Abstract
Brown adipose tissue is a thermogenic organ that dissipates stored energy as heat to maintain body temperature. This process may also provide protection from development of diet-induced obesity. We report that the bioactive lipid mediator lysophosphatidic acid (LPA) markedly decreases differentiation of cultured primary brown adipocyte precursors, whereas potent selective inhibitors of the LPA-generating enzyme autotaxin (ATX) promote differentiation. Transgenic mice overexpressing ATX exhibit reduced expression of brown adipose tissue-related genes in peripheral white adipose tissue and accumulate significantly more fat than wild-type controls when fed a high-fat diet. Our results indicate that ATX and its product LPA are physiologically relevant negative regulators of brown fat adipogenesis and are consistent with a model in which a decrease in mature peripheral brown adipose tissue results in increased susceptibility to diet-induced obesity in mice.
Easy access to food high in calories and the sedentary lifestyle of modern society has produced an escalation in obesity with profound medical and socioeconomic implications (1). Lifestyle plays a leading role in the development of obesity in that consumed, but unexpended, calories are stored as fat. Current pharmacological and surgical strategies for weight loss/weight maintenance are largely aimed at reducing caloric consumption. However, factors that are at least partially under genetic control may also influence the susceptibility to obesity (2, 3). For example, recent evidence suggests that brown adipose tissue (BAT) in adults may protect against the development of obesity, especially with aging (4). BAT is a thermogenic organ that serves to maintain core body temperature in small rodents and infants by dissipating energy as heat (5). This is accomplished by the expression of the uncoupling protein 1 (UCP1) in the inner mitochondrial membrane of brown adipocytes resulting in uncoupling of electron transport from ATP generation. Imaging studies using 18F-fluoro-deoxyglucose positron emission tomography scanning and biochemical analyses have established that metabolically active regions with the functional and histological hallmarks of brown fat are present in adult humans (6–9). These studies implicate BAT as an important variable in the regulation of energy balance in humans, and, as such, a potential target for pharmacological treatment of obesity (4, 10). Because of the potential to harness BAT as a therapeutic strategy to combat obesity, considerable interest has been placed on understanding modulators of BAT development and activity. Prdm16 (11–13) and bone morphogenetic protein (BMP)7 (14) have been identified as master regulators of brown adipocyte differentiation in cell culture systems and animal models. Brown fat-like adipocytes have also been observed in tissues historically defined as white adipose tissue (WAT) and appear to increase in number in response to sympathetic stimulation and cold exposure, leading to the suggestion that there may be inducible forms of BAT (inducible BAT) (15, 16). Brown adipocyte expression can be activated in WAT by cyclooxygenase-2 mediated generation of prostanoids (15), by thiazolidinedione drugs acting thru peroxisome proliferator-activated receptor (PPAR)γ (17), by the BMP family member BMP7 (14, 18), and by the BAT master regulator Prdm16 (13). In particular, Prdm16 and BMP7 promote expression of brown adipocytes in sc depots of WAT and can thereby protect mice from diet-induced obesity. These observations identify potential physiological mechanisms that promote the induction of BAT; however, less is known about endogenous pathways that may suppress brown adipogenesis and that therefore could be important pharmacologic targets to combat obesity.
The bioactive lipid, lysophosphatidic acid (LPA; monoacyl glycerol 3-phosphate) is a candidate mediator of adipocyte differentiation and function. LPA is present in blood and biological fluids and exerts extracellular effects by signaling through a family of G protein-coupled receptors (19, 20). Several observations implicate LPA as a regulator of adipocyte growth and differentiation. Exogenously applied LPA accelerates preadipocyte proliferation in culture (21–23) and inhibits differentiation as measured by triglyceride accumulation and PPARγ2 gene expression (24). Bioactive LPA is primarily generated by the lysophospholipase D enzyme autotaxin (ATX), a member of the ectonucleotidase family encoded by the ENPP2 gene (25). Levels of ATX mRNA increase with adipocyte maturation in culture, and the temporal expression pattern accompanied by the effects in culture are consistent with a paracrine role of ATX in adipose tissue development (26). ATX mRNA levels are higher in adipose tissue of obese db/db mice and in humans with insulin resistance, although ATX expression in fat does not appear higher in mice made diabetic by treatment with streptozotocin or in WAT from mice fed a high-fat diet (HFD) (27). Adipose specific inactivation of ENPP2 results in obesity in mice (28). These studies associate the ATX-LPA axis with adipocyte dynamics; however, essentially nothing is known about the role of the ATX-LPA axis in BAT development. The relative contribution of ATX modulation to both WAT and BAT development needs to be evaluated because the two fat types are both part of a complex system that keeps the body energy balance in check and ultimately controls body weight.
In this work, we report a new role for the ATX-LPA signaling axis as a negative regulator of brown adipocyte differentiation in vitro and provide evidence that this pathway may modulate inducible BAT in sc adipose tissue in adult mice and thereby influence the development of obesity. Our results have important ramifications for therapeutic strategies aimed at harnessing the dissipative power of BAT to combat obesity.
Materials and Methods
Expression and purification of ATX
ATX was expressed by transfection of suspension cultures of Chinese hamster ovary cells with a vector containing human immunoglobulin κ chain leader sequence, a hexa histidine tag sequence, and the cDNA sequence encoding amino acids 57-end of human ATX. Cells were cultured for 4 d in serum-free medium supplemented with 1% fetal bovine serum (FBS). The secreted His6-tagged ATX was purified from the culture medium of the cells by metal ion affinity chromatography and was exchanged into PBS and concentrated to approximately 1 mg/ml by centrifugal filtration (Amicon Ultra, Temecula, CA). Purified ATX hydrolyzed synthetic nucleotide and lipid substrates with a specific activity comparable to that reported for native ATX isolated from human or bovine plasma.
Cell culture and differentiation studies
Primary BAT cell cultures were established as previously reported with some minor modifications (30, 31). Mice (4- to 6 wk of age) were euthanized under aseptic condition and interscapular BAT (IBAT) was dissected, finely minced in digestion buffer containing 123 mm NaCl, 5 mm HCl, 1.3 mm CaCl2, 5 mm glucose, 1.5% (wt/vol) crude BSA fraction V, 100 mm HEPES, 0.2% collagenase type II (Sigma-Aldrich Corp., St. Louis, MO), pH 7.4, and digested in 10 ml of the same buffer for 30 min at 37 C. The supernatant was then filtered through a 250-μm nylon mesh and kept in ice for 15 min to allow fat droplets and mature cells to float. The resulting infranatant was filtered through a 30-μm nylon mesh and centrifuged at 700 × g for 10 min. The pellet obtained was washed once in warm DMEM and resuspended in DMEM containing 10% newborn calf serum, 10 mm HEPES, 4 mm glutamine, 25 μg/ml sodium ascorbate (Sigma-Aldrich Corp.), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were seeded onto 35-mm dish plates. Insulin treatment was started on d 1 at 50 nm, and the concentration was increased to 100 nm at d 5 and 200 nm at d 7. Differentiated/confluent cells were collected for biochemical analysis (∼d 9).
Primary mouse embryonic fibroblast (MEF) cultures were performed as previously described (38). MEF were isolated from 13.5-d-old mouse embryos and cultured to confluence in AmnioMAX-C100 basal medium supplemented with 7.5% AmnioMAX-C100 supplement, 7.5% fetal bovine serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 2 mm l-glutamine. Differentiation was induced in 2-d postconfluent cells by treatment with growth medium containing 1 μm dexamethasone, 0.5 mm methylisobutylxanthine, 5 μg/ml insulin (Sigma-Aldrich Corp.), and 0.5 μm rosiglitazone (Biovision, Mountain View, CA) for 48 h; every other day, cells were refed fresh medium containing 5 μg/ml insulin and 0.5 μmol/liter rosiglitazone. From d 6 onward, cells were deprived of insulin and rosiglitazone, and adipogenesis was examined morphologically using phase contrast microscopy. All-trans retinoic acid (ATRA; 1 μg/ml) was added to the differentiated cells on d 8, a time at which more than 80% of the cells had acquired an adipose phenotype; control cells received an equal volume of vehicle (dimethyl sulfoxide). UCP1 expression was measured 24 h after treatment.
