Abstract
Steroid hormone and MAPK signaling pathways functionally intersect, but the molecular mechanisms of this cross talk are unclear. Here, we demonstrate a functional convergence of the estrogen and c-Jun N-terminal kinase 1 (JNK1) signaling pathways at the genomic level in breast cancer cells. We find that JNK1 binds to many promoters across the genome. Although most of the JNK1-binding sites are constitutive, a subset is estrogen regulated (either induced on inhibited). At the estrogen-induced sites, estrogen receptor (ER)α is required for the binding of JNK1 by promoting its recruitment to estrogen response elements or other classes of DNA elements through a tethering mechanism, which in some cases involves activating protein-1. At estrogen-regulated promoters, JNK1 functions as a transcriptional coregulator of ERα in a manner that is dependent on its kinase activity. The convergence of ERα and JNK1 at target gene promoters regulates estrogen-dependent gene expression outcomes, as well as downstream estrogen-dependent cell growth responses. Analysis of existing gene expression profiles from breast cancer biopsies suggests a role for functional interplay between ERα and JNK1 in the progression and clinical outcome of breast cancers.
Diverse signaling pathways regulate a wide variety of cellular processes in mammalian cells, including global transcription programs, to control both physiological and disease states (1, 2). The signaling pathways controlled by estrogens, such as the predominant natural form 17β-estradiol (E2), are good examples of the signal-dependent transcriptional control of cellular outcomes. Estrogens bind to cognate nuclear estrogen receptor (ER) proteins, ERα and ERβ, which function as sequence-specific, DNA-binding transcription factors in the nucleus to directly regulate the transcription of estrogen-responsive genes (3–5). ER bind directly to genomic DNA through estrogen response element (ERE) sequences (6) or indirectly through other transcription factors [e.g. activating protein-1 (AP-1)] using a tethering mechanism (7–10), where they recruit a variety of coregulator proteins that mediate transcriptional outcomes (11, 12). The genes regulated by estrogens play key roles in the sexual development and fertility of both males and females (13, 14), as well as the regulation of metabolic processes in fat, liver, and bone tissues (15–19). They also play important roles in the aberrant mitogenic and proliferative processes that underlie breast and uterine cancer (20–22). In this regard, the expression of ERα in cells is a well-known prognostic indicator for breast cancers, and a variety of synthetic estrogen antagonists that target ER are used as therapeutic agents for breast cancers to reverse the mitogenic actions of estrogens (23–25).
In contrast to the nuclear actions of estrogens, growth factors act through cytoplasmic membrane receptors to stimulate intracellular signaling pathways, including MAPK cascades, which indirectly regulate gene expression through a variety of target transcription factors (26). The MAPK family comprises a conserved set of proteins that are activated by a series of upstream kinases that form a phosphorylation relay. Activated MAPK, including the c-Jun N-terminal kinases (JNK) and the ERK, phosphorylate downstream effectors at serine or threonine residues to control a variety of cellular processes (27–30). In addition to the direct stimulation of proliferation and cellular survival programs, growth factor signaling pathways functionally interact with estrogen signaling pathways to promote endocrine therapy-resistant growth of cancer cells. Indeed, functional cross talk between steroid hormone and growth factor/MAPK signaling pathways was demonstrated nearly two decades ago in steroid hormone-dependent cancers (31, 32), but our understanding of how these pathways converge at the genomic level to regulate gene expression remains incomplete.
AP-1, which is a heterodimer of c-Jun and c-Fos or related transcription factors, functions as a terminal downstream target of MAPK pathways (33, 34). The JNK family of MAPKs was first identified by its ability to specifically phosphorylate c-Jun to modulate the transcriptional activity of AP-1 (35–37). Subsequent studies have shown that JNK also phosphorylates and regulates the activity of other transcription factors in response to a variety of extracellular stimuli (27, 28). A number of previous studies have described considerable functional interplay between AP-1 and ER (10, 38–41), including interactions at the level of chromatin through the aforementioned ER tethering pathway. The extent to which JNK family members, such as JNK1, play a role in estrogen signaling pathways and where such potential functional interactions might occur in the cell has not been examined in detail.
The prevailing view in the literature has been that the kinase-mediated phosphorylation events regulating transcriptional outcomes do not occur at the genes that they ultimately regulate. Nonetheless, the terminal kinases of various signaling pathways are found in the nucleus under activating conditions (26, 42). In addition, gene-specific and genomic analyses in yeast (43, 44), Drosophila (45), and mammalian cells (42, 46–52) have shown that a number of signaling kinases bind to the promoters of genes whose expression they regulate. For example, AMPK activates transcription in response to cellular stress through direct association with chromatin and phosphorylation of histone H2B at serine 36 (50). Likewise, cyclin A/cyclin-dependent kinase 2 is recruited to gene promoters where it functions as a progesterone receptor coactivator (49). In addition, ERK2 is recruited to ERα-binding sites across the genome where it supports E2-induced gene expression (51). The extent to which other transcription factors and other kinase families collaborate in the nucleus in a similar manner remains to be determined.
In this study, we characterized the genomic relationships between ERα and JNK1 with respect to their binding to chromatin and subsequent transcriptional outcomes. Our results support a model for the estrogen- and ERα-dependent recruitment of preactivated JNK1 to the promoters of estrogen target genes. JNK1, in turn, serves as a coregulator of ERα required for efficient estrogen-dependent transcription of these genes and for downstream cell growth responses. Our study has identified a genomic nexus between the estrogen and JNK1 signaling pathways. Similar genomic systems are likely to integrate the signaling pathways for other steroid hormones and signal-regulated nuclear kinases in broader cellular processes.
Results
Activated/phosphorylated JNK1 localizes to the nuclei of MCF-7 cells
To explore the nuclear actions of JNK1 and its potential role in the estrogen signaling pathway, we used the ERα-positive MCF-7 human breast cancer cell line. We first examined the extent to which JNK1 localizes to the nucleus in MCF-7 cells and whether the natural ERα ligand E2 affects the activation (i.e. phosphorylation) and localization of JNK1. Like other MAPKs, JNK1 is regulated by the phosphorylation of a Thr-Pro-Tyr motif by upstream MAPK kinases (26, 27). Phosphorylation of JNK1 promotes its translocation into the nucleus and activation of its enzymatic activity (26, 27).
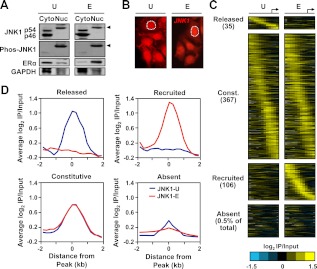
Fractionation of MCF-7 cells followed by Western blotting revealed that although JNK1 is present in both the cytoplasm and nucleus, only the phosphorylated form of JNK1 is detected in the nucleus (Fig. 1A; note the slower migrating JNK1 band in the nuclear samples shown in the top row, as well as the phosphorylated JNK band in the nuclear samples shown in the second row). Treatment of the cells with E2 did not alter the localization of JNK1 or the fraction of phosphorylated JNK1 (Fig. 1A). Immunofluorescent staining of the MCF-7 cells confirmed that JNK1 is located in the cytoplasm and nucleus and that E2 does not alter the localization of JNK1 (Fig. 1B). The constitutive JNK1 phosphorylation may be the result of human epidermal growth factor receptor 2- dependent MAPK hyperactivation (53), or it may be related to the elevated kinase activity associated with breast cancer cell lines (54). In either case, our results show that activated JNK1 is located in the nuclei of MCF-7 cells.
