Abstract
Acute pancreatitis is a major health burden for which there are currently no targeted therapies. Premature activation of digestive proenzymes, or zymogens, within the pancreatic acinar cell is an early and critical event in this disease. A high-amplitude, sustained rise in acinar cell Ca2+ is required for zymogen activation. We previously showed in a cholecystokinin-induced pancreatitis model that a potential target of this aberrant Ca2+ signaling is the Ca2+-activated phosphatase calcineurin (Cn). However, in this study, we examined the role of Cn on both zymogen activation and injury, in the clinically relevant condition of neurogenic stimulation (by giving the acetylcholine analog carbachol) using three different Cn inhibitors or Cn-deficient acinar cells. In freshly isolated mouse acinar cells, pretreatment with FK506, calcineurin inhibitory peptide (CiP), or cyclosporine (CsA) blocked intra-acinar zymogen activation (n = 3; P < 0.05). The Cn inhibitors also reduced leakage of lactate dehydrogenase (LDH) by 79%, 62%, and 63%, respectively (n = 3; P < 0.05). Of the various Cn isoforms, the β-isoform of the catalytic A subunit (CnAβ) was strongly expressed in mouse acinar cells. For this reason, we obtained acinar cells from CnAβ-deficient mice (CnAβ−/−) and observed an 84% and 50% reduction in trypsin and chymotrypsin activation, respectively, compared with wild-type controls (n = 3; P < 0.05). LDH release in the CnAβ-deficient cells was reduced by 50% (n = 2; P < 0.05). The CnAβ-deficient cells were also protected against zymogen activation and cell injury induced by the cholecystokinin analog caerulein. Importantly, amylase secretion was generally not affected by either the Cn inhibitors or Cn deficiency. These data provide both pharmacological and genetic evidence that implicates Cn in intra-acinar zymogen activation and cell injury during pancreatitis.
Keywords: calcium targets
acute pancreatitis (AP) is a painful life-threatening disease that accounts for over 200,000 hospitalizations annually (7). However, unlike other gastrointestinal illnesses, treatments for AP are still largely supportive (9). Aberrant Ca2+ signals are necessary to initiate early events in pancreatitis, particularly the pathological activation of digestive proenzymes, or zymogens, within the acinar cell (19, 31, 35). These Ca2+ signals are nonoscillatory, high-amplitude transients that initiate in the apical pole and propagate to the basolateral region of the acinar cell (30). A key feature is that the Ca2+ signals are followed by a sustained Ca2+ plateau (29). It is of keen interest to know which proteins are directly targeted by the pathological Ca2+ signals. Calcineurin (Cn) is a Ca2+/calmodulin (CaM)-activated phosphatase that responds to sustained Ca2+ signals (8, 44). On the basis of these observations in other cell types, we hypothesized that an important target of aberrant acinar cell Ca2+ is Cn. We previously showed in a cholecystokinin (CCK)-induced murine model of pancreatitis that Cn mediates pathological intra-acinar protease activation (15, 38) and pancreatitis. However, several investigations have questioned whether CCK hyperstimulation is relevant to human acinar cells (34, 36). Whereas CCK receptors are abundant on the murine acinar cells, they have low expression in the human acinar cell (17, 49). Except for one notable recent report (26), CCK failed to elicit a Ca2+ signal or a secretory response in isolated human acini (17, 25, 40). By contrast, neurogenic stimulation using acetylcholine (ACh) or its long-acting analog carbachol causes robust physiological responses in both species (21, 28). It appears that, even in murine models, CCK signals through a neurogenic pathway to affect acinar cell secretion and pathology. Finally, there are numerous clinical reports of pancreatitis associated with cholinergic overload, primarily from exposure with organophosphates or other cholinesterase inhibitors (32, 39, 45, 48).
In this study, we demonstrate using a carbachol-induced model of mouse acinar cell injury that Cn inhibitors reduce intra-acinar zymogen activation and acinar cell injury, without affecting the secretory function of the cell. In addition, β-isoform of the catalytic A subunit (CnAβ)-deficient acinar cells were protected against both carbachol and CCK hyperstimulation.
MATERIALS AND METHODS
Reagents and animals.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. CnAβ−/− mice were of the B6129J/F1 strain (Harlan Laboratories, Boston, MA). Age-, sex-, and strain-matched control mice (generated by JDM; Ref. 1) were used as wild-type (WT) controls. All mice weighed 20–25 g at the time of use and were supplied with food and water ad libitum and maintained on a 12-h:12-h light/dark cycle. The complete protocol was approved by the Institutional Animal Care and Use Committee.
Preparation of pancreatic acini.
