially required for E2-induced expression of several target genes of ERα (22, 23). Altogether this set represents a broad range of coregulator types that contribute to transcription complex formation by a variety of mechanisms (Fig. 1C).
Fig. 1.
Effect of depletion of endogenous coregulators on the expression of ERα target genes. A, MCF-7 cells were transfected with siRNA specific for each mRNA listed on top of the matrix or with siNS and grown in hormone-free media for 72 h. Cells were then treated with E2 for 24 h before harvesting, and total RNA was analyzed for each target gene mRNA by qRT-PCR and normalized to the level of GAPDH mRNA. Each colored square represents the relative expression level of the target gene in E2-treated, coregulator-depleted cells compared with siNS-transfected MCF-7 cells after E2 treatment (first column on the left). The color code table on the right shows the percent change in the hormone-induced expression level indicated by each color. The formula used to calculate the percent change is explained in Supplemental Fig. 3. In the siNS-treated cells, the fold induction by E2 was: GREB1, 8.0-fold; TFF1, 8.6-fold; PgR, 6.9-fold; CTSD, 2.3-fold; CCND1, 2.2-fold; MYC, 1.8-fold. Results shown are representative of at least three independent experiments. B, Depletion of coregulators in MCF-7 cells by siRNA. MCF-7 cells were transfected with siRNA against coregulators or siNS. Levels of the indicated proteins were assessed by immunoblotting. C, Summary of nuclear receptor coregulators that were used in this study (right column) along with their functional class (left column). HMT, Histone methyltransferase; HAT, histone acetyltransferase. D, Effect of MLL1 or G9a depletion on estrogen-induced levels of PgR pre-mRNA. MLL1 or G9a was depleted from MCF-7 cells as in A, and pre-mRNA levels for PgR were determined by qRT-PCR as in Supplemental Fig. 1B, after 0, 3, and 6 h of treatment with E2.
After transfection with siNS, expression of the six target genes of ERα was determined by quantitative RT-PCR (qRT-PCR) before and after 24 h of E2 treatment, and the level of mRNA observed after E2 treatment was used as a baseline (indicated by the red color in Fig. 1A, left column) for comparison with the level of mRNA observed after E2 treatment of cells transfected with the coregulator-directed siRNA. The color code in Fig. 1A indicates the extent to which each coregulator-directed siRNA caused the E2-induced level of mRNA to increase (Fig. 1, black) or decrease (Fig. 1, shades of orange and yellow, representing different percentages of change) relative to the E2-treated siNS control. Calculation of the percent change was performed as described in Supplemental Fig. 3. As an additional control to test this system of evaluation, we depleted ERα and observed a dramatic decrease in the E2-induced mRNA level for all six target genes (Fig. 1A, second column from the left).
Among the 10 coregulators tested, only the depletion of the histone H3 Lys-4 (H3K4) methyltransferases MLL1 or SET1A or depletion of the pioneer factor FOXA1 reduced E2-induced expression of all six target genes of ERα. Depletion of each of the other seven coregulators decreased E2-induced expression of some but not all target genes. The three SWI/SNF subunits (BRG1, BAF53, or BAF57) were required for full E2-induced expression of all target genes except PgR. SRC-3 was required for induction of all target genes except MYC; in agreement, a previous study also found that SRC-3 is required for induction of TFF1, PgR, and CCND1 but not MYC (22). FLII was required for induction of four of six genes, whereas TIP60 and G9a were required only for induction of two of six genes (GREB1 and TFF1). As reported previously (23), the depletion of G9a reduced induction of some target genes (TFF1 and GREB1) but enhanced induction of the PgR gene, indicating that endogenous G9a functions as a ERα coactivator for some target genes but serves as a corepressor to limit induction of other target genes. For most of the 10 coregulators, an additional siRNA directed against a different sequence in the mRNA was tested and produced similar results (Supplemental Fig. 4) to those represented in Fig. 1A.
Assessing the coregulator requirements of the six different target genes, each gene had a unique pattern of coregulator requirements. GREB1 and TFF1 required all 10 coregulators for efficient induction, whereas PgR induction required only four of the 10 coregulators, thus establishing extremely diverse coregulator requirements for these genes. Yu et al. (25) recently reported that the coregulators Deleted in Breast Cancer 1 (DBC1) and Cell division Cycle and Apoptosis Regulator 1 (CCAR1) were also required for induction of TFF1 but not for induction of PgR in MCF-7 cells, further reinforcing the conclusion that induction of PgR expression by E2 involves a dramatically different set of coregulators than induction of TFF1 and GREB1.
Because G9a depletion enhanced the E2-induced level of PgR mRNA whereas depletion of MLL1 reduced the E2-induced PgR mRNA level, we examined the effect of these depletions on the level of PgR pre-mRNA to test whether these coregulators are acting on the rate of transcription rather than mRNA stability. As observed for the mRNA levels, depletion of MLL1 reduced the E2 induction of PgR pre-mRNA, whereas depletion of G9a enhanced the E2-induced level of PgR pre-mRNA (Fig. 1D).