Fig. 1.
Estrogen signaling regulates the JNK1 genomic localization program in MCF-7 cells. A, MCF-7 cells were treated with vehicle (U) or E2 (E) for 45 min. Cytoplasmic (Cyto) and nuclear (Nuc) extracts were made from both conditions and analyzed by Western blotting for JNK1 and phospho-JNK1. Arrows indicate the migration of phosphorylated JNK1. ERα was used as a control for cytoplasmic and nuclear fractionation, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, Immunofluorescent staining of JNK1 (red signal) in MCF-7 cells before and after E2 treatment. One of the nuclei in each panel is denoted by a white dotted line. C, Analysis of promoter-proximal JNK1-binding sites in MCF-7 cells before and after E2 treatment by ChIP-chip using Nimblegen promoter arrays containing approximately 19,000 unique promoters tiled from 2200 bp upstream to 500 bp downstream of the TSS (arrow). The data are shown as heatmaps of the JNK1 ChIP-chip log2 enrichment ratios in both treatment conditions for all promoters with significant binding in either condition, and for 0.5% of JNK1-absent promoters. They are shown in categories of released, constitutive (Const.), recruited, and absent based on the fold changes of ChIP-chip signals between the E and U conditions. The promoters in each category are aligned by their positions relative to the TSS and ordered from those with the 5′-most JNK1 peak to those with the 3′-most JNK1 peak. D, Peak-centered averaging graphs (metagene analyses) of the log2 enrichment ratios from regions in the categories shown in C. The probe signals are centered on JNK1 peaks and averaged for all promoters across the region from −2 to + 2 kb relative to the JNK1 peak.
Estrogen signaling regulates the JNK1 genomic localization program in MCF-7 cells
Based on previous studies showing that a number of signaling kinases associate with chromatin, we considered the possibility that nuclear JNK1 may also associate with chromatin in MCF-7 cells. Furthermore, even though E2 treatment did not alter the subcellular distribution of JNK1, we also considered the possibility that E2 might alter the genomic localization of JNK1. To address these questions, we determined the genomic localization of JNK1 at all annotated promoters across the MCF-7 cell genome using chromatin immunoprecipitation (ChIP) coupled with hybridization to RefSeq promoter microarrays (i.e. ChIP-chip) spanning approximately −2 to +0.5 kb relative to the transcription start site. The ChIP DNA was prepared from MCF-7 cells treated with or without 100 nm E2.
Using stringent peak definition criteria, we identified more than 500 promoters with a significant peak of JNK1 in either treatment condition (∼2% of all promoters on the array) (Fig. 1C). Gene-specific ChIP-quantitative PCR (qPCR) of peak and nonpeak regions confirmed the ChIP-chip results (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Using a similar approach in gene-specific assays, we were unable to reliably ChIP JNK2 with two different antibodies (data not shown); whether this represents the biology of the system or a limitation of the antibodies is not clear. Interestingly, E2 treatment caused a redistribution of the JNK1 localization pattern, with 141 peaks changing upon E2 treatment. Thirty-five promoters showed a release of JNK1 upon E2 treatment, and 106 promoters showed a recruitment of JNK1 upon E2 treatment, whereas the majority of JNK1-bound promoters (367) were unaffected by E2 treatment (i.e. constitutively bound by JNK1) (Fig. 1C). Averaging of JNK1 peak-centered ChIP-chip data across these classes illustrates the distinct patterns of JNK1 promoter binding in response to E2 (Fig. 1D). These data show that JNK1 localizes to discrete genomic binding sites and that E2 regulates the JNK1 genomic localization program. Together with the data in Fig. 1, A and B, we conclude that E2 treatment alters the occupancy of activated JNK1 on gene promoters in MCF-7 cells without altering the overall nuclear pool of JNK1.
Gene ontology (GO) analyses showed that JNK1 binding is enriched in the promoters of genes that code for proteins involved in responses to stimuli, signal transduction, and RNA processing (Supplemental Table 1). These GO categories are driven largely by the group of genes with JNK1 constitutively bound at the promoter. A separate analysis of the genes associated with the JNK1-released and JNK1-recruited promoters also showed an enrichment of genes that code for proteins involved in transcriptional regulation and metabolism of steroids (Supplemental Table 1). The GO terms that we have identified here are similar to those that we have identified previously for a set of genes representing the most immediate transcriptional targets of the estrogen signaling pathway (55, 56). Together, these results point to a role for JNK1 in cell growth-controlling transcriptional responses, much like those observed in response to the mitogenic actions of estrogens.
E2-recruited JNK1 colocalizes with ERα at many target promoters
Given the estrogen-dependent alterations in the JNK1 genomic localization program, we tested the possibility that some JNK1 peaks might correspond to sites of ERα binding. To do so, we performed an ERα ChIP-chip analysis using the same array platform that we used for the JNK1 ChIP-chip in Fig. 1. Of the 508 significant peaks of JNK1 binding that we identified in the three groups (i.e. released, constitutive, and recruited), approximately 15% overlapped with an ERα-binding site (Supplemental Table 2). This increased dramatically to approximately 40% of the significant peaks of JNK1 when the analysis was limited to the 106 promoters with E2-induced binding of JNK1, suggesting the existence of two populations of E2-induced JNK1-binding sites: those that overlap with an ERα-binding site (ERα-positive, ∼40%) and those that do not overlap with an ERα-binding site (ERα-negative, ∼60%), based on direct positional overlap of significant JNK1 and ERα peaks (Supplemental Table 2). The latter group of E2-induced JNK1-binding sites (i.e. JNK1+/ERα−) may represent endpoints of membrane-initiated estrogen signaling pathways that do not drive ERα to chromatin, but instead connect to other transcription factors that may recruit JNK1 (57). In general, the binding of JNK1 at the ERα-negative sites was lower than at the ERα-positive sites, based on the ChIP-chip signals (Supplemental Fig. 2). In contrast to the E2-induced JNK1-binding sites, none of the 35 E2-released JNK1-binding sites overlapped with an ERα peak (Supplemental Table 2). For the purposes of this study, we focused on those promoters with overlapping peaks of JNK1 and ERα.
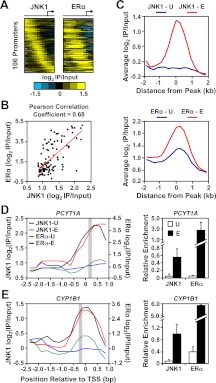
In a separate correlation analysis of the 106 promoters with E2-induced binding of JNK1, we observed a striking positive correlation between JNK1 and ERα binding (Fig. 2, A and B; Pearson correlation coefficient of 0.68). This analysis, which is more inclusive than the direct peak-to-peak comparison described above, because it considers all detectable ChIP-chip signals regardless of significance, suggests that there may be even greater colocalization of JNK1 and ERα binding. This result is further illustrated by 1) averaging ERα ChIP-chip signals centered on the E2-recruited JNK1 peaks (Fig. 2C) and 2) examining JNK1 and ERα ChIP signals from ChIP-chip and ChIP-qPCR data for the promoters of two target genes (PCYT1A and CYP1B1; Fig. 2, D and E, respectively). These analyses show a pattern of ERα promoter binding that is induced in response to E2, much like JNK1 binding, suggesting that JNK1 and ERα are corecruited to these genomic binding sites.
Fig. 2.