Groups of pancreatic acinar cells were isolated as previously described (27) with modifications. Briefly, the pancreas was removed from euthanized mice and minced for 5 min in DMEM/F12 1× buffer without phenol (GIBCO Invitrogen, Carlsbad, CA) containing 15 mM HEPES (pH 7.4), 120 mM NaCl, 4 mM KCl, 0.3 mM MgCl2, 1 mM CaCl2, 18 mM dextrose, and 3 mM glutamine, plus 0.1% BSA, and 2 mg/ml type-4 collagenase (Worthington, Freehold, NJ). The tissue was briefly oxygenated and incubated for 5 min at 37°C with shaking (90 revolution/min). Buffer was removed and replaced with fresh collagenase buffer, briefly oxygenated, and incubated for 35 min. The tissue digest was resuspended to obtain dispersed acini and then filtered through a 300-μm mesh (Sefar American, Depew, NY). Acinar cells were washed three times with collagenase-free buffer and then allowed to equilibrate for 5 min at 37°C before treatment.
Stimulation of acini and enzyme activity assays.
Acini were pretreated with Cn inhibitors (FK506, 1–20 μM) or Cn inhibitory peptide (CiP, 1–20 μM), or cyclosporine A (CsA, 0.1–10 μM) at 37°C for 30 min. They were then stimulated with carbachol (0.1 μM-1 mM) for 30 min to 2 h. Zymogen activity assays were performed at room temperature using fluorogenic substrates as previously described (4) with modifications. Briefly, 50 μl of 400 μM enzyme substrate were added to each homogenized sample, and accumulation of fluorescence was measured over 10 min using a fluorescent plate reader (Infinite M200; Tecan, Gratz, Austria) at 380-nm excitation and 440-nm emission wavelengths. The trypsin substrate was supplied by Peptides International (Louisville, KY) and had the amino acid sequence Boc-Gln-Ala-Arg-MCA. Chymotrypsin substrate was supplied by CalBiochem (Gibbstown, NJ) and had the amino acid sequence Suc-Ala-Ala-Pro-Phe-AMC. Zymogen activity was normalized to total amylase content using a Phadebas Kit (Magle Life Sciences, Lund, Sweden) and expressed as relative fluorescent units per second. Acinar cell injury was quantified by the amount of lactate dehydrogenase (LDH) released into the media using a nonradioactive cytotoxicity assay (Promega, Madison, WI). Absorbance was measured at 490 nm within 15 min of stopping the enzyme reaction. Results were expressed as a percentage of the total LDH that is released into the media.
Genomic DNA preparation and genotyping.
For genotyping, 1–2-mm sections of mouse tail were dissolved in 0.1 ml of 50 mM Tris (pH 8.0), 15 mM EDTA, 0.5% SDS, and 0.5 mg/ml proteinase K (Roche, Mannheim, Germany) solution at 55°C for at least 4 h. The DNA was purified with DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA). Briefly, tail digest was purified once with phenol/chloroform-isoamyl alcohol, and the DNA was precipitated by adding 1.5 ml of 100% ethanol and then dissolved in 0.2 ml of Tris-EDTA solution. CnAβ−/− mice were confirmed by tail cDNA genotyping using the following primers obtained from Ref. 1. 636 (3′) 5′ TCA GTA AGG TGT CTG CAT TC, 829 (5′) 5′ TTA GGC TAT ATT AAG CAA TCT G, 830 (3′) 5′ GAA GGA GCC AAG CTG CTA TT. PCR (34 cycles) consisted of 80°C for 10 min, 94°C for 15 s, optimal annealing temperature 54°C for 1 min, 72°C for 1 min followed by incubation at 72°C for 10 min. The amplified products were fractioned on a 2% agarose gel and visualized by ethidium bromide staining.
Semi-quantitative measurement of Cn expression by RT-PCR.
RNA was isolated from acinar cells using TRIzol Reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. RNA was further purified using the RNeasy kit (Qiagen), with genomic DNA elimination columns and subsequent on-column DNase treatment to eliminate genomic DNA contamination. The quality of RNA was confirmed by detecting the presence of ribosomal RNA bands and the absence of genomic DNA by gel electrophoresis. Only samples having spectrophotometric ratio of optical density (260/280 nm) greater than 1.8 were used. Total RNA (2 μg) was reverse transcribed using the Transcriptor First-Strand cDNA Synthesis Kit (Roche). The resultant cDNA was amplified using PCR SuperMix (Invitrogen, San Diego, CA) with the following set of mouse-specific primers: CnAα, 5′-GTGGAATTCTCTGGAGACAAACCACC-3′ and 5′-GGCAGGAGAGTAAATTCTTGC-3′; CnAβ, 5′-CAGGGACTCTCACATCTTGG-3′ and 5′-TCCTCTGTGGCAGCGATTAC-3′; CnAγ, 5′-TCACTCCCACAGGCACACTC-3′ and 5′-TGAAGCCTCTTTTCGGGGTG-3′; CnB1, 5′-AGGAGTGTCTCAGTTCAGTG-3′ and 5′-CCCACCATCATCTTCAACAC-3′; CnB2, 5′-CGGGTGGAGTGAGAAGATAC-3′ and 5′-TCTGCTAGGGACCAGATCTG-3′; β-actin, 5′-TTCTACAATGAGCTGCGTGTGGC-3′ and 5′-CTCATAGCTCTTCTCCAGGGAGGA-3′. PCR was conducted with the optimal numbers of cycles (Cn isoforms: 34 cycles; β-actin: 32 cycles) consisting of 80°C for 10 min, 94°C for 15 s, optimal annealing temperature 60°C for 1 min, 72°C for 1 min followed by incubation at 72°C for 10 min. The amplified products were fractioned on a 2% agarose gel and visualized by ethidium bromide staining.