Differential recruitment of coregulators to enhancer and promoter regions of the PgR and TFF1 genes
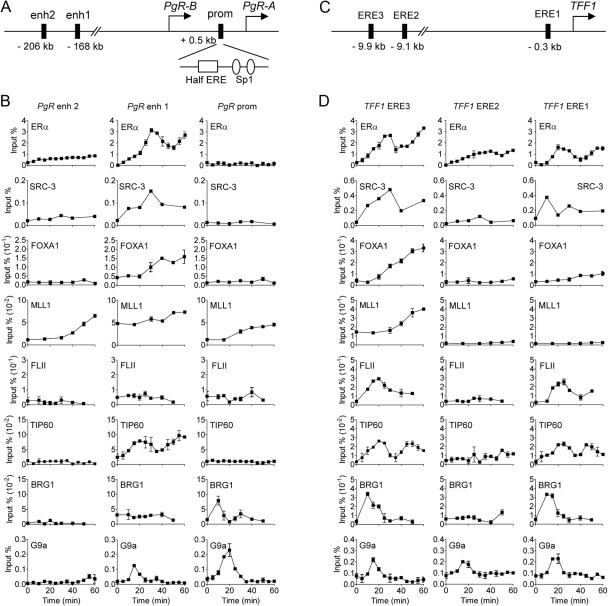
To explore the molecular mechanisms responsible for differential coregulator requirements of different ERα target genes, we compared the recruitment of coregulators to the defined regulatory regions of the TFF1 and PgR genes in MCF-7 cells within 60 min after E2 treatment. In the PgR gene, we focused on the ERα binding sites located at −168 and −206 kb upstream from the PgR-B TSS (5, 15); and in the proximal promoter region, we also examined the ERE half-site located between the PgR-A TSS and the PgR-B TSS (12, 14) (Fig. 2A). In the TFF1 gene, we examined the major ERα binding site at −9.9 kb (relative to the TSS, also called ERE3), the secondary promoter-proximal binding site at −0.3 kb (also called ERE1), and the minor ERα binding site at −9.1 kb (also called ERE2) (Fig. 2C), as in our previous studies of TFF1 (24, 31). In the PgR gene, we failed to observe any significant recruitment of ERα to the promoter region during 60 min of E2 treatment. However, a high level of ERα occupancy occurred at the −168 kb site (also called enhancer 1), and much weaker binding was observed at the −206 kb region (also called enhancer 2) (Fig. 2B). Moreover, ERα occupancy at the −168 kb region occurred in a cyclical manner with peaks at 30 and 60 min or later after the addition of E2, a timing very similar to that observed for ERα binding to the two major ERα binding sites associated with the TFF1 gene (Fig. 2D) (32–34).
Fig. 2.
Recruitment of nuclear receptor coregulators to PgR and TFF1 genes in E2-treated MCF-7 cells. A and C, Schematic diagrams of PgR gene (A) or TFF1 gene (C). Shown are the locations of upstream ERα binding sites for the PgR gene (5), the ERα binding site in the PgR promoter region (half ERE) (12), and ERα binding sites near the TFF1 gene (58). B and D, Time-course ChIP assays were performed with MCF-7 cells in 15-cm dishes treated with 100 nm E2 for the indicated time. The amount of the indicated region of PgR gene precipitated by antibodies specific for each coregulator was determined by quantitative PCR. Results are expressed as a percent of input chromatin (before immunoprecipitation). Results shown are mean and range of variation of duplicate PCR performed on the same DNA sample, are from a single experiment, and are representative of at least three independent experiments performed on different days.
A comparison of coregulator recruitment to the regulatory sites on the PgR gene (Fig. 2B) and TFF1 gene (Fig. 2D) revealed some patterns that were essentially identical and some that were dramatically different. First, SRC-3 and FOXA1, which were required for E2 induction of both genes, were recruited in an E2-dependent manner to the major ERα binding sites associated with both genes and not to the sites at which ERα did not bind. According to Carroll et al. (5), there are overlapping ERα and FOXA1 binding sites at enh1 (−168 kb) and enh2 (−206 kb) of the PgR gene. Since we did not detect FOXA1 binding at enh2 using the same primers used for ERα, we tested additional primer sets that mapped within the FOXA1 footprint reported by Carroll et al. (5) and observed hormone-dependent FOXA1 occupancy just downstream from the ERα binding region (Supplemental Fig. 5). Second, similar to SRC-3 and FOXA1, the major recruitment sites of MLL1, which was required for induction of both TFF1 and PgR, were the major ERα binding sites of both genes; however, E2 also induced MLL1 occupancy to a lesser extent at the −206 kb and promoter-proximal sites of the PgR gene (which bound ERα weakly or not at all) but not at the two minor ERα binding sites on the TFF1 gene. Third, at the other extreme, FLII was recruited transiently to the two major ERα binding sites of the TFF1 gene, in which it was required for induction, but did not exhibit E2-induced occupancy of any of the three regulatory sites associated with the PgR gene, in which it was not required for expression. Fourth, in contrast, TIP60 was recruited in a similar temporal pattern to the major ERα binding sites on both target genes, even though it was required for E2-induced expression of only the TFF1 gene (but not the PgR gene). Fifth, BRG1 was recruited to the two major ERα binding sites of the TFF1 gene, in which it was required for induction; but on the PgR gene, in which it was not required for induction, BRG1 was recruited to the promoter region but not to the major ERα binding site at −168 kb. Sixth and finally, G9a, which was required for the TFF1 induction but inhibited PgR induction, was recruited transiently to the major ERα binding sites and promoter proximal regions of both genes. It is also noteworthy that each coregulator had a specific and unique temporal pattern of recruitment: some were transient, some were cyclical, and some increased steadily during the 60 min of E2 treatment. Furthermore, the diverse patterns of coregulator recruitment and the diverse patterns of correlation and noncorrelation between the coregulator recruitment and the coregulator requirement for E2-induced expression (Table 1) suggest that multiple mechanisms are responsible for the coregulator-specific target gene requirements, as addressed further in Discussion.
Table 1.