Cultures of primary WAT precursors isolated from stromal vascular fraction (SVF) of sc fat were performed as previously described with minor modifications (15, 27). Subcutaneous WAT was dissected, finely minced with scissors, and digested for 45 min at 37 C in DMEM containing 0.5% fatty acid-free BSA, 1.5 mg/ml collagenase type II (Sigma-Aldrich Corp.), and 15 mm HEPES. Cell suspensions were allowed to stand for 10 min, filtered through a 250-μm mesh filter, and spun at 50 × g for 5 min to sediment clumps. The infranatant was further filtered through a 100-μm and then through a 30-μm mesh filter. SFV cells were collected by centrifugation at 700 × g for 10 min, washed in 6 ml plating medium consisting of DMEM, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and plated out at a concentration of 16 × 104/ml in a 35-mm dish. After 24 h cells were washed, and medium was replaced with maintenance medium consisting of DMEM, 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml murine basic fibroblast growth factor (R&D Systems, Minneapolis, MN). Fresh medium was added every other day until cells reached confluence (∼1 wk). At confluence, differentiation was induced by treatment for 48 h with induction medium consisting of DMEM, 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 17 nm insulin, 500 nm dexamethasone, 50 μg/ml l-ascorbate, 1 μm d-biotin, and 17 nm pantothenate (Sigma-Aldrich Corp.). After 48 h the medium was replaced with differentiation medium lacking dexamethasone and containing 5% FBS. Cells were cultured for 5 additional days and monitored for lipid accumulation. For T3-treated cells, 5 nm T3 was added to the media over the full course of the differentiation, and 1 μm norepinephrine (Sigma-Aldrich Corp.) was added 3 h before cell collection.
Immunoblotting analysis
Immunoblot analysis was carried out as previously reported with some minor modifications (38). Brown adipocytes were lysed by addition of lysis buffer (50 mmol/liter Tris HCl, pH 6.8) containing 2.5% sodium dodecyl sulfate, 1% Nonidet P40, 10% glycerol, 10 mmol/liter NaF, 0.1 mmol/liter Na orthovanadate, and protease inhibitors. Western blot was carried out using anti-UCP1 antibody (Abcam, Cambridge, MA), anti-β3 integrin (polyclonal antibody generated against a peptide corresponding to the C terminus of integrin β3 and purified on a peptide affinity column; custom generated by PolyScience), and an anti-β-actin antibody (Sigma-Aldrich Corp.) and visualized using fluorescently conjugated secondary antibodies using a Licor Odyssey system (LI-COR, Lincoln, NE).
Determination of LPA level and autotaxin activity
LPA level in cell culture experiments was measured in conditioned medium from cell treated for 48 h with vehicle control, HA155 inhibitor, and recombinant ATX. Lipids were extracted using acidified organic solvents as described previously. LPA levels were determined using liquid chromatography and tandem mass spectrometry with ABI-4000 Q-TRAP hybrid triple quadrupole/ion trap mass spectrometer coupled with an Agilent 1100 liquid chromatography system using methods that have been described previously. Lipids were separated on Zorbax Eclipse XDB-C8 column, 4.6 × 150 mm, 5 μm using methanol-water-HCOOH (79:20:0.5, vol/vol), with 5 mm NH4COOH as solvent A and methanol-acetonitrile-HCOOH (59:40:0.5, vol/vol), with 5 mm NH4COOH as Solvent B. LPA molecular species were analyzed in negative ionization mode with declustering potential and collision energy optimized for 17:0, 18:0, 18:1, 18:2, and 20:4 LPA. Multiple reaction monitoring parameters for nine other LPA molecular species were selected with the closest possible approximation with available LPA standards. The following transitions were monitored: 407.0/153.0 (16:1 LPA); 409.0/153.1 (16:0 LPA); 423.0/153.1 [17:0 LPA (an unnatural LPA species used as an internal standard/recovery control)]; 431.0/153.0 (18:3 LPA); 433.0/153.0 (18:2 LPA); 435.1/152.9 (18:1 LPA); 437.0/153.0 (18:0 LPA); 455.1/153.0 (20:5 LPA); 457.0/153.0 (20:4 LPA); 459.1/153.0 (20:3 LPA); 461.1/153.0 (20:2 LPA); 481.1/153.0 (22:6 LPA); 483.1/153.0 (22:5 LPA); and 485.1/153.0 (22:4 LPA). Total LPA species content was reported.
RNA expression analysis
To identify genes regulated by ATX and LPA signaling, primary BAT cultures (see Materials and Methods, cell culture and differentiation studies) were treated with the vehicle (dimethylsulfoxide), specific ATX inhibitor HA155 (2.5 μm), or LPA (1-Oleoly-LPA, 5 μm) (Avanti Polar Lipids, AL) during differentiation. Treatment of the BAT cultures did not affect confluence or markers of proliferation. RNA was extracted from three cultures per condition using TRIzol reagent (Invitrogen, Carlsbad, CA) as described previously (39). Agilent Bioanalyzer assessed RNA quality and quantity. Overall rRNA 28s/18s ratios (1.63 ± 0.04), RNA integrity numbers (9.84 ± 0.04), and RNA yield (246 ± 26) were in acceptable ranges and did not differ significantly with treatment (P ≥ 0.1; one-way ANOVA). Extracted material was stored at −80 C until further use. RNA was labeled and hybridized to Affymetrix Mouse Gene 1.0 ST arrays by the Microarray Core at the University of Kentucky according to the manufacturer's protocol. Estimates of signal intensity were output using the PLIER algorithm on the standard gene interpretation of Exon data and copied to flat files for further analysis in Excel.
Data were analyzed as previously described (39, 40). Briefly, microarray data were prestatistically filtered to remove poorly annotated probe sets and low-intensity signals. Filtered data were analyzed by one-way ANOVA to identify significant differences (P ≤ 0.01), and the False Discovery Rate procedure (41) was used to estimate the error of multiple testing. Significant genes were assigned to patterns of expression using a post hoc template matching strategy as previously described (40). Briefly, significant genes were assigned to one of six idealized patterns of expression using Pearson's test and separated by the sign of their correlation. A Monte-Carlo simulation (10,000 iterations) was used to construct a test statistic for number of genes that would be expected by chance in each pattern as previously described (42). Functional categorization for significant genes was determined using the Database for Annotation, Visualization and Integrated Discovery overrepresentation clustering analysis tool (43) on the Gene Ontology (GO) databases of Biological Process, Cellular Component, and Molecular Function (44). The results of the study are uploaded to the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) under accession no. GSE26442
Animals
All procedures conformed to the recommendations of “Guide for the Care and Use of Laboratory Animals” (Department of Health, Education, and Welfare publication number NIH 78-23, 1996) and were approved by the Institutional Animal Care and Use Committee. Transgenic mice expressing the human ENPP2 gene [Tg(ENPP2) mice] under the control of the α1 antitrypsin promoter were generated on an FVB/N genetic background as previously described (33). Mice were weaned at 21 d, maintained on a 14-h light and 10-h dark cycle, and fed water and standard rodent chow (2018 Harlan Tekland Rodent Diet; Harlan Bioproducts for Science, Indianapolis, IN) ad libitum before and during the experiment. At 8–10 wk of age, both Tg(ENPP2) and wild-type (WT) mice were randomly assigned to two groups. One group of mice was fed a HFD containing 45% fat, 20% protein, and 35% carbohydrate by calories (Research Diet D124511, New Brunswick, NJ). The second group was fed a low-fat diet containing 10% fat by calories with equivalent protein-to-energy ratio (Research Diet D12450B). Initial experiments were performed with mice housed in groups (three to four mice per group/cage) and subsequently repeated with animals housed individually. Body weights were measured weekly until the end of experiment (up to 5 months) when composition scans for fat and lean mass were performed using dual-energy X-ray absorptiometry (GE Lunar PIXImus software version 1.45; Lunar, Madison, WI). Fat pads, including interscapular and periaortic BAT, epididymal, and sc WAT were dissected, weighed, frozen in liquid N2, and stored at −80 C for biochemical analysis. CL316, 243 (Sigma Chemical Co., St Louis, MO) was ip administrated (1 mg/kg body weight) to 4- to 6-wk-old male mice. Glucose measurements were carried out 3–4 wk after the mice were assigned to a diet. Intraperitoneal glucose tolerance test was performed in the afternoon after a 5-h fasting. After baseline glucose measurements were taken, mice were injected ip with glucose (2 g/kg body weight) in isotonic saline, and serial measurements of glucose level were taken at 7, 15, 30, and 120 min. Where necessary, animals were euthanized and organs were collected for quantitative RT-PCR analysis as described below.