Cn phosphatase activity.
Whole pancreas and acinar cells were homogenized in lysis buffer containing 50 mM Tris·HCl (pH 7.5), 0.1 mM EGTA, 1 mM EDTA, 0.5 mM dithiothreitol, 0.1% β-mercaptoethanol, 5 mM ascorbic acid, 0.1% NP-40, and a protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Splenic lymphocytes were isolated from splenic homogenates as previously described (3), with modifications. Briefly, homogenate was filtered through a 40-μm nylon mesh. The filtrate was then treated with 0.5 ml red cell lysis solution per ml of filtrate and incubated at room temperature for 5 min. Lymphocytes were filtered through a 40-μm nylon mesh. Cell filtrates and tissue homogenates were triturated in lysis buffer on ice using a 30.5-gauge needle. Homogenates were then centrifuged at 15,000 g for 15 min at 4°C. The resulting supernatants were stored at −80°C until used. Phosphatase activity was measured using the Calcineurin Cellular Activity Assay Kit (EMD Chemicals, Rockland, MA).
Statistics.
Data are represented as means ± SE of three individual experiments with multiple cells from each experiment unless otherwise stated. Where not shown, error bars are within the size of the data point. Statistical significance was determined by Student's t-test.
RESULTS
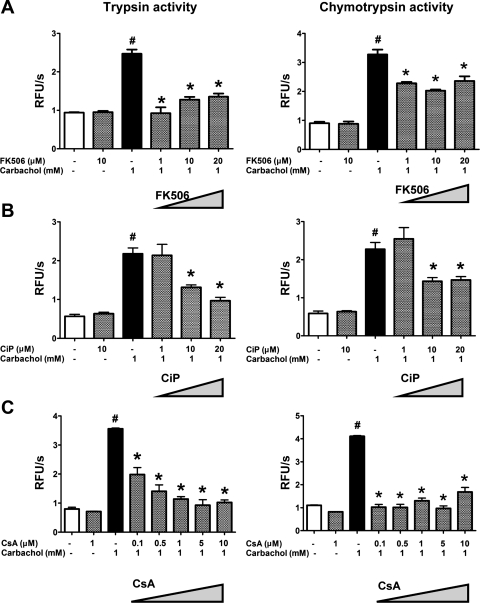
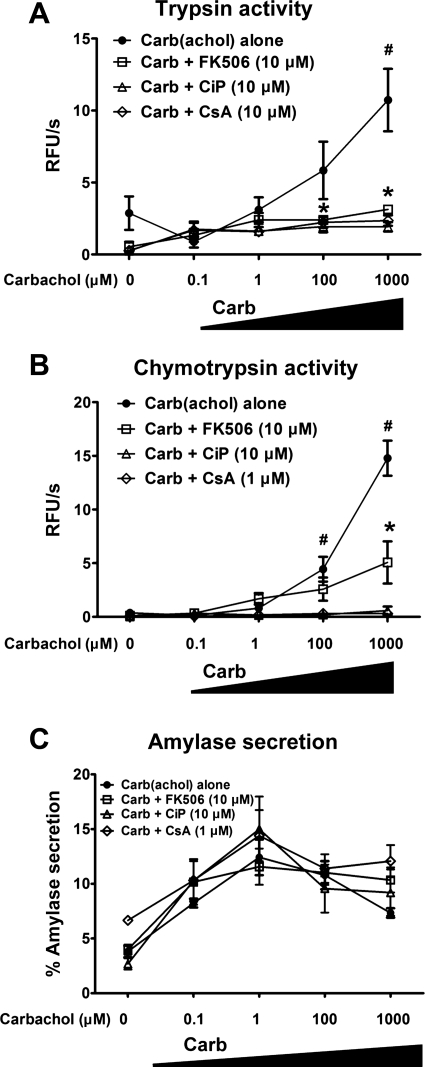
One of the earliest features of pancreatitis is the activation of zymogens, particularly, proteases, within the pancreatic acinar cell (20). These events occur in vivo and can be recapitulated in isolated acinar cells. The long-acting ACh analog carbachol can induce intra-acinar zymogen activation at supraphysiological concentrations in the high-micromolar or low-millimolar range (Figs. 1 and 2). In this situation, muscarinic receptors on the acinar cell signal to cause the opening of intracellular Ca2+ channels, including the inositol 1,4,5-trisphosphate receptor, the ryanodine receptor, and the subsequent opening of store-operated Ca2+ channels (16, 30). To examine whether these Ca2+ events trigger zymogen activation via Cn, freshly isolated mouse acinar cells were pretreated with each of three Cn inhibitors. FK506 and CsA inhibit Cn by forming a complex with FK506 binding protein 12 and cyclophilin, respectively, which then binds Cn and blocks access of substrates to the catalytic site on Cn (24). CiP is a short peptide that mimics the autoinhibitory domain of Cn. We have used a cell-permeable form of CiP that is made by covalently attaching an arginine tail to the peptide (43). Figure 1 provides concentration-dependence data of the Cn inhibitors on zymogen activation, whereas Fig. 2 shows concentration dependence of carbachol. There was a 2.5- and 3.0-fold increase in the intra-acinar activation of the zymogens trypsin and