Coregulator recruitment to PgR gene and requirement for PgR gene expression
| Effect of coregulator depletion on PgR mRNA level | ChIP | Coregulators |
|---|---|---|
| No change | Not recruited | FLII |
| Recruited | TIP60, BRG1 | |
| Decrease | Recruited | MLL1, FOXA1, SRC-3 |
| Increase | Recruited | G9a |
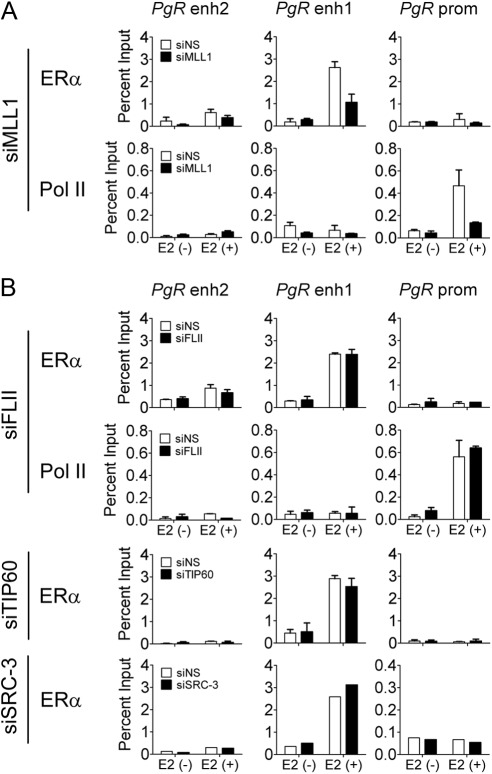
MLL1 is required for ERα binding to PgR enhancer
The distinct effects of MLL1 and G9a on PgR and TFF1 gene expression led us to further investigate and compare the mechanisms by which these two proteins function as coactivators or corepressors for E2 induction of the PgR and TFF1 genes. MLL1 is required for E2 induction of both genes; in contrast, G9a is required for induction of TFF1 but limits the induction of PgR (Fig. 1A). We showed previously that depletion of MLL1 in MCF-7 cells reduces global H3K4 methylation, H3K4 methylation at the TFF1 regulatory sites, ERα binding to the TFF1 gene, and recruitment of coregulators such as TIP60 (24). Depletion of MLL1 by transfection with specific siRNA duplexes also decreased the occupancy by ERα at PgR enhancer 1 (−168 kb) after 60 min of E2 treatment and consequently decreased RNA polymerase II occupancy at the promoter region (Fig. 3A). Thus, MLL1 is required for ERα occupancy on regulatory sites of the PgR and TFF1 genes and for initiation of transcription of both genes.
Fig. 3.
Effect of coregulator depletion on the recruitment of ERα and RNA polymerase II to PgR gene. ChIP assays were performed as in Fig. 2B after transfection with siNS, small interfering (si) RNA against MLL1 (siMLL1) siFLII, siTIP60, or siSRC-3. MCF-7 cells were treated with E2 (100 nm) for 60 min. The amount of the indicated region of PgR gene precipitated by ERα or polymerase II antibodies was determined by quantitative PCR.
In contrast, depletion of FLII or TIP60 (which are not required for E2 induction of the PgR gene) did not affect the binding of ERα to the PgR gene (Fig. 3B). Depletion of FLII also had no effect on RNA polymerase II occupancy on the PgR promoter region. Previously we showed that FLII and TIP60 are both required for induction of the TFF1 gene and that their depletion affects RNA polymerase II occupancy on the TFF1 TSS but does not affect binding of ERα to the TFF1 EREs (24, 31). Depletion of SRC-3, a coregulator that is required for full E2 induction of PgR gene expression, also had no effect on binding of ERα to PgR enhancer 1 at −168 kb (Fig. 3B). Thus, on genes in which they are required for induction, MLL1 is required for binding of ERα to the ERE, whereas FLII, TIP60, and SRC-3 are required for steps downstream from the binding of ERα to the ERE. This is consistent with previous findings in E2-inducible gene systems that SRC proteins serve as scaffolds for recruitment of other coregulators (16–18), FLII is involved in the recruitment of the SWI/SNF complex (31), and TIP60 acetylates histone H2A at Lys-5 (24). Thus, each coregulator makes specific contributions to the transcription initiation process, and the contributions of each coregulator are required for some target genes but not for others.
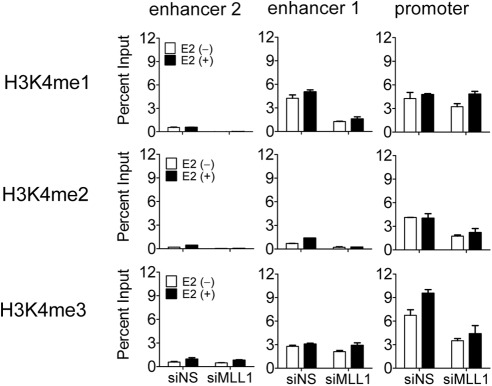
MLL1 deposits activation-associated histone H3K4 methylation marks on the PgR gene
To investigate the function of MLL1 at the PgR gene, we tested the effect of MLL1 depletion on H3K4 methylation at the regulatory sites of the PgR gene. H3K4 monomethylation peaks are generally observed at enhancer elements of active or potentially active genes, and H3K4 trimethylation peaks are generally associated with the TSS regions of such genes (35–39). As expected, high levels of H3K4 monomethylation at enhancer 1 and H3K4 trimethylation at the promoter region were observed before E2 treatment, and trimethylation at the promoter region was further increased after 60 min of E2 treatment (Fig. 4). A slight but marginal increase of monomethylation at enhancer 1 was also observed after E2 treatment. Both basal and E2-induced monomethylation at enhancer 1 and trimethylation at the promoter region were decreased by depletion of MLL1. H3K4 dimethylation was also decreased at these regulatory sites of the PgR gene. These results indicate that MLL1 is at least partially responsible for maintaining the basal H3K4 methylation status at enhancer and promoter regions of the PgR gene and is also involved in E2-induced increases of H3K4 methylation at these sites.