Quantitative RT-PCR measurements
Total RNA was extracted from cells or tissues using Trizol reagent (Invitrogen, Carlsbad, CA) and quantitative, real-time PCR was conducted using a cDNA synthesis kit (Applied Biosystems, Foster City, CA) and gene-specific primers according to the manufacturer's directions. The threshold cycles (CT value), corresponding to exponential amplification of PCR product during the log-linear phase for both the target genes and internal reference gene (Eukaryotic 18S rRNA Endogenous Control from Applied Biosystems), were analyzed for each sample in duplicate using the ΔCT comparative method to determine relative expression levels. The relative quantification values were calculated for each sample using the 2-ΔΔCt formula. In cell culture experiments the reference sample was taken from the untreated group whereas in the in vivo experiments the reference samples were taken from the cohort of wild-type animals. The list of primers is available in the supplemental data section published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org.
Measurement of plasma adipokines
Mice were anesthetized with isoflurane, and blood was collected into EDTA-coated tubes. Plasma was obtained by centrifugation (30 sec at 14, 000 × g) and immediately frozen and stored at −20 C. Circulating inflammatory markers, insulin, leptin and tissue-plasminogen activator inhibitor (tPAI) measurements were performed on a Bio-plex 200 Suspension array System (Bio-Rad Laboratories, Inc., Hercules, CA) using Mouse Serum Adipokine LINCOplex Kit (Linco Research, St. Charles, MO). Plasma triglycerides were measured by Wako L-Type TG M microtiter plate assay kit (WAKO Diagnostic, Richmond, VA) according to manufacturer's instructions.
Cold tolerance studies
Body temperature of mice was measured at room temperature with an electronic thermistor equipped with a rectal probe and transferred to a cold room maintained at 4 C. Body core temperature was measured at different intervals up to 6 h.
Statistics
For cell culture, results were representative of at least two independent experiments and expressed as mean ± sd. Differences were analyzed using unpaired one-tailed Student's t test or ANOVA and considered statistically significant at P < 0.05. In vivo data were analyzed using one-way ANOVA adjusted for multiple comparisons or unpaired one-tailed Student's t test. Statistical analysis was performed using SigmaStat.
Results
ATX and its product LPA inhibit primary brown preadipocyte differentiation in culture
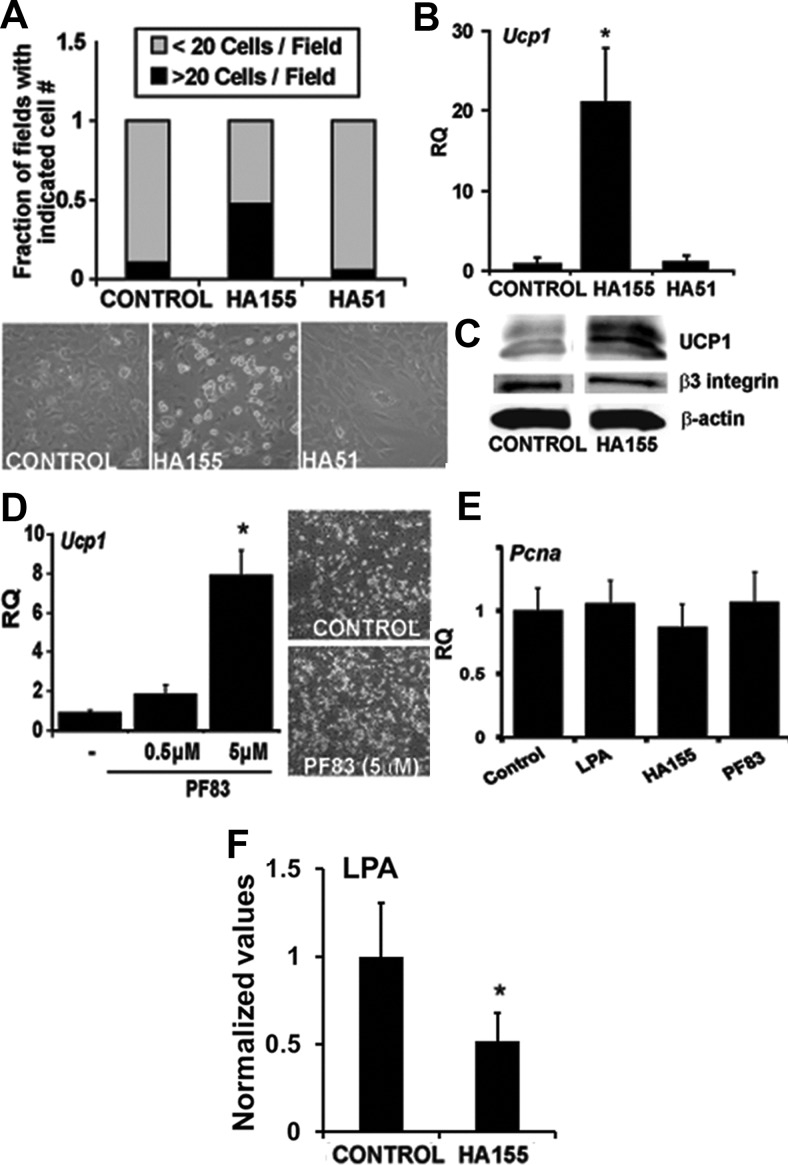
Previous investigations demonstrated that exogenously added LPA promoted the proliferation and inhibited the differentiation of white preadipocytes (21, 24). The effects of LPA on 3T3 preadipocyte proliferation were mimicked by the addition of conditioned media from COS cells overexpressing ATX (26). However, it remains to be determined whether the ATX-LPA axis plays a normal role in adipogenesis. To investigate a role for ATX-catalyzed generation of LPA in adipogenesis, we determined whether recently described potent and selective inhibitors of ATX activity (29) have effects on primary cultures of brown preadipocytes isolated from the SVF of IBAT (30, 31). The addition of the ATX inhibitor HA155 increased by the number of differentiated brown adipocytes by 4.5 fold (Fig. 1A), up-regulated Ucp1 gene expression by 20-fold (Fig. 1B), and correspondingly increased UCP1 protein in the cells (Fig. 1C). HA51, a close structural analog of HA155 that contains a carboxylic acid rather than boronic acid moiety and is an approximately 100-fold less potent ATX inhibitor, had no effect (Fig. 1, A and B). The structurally unrelated ATX inhibitor, PF8389, also promoted a slight increase in brown adipocyte number and an 8-fold up-regulation in Ucp1 expression (Fig. 1D). In contrast to reported stimulatory effects of LPA and ATX on preadipocyte proliferation, in this in vitro system neither LPA nor ATX inhibitors had an effect on the proliferation marker proliferating cell nuclear antigen (PCNA) in brown adipocytes (Fig. 1E). Under conditions in which an effect of ATX inhibition on differentiation was observed, LPA levels were significantly reduced in conditioned medium from cells treated with HA155 (Fig. 1F).