chymotrypsin, respectively, with carbachol (1 mM). FK506, CiP, and CsA reduced the pathological activation of trypsin by 81%, 50%, and 88%, respectively (P < 0.05; Fig. 1). The reduction was observed over a range of carbachol concentrations that are known to induce zymogen activation (Fig. 2). Carbachol stimulates the secretion of pancreatic enzymes from the acinar cell in a biphasic fashion, with maximal secretion at 1 μM (Fig. 2C) (51). However, none of the Cn inhibitors significantly affected the secretory pattern of carbachol-stimulated amylase release. The results with the Cn inhibitors indicate that Cn mediates carbachol-induced zymogen activation and cell injury but does not affect the primary physiological function of the cell in secreting pancreatic enzymes.
Fig. 1.
Calcineurin (Cn) inhibitors at varying concentrations reduce intra-acinar zymogen activation. Acinar cells were treated with FK506 (A), Cn inhibitory peptide (CiP) (B), or cyclosporine A (CsA) (C) at the indicated concentrations for 30 min before carbachol (1 mM) stimulation. Activities of trypsin (left) and chymotrypsin (right) were measured 30 min after carbachol stimulation and normalized to total amylase content (n = 3). *P < 0.05 with respect to control or carbachol alone, respectively. Data are means ± SE. RFU, relative fluorescence units.
Fig. 2.
Cn inhibitors reduce intra-acinar zymogen activation over a range of carbachol concentrations, but they do not affect acinar cell enzyme secretion. Acinar cells were pretreated with FK506 (10 μM), CiP (10 μM), or CsA (1 μM) for 30 min before carbachol (0.1 μM-1.0 mM) stimulation. Activities of trypsin (A) and chymotrypsin (B) were measured 30 min after carbachol stimulation and normalized to total amylase content (n = 3). C: biphasic amylase secretion curve with or without the Cn inhibitors (n = 3). *P < 0.05 with respect to control or carbachol alone, respectively. Data are means ± SE.
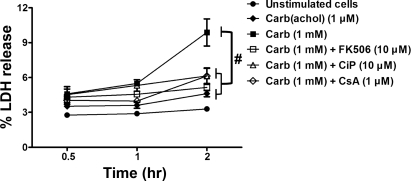
The intra-acinar activation of zymogens is followed by acinar cell injury, which can be followed over several hours in cultured acini by measuring leakage of LDH into the media (35). Carbachol (1 mM) induced 10 ± 1.2% LDH release over a 2-h period. Compared with 3 ± 0.17% in unstimulated cells and 5 ± 0.27% in cells receiving a generally noninjurious concentration of carbachol (1 μM; Fig. 3). Pretreatment with the Cn inhibitors each reduced cell injury on average by 68% (P < 0.05).
Fig. 3.
Cn inhibitors reduce cell injury induced by carbachol. Acinar cells were pretreated with FK506 (10 μM), CiP (10 μM), or CsA (1 μM) for 30 min before a 2-h carbachol (1 μM or 1 mM) incubation. Cell injury was measured as percent lactate dehydrogenase (LDH) released from acinar cells (n = 3). #P < 0.001 with respect to 1 mM carbachol. Data are means ± SE.