JNK1 recruitment correlates with ERα occupancy at target promoters in MCF-7 cells. A, Analysis of promoter-proximal JNK1 and ERα binding in MCF-7 cells. JNK1 and ERα ChIP-chip data from E2-treated MCF-7 cells for the 106 “JNK1-recruited” promoters from Fig. 1C are shown as heatmaps of log2 recruitment ratios aligned and ordered as in Fig. 1C. B, Pearson correlation analysis of the JNK1 and ERα ChIP-chip log2 recruitment ratios from A. C, Metagene analyses of the log2 enrichment ratios of JNK1 (top) and ERα (bottom) ChIP-chip data for the JNK1-recruited promoters from Fig. 1C before (U, in blue) and after (E, in red) E2 treatment. The probe signals are centered on the JNK1 peaks in both the JNK1 and ERα graphs. D and E, ChIP-chip (left) and ChIP-qPCR (right) analyses of JNK1 and ERα at two JNK1-recruited gene promoter regions (PCYT1A in D and CYP1B1 in E) in MCF-7 cells treated with vehicle (U) or E2 (E). The average JNK1 ChIP-qPCR signals (right) of peak regions defined by ChIP-chip (gray boxes in left panels) are consistent with the array profiles. For the ChIP-qPCR analyses, each bar represents the mean ± sem, n = 3.
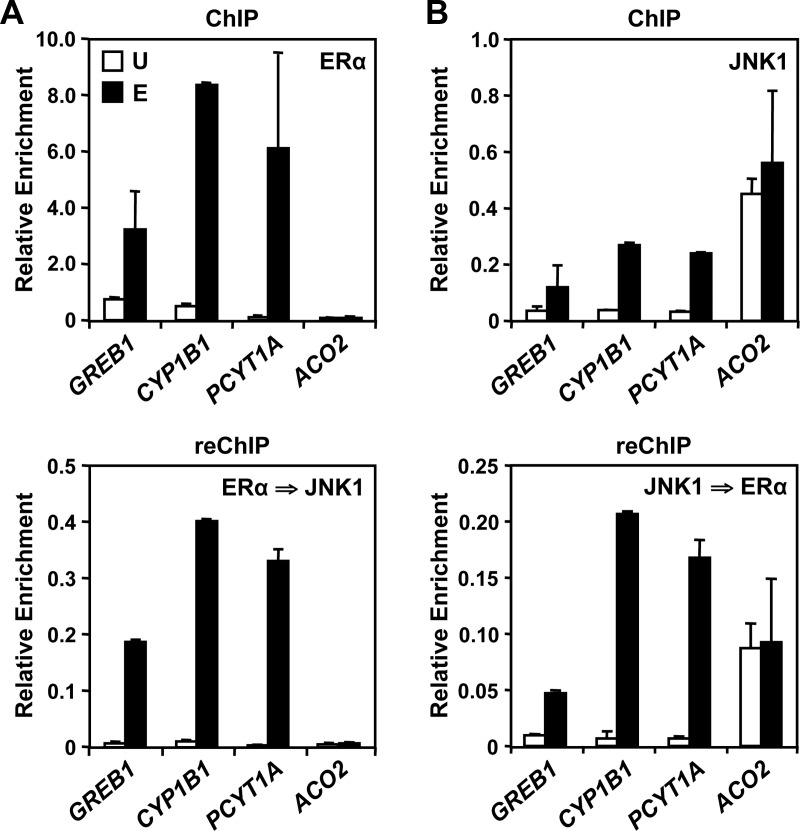
To confirm that JNK1 and ERα are present simultaneously at these binding sites, we performed ChIP-reChIP experiments for a selected set of JNK1-bound promoters (JNK1-recruited and constitutive). Whether we first immunoprecipitated ERα (Fig. 3A, top) or JNK1 (Fig. 3B, top), we were able to detect both proteins in the reChIP for three JNK1-recruited promoters (GREB1, CYP1B1, and PCYT1A; Fig. 3, bottom). In contrast, ERα did not show strong binding to the JNK1 constitutively bound promoter, ACO2, and we were thus unable to reChIP JNK1 at this promoter, as expected (Fig. 3A, bottom). These results demonstrate that, for these promoters, E2-recruited JNK1 cooccupies its binding sites with ERα, indicating that E2 signaling causes the convergence of ERα and JNK1 pathways on chromatin.
Fig. 3.
JNK1 and ERα colocalize at promoters of “JNK1-recruited” genes. A and B, ChIP-qPCR (top) and reChIP-qPCR (bottom) were performed reciprocally for ERα and JNK1 for three JNK1-recruited gene promoters (GREB1, CYP1B1, and PCYT1A) and one “JNK1-consititutive” gene promoter (ACO2) before (U) and after (E) E2 treatment. The initial ChIP (top) was performed using antibodies to ERα (A) or JNK1 (B). The recovered ChIP DNA was then immunoprecipitated using antibodies to JNK1 (A) or ERα (B) in a reChIP experiment (bottom). The ChIP and reChIP DNAs were analyzed by RT-qPCR. Each bar represents the mean ± sem, n = 3.
E2-dependent binding of JNK1 to many target promoters is dependent on ERα
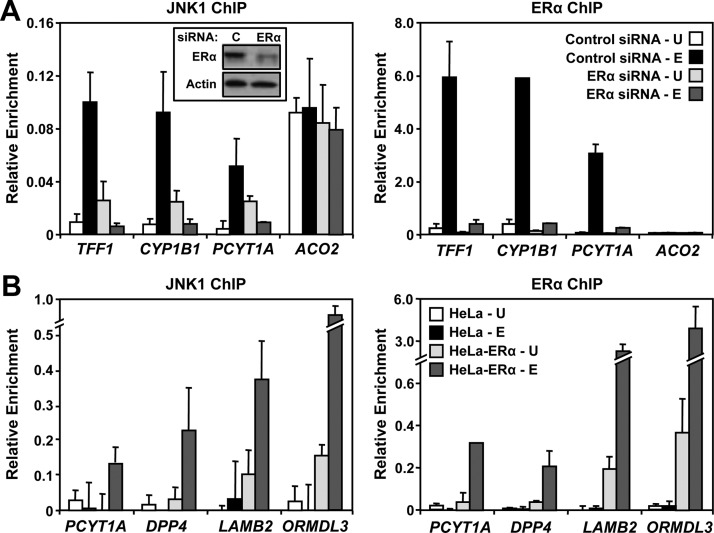
Corecruitment of JNK1 and ERα to specific sites in the genome after E2 treatment suggests a role for ERα in mediating the E2-induced genomic localization of JNK1 at these sites. To explore the dependency of E2-dependent JNK1 recruitment on ERα, we used small interfering RNAs (siRNAs) to knockdown ERα in MCF-7 cells. A pool of ERα-targeting siRNA, but not a control pool, effectively depleted ERα in the cells (Fig. 4A, left, inset), without affecting the cellular levels of JNK1 (Supplemental Fig. 3). As shown in Fig. 4A, it also blocked E2-dependent recruitment of ERα (right) and JNK1 (left) to target gene promoters (e.g. TFF1, CYP1B1, PCYT1A). In contrast, depletion of ERα did not affect the localization of JNK1 to the constitutive JNK1-bound promoter of ACO2 (Fig. 4A).
Fig. 4.