Fig. 1.
Inhibition of ATX activity promotes brown preadipocyte differentiation. A, Representative images and quantification of the effect of the ATX inhibitor HA155 (2.5 μm) or the structurally related but markedly less potent analog HA 51 (2.5 μm) on differentiation of primary BAT preadipocytes. Differentiated adipocytes were identified morphologically by lipid content, and the numbers per field were recorded (n = 20 fields). B, Real-time PCR measurement of Ucp-1 mRNA in primary BAT preadipocytes differentiated in presence of HA155, HA51, or vehicle control. Relative quantification (RQ) from each condition is shown. *, P < 0.02 (one-way ANOVA). C, Immunoblot analysis using antibodies to UCP1, β3 integrin, and β-actin (loading control) on lysates from BAT preadipocytes differentiated in presence of HA155 inhibitor or vehicle (dimethylsulfoxide). D, Real-time PCR measurement of Ucp-1 mRNA in primary BAT preadipocytes differentiated in presence of increasing doses of the ATX inhibitor PF83. *, P < 0.05 (unpaired Student's t test). D (right panel), Representative images of primary BAT preadipocytes incubated with the ATX inhibitor PF83. E, Expression of the proliferation marker Pcna in brown preadipocytes grown in the presence of LPA (5 μm), HA155 (2.5 μm), or PF83 (5 μm). F, LPA levels in conditioned medium treated with HA155 (2.5 μm) or vehicle control. *, P < 0.05 (unpaired Student's t test). Data were obtained from at least three independent experiments. Values shown are means ± sd.
ATX is present in serum and can be produced by adipocytes during differentiation (26). Therefore, both serum and cell-derived sources of ATX may be biologically important in this model system. Heat inactivation of serum at 56 C reduced ATX-related activity by more than 90% (as measured by p-nitrophenylphosphorylcholine hydrolysis; Supplemental Fig. 1B) and increased adipocyte differentiation and Ucp1 expression by about 50-fold, an effect that was reversed by LPA (Supplemental Fig. 1A). The combination of heat-inactivated serum and HA155 did not have an additive effect on adipocyte differentiation (Supplemental Fig. 1A), indicating that serum-derived ATX may be largely responsible for the inhibitory effect. Serum contains other heat-sensitive factors that may contribute to the increase in differentiation. Therefore, we immunodepleted ATX from serum to test the effects of selectively decreasing serum ATX on brown adipogenesis in culture. In comparison with serum incubated with an isotype control antibody, serum immunodepleted with anti-ATX IgG increased brown preadipocyte number (Supplemental Fig. 1C) and promoted higher expression of Pparγ (P < 0.05), Ucp1 (P = 0.076), and Cidea (P = 0.064) (Supplemental Fig. 1D). These results support a role for serum-derived ATX as a negative regulator of brown preadipocyte differentiation.
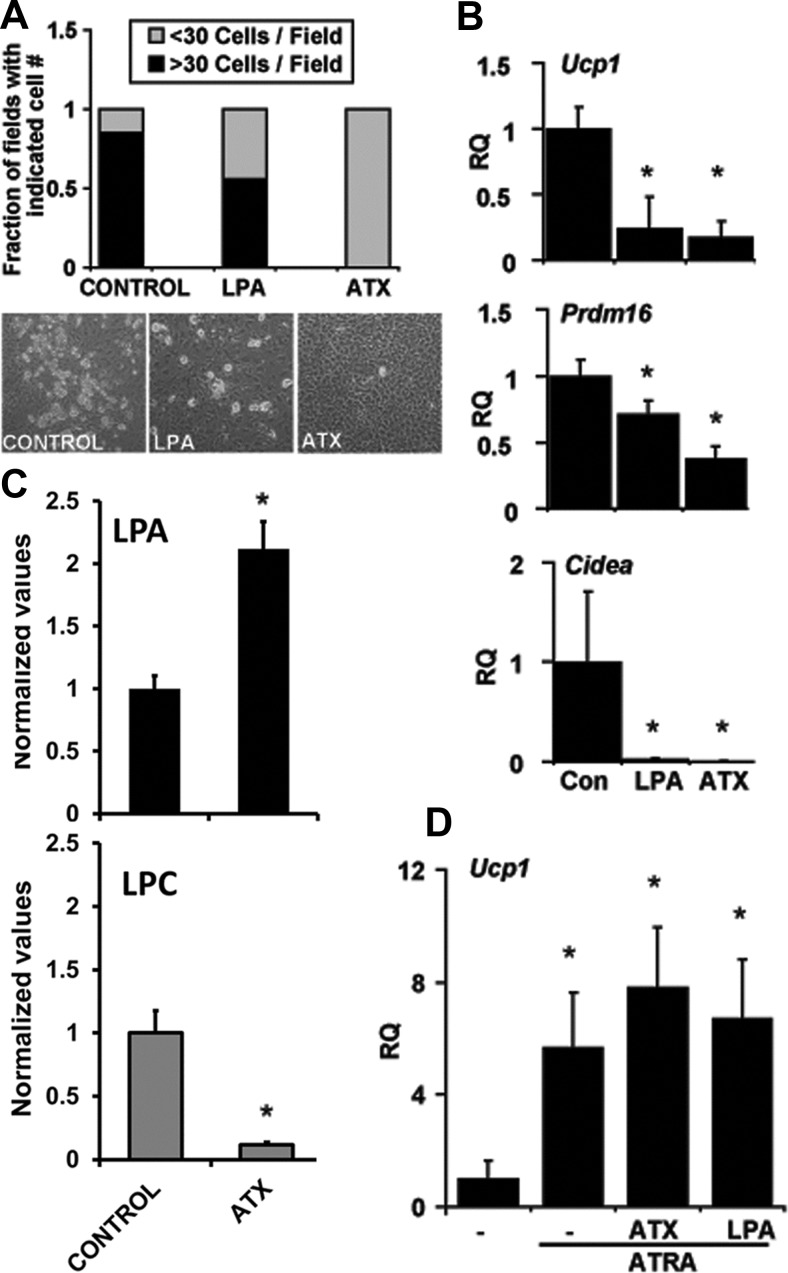
In keeping with these observations, the addition of recombinant ATX to primary brown adipocyte precursors resulted in a near-complete inhibition of lipid accumulation in these cells (Fig. 2A), accompanied by an approximately 83% reduction in Ucp1 expression (Fig. 2B). Expression of other BAT-specific markers, including Pdrm16, and Cidea was also significantly lower in the presence of ATX (Fig. 2B). The effects of recombinant ATX were recapitulated by the addition of exogenous LPA to the culture media (Fig. 2, A and B). LPA concentration was significantly increased, and LPC markedly decreased in the media from cells treated with recombinant ATX (Fig. 2C). However, neither ATX nor LPA had an effect on direct induction of Ucp1 expression by ATRA in MEF-derived adipocytes (Fig. 2D), confirming that the ATX-LPA axis interferes with the biochemical events resulting in brown adipocyte differentiation rather than having a direct effect on Ucp1 expression per se.
Fig. 2.
Effect of ATX and LPA treatment on brown preadipocyte differentiation. A, Quantification and representative images of primary BAT preadipocyte differentiation in the absence (control) or presence of LPA (5 μm) or recombinant ATX (3 μg/ml). Differentiated adipocytes were identified morphologically by lipid content, and the numbers per field were recorded (n = 20 fields). B, Real-time PCR measurement of Ucp-1, Prdm16, and Cidea expression in primary BAT preadipocytes differentiated in presence of LPA (5 μm) or recombinant ATX (3 μg/ml). *, P < 0.01 (one-way ANOVA). C, LPA (top) and LPC (bottom) levels in conditioned medium treated with recombinant ATX (3 μg/ml) or vehicle control. *, P < 0.05 (unpaired Student's t test). D, The effect of recombinant ATX and LPA treatment on ATRA-induced Ucp1 expression in MEF-derived adipocytes. *, P < 0.05 (one-way ANOVA). Data are representative of at least three independent experiments. Values shown are means ± sd. Con, Control; RQ, relative quantification.