Fig. 4.
Histone H3K4 methylation status in MLL1-depleted MCF-7 cells. ChIP assays were performed as in Fig. 2B after transfection with siNS or siMLL1. MCF-7 cells were treated with E2 (100 nm) for 0 or 60 min. The amount of the indicated region of PgR genes precipitated by H3K4 methyl-specific antibodies was determined by quantitative PCR.
Mechanism of corepressor function by G9a on E2-induced PgR gene expression
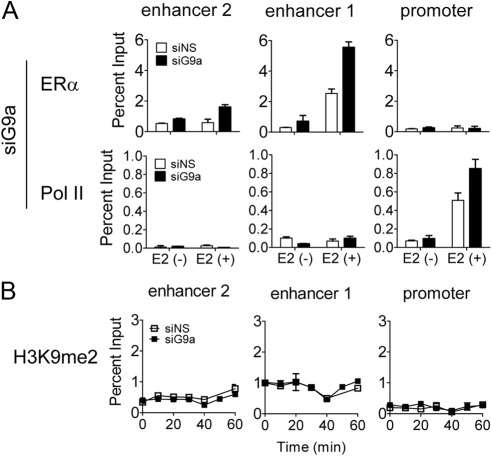
G9a maintains the repression of some genes by recruiting transcriptional repressors and corepressors such as CDP (CCAAT displacement protein)/cut, PRDM1 (PR domain containing 1), and REST (RE1-silencing transcription factor) (40–42). However, in the case of the PgR gene, G9a acts as a corepressor in a different scenario, in which it limits the extent of E2-induced gene expression. We therefore explored the mechanism by which G9a exerts this different type of transcriptional repression. First, we tested whether G9a depletion affects the occupancy of ERα and RNA polymerase II on the PgR gene. Transfection of siRNA directed against G9a increased the basal and E2-induced occupancy of ERα at 1; RNA polymerase II occupancy at the PgR promoter region was also increased (Fig. 5A), consistent with the increased E2-induced expression of PgR observed when G9a is depleted (Fig. 1A). To determine whether histone H3K9 methylation by G9a is required for its corepressor function on the PgR gene, we performed ChIP with antibody specific for histone H3K9 dimethylation (the characteristic mark made by G9a) after transfecting MCF-7 cells with nonspecific or G9a-directed siRNA and treating cells with E2 for various time periods (Fig. 5B). G9a depletion caused no significant change of histone H3K9 dimethylation at the regulatory sites of the PgR gene. Therefore, it appears that G9a limits E2-induced expression of the PgR gene by interfering with the binding of ERα to enhancer 1 and thereby inhibiting subsequent assembly of an active transcription complex, including recruitment of RNA polymerase II. The inhibition of ERα binding by G9a is apparently not caused by H3K9 methylation and thus involves a different mechanism of repression by G9a.
Fig. 5.
Effect of depletion of G9a on ERα and RNA polymerase II recruitment to PgR gene. ChIP assays were performed as in Fig. 2B after transfection with siNS or siG9a. MCF-7 cells were treated with E2 (100 nm) for 0 or 60 min (A) or for the indicated time (B). The amount of the indicated region of PgR genes precipitated by ERα, RNA polymerase II or H3K9me2 specific antibodies was determined by quantitative PCR.
Discussion
Gene-specific coregulator requirements and their significance
There are now many examples of individual coregulators that are differentially required for hormone-induced expression of multiple target genes of the same nuclear receptor (21–24). In the current study, we conducted a more broad and systematic analysis with 10 coregulators and six different target genes of ERα. Our results demonstrated that each of the six different E2-induced genes has a substantially different pattern of coregulator requirements in the same cell line. Moreover, E2 induction of PgR gene expression has a dramatically different pattern of coregulator requirement than induction of the other five ERα target genes. For example, PgR gene expression required only four of the 10 coregulators tested, whereas efficient induction of TFF1 and GREB1 required all 10 coregulators. The previous finding that two additional coregulators (DBC1 and CCAR1) are required for E2 induction of TFF1 expression but not PgR expression (25) further emphasizes this difference. Because coregulators are responsible for remodeling chromatin, recruiting RNA polymerase II, and activating transcription, the mechanism for accomplishing these tasks must be dramatically different for these two genes, i.e. the process is conducted by largely different sets of coregulators on these two genes.
Recent evidence suggests that specific physiological programs depend on the differential coregulator requirements of specific target genes of ERα. For example, a study of the three p160 coactivators revealed that depletion of SRC-2 or SRC-3, but not SRC-1, decreased proliferation of MCF-7 cells; and PgR mRNA expression was significantly decreased only by SRC-3 depletion, not by depletion of SRC-1 or SRC-2 (22). It has now been well established that the SRC proteins undergo a variety of posttranslational modifications, which result from activation of various signaling pathways, and different modifications are connected with the roles of these coregulators in different physiological responses (43). These examples indicate that the overall set of genes regulated by E2 (or any nuclear receptor ligand) can be altered by modulating the activity of a specific coregulator, which is required for the hormonal regulation of a subset of the ERα target genes. Because all coregulators are probably targets for posttranslational modifications, it seems likely that gene-specific coregulator requirements represent a vast and largely unexplored mechanism for, in essence, fine-tuning the set of genes that respond to E2.