ERα binding at target promoters is required for JNK1 recruitment. A, MCF-7 cells were transfected with control or ERα siRNAs. Sixty hours after transfection, the cells were treated with vehicle (U) or E2 (E) for 45 min and collected for Western blotting or ChIP-qPCR analyses. ChIP-qPCR analyses of the E2-dependent JNK1 (left) and ERα (right) binding to three “JNK1-recruited” gene promoters (TFF1, CYP1B1, and PCYT1A) and one “JNK1-constitutive” gene promoter (ACO2) are shown. Each bar represents the mean ± sem, n = 3. Inset in left panel, Western blot analysis of siRNA-mediated ERα knockdown vs. control (C) is shown with β-actin as a loading control. B, ChIP-qPCR analyses of E2-dependent JNK1 (left) and ERα (right) binding to four gene promoters (PCYT2, DPP4, LAMB2, and ORMDL3) in parental HeLa cells and HeLa cells stably expressing ERα (HeLa-ERα). The cells were treated with vehicle (U) or E2 (E) for 45 min and analyzed by ChIP-qPCR. Each bar represents the mean ± sem, n = 3.
To further explore the dependency of E2-dependent JNK1 recruitment on ERα, we used HeLa cells lacking (i.e. HeLa) or stably expressing (i.e. HeLa-ERα) ERα. Using these two cell lines, we examined the promoter localization of ERα and JNK1 in response to E2 treatment. Although no promoter localization of ERα or JNK was observed in the ERα-negative HeLa cells, E2-induced ERα and JNK1 recruitment was observed at specific gene promoters in the HeLa-ERα cells (Fig. 4B), consistent with the results from the MCF-7 cells. Together, these experiments using two different strategies show definitively that functionally active ERα is required for the E2-dependent recruitment of JNK1 to target promoters where they colocalize.
Transcription factor-binding sites are found under JNK1 peaks
As noted above, ERα exhibits two distinct modes of genomic binding: 1) direct binding to genomic DNA through EREs or 2) indirect binding through other transcription factors (e.g. AP-1) using a tethering mechanism (10). To determine which mode of binding might direct the corecruitment of ERα and JNK1, we employed a series of bioinformatic analyses. First, we used Multiple Em for Motif Elicitation (MEME) and Motif Alignment and Search Tool (MAST) in an unbiased search for DNA sequence motifs enriched under the JNK1 peaks in E2-treated condition. These results yielded a number of high-confidence motifs (Fig. 5A and Supplemental Table 3). Using Transcription Element Search Software (TESS) to predict the transcription factors that might bind to these sequences, we identified AP-1, as well as other transcription factors not previously associated with ERα-dependent gene regulation (e.g. the POU homeodomain transcription factor POU1F1/pituitary-specific transcription factor 1; Fig. 5A). Of note, the motifs did not include a canonical ERE, but did include an ERE half-site.
Fig. 5.
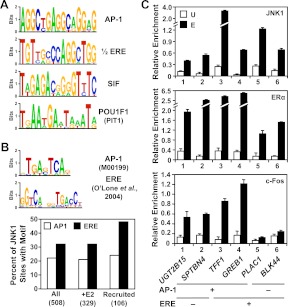
Motif analysis of JNK1 peaks. A, Unbiased search for DNA sequence motifs enriched under the JNK1-bound regions in the E2-treated condition using MEME. A selection of some of the most significantly enriched motifs are shown as web logos of the position weight matrices. Motif predictions were examined by TESS, as well visual inspection, to determine transcription factors that are most likely to bind to the indicated sequence, as indicated. B, Targeted search for AP-1 and ERα (i.e. ERE) binding motifs 1) under all JNK1 peaks in both vehicle- and E2-treated conditions, 2) JNK1 peaks in the E2-treated condition, and 3) under all JNK1-recruited peaks. Top, The position weight matrices used in the targeted search for AP-1 and ERα binding motifs are shown as web logos. The AP-1 position weight matrix is from TRANSFAC, whereas the ERα position weight matrix is based on information from O'Lone et al. (70). Bottom, The position weight matrices for the AP-1 and ERα motifs were used with MAST to map the location of the motifs under JNK1 peaks in a directed search. The percent of JNK1-binding sites in each of the indicated groups with an AP-1 or ERα motifs motif is shown. C, c-Fos localizes with JNK1 and ERα at target promoters containing an AP-1 binding motif. ChIP-qPCR analyses of JNK1, ERα, and c-Fos binding at JNK1- and ERα-recruited regions before (U) and after estrogen (E) treatment are shown. The UGT2B15, SPTBN4, TFF1, and GREB1 gene promoters contain at least one predicted AP-1 motif under the JNK1 peak. The TFF1, GREB1, and PLAC1 promoters, as well as the BLK44 distal enhancer (58), contain at least one ERE under the JNK1 peak. Each bar represents the mean ± sem, n = 3.
Next, in a directed search, we mapped probability weight matrices (obtained from TRANSFAC) for AP-1 and ERα binding motifs to the JNK1 peaks. Although full EREs were not identified in the unbiased search, we included the ERE probability weight matrix in this directed search based on our results showing a role for ERα in the E2-dependent recruitment of JNK1 to promoters. This analysis yielded high-confidence sites for the AP-1 and ERα binding motifs (Fig. 5B, top). For all groups tested, we observed a greater enrichment of ERα binding motifs (i.e. ERE) than AP-1 binding motifs (Fig. 5B, bottom). The results of these bioinformatic analyses, together with the ChIP-chip results described above, suggest that the E2-dependent recruitment of JNK1 occurs through ERα using both 1) direct binding to EREs and 2) a tethering mechanism mediated by AP-1 and possibly other DNA-binding transcription factors.
We tested the validity of our bioinformatics analyses using gene-specific ChIP-qPCR assays. For this analysis, we focused on JNK1-recruited promoters (and one ERα enhancer, BLK44) (58) containing high-confidence AP-1 motifs or EREs. Although these JNK1-recruited genomic regions showed E2-induced binding of JNK1 and ERα, as expected, only those with a high-confidence AP-1 motif showed binding of c-Fos, a component of the AP-1 heterodimer (Fig. 5C). Interestingly, the binding of c-Fos was also stimulated by E2 treatment (Fig. 5C, bottom), as we have reported previously (59), suggesting E2-induced binding of a complex containing ERα, AP-1, and JNK1 at target promoters. Together, these results support the validity of our bioinformatic analyses by demonstrating the recruitment of JNK1 and c-Fos to regions containing predicted AP-1 sites.