An unbiased, microarray-based informatics approach was used to further examine the phenotypic effects of manipulation of the ATX-LPA axis on brown preadipocyte differentiation. Gene expression patterns were categorized based on reciprocal effects of inhibitors of LPA production as compared with exogenous LPA (patterns 4 and −4; red boxes in Supplemental Fig. 2). In the first category were genes the expression of which was increased when differentiation occurred in the presence of ATX inhibitors for about 5 d and was decreased by the addition of exogenous LPA (Genes down-regulated by ATX-LPA axis; pattern 4 in Supplemental Fig. 2). Several genes in this category encoded proteins involved in mitochondrial function and lipid metabolism (Supplemental Fig. 3A and Supplemental Table 1), confirming the ability of ATX inhibitors to promote brown adipocyte differentiation. In the second category were genes the expression of which increased when differentiation occurred in the presence of LPA for approximately 5 d and decreased with ATX inhibition (genes up-regulated by ATX-LPA axis; pattern −4 in Supplemental Fig. 2). Interestingly, this category was enriched in genes encoding proteins involved in extracellular matrix interactions (Supplemental Fig. 3B and Supplemental Table 2). Overall these data indicate that activation of the ATX-LPA axis inhibits brown adipogenesis in vitro and that increased ATX-LPA signaling might be associated with changes in cell-extracellular matrix interactions that could in turn participate in regulation of differentiation processes.
Overexpression of ATX in mice abrogates peripheral Ucp1 expression
Our results extend previous findings by establishing a normal role for ATX in BAT preadipocyte differentiation in a primary culture model system. However, the artificial experimental conditions, in which serum containing ATX is present, limits the utility of culture model systems to establish a definitive physiological role for the ATX-LPA axis in adipogenesis. Therefore, we sought to determine whether the effects of ATX observed in the preadipocyte culture system could be recapitulated in an in vivo setting. To do so, we took advantage of transgenic mice [Tg (ENPP2) mice] expressing the human ATX gene ENPP2 under control of the α1-antitrypsin promoter, which from previous reports is expected to drive transgene expression in later embryonic stages through adulthood in mice (32). We reasoned that the later embryonic expression pattern for the promoter might exclude a developmental effect of ATX. The promoter drives expression in several tissues, including liver, lung, and possibly macrophages, and results in modest increases in circulating levels of ATX and LPA in adult mice (33). The expression of the human ENPP2 mRNA transgene in sc and brown fat tissues of adult Tg (ENPP2) mice (Supplemental Fig. 4, A and B) did not result in measurable changes in LPA level in the tissues (data not shown). These results are in contrast with our observations in plasma (33) and probably reflect the fact that total tissue LPA contains small amounts of extracellular LPA generated by ATX and intracellular LPA generated by non-ATX-dependent pathways. Similar to the effect observed in cultured brown preadipocytes (Fig. 2), increased ATX expression was associated with a 75% decrease in Ucp1 expression in sc fat (P < 0.01) (Supplemental Fig. 5A). This effect was not due to a defective Ucp1 locus, because administration of the β3-adrenergic agonist CL-316,243 (1 mg/kg, ip) for 2 wk increased Ucp1 expression in WAT from both wild-type and transgenic mice (Supplemental Fig. 4C) Expression of other BAT-associated genes, including Prmd16, Cidea, and Pgc1a, was also significantly reduced in sc fat of Tg(ENPP2) mice (Supplemental Fig. 5A), suggesting that ATX expression may lower differentiation or expression of inducible BAT in sc WAT. The histological appearance of the sc WAT was similar in WT and Tg(ENPP2) mice (Supplemental Fig. 5B). No differences were observed in body weight (Supplemental Fig. 5C) or fat composition (Supplemental Fig. 5D) of young adult (8–10 wk of age) WT and Tg(ENPP2) mice on low-fat diet (10% kcal from fat).
Human ENPP2 transgene mRNA was also detected in IBAT of adult Tg(ENPP2) mice but was not present in neonatal mice (Supplemental Fig. 6A). Weight (Supplemental Fig. 6B), histological appearance (Supplemental Fig. 6C), and Ucp1 and Cidea expression in IBAT of Tg(ENPP2) mice (Supplemental Fig. 6D), were the same as WT mice at 8–10 wk of age. Thus, the transgene is not expressed during the critical developmental period for IBAT, and the delayed expression of the transgene in adult mice may functionally limit the phenotypic effect of ATX overexpression on IBAT. Taken together, these results indicate that, in this mouse model of ATX overexpression, the inhibitory effects of ATX and LPA on brown adipocyte differentiation observed in vitro are paralleled in vivo by the suppression of Ucp1 and other inducible BAT-related genes in sc fat.
Overexpression of ATX in mice promotes HFD-induced obesity
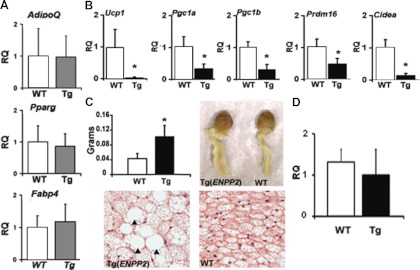
To determine whether the changes observed in BAT in sc adipose tissue could be exacerbated by conditions that predispose to obesity, wild-type and Tg(ENPP2) mice were fed an HFD (45% kcal from fat). The expression profile of classic adipocyte markers such as AdipoQ, Pparγ, and Fabp4 was normal in sc fat from Tg(ENPP2) mice on HFD (Fig. 3A). However, the BAT-specific genes Ucp1, Prmd16, Pgc1a, Pgc1β, and Cidea were 10-, 2-, 3-, and 7-fold lower in the Tg(ENPP2) mice as compared with WT mice on HFD (Fig. 3B), which was a more dramatic lowering than that observed in mice on standard diet (SD). In addition to changes in sc fat depots, we also observed that the periaortic BAT in Tg(ENPP2) mice on HFD was enlarged by approximately 50% (P < 0.05) in comparison with that in wild-type mice (Fig. 3C). Histological examination revealed that the normal multilocular lipid appearance of the periaortic BAT was often replaced by large deposits of intracellular lipid resembling WAT (Fig. 3C, bottom panels).
Fig. 3.
Impact of ATX overexpression in mice on HFD. A and B, Levels of expression of (A) AdipoQ, Pparg, and Fabp4 and (B) BAT-related genes Ucp1, Pgc1a, Pgc1b, Prdm16, and Cidea in sc fat from Tg(ENPP2) and WT mice (n = 5–10 mice/group) on HFD for 3 wk. *, P < 0.01 (unpaired Student's t test). C, Periaortic BAT depots (pictures in the right panel and weights in the left panel) in 8- to 10-wk-old Tg(ENPP2) and WT mice (n = 5). *, P < 0.05 (unpaired Student's t test). C (bottom), Hematoxylin and eosin staining of periaortic fat: large collections of intracellular lipid resembling WAT in the Tg(ENPP2) are indicated with black arrowhead (×40 magnification). *, P < 0.05 (unpaired Student's t test). D, Levels of expression of Ucp1 in periaortic BAT of Tg(ENPP2) and WT mice on HFD for 3 wk (n = 4 mice/group). Values are reported as means ± sd. RQ, Relative quantification.