Mechanisms of coregulator specificity
What are the mechanisms responsible for the different coregulator requirements for E2 induction of the TFF1 and PgR genes? Our results indicate at least two different mechanisms at work, the first of which is differential coregulator recruitment. FLII is required for E2 induction of TFF1 and is recruited by ERα to the TFF1 gene regulatory sites (Figs. 1A and 2D) (31). However, FLII is not required for E2-induced expression of PgR and is not recruited to the PgR gene regulatory sites (Figs. 1A and 2B). A second mechanism is illustrated by TIP60 and BRG1, which are recruited to the regulatory sites of both genes in response to E2 but are required only for E2-induced expression of TFF1, not PgR (Figs. 1 and 2). TIP60 is recruited to the primary ERα binding sites of both genes; however, BRG1 is recruited to the ERα binding sites of the TFF1 gene but only to the promoter region of the PgR gene (Fig. 2). In these cases the coregulators are recruited to the PgR gene but are not required for gene activation, possibly because they are redundant with other coregulators that are also present.
The reasons for target gene-specific recruitment and requirement of coregulators are currently unclear but presumably involve differences in promoter environment of each target gene. These promoter differences could explain why coregulators are recruited to some target genes but not others, why there are different spatial or temporal patterns of coregulator occupancy on different target genes, and why the specific services of a particular coregulator are needed for some target genes but not others. What could be the nature of these differences in promoter environment that explain differential coregulator recruitment and requirements? It is now well established that the specific DNA sequence in a particular steroid receptor binding site serves as an allosteric modulator of steroid receptor conformation and thereby alters the coregulator binding specificity and transcriptional activity of the steroid receptor (44–50). In addition, each gene has a specific combination of regulatory sites, which are occupied by various transcription factors; thus, the different complement of transcription factors associated with different target genes, along with the coregulators that each transcription factor recruits, provides a different regulatory environment for each gene that could help to explain differential recruitment and requirements of coregulators for different target genes. Finally, chromatin architecture, which is still poorly understood, could vary on different target genes, creating variable environments that influence what types of coregulators are recruited or are required to remodel the chromatin into an active conformation and assemble an active transcription complex.
Mechanisms of coregulator action
The current study demonstrated several different mechanisms by which coregulators contribute to the process of transcriptional activation, including the following: 1) maintenance of the chromatin architecture of regulatory sites; 2) regulation of ERα binding to the ERE; and 3) acting downstream from ERα binding to make or read histone modifications, recruit other coregulators, or recruit RNA polymerase II and other components of the basal transcription machinery. MLL1, which makes H3K4 methylation, provided examples of the first and second mechanisms. Similar to our previous findings for the TFF1 gene (24), depletion of MLL1 in MCF-7 cells reduced basal and hormone-induced levels of H3K4 methylation at key regulatory sites associated with the PgR gene (Fig. 4). Because H3K4 monomethylation peaks are present on all active and potentially active enhancer elements (35–39), it is likely that they are involved in maintaining the chromatin conformation required to facilitate efficient transcription factor binding; and indeed, depletion of MLL1 dramatically reduced ERα binding to the PgR gene (Fig. 3A) and the TFF1 gene (24). H3K4 methylation at ERE has also been shown to be important for binding of FOXA1, a pioneer factor that binds to enhancer elements located near many ERE and facilitates ERα recruitment upon hormone treatment (5).
Examples of the third mechanism, acting downstream from ERα binding, were previously illustrated for the TFF1 gene in MCF-7 cells by the role of coregulator FLII in the E2-depedent recruitment of SWI/SNF complex (31) and the role of TIP60 in E2-induced acetylation of histone H4 at Lys-5 and recruitment of RNA polymerase II (24).
G9a exemplified the second mechanism of coregulator action mentioned above. G9a is one of the major histone H3K9 methyltransferases in higher eukaryotes and is responsible for H3K9 mono- and dimethylation in euchromatin (51, 52). G9a is known best as a corepressor, but G9a also has a positive effect on the expression of some genes, by either direct or indirect mechanisms (23, 53–55). Depletion of G9a in MCF-7 cells enhanced E2-induced expression of the PgR gene (Fig. 1A), suggesting that endogenous G9a functions as a corepressor to limit the E2-induced expression of PgR. Depletion of G9a also increased binding of ERα to the enhancer 1 site of the PgR gene and the binding of RNA polymerase II to the PgR promoter region (Fig. 5A). The enhanced E2-dependent recruitment of ERα to the PgR gene presumably accounts partially or fully for the enhanced E2-induced expression of PgR after G9a depletion (Fig. 1A). Because there was no change in H3K9 dimethylation after G9a depletion, the corepressor action of G9a on the PgR gene apparently involves other repression domains of G9a (42, 56, 57) rather than the histone methylation domain. In contrast to its negative effect on E2-induced expression of PgR, endogenous G9a was required for E2-induced expression of TFF1 and GREB1 (Fig. 1A) (23). This illustrates the ability of G9a to function as either a coactivator or corepressor, depending on the regulatory context of the target gene promoter.
Materials and Methods
Chemicals and cell culture
E2 and ICI were purchased from Sigma-Aldrich (St. Louis, MO). All siRNA were synthesized by Dharmacon (Lafayette, CO) as oligonucleotide duplex. MCF-7 cells were maintained in DMEM with 10% fetal bovine serum. Before E2 treatment, the cells were transferred to phenol red-free DMEM containing 5% charcoal dextran-treated fetal bovine serum for 3 d.