JNK1 acts as an ERα coregulator at E2-responsive genes
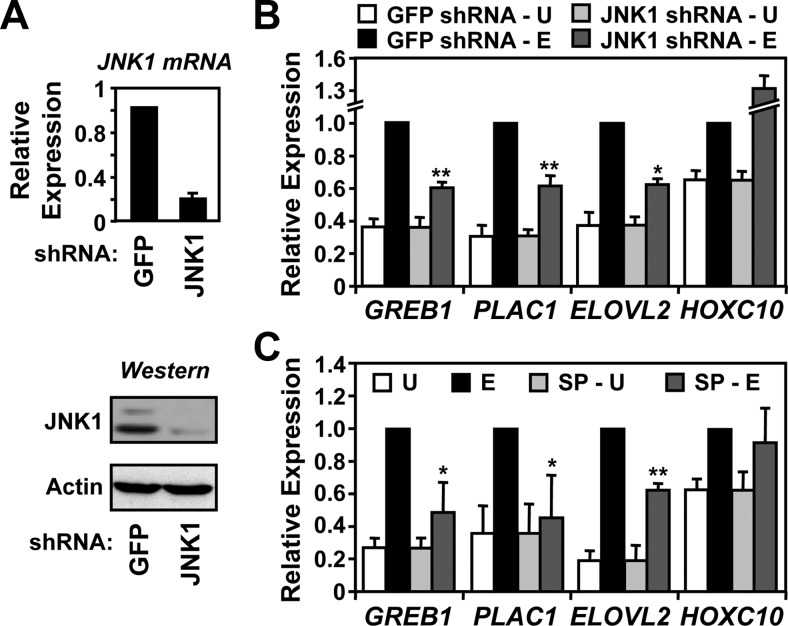
Next, we determined the role of JNK1 in the E2-dependent expression of target genes in MCF-7 cells using RT-qPCR coupled with knockdown of JNK1 or chemical inhibition of JNK1 kinase activity. As shown in Fig. 6A, stable expression of a short hairpin RNA (shRNA) resulted in efficient knockdown of both JNK1 mRNA (Fig. 6A, top) and protein (Fig. 6A, bottom). In parallel experiments, however, JNK1 knockdown did not reduce the total cellular levels of ERα (Supplemental Fig. 3). As expected, JNK1 resulted in reduced occupancy of JNK1 at the promoters of estrogen-regulated genes (Supplemental Fig. 4A), without an appreciable reduction in ERα binding (Supplemental Fig. 4B). Knockdown of JNK1 (Fig. 6B) or chemical inhibition of JNK catalytic activity using SP600125 (SP) (Fig. 6C) inhibited the E2-stimulated expression of some (e.g. GREB1, PLAC1, ELOVL2), but not all (e.g. HOXC10), estrogen target genes tested. The latter indicates a gene for which JNK1 is dispensable for transcription (i.e. HOXC10). In all cases tested, the results with JNK1 knockdown were consistent with those using the JNK inhibitor SP, including the HOXC10 gene, whose expression was not inhibited by either (Fig. 6, B and C). Importantly, treatment with SP had no appreciable effect on the binding of JNK1 or ERα to the promoters of the target genes (Supplemental Fig. 5). Thus, JNK1 protein and its kinase activity are required for full E2-dependent regulation of estrogen target genes in MCF-7 cells, implicating JNK1 as a hormone-dependent transcriptional coregulator of ERα. Interestingly, the effects of JNK1 knockdown on E2-dependent gene expression were observed even in the face of a compensatory increase in JNK2 mRNA (Supplemental Fig. 6).
Fig. 6.
JNK1 activity is required for full estrogen-dependent transcriptional responses at estrogen target promoters. A, JNK1 was stably knocked down in MCF-7 cells by retroviral-mediated delivery of an shRNA construct followed by drug selection. An shRNA construct targeting GFP was used as a control. Top, Analysis of JNK1 mRNA expression by RT-qPCR. β-Actin mRNA was used as an internal control. Each bar represents the mean ± sem, n = 3. Bottom, Analysis of JNK1 protein levels by Western blotting. β-Actin was used as a loading control. B, Effect of JNK1 knockdown on estrogen-dependent gene expression. The E2-regulated expression of four JNK1-recruited genes (GREB1, PLAC1, ELOVL2, and HOXC10) in control (GFP) and JNK1 knockdown MCF-7 cells was monitored by RT-qPCR before (U) and after (E) a 3-h treatment with E2. Each bar represents the mean ± sem, n = 3. *, P < 0.05; **, P < 0.01; Student's t test vs. corresponding E2 control. C, Effect of inhibiting JNK catalytic activity on estrogen-dependent gene expression. The E2-regulated expression of the four JNK1-recruited genes shown in B was examined by RT-qPCR in the absence or presence of the JNK inhibitor SP for 1 h, before a 3-h treatment with vehicle (U) or E2 (E). Each bar represents the mean ± sem, n = 3. *, P < 0.05; **, P < 0.01; Student's t test vs. corresponding E2 control.
JNK1 is required for E2-dependent growth of MCF-7 cells
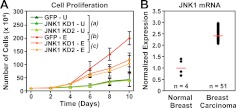
E2 regulates the transcription of estrogen-responsive genes, including a set of genes involved in cell growth control (2). This transcriptional program underlies the potent mitogenic effects of E2 on estrogen-responsive cells, such as MCF-7 cells. To determine the role of JNK1 in E2-dependent mitogenic responses, we determined the proliferation of MCF-7 cells in response to E2 treatment with or without JNK1 knockdown. Two different shRNAs targeting JNK1, expressed individually in the cells, reduced the E2-dependent proliferation of MCF-7 cells by about half (Fig. 7A). These results suggest that the impaired E2-dependent transcriptional responses that we observed upon JNK1 knockdown (Fig. 6B) are reflected in a corresponding loss of cell growth (Fig. 7A). Cell proliferation assays in another ERα-positive human breast cancer cell line, ZR75T, showed no effect of JNK1 knockdown on cell growth when performed at the same cell density where effects were observed in MCF-7 cells (Supplemental Fig. 7). These results may indicate cell type-specific effects or may reflect differences in the biology of these two breast cancer cell lines (e.g. ZR75T cells proliferate faster than MCF-7 cells, perhaps indicating less dependence on JNK1 for growth-promoting effects).
Fig. 7.
JNK1 is required for full estrogen-dependent growth responses in MCF-7 cells. A, Analysis of E2-dependent cell growth in control (GFP) and JNK1 knockdown (KD) MCF-7 cells grown for 10 d in the absence (U) or presence (E) of E2. Two independent JNK1 shRNA (KD1 and KD2) constructs were used, as shown, and the number of cells in each condition was counted every 2 d. Each point represents the mean ± sem, n = 3. Statistical analysis was performed by ANOVA for each time point (P < 0.05). Groups a–c are not statistically different from each other at d 0, 2, and 4. Groups b and c are statistically different from group a, but not each other, at d 6. Groups a–c are statistically different from each other at d 8 and 10. B, JNK1 expression is elevated in breast carcinomas. Data obtained from gene expression analyses were analyzed using Oncomine. The relative expression of MAPK8 (i.e. JNK1) from four normal breast stroma samples and 51 breast tumor samples is shown. The Oncomine-reported P value was less than 3.0 × 10−4. The values were normalized to an average expression level of 1 for the normal breast samples. Red lines represent the average signal in each category.
The observed link between JNK1 and estrogen signaling may have relevance for the growth and clinical outcomes of estrogen-dependent breast cancers. In this regard, note that the expression of JNK1 is up-regulated in breast cancers (Fig. 7B). In addition, the expression of the JNK1 phosphatase, mitogen-activated protein kinase phosphatase 1, a negative regulator of JNK1 activity, is reduced in high-grade malignant breast cancers (Supplemental Fig. 8). Both of these cancer-related changes would increase the net JNK1 activity and, hence, have the potential to modulate estrogen-dependent growth responses in those cells.
Discussion
Our genomic and gene-specific analyses of the nuclear functions of JNK1 have revealed new facets of JNK1 biology, including functional interplay with the estrogen signaling pathway. Collectively, our results indicate that 1) activated nuclear JNK1 binds to specific sites in the genome (Fig. 1), 2) E2 induces a redistribution of JNK1 binding at promoters (Fig. 1), 3) E2-induced binding of JNK1 at many target genes is mediated by the E2-induced formation of promoter-bound complexes containing ERα and, in some cases, tethering proteins such as AP-1 (Figs. 2–5), 4) JNK1 can act as a coregulator of ERα-dependent transcriptional outcomes (Fig. 6), and 5) the estrogen and JNK1 signaling pathways collaborate to control the proliferation of breast cancer cells (Fig. 7). Thus, the functional interplay between the estrogen and MAPK signaling pathways that has been observed previously is manifested in a molecular cross talk at the genomic level. These results help to define the molecular mechanisms underlying estrogen signaling in the nucleus and estrogen-dependent gene regulation, as well as the nuclear functions of JNK1. Interestingly, JNK1/3 interactions with the genome have recently been reported for a set of actively transcribed promoters during the differentiation of stem cells into neurons (60), suggesting that JNK-dependent gene regulation occurs across diverse set of cell types.