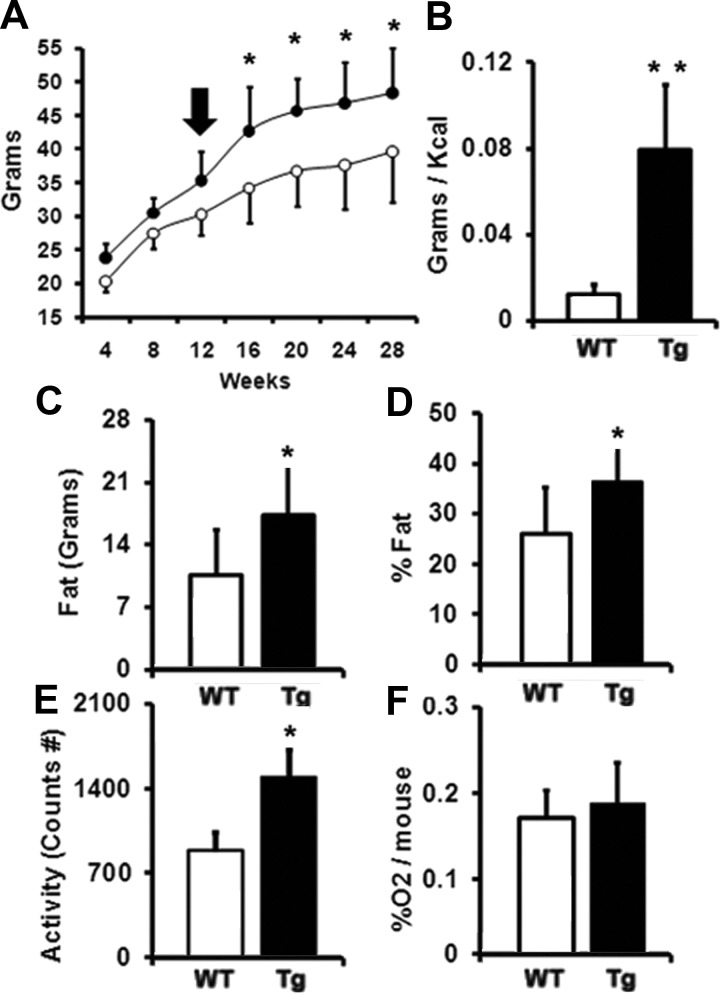
Semiquantitaive PCR analysis of periaortic BAT depots revealed that the levels of Ucp1 transcripts were similar between WT and transgenic tissues (Fig. 3D), indicating that the enlarged cells observed in transgenic mice are brown adipocytes that have accumulated more fat. After the switch from SD to HFD (Fig. 4A, arrow), the average body weight of Tg(ENPP2) mice increased substantially more than WT animals (Fig. 4A, 20.4% body weight increase in Tg(ENPP2) vs. 12.4% increase in WT mice; P < 0.05). Calories-to-body mass conversion efficiency, defined as grams of body weight gained per kcal food consumed, was significantly higher in Tg(ENPP2) mice (Fig. 4B), indicating that the transgenic mice were more efficient at turning energy into body mass. Dual-energy x-ray absorptiometry measurements revealed that the increase in weight was due to fat accumulation (Fig. 4, C and D), with the Tg(ENPP2) mice having on average 17.2 ± 5.8 g of fat and the WT controls 10.5 ± 5 g (Fig. 4C, P < 0.05). Interestingly, transgenic mice showed significantly more motor activity than WT mice (Fig. 4E), although no difference in O2 consumption was observed (Fig. 4F).
Fig. 4.
Impact of ATX overexpression on weight gain and metabolism. A, Average body weight of Tg(ENPP2) (•) and WT mice (○) before and after the switch from SD to HFD (black arrow) (n = 7) *, P < 0.05 (two-way ANOVA). B, Calories-to-body mass conversion efficiency (grams of body weight gained per kcal of food consumed). **, P < 0.01 (unpaired Student's t test). C and D, Total body fat (C) and body fat % (D), (n = 6/group). *, P < 0.05 (unpaired Student's t test). E and F, motor activity (E), and oxygen consumption (% oxygen consumed per mouse) (F) in Tg(ENPP2) and WT mice (n = 6–12 mice/group). *, P < 0.05 (unpaired Student's t test). Values are reported as means ± sd.
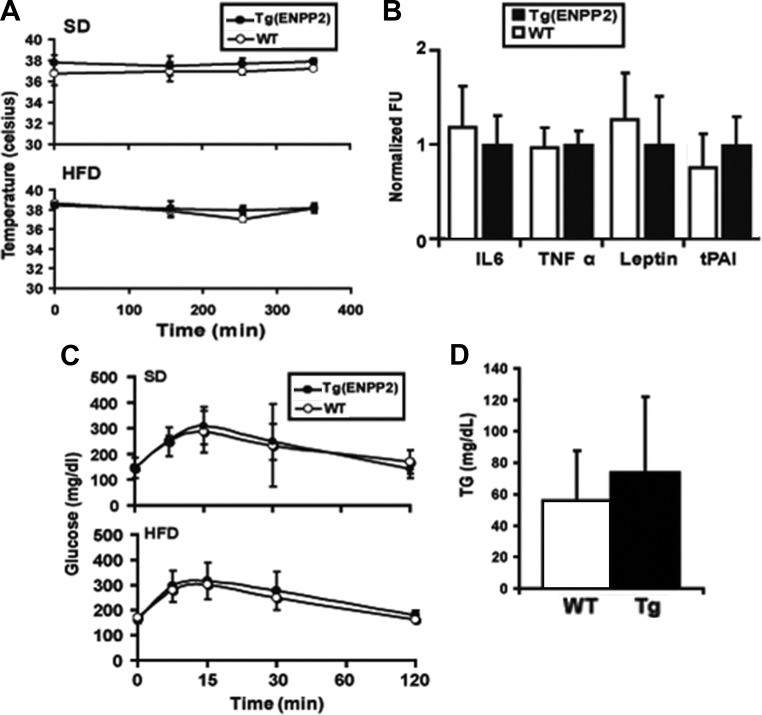
The peripheral changes in BAT markers did not alter the cold sensitivity of Tg(ENPP2) mice during a 6-h cold exposure (Fig. 5A). Moreover, Tg(ENPP2) and WT mice exhibited similar levels of plasma inflammatory markers and adipokines, including IL-6, TNFα, leptin, and tPAI (Fig. 5B), identical glycemic profiles after ip administration of glucose (Fig. 5C), and similar plasma triglyceride concentrations (Fig. 5D).
Fig. 5.
Cold tolerance, plasma inflammatory markers, plasma triglycerides, and glucose handling in Tg(ENPP2) mice. A, Body temperature of Tg(ENPP2) and WT mice (n = 4) was measured at room temperature and at different intervals up to 6 h at 4 C. Values are means ± sd. B, Circulating inflammatory markers, IL6, TNFα, insulin, leptin, and tPAI were measured in plasma from Tg(ENPP2) and WT mice (n = 10) on a Bio-plex 200 Suspension array System (Bio-Rad) using Mouse Serum Adipokine LINCOplex Kit. Normalized fluorescent unit (FU) values are reported. C, Intraperitoneal glucose tolerance test was performed by ip injection of glucose (2 g/kg body weight in isotonic saline) (n = 4 per group). D, Plasma triglycerides were measure by Wako L-Type TG M microtiter plate assay kit (n = 10). Values are means ± sd.
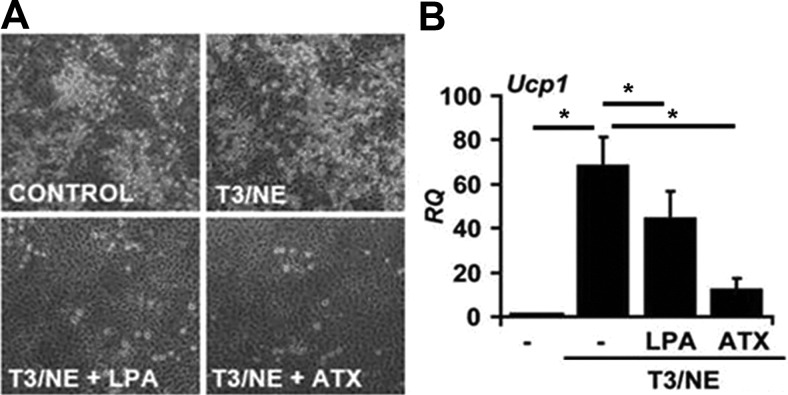
The observation that BAT genes were down-regulated in sc fat of Tg(ENPP2) mice is consistent with a negative impact of ATX on inducible brown adipocyte expression in sc WAT. To investigate this possibility, we used a culture model to simulate induction of BAT from WAT. Adipocyte precursors were isolated from the SVF of sc WAT and cultured in the presence of T3 and norepinephrine (NE) to promote the differentiation of progenitors toward a brown adipocyte phenotype (Fig. 6A). As expected, during differentiation of WAT-SVF preadipocytes, T3/NE treatment markedly increased Ucp1 expression (Fig. 6B). Adipocyte differentiation and Ucp1 expression were significantly inhibited by the addition of either ATX or LPA to the cells (Fig. 6, A and B). Together, our results suggest that the ATX-LPA signaling axis inhibits the expansion of inducible BAT in culture models and white adipose depots and thereby alters metabolic efficiency and adiposity in the setting of excessive caloric intake.