Antibodies and immunoblot
Immunoblotting was performed as described previously (31). Briefly, MCF-7 cells were plated into a six-well plate and then transfected with siRNA using oligofectamine (Invitrogen, Carlsbad, CA). Seventy-two hours later, cells were harvested and lysed with radioimmunoprecipitation assay buffer [20 mm Tris-Cl (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 2 mm EDTA]. Total cell lysates were used for immnoblotting, using the following antibodies: anti-ERα, anti-FLII, anti-TIP60, anti-BRG1, anti-BAF53, anti-MLL1, anti-SET1A, anti-SRC-3 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-FOXA1, anti-G9a (Abcam, San Francisco, CA); anti-SET1A (Bethyl, Montgomery, TX). Anti-BAF57 antibody was kindly provided by Karen E. Knudsen (Thomas Jefferson University, Philadelphia, PA).
RNA interference
Small interfering RNA experiments were performed according to previously published methods (31). The sequences of siRNA used were: siNS, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (sense), 5′-ACGUGACACGUUCGGAGAAdTdT-3′ (antisense); small interfering ERα, 5′-CUACAGGCCAAAUUCAGAUdTdT-3′ (sense), 5′-AUCUGAAUUUGGCCUGUAGdTdT-3′ (antisense); small interfering FLII, 5′-CAACCUGACCACGCUUCAUdTdT-3′ (sense), 5′-AUGAAGCGUGGUCAGGUUGdTdT-3′ (antisense); small interfering TIP60, 5′-CCUCAAUCUCAUCAACUACdTdT-3′ (sense), 5′-GUAGUUGAUGAGAUUGAGGdTdT-3′ (antisense); small interfering BAF53, 5′-GCUUUCCUUGAAAUGCACUdTdT-3′ (sense), 5′-AGUGCAUUUCAAGGAAAGCdTdT-3′ (antisense); small interfering BAF57, 5′-CCGCGUACCUUGCUUACAUdTdT-3′ (sense), 5′-AUGUAAGCAAGGUACGCGGdTdT-3′ (antisense); small interfering BRG1, 5′-CAUGCACCAGAUGCACAAGdTdT-3′ (sense), 5′-CUUGUGCAUCUGGUGCAUGdTdT-3′ (antisense); small interfering MLL1, 5′-GAUUCGAACACCCAGUUAUdTdT-3′ (sense), 5′-AUAACUGGGUGUUCGAAUCdTdT-3′ (antisense); small interfering SET1A, 5′-CGGAAGAAGAAGCUCCGAUdTdT-3′ (sense), 5′-AUCGGAGCUUCUUCUUCCGdTdT-3′ (antisense); small interfering FOXA1, 5′-GCGAAGUUUAAUGAUCCACdTdT-3′ (sense), 5′-GUGGAUCAUUAAACUUCGCdTdt-3′ (antisense); small interfering SRC-3, 5′-GAUAUAAUCCGAAGGUGUAUU-3′ (sense), 5′-UACACCUUCGGAUUAUAUCUU-3′ (antisense); small interfering G9a, SMARTpool siRNA targeting G9a (Dharmacon). Transfections in MCF-7 cells were performed using Oligofectamine (Invitrogen) according to the manufacturer's protocol.
Quantitative RT-PCR
Total RNA was isolated from MCF-7 cells with Trizol (Invitrogen), after hormone treatment as indicated, and subjected to reverse transcription by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Then 0.2 μl of diluted reverse transcription product was used for quantitative PCR analysis in the Stratagene Mx3000P system with SYBRGreen dye (Roche, Indianapolis, IN). The primers used were: TFF1, 5′-GAACAAGGTGATCTGCG-3′ (forward) and 5′-TGGTATTAGGATAGAAGCACCA-3′ (reverse); GREB1, 5′-CAAAGAATAACCTGTTGGCCCTGC-3′ (forward) and 5′-GACATGCCTGCGCTCTCATACTTA-3′ (reverse); PgR, 5′-GTGCCTATCCTGCCTCTCAATC-3′ (forward) and 5′-CCCGCCGTCGTAACTTTCG-3′ (reverse); PgR pre-mRNA, 5′-CAGGTCTACCCGCCCTATCT-3′ (forward) and 5′-TATATTCTGCGCCCACCTTC-3′ (reverse); CTSD, 5′-GTACATGATCCCCTGTGAGAAGGT-3′ (forward) and 5′-GGGACAGCTTGTAGCCTTTGC-3′ (reverse); CCND1, 5′-AAGCTCAAGTGGAACCT-3′ (forward) and 5′-AGGAAGTTGTTGGGGC-3′ (reverse); MYC, 5′-CTCTCAACGACAGCAGCTCG-3′ (forward) and 5′-CAACATCGATTTCTTCCTCATCTTC-3′ (reverse); β-actin, 5′-ACCCCATCGAGCACGGCATCG-3′ (forward) and 5′-GTCACCGGAGTCCATCACGATG-3′ (reverse); GAPDH, 5′-TCTGGTAAAGTGGATATTGTTG-3′ (forward) and 5′-GATGGTGATGGGATTTCC-3′ (reverse). Relative expression levels were normalized to the GAPDH mRNA levels. Results shown are mean and range of variation for duplicate PCR reactions performed on the same cDNA sample; the results are from a single experiment that is representative of at least three independent experiments conducted on different days.