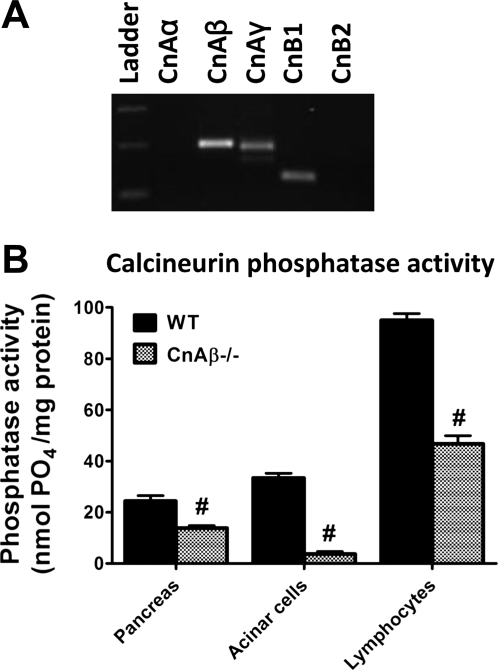
To confirm the results with the three different inhibitors, we also pursued a genetic approach to provide complimentary evidence for a role of Cn in acinar cell pathology. Of the CnA subunit isoforms, we found by semi-quantitative PCR that CnAβ is strongly expressed in the acinar cell (Fig. 4A). For this reason, we obtained acinar cells from CnAβ−/− mice. These mice have no overt phenotypic defects at baseline, but they display defective T cell development (1) and have a reduced ability to mount a cardiac hypertrophy response (2). Tail genotyping confirmed the presence or absence of CnAβ in WT or CnAβ−/− mice, respectively (data not shown). Although CnAγ is also expressed in the acinar cell, the CnAβ−/− acinar cells had an 89% reduction in Cn phosphatase activity compared with WT controls (P < 0.05; Fig. 4B). This represented a greater reduction in activity than the 43% and 51% seen in the whole pancreas or splenic lymphocytes, respectively, from the CnAβ−/− mice. The findings indicate that CnAβ−/− acinar cells can be used as a model of Cn deficiency to examine the role of Cn in acinar cell events leading to pancreatitis.
Fig. 4.
The β-isoform of the catalytic A subunit (CnAβ) isoform is expressed in pancreatic acinar cells, and CnAβ-deficient mice have reduced pancreatic and acinar cell Cn phosphatase activity. A: DNA gel of PCR from acinar cell cDNA using Cn isoform-specific primers. B: Cn phosphatase activity from whole pancreas, acinar cells, and splenic lymphocytes. #P < 0.01 with respect to wild-type (WT). Data are means ± SE.
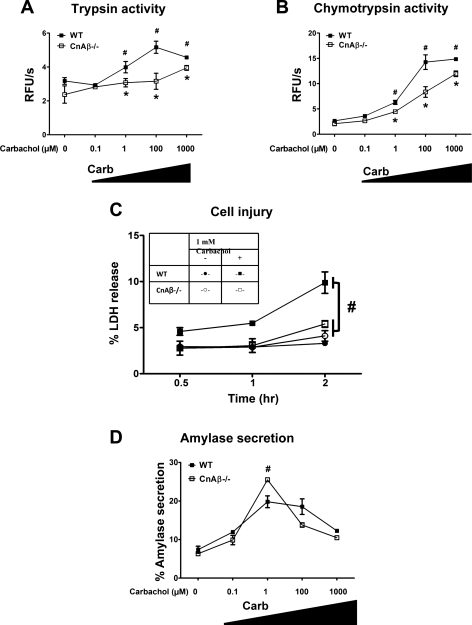
Compared to WT acinar cells, CnAβ-deficient acinar cells had an 84% and 50% reduction in trypsin and chymotrypsin activation, respectively (Fig. 5, A and B). Cell injury after a 2-h incubation with 1 mM carbachol was reduced by 63% compared with the WT cells (P < 0.05; Fig. 5C). Differences in amylase secretion were modest although secretion in the CnAβ−/− cells was significantly higher at maximal stimulatory concentrations of carbachol (1 mM). However, there was no particular shift in the biphasic secretion curve in the CnAβ-deficient cells compared with WT.
Fig. 5.
CnAβ-deficient mice have reduced intra-acinar zymogen activation and cell injury induced by carbachol. Acinar cells from WT or CnAβ−/− mice were treated with carbachol (0.1 μM-1.0 mM) for 30 min, and trypsin (A) and chymotrypsin (B) activities were normalized to total amylase content (n = 3). #*P < 0.05 with respect to the unstimulated condition or WT acinar cells, respectively. C: acinar cells were treated with 1 mM carbachol for 2 h, and cell injury was measured as percent LDH released (n = 2). #P < 0.001, with respect to WT acinar cells receiving 1 mM carbachol. D: biphasic amylase secretion curve (n = 3). Data are means ± SE.