Fig. 6.
Effect of recombinant ATX and LPA on induction of brown adipogenesis in stromal vascular preadipocyte isolated from sc WAT. A, Images of cells from SVF differentiated in presence of insulin and dexamethasone (see Materials and Methods for details) with or without T3-norepinephrine (NE) treatment (5 nm T3 for 7 d followed by 1 μm NE 3 h before collection). The effect of LPA (5 μm) or ATX (3 μg/ml) treatment on differentiation is shown. B, Real-time PCR measurement of Ucp1 mRNA transcripts. *, P < 0.05 (ANOVA with Bonferroni correction). Values are means ± sd. RQ, Relative quantification.
Discussion
The pathological and physiological roles for ATX and its enzymatic product LPA have been investigated in cancer progression and metastasis (34), in the cardiovasculature (35), and in nervous system development (36). Much less is known about the involvement of the ATX-LPA signaling axis in adipogenesis and metabolism. In the present study, we identify a new role of the ATX-LPA axis in brown adipocyte differentiation. Our work establishes that ATX normally attenuates brown adipogenesis in vitro. The ability of specific and structurally unrelated ATX inhibitors, HA155 and PF83, to promote differentiation and Ucp1 and other BAT gene expression strongly implicates ATX as a negative regulator of brown adipocyte maturation in culture. Recombinant ATX and exogenous LPA blunted the differentiation of brown adipocytes, suggesting that dynamic regulation of their levels might normally affect maturation of brown adipocytes. Our observations are in keeping with reports of the effects of exogenously added LPA on white adipocyte differentiation, a response mediated by LPA1 receptor and accompanied by a decrease in Pparγ2 gene expression (24). Previous studies have also examined the effect of LPA on brown adipocyte proliferation through the Erk pathway (45, 46). Although no direct evidence of preadipocyte proliferation was reported, those studies suggest that the effect of the ATX/LPA axis on differentiation observed in our experiments could be explained by the growth promoting actions of these mediators. We did not observe, however, any noticeable difference in proliferation during the treatment period and the expression level of the proliferation marker Pcna was unaltered by LPA treatment. The inhibitory effect of ATX on adipocyte differentiation was greater than LPA alone, most likely because the additional ATX continuously generates bioactive LPA, whereas exogenously added LPA is degraded and inactivated by cell-associated activities. Further, ATX may be recruited to the cell surface, in close proximity of its lipid substrate and LPA receptors, and generate elevated local LPA concentrations in the vicinity of receptors for LPA. The existence of structural elements of ATX with integrin binding capability, such as the somatomedin B-like domain (37), indicates that integrin-mediated ATX binding to the cell surface could be a possible mechanism of enzymatic activity recruitment in the region in which LPA biological activity is required (48). It is therefore interesting to note that our microarray data indicate that increased ATX-LPA signaling associates with changes in expression of genes involved in cell-extracellular matrix interactions, which may, in turn, regulate adipose tissue development.
To assess the functional consequences of ATX modulation on adipogenesis at an organismal level, the effects of systemic elevation of ATX were examined in Tg(ENPP2) mice previously demonstrated to have elevated ATX and LPA levels (33). In this model the expression of the ENPP2 transgene is driven by the α1-antitrypsin promoter that is prevalently expressed in the liver, an organ with substantially low level of LPA receptors expression. Although the likelihood of an organ-related effect is expected to be reduced, it is likely that the systemic increase in ATX levels is the primary responsible for the observed phenotype. A nonautonomous effect of ATX is also supported by the marked effect that serum-derived ATX has on adipocyte cultures. However, we presently cannot exclude the possibility that overexpression of ATX is altering levels of an intermediate mediator that is ultimately responsible for the phenotype. With age, a subset of mice overexpressing ENPP2 do appear to gain more weight (Liu, S., S. Smyth, and G. Mills, unpublished observations), although it is not clear whether this phenotype emerges from a direct effect on WAT or on BAT depots. At a young age, Tg(ENPP2) mice appeared phenotypically indistinguishable from their WT counterparts, and their IBAT displayed normal development. The lack of an effect on IBAT may relate to the late onset of transgene expression, occurring after BAT development. In contrast, Tg(ENPP2) mice exhibit a significant decrease in Ucp1 and other BAT-related genes including Prdm16, Pgc1α, and Cidea in sc WAT. The down-regulation was amplified by HFD feeding and was accompanied by an increase in diet-induced obesity. These findings are strikingly similar to recent observations in mice overexpressing Prdm16 that demonstrated an increase in inducible BAT in sc, but not epididymal, adipose tissue and an accompanying increase in body weight on HFD but not SD (13). Our results are also consistent with the observation that a subpopulation of Sca1+/CD45−/Mac1− progenitor cells residing in murine sc fat can be induced to differentiate into brown fat-like cells, whereas the brown adipogenic capacity of this progenitor pool is substantially absent in epididymal and interscapular BAT (18). Interestingly, the increase in fat accumulation in the Tg(ENPP2) mice was not associated with heightened levels of systemic inflammatory mediators or metabolic dysregulation. Altogether, our results indicate that the ATX-LPA signaling axis exerts inhibitory control over inducible BAT in vitro and in vivo. Dusaulcy et al. (28) recently reported the phenotype of adipose-specific ablation of the ATX (Enpp2) gene in mice (FATX-KO), and showed that FATX-KO mice gained more weight than their wild-type counterparts. There are several potential explanations for the apparent disagreement between these observations and those reported here. First, temporal and spatial expression of ATX may have distinct consequences. Our data show that transgenic the ENPP2 mRNA transcripts are detectable only during adulthood in IBAT when fat tissues are already developed (Supplemental Fig. 6A). Postnatal up-regulation of ENPP2 transgene expression may therefore have distinct consequences from those that occur when Enpp2 is deleted by the AP2-Cre transgene in the FATX-KO model. Second, FATX-KO mice were on a C57BL/6 background whereas the mice used in our study were on a FVB/N background. This is of importance because these two strains show different susceptibility to diet-induced obesity (47), and genetic modifiers can impact the metabolic phenotype in unforeseeable ways. Finally, our observations support a role for extracellular ATX, which may be distinct from a developmental effect of adipocyte-generated ATX. It is interesting, however, to note that FATX-KO mice display an increase in weight of IBAT, which is in keeping with our observations that ATX may serve as an intrinsic negative regulator of brown fat adipogenesis. Although we cannot exclude a direct effect of the ATX transgene on white adipose cells, our results are consistent with phenotypes observed in mice in which genetic approaches have been used to up- or down-regulate inducible BAT. Finally, the ATX/LPA pathway is an attractive pharmacological candidate because of the ability of ATX inhibitors to potently promote brown adipogenesis in vitro. The results suggest that potent and orally available ATX inhibitors could be a new pharmacological tool in the armamentarium to combat obesity.