ChIP assay
ChIP assays were performed according to previously described protocols (31). Briefly, MCF-7 cells were transfected with siRNA and then cultured for 3 d in phenol red-free DMEM supplemented with 5% charcoal-dextran-stripped fetal bovine serum. At approximately 90% confluency, cells were treated with 100 nm E2 for the indicated time. After cross-linking with formaldehyde, cell extracts were prepared from control and E2-treated MCF-7 cells. Immunoprecipitation of sonicated chromatin solutions was conducted by overnight incubation at 4 C with normal mouse or rabbit IgG, anti-ERα, anti-TIP60, anti-BRG1, anti-FLII (Santa Cruz Biotechnology); anti-MLL1, anti-SRC-3 (Bethyl); anti-G9a (Sigma-Aldrich); anti-FOXA1, anti-H3K4me1, anti-H3K9me2 (Abcam); anti-H3K4me2, anti-H3K4me3 (Active Motif, Carlsbad, CA); or anti-RNA Polymerase II (Millipore, Billerica, MA). Cross-linking was reversed by heating, and immunoprecipitated DNA was purified by phenol-chloroform extraction and ethanol precipitation. The purified DNA was dissolved in 100 μl of a buffer of 10 mm Tris-HCl (pH 8.0) and 1 mm EDTA and analyzed by quantitative PCR using the Stratagene Mx3000P system with SYBRGreen dye (Roche). The primers used were: PgR promoter region, 5′-AGGGAGGAGAAAGTGGGTGT-3′ (forward) and 5′-GGAGAACTCCCCGAGTTAGG-3′ (reverse); PgR enhancer 1 region, 5′-GCCTGACCTGTTGCTTCAAT-3′ (forward) and 5′-GCAGGACGACTTCTCAGACC-3′ (reverse); PgR enhancer 2 region, 5′-AACGTGTTTGCATCTTGCTG-3′ (forward) and 5′-GGGCTGGCTTTTATCATTCA-3′ (reverse); TFF1 ERE1, 5′-CCGGCCATCTCTCACTATGAA-3′ (forward) and 5′-CCTTCCCGCCAGGGTAAATAC-3′ (reverse); TFF1 ERE2 5′-CCTCCCCAGCTCACGTTGT-3′ (forward) and 5′-GGGTTGCATTTAAGGGACCTT-3′ (reverse); TFF1 ERE3 5′-GTCGTTGCCAGCGTTTCC-3′ (forward) and 5′-CTTCTCCACGCCCTGTAAATTT-3′ (reverse).
Supplementary Material
Acknowledgments
We thank Dan Gerke and Kelly Chang (University of Southern California) for expert technical assistance and Karen E. Knudsen (Thomas Jefferson University, Philadelphia, PA) for anti-BAF57 antibody.
This work was supported by Grants R01 DK043093 and R37 DK055274 from the National Institutes of Health.
Disclosure Summary: The authors declare no conflict of interest.
NURSA Molecule Pages†:
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- BAF
- BRG1 associated factor
- BRG1
- Brahma Related Gene 1
- CCAR1
- Cell division Cycle and Apoptosis Regulator 1
- ChIP
- chromatin immunoprecipitation
- DBC1
- Deleted in Breast Cancer 1
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- FOXA1
- Forkhead box A1
- H3K9
- histone H3 Lys-9
- ICI
- ICI 182.780
- MLL1
- myeloid/lymphoid or mixed-lineage leukemia
- PgR
- progesterone receptor
- qRT-PCR
- quantitative RT-PCR
- SET1A
- SET domain containing 1A
- siNS
- nonspecific siRNA
- si
- small interfering
- SRC
- steroid receptor coactivator
- SWI/SNF
- SWItch/Surcrose NonFermentable
- TIP60
- tat-interactive protein 60.
References
- 1. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. 2001. Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- 2. Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. 2003. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- 3. Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. 2005. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat 92:141–149 [DOI] [PubMed] [Google Scholar]
- 4. Lin CY, Ström A, Li Kong S, Kietz S, Thomsen JS, Tee JB, Vega VB, Miller LD, Smeds J, Bergh J, Gustafsson JA, Liu ET. 2007. Inhibitory effects of estrogen receptor β on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res 9:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 6. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y. 2009. An oestrogen receptor α-bound human chromatin interactome. Nature 462:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 9. Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL. 1995. Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. J Biol Chem 270:30693–30700 [DOI] [PubMed] [Google Scholar]
- 12. Xu X, Murdoch FE, Curran EM, Welshons WV, Fritsch MK. 2004. Transcription factor accessibility and histone acetylation of the progesterone receptor gene differs between parental MCF-7 cells and a subline that has lost progesterone receptor expression. Gene 328:143–151 [DOI] [PubMed] [Google Scholar]
- 13. Petz LN, Ziegler YS, Loven MA, Nardulli AM. 2002. Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology 143:4583–4591 [DOI] [PubMed] [Google Scholar]
- 14. Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. 2004. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol 88:113–122 [DOI] [PubMed] [Google Scholar]
- 15. Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. 2010. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol 24:346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JH, Li H, Stallcup MR. 2003. CoCoA, a nuclear receptor coactivator which acts through an N-terminal activation domain of p160 coactivators. Mol Cell 12:1537–1549 [DOI] [PubMed] [Google Scholar]
- 17. Lee YH, Campbell HD, Stallcup MR. 2004. Developmentally essential protein flightless I is a nuclear receptor coactivator with actin binding activity. Mol Cell Biol 24:2103–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YH, Kim JH, Stallcup MR. 2005. GAC63, a GRIP1-dependent nuclear receptor coactivator. Mol Cell Biol 25:5965–5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lonard DM, O'Malley BW. 2005. Expanding functional diversity of the coactivators. Trends Biochem Sci 30:126–132 [DOI] [PubMed] [Google Scholar]
- 20. Lonard DM, O'malley BW. 2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 21. Paul BD, Buchholz DR, Fu L, Shi YB. 2005. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J Biol Chem 280:27165–27172 [DOI] [PubMed] [Google Scholar]