Our results support a model for the estrogen- and ERα-dependent recruitment of preactivated JNK1 from the nuclear compartment (i.e. nucleoplasm or chromatin) to the promoters of estrogen target genes. JNK1, in turn, serves a coregulator function required for efficient estrogen-dependent transcription of these genes. This role of JNK1 in the genomic estrogen signaling pathway is supported by JNK1's kinase activity, which likely targets histones or other proteins in the promoter-assembled transcription complexes, as described for other cellular kinases in the nucleus (61). Indeed, two well-characterized ERα coregulators, p300 and steroid receptor coactivator 1, are strongly phosphorylated by JNK1 in vitro, whereas ERα is only weakly phosphorylated (Supplemental Fig. 9). A recent study found that histone H3 Ser10 is a substrate for JNK, and JNK-bound promoters are enriched for histone H3 Ser10 phosphorylation (60). We also observed that nucleosomal histone H3 is phosphorylated by JNK1, but only when JNK1 is recruited to the nucleosomes by a DNA-bound transcription factor, such as AP-1 (Supplemental Fig. 10). Determining the functional relevance of these and other potential targets of JNK1 will be an important question to address in future studies.
Our results fit well with the growing evidence supporting a role for cellular kinases in the nucleus and across the genome (61). A recent study by Madak-Erdogan et al. (51) showed that ERK2 is recruited to ERα-binding sites across the genome where it supports E2-induced gene expression. There are a number of parallels between this study and ours, which suggests that there may be some universal features for cellular kinase actions across the genome, at least in the estrogen signaling pathway. These include the following: 1) estrogen-induced binding and colocalization of the kinase and ERα at specific sites in the genome, 2) a requirement for ERα to drive the recruitment of the kinase to chromatin, 3) interplay with other transcription factors (e.g. AP-1 for JNK1 and cAMP response element-binding protein 1 for ERK2), possibly through a tethering mechanism, 4) a requirement for the kinase to be activated, and 5) a role for the kinase in estrogen-dependent gene regulation. The extent to which these features apply to other kinases and other transcription factors, especially other nuclear receptors, remains to be determined.
In summary, our studies have identified significant molecular cross talk between the estrogen and JNK1 signaling pathways that regulates target gene expression and downstream cell growth responses. As noted above, similar genomic systems are likely to integrate the signaling pathways for other steroid hormones and signal-regulated nuclear kinases. Although we have not established a direct causal link between JNK1 actions at the promoters of estrogen target genes and the effects of JNK1 knockdown on estrogen-dependent cell growth, our data are highly correlative and provide a strong circumstantial argument for such an effect. Further studies using an approach that selectively interferes with JNK1 recruitment to promoters without affecting overall JNK level or activity will be required to address this issue in more detail.
Materials and Methods
Additional details about the materials and methods can be found in the Supplemental data.
Cell culture and treatments
MCF-7 cells were maintained in MEM with Hanks' salts (M1018; Sigma, St. Louis, MO) supplemented with 5% calf serum. Before all experimental procedures and treatments, the cells were grown for at least 3 d in phenol red-free MEM Eagle medium with Earle's salts (M3024; Sigma) supplemented with 5% charcoal-dextran-treated calf serum, as described previously (62). Adherent HeLa and HeLa-ERα cells were maintained in DMEM/F12 (D2906; Sigma) supplemented with 10% charcoal-dextran-stripped calf serum, as described previously (59). The cells were treated with control vehicle (ethanol) or E2 (100 nm) for the times specified in the figure legends. For the JNK inhibition experiments, the cells were pretreated with control vehicle or 20 μm SP (Biomol, Hamburg, Germany) for 1 h before treatment with E2.
Antibodies
The antibodies used were as follows: JNK1/3 (sc-474; note that JNK3 is not expressed in MCF-7 cells, so we have unambiguous identification of JNK1 using this antibody; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), pan-JNK (sc-7345; Santa Cruz Biotechnology, Inc.), phosphorylated pan-JNK (sc-6254; Santa Cruz Biotechnology, Inc.), ERα (rabbit polyclonal generated in the Kraus Laboratory, University of Texas Southwestern Medical Center), and c-Fos (rabbit polyclonal generated in the Kraus Laboratory).
JNK1 subcellular localization
Estrogen-starved MCF-7 cells were treated with ethanol or 100 nm E2 for 45 min and then fractionated into cytoplasmic and nuclear extracts in the presence of phosphatase inhibitors (5 mm NaF and 1 mm sodium vanadate). The extracts were subjected to immunoblotting using antibodies against JNK1, phosphorylated JNK1, ERα, and glyceraldehyde-3-phosphate dehydrogenase.
Immunofluorescent staining of cells for JNK1
Estrogen-starved MCF-7 cells were grown on coverslips and treated with ethanol or 100 nm E2 for 45 min. The cells were fixed with 3% formaldehyde, permeabilized with 0.1% Triton X-100, blocked with 5% BSA, and subjected to staining with primary (anti-JNK1) and secondary (fluorescein-conjugated antigoat IgG) antibodies. The coverslips were then washed five times with Tris-buffered saline with Tween 20, mounted on slides using Vectashield (H-1000; Vector Laboratories, Burlingame, CA), and visualized using a Leica Confocal Microscope System (Leica, Heerbrugg, Switzerland).
Chromatin immunoprecipitation
ChIP assays for JNK1, ERα, and c-Fos were performed using a ChIP protocol described previously (62), with minor modifications. The key difference in the protocol was the inclusion of a cross-linking step with 10 mm dimethyl suberimidate·HCl (20700; Pierce, Rockford, IL) for 10 min at room temperature before cross-linking with 1% formaldehyde for 10 min at 37 C. The ChIP DNA was dissolved in water and analyzed by qPCR using a set of gene-specific primers. Each ChIP experiment was conducted with at least three independent chromatin isolates to ensure reproducibility.
ChIP-reChIP
After the primary ChIP, JNK1- and ERα-precipitated complexes were eluted with 10 mm dithiothreitol twice for 20 min at 37 C. Eluates were diluted 20 times in ChIP dilution buffer, incubated with a second antibody at 4 C overnight, followed by the addition of the protein-A/G-agarose bead mixture. After this secondary ChIP, washing, elution, reversal of the cross-links, and analysis by qPCR were carried out as described for the standard ChIP protocol described above.
ChIP-chip
JNK1- and ERα-precipitated genomic DNA was blunted, amplified by ligation-mediated-PCR, and labeled as described previously (63). The labeled samples were combined and hybridized to human HG18 RefSeq Promoter Arrays (C4226-00-01; NimbleGen, Indianapolis, IN), which contain approximately 19,000 well-characterized RefSeq promoters tiled with 50- to 75-mer probes every 100 bp. The tiled regions cover approximately 2200 bp upstream and approximately 500 bp downstream of each transcription start site (TSS). The ChIP-chip experiments were performed using three independent ChIP DNA isolates from cells treated with or without E2. The ChIP-chip data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE13200 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13200).