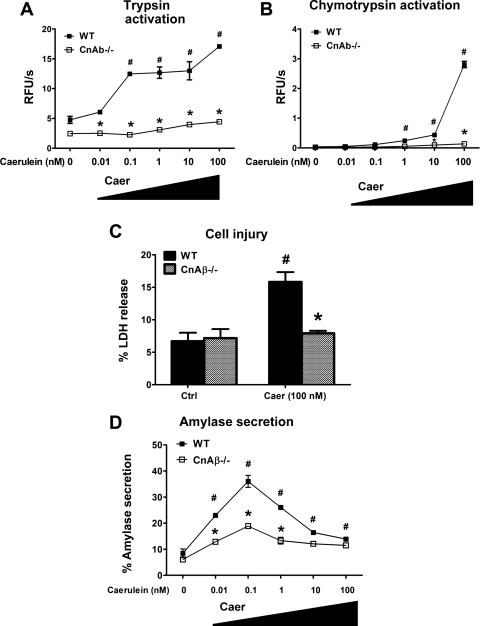
We have previously demonstrated that pharmacological Cn inhibition reduces CCK-induced intra-acinar zymogen activation (15, 38). In the present study, we now examined the effects of CnAβ deficiency on CCK responses. As with carbachol, the CCK analog caerulein caused intra-acinar zymogen activation in a concentration-dependent manner in WT cells (Fig. 6, A and B). Interestingly, the CnAβ−/− cell responses to caerulein were comparatively flat-lined, and cell injury was virtually abrogated (Fig. 6C). In contrast to the secretory response with carbachol, maximal secretion was reduced in the CnAβ−/− cells. Overall, the results with the CnAβ−/− cells provide compelling evidence that Cn influences carbachol- and caerulein-induced zymogen activation and cell injury.
Fig. 6.
CnAβ-deficient mice have reduced intra-acinar zymogen activation and cell injury induced by caerulein. Acinar cells from WT or CnAβ−/− mice were treated with caerulein (0.01–100 nM) for 30 min, and trypsin (A) and chymotrypsin (B) activities were normalized to total amylase content (n = 3). #*P < 0.05 with respect to the unstimulated condition or WT acinar cells, respectively. C: acinar cells were treated with 100 nM caerulein for 2 h, and cell injury was measured as percent LDH released (n = 2). #P < 0.001, with respect to WT acinar cells receiving 100 nM caerulein. D: biphasic amylase secretion curve (n = 3). Data are means ± SE.
DISCUSSION
The key findings of the present study are novel because previous studies implicated a role for Cn in mediating CCK-induced zymogen activation (15, 38). Here, we demonstrate that either Cn inhibition or genetic deficiency of a predominant Cn isoform (CnAβ) protects the pancreatic acinar cell from neurogenic-induced zymogen activation as well as cell injury. Cn (also known as PP2B) is a CAM-dependent serine/threonine phosphatase. It was first identified in brain, but its importance is now recognized in a diverse number of physiological and pathological states ranging from neuronal and muscle development, lymphocyte activation, cardiac hypertrophy, and growth of the pancreas (33). Regarding the latter, Williams and colleagues (42) demonstrated that the adaptive growth of the pancreas in mice fed camostat, a soybean trypsin inhibitor, could be blocked by CsA and FK506 (42), as well as in mice that conditionally overexpressed in acinar cells an endogenous Cn inhibitor, Rcan1 (regulator of calcineurin 1) (13, 14). The role of Cn in lymphocyte activation has been clinically exploited with the advent of the Cn inhibitors CsA and FK506 (18). They are widely used as immunosuppressants after solid organ transplantation and have other indications in the treatment of several autoimmune disorders. Thus our findings in a neurogenic model of acinar cell injury suggest the Cn inhibitors could have a multimodal effect on acute pancreatitis at both the level of early acinar cell events and the ensuing inflammatory cascade. It is important to note, however, that the Cn inhibitors were given for brief periods before or during the induction of acinar cell injury. However, chronic administration of Cn inhibitors exacerbates pancreatitis or pancreatic fibrosis in several clinical (37, 46) and experimental reports (6, 12, 47).
