Abstract
Beclin-1 has a central role in the regulation of autophagy. Barrett's esophagus (BE) is associated with a significantly increased risk for the development of esophageal adenocarcinoma (EAC). In the current study, we evaluated the role of Beclin-1 and autophagy in the EAC. Biopsies obtained from patients with BE and EAC, tissues from a rat model of BE and EAC, and esophageal cell lines were evaluated for the expression of Beclin-1 by immunohistochemistry, immunoblotting, or RT-PCR. Since reflux of bile acids is important in EAC, we also evaluated the effect of exposure to deoxycholic acid (DCA) on autophagy and Beclin-1 expression. Beclin-1 expression was high in squamous epithelium and nondysplastic BE, whereas its expression was low in dysplastic BE and EAC. The same pattern of expression was observed in rat tissues and in esophageal cell lines. Normal esophageal epithelium and HET-1A cells (derived from normal squamous epithelium) show high levels of Beclin-1, but lower levels of Beclin-1 were found in BE and EAC cell lines (CP-A, CP-C, and OE33). Acute exposure to DCA led to increased Beclin-1 expression and increased autophagy as evaluated by electron microscopy and counting percentage of GFP-LC3-positive BE cells with punctate pattern. In contrast, chronic exposure to DCA did not result in the alteration of Beclin-1 levels or autophagy. In summary, these data suggest that autophagy is initially activated in response to bile acids, but chronic exposure to bile acids leads to decreased Beclin-1 expression and autophagy resistance.
Keywords: bile acids, autophagy
barrett's esophagus (BE) is a condition where normal squamous epithelium is replaced by metaplastic columnar epithelium containing goblet cells. This condition is associated with a 40-fold increased risk for the development of esophageal adenocarcinoma (EAC) (14). Now, there is overwhelming evidence that BE arises as a consequence of chronic gastroesophageal reflux disease (GERD) (19, 20). Although the importance of GERD in the pathogenesis of BE is undisputed, it is not yet clear which are the key elements in the refluxate responsible for metaplastic change to intestinal epithelium (38). In addition to gastric acid, bile acids (secreted into duodenum in response to a high-fat diet) are implicated in the development of BE and EAC (9). It was shown that 22.2% of patients with esophagitis, 54.5% of patients with BE, and 78.6% of patients with EAC exhibit pathological exposure to duodenal refluxate, indicating the importance of bile acids in EAC pathogenesis (42).
Autophagy is a major physiological lysosome-dependent mechanism for degrading and recycling cellular proteins and organelles (50). Basal level of autophagy contributes to the maintenance of intracellular homeostasis and is required for cell cleansing and remodeling (12). Under conditions of starvation, the autophagic pathway supplies cells with metabolic substrates and represents a prosurvival mechanism. In response to stress, autophagy is an important mechanism to prevent the accumulation of damaged organelles and proteins. However, excessive autophagy induced by cellular stress leads to cell death, characterized by the massive accumulation of autophagosomes (21). Experimental data and animal studies indicate that autophagy has an adaptive role to protect organisms against diverse pathologies, including cancer, neurodegeneration, and aging (28). Tumor suppressor genes activate autophagy, whereas oncogenes usually inhibit autophagy (49). However, once cancer develops, many cancer cells upregulate autophagy to survive hypoxia and nutrient limitation (33). Autophagy is thus considered a double-edged sword since it is a tumor-suppression mechanism but also enables survival of tumor cell during stress (32).
Beclin-1 has a central role in the regulation of autophagy in mammals (28). Autophagic triggers upregulate Beclin-1, which in turn binds to class III phosphatidylinositol 3-kinase (PI3KC3) and activates autophagosome formation and maturation. Beclin-1 acts as a tumor suppressor in mammalian systems, and the deletion of Beclin-1 was observed in various cancers, including prostate, ovarian, breast, brain, and lung cancers (30, 36).
There are only limited studies on esophageal cancer and autophagy. It was shown that increased expression of Beclin-1 is an important determinant of survival in patients with esophageal squamous cell carcinoma (7). However, no studies on Beclin-1 expression and EAC have been reported yet, and it is not clear what role autophagy plays in EAC. In this study, we tested the hypothesis that exposure to an environment that induces cellular stress (such as bile acids present in refluxate) first leads to increased Beclin-1 expression and autophagy activation. However, long-term repeated exposures lead to decreased Beclin-1 expression, autophagy inhibition, and cancer progression.
METHODS
Cell lines.
HET-1A cells were provided by Dr. Curtis C. Harris (National Cancer Institute, Bethesda, MD). HET-1A is a normal human esophageal epithelial cell line immortalized by transfection of the SV40 T antigen early region gene (43). The cells were cultured in BRFF-EPM2 medium (Athena Environmental Sciences, Baltimore, MD) supplemented with 50 μg/ml gentamicin and 0.25 μg/ml Fungizone. BE-derived CP-A and CP-C cells were kindly provided by Dr. Rabinovitch (Fred Hutchinson Cancer Research Center, University of Washington). The CP-A cells were derived from patients with nondysplastic BE, and CP-C cells were derived from patients with dysplastic BE. The cells were maintained in MCDB 153 medium as described previously (22). JH-EsoAd1 EAC cells were a kind gift from Dr. James R. Eshleman (Johns Hopkins University, Baltimore, MD) (2). The cells were cultured in RPMI medium containing 10% FBS. All experiments were performed in cells passaged less than 13 times. The cells were exposed to control medium (pH 7.4) or medium containing 0.2 mM deoxycholic acid (DCA).