ChIP-chip data analysis
Data processing was done essentially as described previously (63) using the statistical programming language R (64). All R scripts are available upon request. The analysis included three components: 1) a moving window analysis using a 1000-bp moving window with 250-bp steps in which both the mean probe log2 ratio and P value from a nonparametric Wilcoxon signed-rank test were calculated for each window, 2) definition of regions with significant JNK1 or ERα binding or significant fold changes in JNK1 or ERα binding according to a set of definitions and criteria elaborated in Supplemental Materials, and 3) visual representation of the data by generating TSS-anchored heat maps using Java Treeview (65). To call overlap between significant JNK1 and ERα peaks, we defined a region ± 750 bp from the center of the JNK1 peak. If that region overlapped any part of a significant ERα peak, regardless of E2 treatment, the peaks were called overlapping.
Knockdown of ERα and JNK1 in MCF-7 cells
Transient RNA interference-mediated knockdown of ERα or JNK1 was achieved by transfection of SMARTpool siRNA with an appropriate control siRNA pool (Dharmacon, Lafayette, CO), as recommended by the manufacturer. Sixty hours after transfection, the cells were treated with E2 and collected for experiments. Stable RNA interference-mediated knockdown of JNK1 was performed using retroviral-mediated gene transfer of shRNA sequences specifically targeting the JNK1 mRNA with the pSUPER.retro system under appropriate drug selection (Oligoengine, Seattle, WA). The JNK1 target sequences are as follows: 5′-CAGAGAGCTAGTTCTTATGAA-3′ and 5′-CCTACAGAGAGCTAGTTCTTA-3′. As a control, we used an shRNA sequence directed against green fluorescent protein (GFP). Knockdown was verified by immunoblotting and RT-qPCR.
Gene-specific expression analyses by RT-qPCR
The expression of endogenous target genes was determined by RT-qPCR, as described previously (62), with minor modifications. The cDNA products from the RT reactions were analyzed by qPCR using a set of gene-specific primers. Each experiment was conducted with at least three independent RNA isolates to ensure reproducibility.
Cell proliferation assays
MCF-7 cells with stable knockdown of JNK1, or controls cells stably transfected with an shRNA targeting GFP, were plated at a density of 1 × 105 per well of a six-well dish. After attachment, the cells were treated with E2 for a 10-d time course. At 2-d intervals, the cells were collected, stained with trypan blue, and counted.
Bioinformatic analyses
De novo motif predictions were performed on gene lists that show JNK1 binding at their promoters in the E2-treated condition. These lists were formulated using the tools on the Galaxy browser (66), so genomic locations from JNK1-bound regions would not be present in the background regions. De novo motif detection was carried out using MEME (67) on repeat-masked sequences. The top 20 motifs in each peak class were retained for further analysis. MAST (67) was used to scan for the locations of all motif instances within both bound and unbound sequences, using a P value threshold of 1.5 × 10−4, as previously reported (67). Fisher's exact tests were used to determine enrichments relative to background with P values corrected for multiple testing using the Holm method in R.
TESS (68) was used to predict the transcription factors that might bind to the enriched sequences from MEME. Position weight matrices for the predicted transcription factors were obtained from the TRANSFAC database (69). Adjusted matrices for the predicted transcription factors were mapped to the JNK1-bound and JNK1-negative regions with MAST using a sixth order Markov model. Fisher's exact tests were used to determine the enrichments for each motif, as described above. In addition, promoters were scanned for the presence of ERE in the same manner, and the enrichment was calculated.
Supplementary Material
Acknowledgments
We thank members of the Kraus Laboratory for technical advice, helpful discussions, and critical comments on this study and manuscript. We also thank Dr. Matt Gamble and Dr. Miltos Kininis for assistance with the genomic data analyses, Adam Diehl and Dr. Adam Siepel for assistance with the bioinformatic analyses, Dr. David Shapiro for the HeLa-ERα cells, and Dr. Melanie Cobb for the constructs for producing activated JNK1.
Present address for G.D.I.: Department of Chemistry and Biology, Liberty University, Lynchburg, Virginia 24502.
Present address for N.Ha.: Gene Expression Laboratory, The Salk Institute, La Jolla, California 92037.
Present address for N.He.: Department of Neuroscience, Karolinska Institute, 17177 Stockholm, Sweden.
This work was supported by a predoctoral fellowship from the American Heart Association (M.S.), a predoctoral fellowship from the United States Department of Defense Breast Cancer Research Program (G.D.I.), a Marie Curie Outgoing International fellowship (N.He.), and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK058110 (to W.L.K.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: ER-α;
Ligands: 17β-estradiol.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- AP-1
- Activating protein-1
- ChIP
- chromatin immunoprecipitation
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- GFP
- green fluorescent protein
- GO
- gene ontology
- JNK
- c-Jun N-terminal kinase
- MAST
- Motif Alignment and Search Tool
- MEME
- Multiple Em for Motif Elicitation
- qPCR
- quantitative PCR
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- SP
- SP600125
- TESS
- Transcription Element Search Software
- TSS
- transcription start site.
References
- 1. Cheung E, Kraus WL. 2010. Genomic analyses of hormone signaling and gene regulation. Annu Rev Physiol 72:191–218 [DOI] [PubMed] [Google Scholar]
- 2. Kininis M, Kraus WL. 2008. A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Recept Signal 6:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- 5. Warner M, Nilsson S, Gustafsson JA. 1999. The estrogen receptor family. Curr Opin Obstet Gynecol 11:249–254 [DOI] [PubMed] [Google Scholar]
- 6. Kumar V, Chambon P. 1988. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell 55:145–156 [DOI] [PubMed] [Google Scholar]
- 7. Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. 1990. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell 63:1267–1276 [DOI] [PubMed] [Google Scholar]
- 8. Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T. 1994. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269:16433–16442 [PubMed] [Google Scholar]
- 9. Weisz A, Rosales R. 1990. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res 18:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. 2000. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- 11. Acevedo ML, Kraus WL. 2004. Transcriptional activation by nuclear receptors. Essays Biochem 40:73–88 [DOI] [PubMed] [Google Scholar]
- 12. Glass CK, Rose DW, Rosenfeld MG. 1997. Nuclear receptor coactivators. Curr Opin Cell Biol 9:222–232 [DOI] [PubMed] [Google Scholar]
- 13. Hess RA. 2003. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol 1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Findlay JK, Liew SH, Simpson ER, Korach KS. 2010. Estrogen signaling in the regulation of female reproductive functions. Handb Exp Pharmacol:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- 16. Hewitt SC, Harrell JC, Korach KS. 2005. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 67:285–308 [DOI] [PubMed] [Google Scholar]
- 17. Li R, Shen Y. 2005. Estrogen and brain: synthesis, function and diseases. Front Biosci 10:257–267 [DOI] [PubMed] [Google Scholar]
- 18. Pallottini V, Bulzomi P, Galluzzo P, Martini C, Marino M. 2008. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infect Disord Drug Targets 8:52–60 [DOI] [PubMed] [Google Scholar]
- 19. Murphy E, Korach KS. 2006. Actions of estrogen and estrogen receptors in nonclassical target tissues. Ernst Schering Found Symp Proc:13–24 [DOI] [PubMed] [Google Scholar]