Cn activity is dependent on the formation of a heterodimer, a catalytic A subunit (CnA) and a regulatory B subunit (CnB). Of the three CnA isoforms, we saw strong expression in the pancreatic acinar cell of CnAβ. Even though CnAγ was also expressed, Cn phosphatase activity in the CnAβ−/− acinar cell was reduced by 89%, demonstrating that CnAβ is a functionally important acinar cell Cn isoform. In addition, CnAβ may have selective effects as a stress-response isoform, as described in heart (2, 41). The findings in these cells compared with WT mostly paralleled those seen with administration of the Cn inhibitors. With regard to amylase secretion, however, both the present study and previous reports expose a heterogeneity of findings. Doi et. al (5) and Waschulewski et. al (50) reported that both FK506 and CsA reduced CCK-stimulated amylase secretion. Later, Groblewski et. al (11) demonstrated a reduction with CsA but not FK506, and this discrepancy roughly correlated with the degree of pancreatic Cn phosphatase inhibition. In a previous study, we also saw no reduction in caerulein-stimulated amylase secretion with FK506 (15), consistent with the present findings of the Cn inhibitors with carbachol stimulation. However, it is not clear why there was a marked reduction in amylase secretion observed in the CnAβ−/− acini stimulated with caerulein but no decrease with carbachol. It is possible that, compared with muscarinic receptor stimulation, CCK receptor-mediated Ca2+ release selectively signals to the Cn complex containing CnAβ to induce pancreatic enzyme secretion. Another explanation for the difference could be that CCK receptors, but not muscarinic receptors, are coupled to adenylate cyclase (23). Thus the cAMP rise from the CCK receptor stimulation could potentiate the effects of Cn on downstream targets such as the phosphatase PP1 by posttranslationally modifying a Cn substrate DARPP-32 (10). It is also notable that CnAβ deficiency caused a more profound reduction in trypsin and chymotrypsin activation with caerulein than with carbachol, indicating the Cn plays a more prominent role in the CCK receptor pathway.
In previous work, we showed that Cn inhibition does not modulate acinar cell Ca2+ signals, suggesting that Cn is downstream to Ca2+ (15). Together with the present work, however, the findings raise several questions for further study, including what pattern of Ca2+ signaling would favor Cn activation in the acinar cell and what modulates this response to Ca2+. Further downstream, what are the targets of Cn activation in the acinar cell that lead to pathological zymogen activation and cell injury? Cn has a number of known phosphoprotein targets. The most well-described is the transcription factor nuclear factor of activated T cells (NFAT) (22). However, our zymogen activation and injury responses were assayed within 2 h of administering carbachol, and thus NFAT is unlikely a target of these early events. Nonetheless, its role in vivo, hours after the onset of pancreatitis, either within the acinar cell or in other cell types, has not been determined. In summary, we have used a model of acinar cell injury with carbachol that mimics neurogenic hyperstimulation and demonstrates that both pharmacological inhibition and genetic deficiency of Cn protect against intra-acinar zymogen activation and cell injury. The findings implicate a role of Cn in clinically relevant models of acinar cell injury leading to acute pancreatitis and the potential role of Cn inhibitors in treating pancreatitis.
GRANTS
This work was supported by National Institutes of Health Grants DK093491, DK083327, HD001401 (Yale Child Health Research Center), DK34989 (Yale Liver Center), and a Children's Digestive Health and Nutrition Young Investigator Award (to S. Husain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.A.M., M.U.A., S.M.M., and A.U.S. performed experiments; K.A.M., M.U.A., S.M.M., A.U.S., and S.Z.H. analyzed data; K.A.M., M.U.A., A.I.O., S.M.M., A.U.S., and S.Z.H. interpreted results of experiments; K.A.M. and A.I.O. prepared figures; K.A.M., A.I.O., J.D.M., and S.Z.H. edited and revised manuscript; K.A.M., M.U.A., A.I.O., S.M.M., A.U.S., J.D.M., and S.Z.H. approved final version of manuscript; S.Z.H. conception and design of research; S.Z.H. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Fred Gorelick, Michael Nathanson, Mark Lowe, Vijay Singh, and Vineet Bhandari for helpful discussion.
REFERENCES
- 1. Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin Abeta-deficient mice. Proc Natl Acad Sci USA 99: 9398–9403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci USA 99: 4586–4591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casareale D, Pottathil R, Diaco R. Improved blood sample processing for PCR. PCR Methods Appl 2: 149–153, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 292: G1403–G1410, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doi R, Tangoku A, Inoue K, Chowdhury P, Rayford PL. Effects of FK506 on exocrine pancreas in rats. Pancreas 7: 197–204, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Echigo Y, Inoue K, Kogire M, Doi R, Higashide S, Sumi S, Kaji H, Imamura M. Effects of cyclosporine and tacrolimus (FK 506) on acute pancreatitis in mice. Arch Surg 130: 64–68, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 136: 1134–1144, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 371: 143–152, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23: 435–447, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Groblewski GE, Wagner AC, Williams JA. Cyclosporin A inhibits Ca2+/calmodulin-dependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem 269: 15111–15117, 1994 [PubMed] [Google Scholar]