Supplementary Material
Acknowledgments
We thank Tracy Drennan (Division of Cardiovascular Medicine, University of Kentucky) and Wendy Katz (Graduate Center for Nutritional Sciences, University of Kentucky) for technical assistance and advice and Craig Vander Kooi (Department of Molecular and Cellular Biochemistry, University of Kentucky) for providing reagents.
This research was supported by grants from the NIH grants HL078663, GM050388, 1P20RR021954, and UL1RR033173 (to S.S.S. and A.J.M), a Veterans Affairs Merit Award (to S.S.S.), and fellowships from the American Heart Association (to L.F. and T.W.). This material is the result of work supported with the resources and use of the facilities at the Lexington VA Medical Center.
Disclosure Summary: The authors have no first-tier potential conflicts of interest with the submitted work to report. S.S.S has received investigator-initiated research/grant support from The Medicines Company in excess of $50,000 for unrelated work, and her laboratory serves as a core laboratory for biomarker analysis that is part of a preplanned substudy analysis of the ongoing TRACER trial being overseen by CirQuest Laboratories.
Footnotes
- ATRA
- All-trans-retinoic acid
- ATX
- autotaxin
- BAT
- brown adipose tissue
- BMP
- bone morphogenetic protein
- FBS
- fetal bovine serum
- IBAT
- interscapular BAT
- HFD
- high-fat diet
- LPA
- lysophosphatidic acid
- MEF
- mouse embryonic fibroblasts
- PCNA
- proliferating cell nuclear antigen
- PPAR
- peroxisome proliferator-activated receptor
- SD
- standard diet
- SVF
- stromal vascular fraction
- tPAI
- tissue-plasminogen activator inhibitor
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue
- WT
- wild type.
References
- 1. James WP. 2008. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 32(Suppl 7):S120–S126 [DOI] [PubMed] [Google Scholar]
- 2. Herrera BM, Lindgren CM. 2010. The genetics of obesity. Curr Diab Rep 10:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Speliotes EK, Willer CJ, Bernd SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, et al. 2010. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nedergaard J, Cannon B. 2010. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11:268–272 [DOI] [PubMed] [Google Scholar]
- 5. Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev Physiol Re 84:277–359 [DOI] [PubMed] [Google Scholar]
- 6. Nedergaard J, Bengtsson T, Cannon B. 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–E452 [DOI] [PubMed] [Google Scholar]
- 7. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. 2009. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 8. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. 2009. Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 9. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cypess AM, Kahn CR. 2010. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab 6:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM., Meyer CW, Wahli W, Klingenspor M, Herzig S. 2010. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328:1158–1161 [DOI] [PubMed] [Google Scholar]
- 16. Frontini A, Cinti S. 2010. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 11:253–256 [DOI] [PubMed] [Google Scholar]
- 17. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. 2010. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz TJ, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. 2011. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA 108:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills GB, Moolenaar WH. 2003. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3:582–591 [DOI] [PubMed] [Google Scholar]
- 20. Lin ME, Herr DR, Chun J. 2010. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 91:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pages C, Daviaud D, An S, Krief S, Lafontan M, Valet P, Saulnier-Blache JS. 2001. Endothelial differentiation gene-2 receptor is involved in lysophosphatidic acid-dependent control of 3T3F442A preadipocyte proliferation and spreading. J Biol Chem 276:11599–11605 [DOI] [PubMed] [Google Scholar]
- 22. Maumus M, Sengenès C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, Galitzky J, Bouloumié A. 2008. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab 93:4098–4106 [DOI] [PubMed] [Google Scholar]
- 23. Nobusue H, Kondo D, Yamamoto M, Kano K. 2010. Effects of lysophosphatidic acid on the in vitro proliferation and differentiation of a novel porcine preadipocyte cell line. Comp Biochem Physiol B Biochem Mol Biol 157:401–407 [DOI] [PubMed] [Google Scholar]
- 24. Simon MF, Daviaud D, Pradère JP, Grès S, Guigné C, Wabitsch M, Chun J, Valet P, Saulnier-Blache JS. 2005. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem 280:14656–14662 [DOI] [PubMed] [Google Scholar]
- 25. van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradère JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26:5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferry G, Tellier E, Try A, Grés S, Naime I, Simon MF, Rodriguez M, Boucher J, Tack I, Gesta S, Chomarat P, Dieu M, Raes M, Galizzi JP, Valet P, Boutin JA, Saulnier-Blache JS. 2003. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem 278:18162–18169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boucher J, Quilliot D, Pradères JP, Simon MF, Grès S, Guigné C, Prévot D, Ferry G, Boutin JA, Carpéné C, Valet P, Saulnier-Blache JS. 2005. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia 48:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dusaulcy R, Rancoule C, Grès S, Wanecq E, Colom A, Guigné C, van Meeteren LA, Moolenaar WH, Valet P, Saulnier-Blache JS. 2011. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res 52:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albers HM, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris AJ, Smyth SS, Moolenaar WH, Ovaa H. 2010. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci USA 107:7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Néchad M, Kuusela P, Carneheim C, Björntorp P, Nedergaard J, Cannon B. 1983. Development of brown fat cells in monolayer culture. I. Morphological and biochemical distinction from white fat cells in culture. Exp Cell Res 149:105–118 [DOI] [PubMed] [Google Scholar]
- 31. Cannon B, Nedergaard J. 2001. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol Biol 155:213–224 [DOI] [PubMed] [Google Scholar]
- 32. Kelsey GD, Povey S, Bygrave AE, Lovell-Badge RH. 1987. Species- and tissue-specific expression of human α1-antitrypsin in transgenic mice. Genes Dev 1:161–171 [DOI] [PubMed] [Google Scholar]
- 33. Pamuklar Z, Federico L, Liu S, Umezu-Goto M, Dong A, Panchatcharam M, Fulkerson Z, Berdyshev E, Natarajan V, Fang X, van Meeteren LA, Moolenaar WH, Mills GB, Morris AJ, Smyth SS. 2009. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem 284:7385–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu S, Murph M, Panupinthu N, Mills GB. 2009. ATX-LPA receptor axis in inflammation and cancer. Cell Cycle 8:3695–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris AJ, Panchatcharam M, Cheng HY, Federico L, Fulkerson Z, Selim S, Miriyala S, Escalante-Alcalde D, Smyth SS. 2009. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thromb Haemost 7:(Suppl 1):38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y, Kontoyiannis DL, Remboutsika E, Aidinis V. 2010. ATX expression and LPA signalling are vital for the development of the nervous system. Dev Biol 339:451–464 [DOI] [PubMed] [Google Scholar]
- 37. Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. 2000. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol 35:393–432 [DOI] [PubMed] [Google Scholar]
- 38. Mercader J, Palou A, Bonet ML. 2010. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity (Silver Spring) 18:655–662 [DOI] [PubMed] [Google Scholar]
- 39. Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. 2003. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 23:3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. 2009. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci 29:1805–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hochberg Y, Benjamini Y. 1990. More powerful procedures for multiple significance testing. Stat Med 9:811–818 [DOI] [PubMed] [Google Scholar]
- 42. Hulshizer R, Blalock EM. 2007. Post hoc pattern matching: assigning significance to statistically defined expression patterns in single channel microarray data. BMC Bioinformatics 8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4:3. [PubMed] [Google Scholar]
- 44. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. 2000. Ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holmström TE, Mattsson CL, Wang Y, Iakovleva I, Petrovic N, Nedergaard J. 2010. Non-transactivational, dual pathways for LPA-induced Erk1/2 activation in primary cultures of brown pre-adipocytes. Exp Cell Res 316:2664–2675 [DOI] [PubMed] [Google Scholar]
- 46. Mattsson CL, Andersson ER, Nedergaard J. 2010. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired β3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim Biophys Acta 1803:983–989 [DOI] [PubMed] [Google Scholar]
- 47. Hu CC, Qing K, Chen Y. 2004. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res 12:1264–1270 [DOI] [PubMed] [Google Scholar]
- 48. Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS. 2011. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem 286:34654–34663 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.