- 20. Foster JS, Henley DC, Ahamed S, Wimalasena J. 2001. Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab 12:320–327 [DOI] [PubMed] [Google Scholar]
- 21. Prall OW, Rogan EM, Sutherland RL. 1998. Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol 65:169–174 [DOI] [PubMed] [Google Scholar]
- 22. Sommer S, Fuqua SA. 2001. Estrogen receptor and breast cancer. Semin Cancer Biol 11:339–352 [DOI] [PubMed] [Google Scholar]
- 23. Kuiper GG, van den Bemd GJ, van Leeuwen JP. 1999. Estrogen receptor and the SERM concept. J Endocrinol Invest 22:594–603 [DOI] [PubMed] [Google Scholar]
- 24. McDonnell DP, Chang CY, Norris JD. 2001. Capitalizing on the complexities of estrogen receptor pharmacology in the quest for the perfect SERM. Ann NY Acad Sci 949:16–35 [DOI] [PubMed] [Google Scholar]
- 25. Johnston SR. 2001. Endocrine manipulation in advanced breast cancer: recent advances with SERM therapies. Clin Cancer Res 7:4376s–4387s; discussion 4411s–4412s [PubMed] [Google Scholar]
- 26. Turjanski AG, Vaqué JP, Gutkind JS. 2007. MAP kinases and the control of nuclear events. Oncogene 26:3240–3253 [DOI] [PubMed] [Google Scholar]
- 27. Davis RJ. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239–252 [DOI] [PubMed] [Google Scholar]
- 28. Vlahopoulos S, Zoumpourlis VC. 2004. JNK: a key modulator of intracellular signaling. Biochemistry 69:844–854 [DOI] [PubMed] [Google Scholar]
- 29. Johnson GL, Lapadat R. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912 [DOI] [PubMed] [Google Scholar]
- 30. Chang L, Karin M. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37–40 [DOI] [PubMed] [Google Scholar]
- 31. Smith CL. 1998. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 58:627–632 [DOI] [PubMed] [Google Scholar]
- 32. Lange CA. 2004. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol 18:269–278 [DOI] [PubMed] [Google Scholar]
- 33. Karin M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486 [DOI] [PubMed] [Google Scholar]
- 34. Hess J, Angel P, Schorpp-Kistner M. 2004. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973 [DOI] [PubMed] [Google Scholar]
- 35. Dai T, Rubie E, Franklin CC, Kraft A, Gillespie DA, Avruch J, Kyriakis JM, Woodgett JR. 1995. Stress-activated protein kinases bind directly to the δ domain of c-Jun in resting cells: implications for repression of c-Jun function. Oncogene 10:849–855 [PubMed] [Google Scholar]
- 36. Ip YT, Davis RJ. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol 10:205–219 [DOI] [PubMed] [Google Scholar]
- 37. Hibi M, Lin A, Smeal T, Minden A, Karin M. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev 7:2135–2148 [DOI] [PubMed] [Google Scholar]
- 38. Qi X, Borowicz S, Pramanik R, Schultz RM, Han J, Chen G. 2004. Estrogen receptor inhibits c-Jun-dependent stress-induced cell death by binding and modifying c-Jun activity in human breast cancer cells. J Biol Chem 279:6769–6777 [DOI] [PubMed] [Google Scholar]
- 39. Teyssier C, Belguise K, Galtier F, Chalbos D. 2001. Characterization of the physical interaction between estrogen receptor α and JUN proteins. J Biol Chem 276:36361–36369 [DOI] [PubMed] [Google Scholar]
- 40. Webb P, Lopez GN, Uht RM, Kushner PJ. 1995. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456 [DOI] [PubMed] [Google Scholar]
- 41. Webb P, Nguyen P, Kushner PJ. 2003. Differential SERM effects on corepressor binding dictate ERα activity in vivo. J Biol Chem 278:6912–6920 [DOI] [PubMed] [Google Scholar]
- 42. Edmunds JW, Mahadevan LC. 2004. MAP kinases as structural adaptors and enzymatic activators in transcription complexes. J Cell Sci 117:3715–3723 [DOI] [PubMed] [Google Scholar]
- 43. Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. 2006. Activated signal transduction kinases frequently occupy target genes. Science 313:533–536 [DOI] [PubMed] [Google Scholar]
- 44. Pascual-Ahuir A, Struhl K, Proft M. 2006. Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods 40:272–278 [DOI] [PubMed] [Google Scholar]
- 45. Suganuma T, Mushegian A, Swanson SK, Abmayr SM, Florens L, Washburn MP, Workman JL. 2010. The ATAC acetyltransferase complex coordinates MAP kinases to regulate JNK target genes. Cell 142:726–736 [DOI] [PubMed] [Google Scholar]
- 46. Bruna A, Nicolàs M, Muñoz A, Kyriakis JM, Caelles C. 2003. Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. EMBO J 22:6035–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. 2009. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature 461:819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M. 2006. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell 24:367–381 [DOI] [PubMed] [Google Scholar]
- 49. Narayanan R, Adigun AA, Edwards DP, Weigel NL. 2005. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol 25:264–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, Berger SL. 2010. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 329:1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu S, Xie Z, Onishi A, Yu X, Jiang L, Lin J, Rho HS, Woodard C, Wang H, Jeong JS, Long S, He X, Wade H, Blackshaw S, Qian J, Zhu H. 2009. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 139:610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. 2001. Hyperactivation of MAPK induces loss of ERα expression in breast cancer cells. Mol Endocrinol 15:1344–1359 [DOI] [PubMed] [Google Scholar]
- 54. Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. 2002. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol 80:239–256 [DOI] [PubMed] [Google Scholar]
- 55. Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL. 2011. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145:622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kininis M, Isaacs GD, Core LJ, Hah N, Kraus WL. 2009. Postrecruitment regulation of RNA polymerase II directs rapid signaling responses at the promoters of estrogen target genes. Mol Cell Biol 29:1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hammes SR, Levin ER. 2007. Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 58. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 59. Heldring N, Isaacs GD, Diehl AG, Sun M, Cheung E, Ranish JA, Kraus WL. 2011. Multiple sequence-specific DNA-binding proteins mediate estrogen receptor signaling through a tethering pathway. Mol Endocrinol 25:564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tiwari VK, Stadler MB, Wirbelauer C, Paro R, Schübeler D, Beisel C. 2012. A chromatin-modifying function of JNK during stem cell differentiation. Nat Genet 44:94–100 [DOI] [PubMed] [Google Scholar]
- 61. Baek SH. 2011. When signaling kinases meet histones and histone modifiers in the nucleus. Mol Cell 42:274–284 [DOI] [PubMed] [Google Scholar]
- 62. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. 2008. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science 319:819–821 [DOI] [PubMed] [Google Scholar]
- 64. R Development Core Team 2006. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing [Google Scholar]
- 65. Saldanha AJ. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248 [DOI] [PubMed] [Google Scholar]
- 66. Elnitski L, King D, Hardison RC. 2006. Computational prediction of cis-regulatory modules from multispecies alignments using Galaxy, Table Browser, and GALA. Methods Mol Biol 338:91–103 [DOI] [PubMed] [Google Scholar]
- 67. Bailey TL, Williams N, Misleh C, Li WW. 2006. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:W369–W373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schug J. 2008. Using TESS to predict transcription factor binding sites in DNA sequence. Curr Protoc Bioinformatics Chapter 2:Unit 2 6 [DOI] [PubMed] [Google Scholar]
- 69. Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, Krull M, Matys V, Michael H, Ohnhäuser R, Prüss M, Schacherer F, Thiele S, Urbach S. 2001. The TRANSFAC system on gene expression regulation. Nucleic Acids Res 29:281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Lone R, Frith MC, Karlsson EK, Hansen U. 2004. Genomic targets of nuclear estrogen receptors. Mol Endocrinol 18:1859–1875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.