- 12. Gukovsky I, Lugea A, Shahsahebi M, Cheng JH, Hong PP, Jung YJ, Deng QG, French BA, Lungo W, French SW, Tsukamoto H, Pandol SJ. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 294: G68–G79, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Gurda GT, Crozier SJ, Ji B, Ernst SA, Logsdon CD, Rothermel BA, Williams JA. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology 139: 609–619; e601–e606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurda GT, Guo L, Lee SH, Molkentin JD, Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19: 198–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 292: G1594–G1599, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci USA 102: 14386–14391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology 121: 1380–1390, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Kapturczak MMH, Meier-Kriesche HHU, Kaplan BB. Pharmacology of calcineurin antagonists. Transplant Proc 36: 25S–32S, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol 157: 43–50, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am 84: 549–563; viii, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lugea A, Gong J, Nguyen J, Nieto J, French SW, Pandol SJ. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res 34: 1768–1781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macian F. NFAT proteins: Key regulators of t-cell development and function. Nat Rev Immunol 5: 472–484, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Marino CR, Leach SD, Schaefer JF, Miller LJ, Gorelick FS. Characterization of cAMP-dependent protein kinase activation by CCK in rat pancreas. FEBS Lett 316: 48–52, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez-Martínez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem 11: 997–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Miyasaka K, Shinozaki H, Jimi A, Funakoshi A. Amylase secretion from dispersed human pancreatic acini: neither cholecystokinin a nor cholecystokinin B receptors mediate amylase secretion in vitro. Pancreas 25: 161–165, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Green GM, Reeve JR, Jr, Petersen OH, Sutton R. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 135: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Orabi AI, Shah AU, Muili K, Luo Y, Mahmood SM, Ahmad A, Reed A, Husain SZ. Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini. J Biol Chem 286: 14090–14097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 127: 957–969, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Parekh AB. Calcium signaling and acute pancreatitis: specific response to a promiscuous messenger. Proc Natl Acad Sci USA 97: 12933–12934, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA 97: 13126–13131, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roeyen G, Chapelle T, Jorens P, de Beeck BO, Ysebaert D. Necrotizing pancreatitis due to poisoning with organophosphate pesticides. Acta Gastroenterol Belg 71: 27–29, 2008 [PubMed] [Google Scholar]
- 33. Rusnak F, Mertz P. Calcineurin: Form and Function. Physiol Rev 80: 1483–1521, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Saluja A, Logsdon C, Garg P. Direct versus indirect action of cholecystokinin on human pancreatic acinar cells: is it time for a judgment after a century of trial? Gastroenterology 135: 357–360, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 276: G835–G842, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol 69: 249–269, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sastry J, Young S, Shaw PJ. Acute pancreatitis due to tacrolimus in a case of allogeneic bone marrow transplantation. Bone Marrow Transplant 33: 867–868, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Shah AU, Liu J, Mistry PK, Jain D, Husain SZ. Protease activation during in vivo pancreatitis is dependent upon calcineurin activation. Am J Physiol Gastrointest Liver Physiol 297: G967–G973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh S, Bhardwaj U, Verma SK, Bhalla A, Gill K. Hyperamylasemia and acute pancreatitis following anticholinesterase poisoning. Hum Exp Toxicol 26: 467–471, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Susini C, Estival A, Scemama JL, Ruellan C, Vaysse N, Clemente F, Esteve JP, Fourmy D, Ribet A. Studies on human pancreatic acini: action of secretagogues on amylase release and cellular cyclic AMP accumulation. Pancreas 1: 124–129, 1986 [PubMed] [Google Scholar]
- 41. Taigen T, DeWindt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA 97: 1196–1201, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784–G790, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Terada H, Matsushita M, Lu YF, Shirai T, Li ST, Tomizawa K, Moriwaki A, Nishio S, Date I, Ohmoto T, Matsui H. Inhibition of excitatory neuronal cell death by cell-permeable calcineurin autoinhibitory peptide. J Neurochem 87: 1145–1151, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383: 837–840, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Tomiyama M, Arai A, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shoji M. Exacerbation of chronic pancreatitis induced by anticholinesterase medications in myasthenia gravis. Eur J Neurol 15: e40–e41, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Uemoto S, Tanaka K, Honda K, Tokunaga Y, Sano K, Katoh H, Yamamoto E, Takada Y, Ozawa K. Experience with FK506 in living-related liver transplantation. Transplantation 55: 288–292, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Vaquero E, Molero X, Tian X, Salas A, Malagelada JR. Myofibroblast proliferation, fibrosis, and defective pancreatic repair induced by cyclosporin in rats. Gut 45: 269–277, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Votanopoulos KI, Lee TC, Dominguez EP, Choi YU, Sweeney JF. Propoxur induced pancreatitis after inhalation of baygon pesticide. Pancreas 34: 379–380, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Wank SA, Pisegna JR, de Weerth A. Cholecystokinin receptor family. Molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann NY Acad Sci 713: 49–66, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Waschulewski IH, Hall DV, Kern HF, Edwardson JM. Effects of the immunosuppressants cyclosporin A and FK 506 on exocytosis in the rat exocrine pancreas in vitro. Br J Pharmacol 108: 892–900, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol 235: 517–524, 1978 [DOI] [PubMed] [Google Scholar]