- 22. Karmakar S, Foster EA, Smith CL. 2009. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-α transcriptional activity. Endocrinology 150:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Purcell DJ, Jeong KW, Bittencourt D, Gerke DS, Stallcup MR. 2011. A distinct mechanism for coactivator versus corepressor function by histone methyltransferase G9a in transcriptional regulation. J Biol Chem 286:41963–41971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong KW, Kim K, Situ AJ, Ulmer TS, An W, Stallcup MR. 2011. Recognition of enhancer element-specific histone methylation by TIP60 in transcriptional activation. Nat Struct Mol Biol 18:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji Yu E, Kim SH, Heo K, Ou CY, Stallcup MR, Kim JH. 2011. Reciprocal roles of DBC1 and SIRT1 in regulating estrogen receptor α activity and co-activator synergy. Nucleic Acids Res 39:6932–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang F, Zhang R, Wu X, Hankinson O. 2010. Roles of coactivators in hypoxic induction of the erythropoietin gene. PLoS One 5:e10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malovannaya A, Lanz RB, Jung SY, Bulynko Y, Le NT, Chan DW, Ding C, Shi Y, Yucer N, Krenciute G, Kim BJ, Li C, Chen R, Li W, Wang Y, O'Malley BW, Qin J. 2011. Analysis of the human endogenous coregulator complexome. Cell 145:787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulynko YA, O'Malley BW. 2011. Nuclear receptor coactivators: structural and functional biochemistry. Biochemistry 50:313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanz RB, Bulynko Y, Malovannaya A, Labhart P, Wang L, Li W, Qin J, Harper M, O'Malley BW. 2010. Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol 24:859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. 2011. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeong KW, Lee YH, Stallcup MR. 2009. Recruitment of the SWI/SNF chromatin remodeling complex to steroid hormone-regulated promoters by nuclear receptor coactivator flightless-I. J Biol Chem 284:29298–29309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. 2003. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- 33. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 34. Burakov D, Crofts LA, Chang CP, Freedman LP. 2002. Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J Biol Chem 277:14359–14362 [DOI] [PubMed] [Google Scholar]
- 35. Robertson AG, Bilenky M, Tam A, Zhao Y, Zeng T, Thiessen N, Cezard T, Fejes AP, Wederell ED, Cullum R, Euskirchen G, Krzywinski M, Birol I, Snyder M, Hoodless PA, Hirst M, Marra MA, Jones SJ. 2008. Genome-wide relationship between histone H3 lysine 4 mono- and tri-methylation and transcription factor binding. Genome Res 18:1906–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- 37. Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. 2002. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci USA 99:8695–8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, Richmond TA, Wu Y, Green RD, Ren B. 2005. A high-resolution map of active promoters in the human genome. Nature 436:876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837 [DOI] [PubMed] [Google Scholar]
- 40. Nishio H, Walsh MJ. 2004. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc Natl Acad Sci USA 101:11257–11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gyory I, Wu J, Fejér G, Seto E, Wright KL. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol 5:299–308 [DOI] [PubMed] [Google Scholar]
- 42. Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. 2004. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell 14:727–738 [DOI] [PubMed] [Google Scholar]
- 43. Han SJ, Lonard DM, O'Malley BW. 2009. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab 20:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heery DM, Kalkhoven E, Hoare S, Parker MG. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736 [DOI] [PubMed] [Google Scholar]
- 45. Hall JM, McDonnell DP, Korach KS. 2002. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol 16:469–486 [DOI] [PubMed] [Google Scholar]
- 46. Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. 2009. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 324:407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. 2004. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol 33:387–410 [DOI] [PubMed] [Google Scholar]
- 48. Wood JR, Greene GL, Nardulli AM. 1998. Estrogen response elements function as allosteric modulators of estrogen receptor conformation. Mol Cell Biol 18:1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wood JR, Likhite VS, Loven MA, Nardulli AM. 2001. Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol Endocrinol 15:1114–1126 [DOI] [PubMed] [Google Scholar]
- 50. Lefstin JA, Yamamoto KR. 1998. Allosteric effects of DNA on transcriptional regulators. Nature 392:885–888 [DOI] [PubMed] [Google Scholar]
- 51. Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. 2002. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 16:1779–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD. 2003. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell 12:1591–1598 [DOI] [PubMed] [Google Scholar]
- 53. Lee DY, Northrop JP, Kuo MH, Stallcup MR. 2006. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem 281:8476–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaturvedi CP, Hosey AM, Palii C, Perez-Iratxeta C, Nakatani Y, Ranish JA, Dilworth FJ, Brand M. 2009. Dual role for the methyltransferase G9a in the maintenance of β-globin gene transcription in adult erythroid cells. Proc Natl Acad Sci USA 106:18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC, Rossi FM, Zaph C. 2010. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med 207:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shinkai Y, Tachibana M. 2011. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev 25:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Epsztejn-Litman S, Feldman N, Abu-Remaileh M, Shufaro Y, Gerson A, Ueda J, Deplus R, Fuks F, Shinkai Y, Cedar H, Bergman Y. 2008. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat Struct Mol Biol 15:1176–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pan YF, Wansa KD, Liu MH, Zhao B, Hong SZ, Tan PY, Lim KS, Bourque G, Liu ET, Cheung E. 2008. Regulation of estrogen receptor-mediated long range transcription via evolutionarily conserved distal response elements. J Biol Chem 283:32977–32988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.