CP-AR cells resistant to cell death induced by DCA were developed from CP-A cells. Initially, CP-A cells were grown in medium containing 0.02 mM DCA for at least 2 passages. As the cells became adapted, the concentration of DCA was increased by 0.02 mM. This procedure was repeated until the cells were able to survive and proliferate in medium with 0.2 mM DCA. It took 15 wk to achieve this resistance.
Patients.
Sixty-two patients with known BE or EAC were included in the present study. All patients gave written informed consent with the approval of the University of Arizona Human Subjects Committee. Biopsies of BE, squamous mucosa, EAC, and colon were taken from patients undergoing regular surveillance procedures and fixed in formalin. Biopsies for microarray analysis were immediately stored in RNAlater solution (Ambion, Austin, TX). Adjacent biopsies were stained with hematoxylin and eosin (H&E) and Alcian blue (pH 2.5) for histological evaluation and assessment of intestinal metaplasia. BE was defined as the presence of intestinal-like metaplastic epithelium containing goblet cells (IM) from the sites above the gastroesophageal (GE) junction.
Experimental model of BE and EAC.
The study protocol was approved by the Animal Care and Use Committee at the North Carolina Central University (Durham, NC). Eight-week-old rats were administered anesthetics premixed in normal saline (80 mg/kg ketamine and 12 mg/kg xylazine ip). Esophagogastroduodenal anastomosis was performed through an upper midline incision as described previously (6). Iron dextran (12 mg−1·kg·wk−1 ip) was administered to promote carcinogenesis. Animals were killed at 40 wk after surgery, and tissues were harvested for histopathological analysis in the future. Twelve animals were examined in total.
Electron microscopy.
Transmission electron microscopy (TEM) was used to detect ultrastructural changes in patient biopsies and CP-A cells. Tissue and cells were fixed with 3% glutaraldehyde in 0.1 mM cacodylate buffer. Samples were postfixed in 1% osmium tetroxide, dehydrated in a graded series of ethanols, and embedded in epoxy resin. Ultrathin sections were evaluated for morphological changes using a Phillips CM12 transmission electron microscope (Eindhoven, The Netherlands) (3).
Cell proliferation.
Cell proliferation was analyzed using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay] from Promega (Madison, WI) according to the manufacturer's instructions as described previously (22). Blank control values were subtracted from experimental and control samples, and the percentage of viable cells was calculated as (A480 experiment − A480 blank control)/(A480 control − A480 blank control) × 100, where A480 is absorbance at 480 nm.
Western blot analysis.
Western blot analysis was performed as previously described (18). Briefly, the cells exposed to different treatments were lysed using lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, pH 8) supplemented with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL). Protein aliquots (30 μg/lane) were loaded on 10–15% SDS-polyacrylamide gels for size fractionation by electrophoresis. The proteins were blotted onto Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA). The membranes were immunostained with antibodies against Beclin-1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (1:20,000; Pierce, Rockford, IL). Antibody complexes were detected using the SuperSignal West Pico chemiluminescence detection system (Pierce). After this, the membranes were stripped using stripping buffer (2% SDS, 100 mM basal medium Eagle, 62.5 mM Tris, pH 6.8) and immunostained with β-actin antibody (1:10,000; Calbiochem, Gibbstown, NJ), or the membranes were stained for 20 min with Bio-Safe Coomassie Stain (Bio-Rad, Hercules, CA) to confirm equal protein loading. The densities of individual bands were determined using QuantiScan software (Biosoft, Cambridge, United Kingdom).
Autophagy evaluation.
During autophagy, the cytoplasmic form of microtubule-associated protein light chain 3 (LC3) is processed by addition of a phosphatidylethanolamine to form LC3-II. This modified form of LC3 translocates rapidly in a punctate pattern to the autophagosome. The change in LC3 localization (from diffuse, cytoplasmic to punctate, membrane-associated) is used as a specific marker to monitor autophagy. cDNA-encoding human LC3 was inserted into the EcoR I site of pEGFP-C2 (Addgene, Cambridge, MA). pEGFP-LC3 was transfected into the CP-A cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Briefly, 2 μl of Lipofectamine 2000 and 0.8 μg of pEGFP-LC3 DNA were incubated separately in 50 μl of serum-free and antibiotic-free medium. After 5 min of incubation, DNA and Lipofectamine 2000 were mixed and incubated for another 20 min. This transfection medium (100 μl) was then added to cells growing in a 500-μl 4-chamber slide. After 4 h, the media were replaced with fresh antibiotic-free media and allowed to grow for 24 h. CP-AR cells were transfected in a similar way, however, during transfection, DCA was not added into the media. The cells were then exposed to control medium, medium supplemented with 0.2 mM DCA and HBSS. Immediately following treatments, cells were washed with PBS, fixed in ice-cold methanol for 6 min, mounted with ProLong Gold antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI), and analyzed by fluorescent microscopy. The percentage of cells displaying punctate distribution of GFP-LC3 was determined for at least 200 cells per sample. The experiment was repeated at least 3 times.
Immunohistochemistry.
The expression of Beclin-1 was determined in BE tissues with no dysplasia (BE), in low-grade dysplasia (LGD) and high-grade dysplasia (HGD) EAC, and in squamous epithelium by immunohistochemistry as described previously (15). The Beclin-1 antibody was from ProSci (2 μg/ml; Poway, CA). A simple grading system (0–4) was employed to evaluate the level of Beclin-1 expression. Immunocontrol slides were prepared by replacing the primary antibody with rabbit IgG at the same protein concentration as the primary antibody (2 μg/ml). Staining was evaluated independently by two experienced investigators. Beclin-1 in rat tissues was evaluated by fluorescent microscopy as described previously (34).
For confocal microscopy, the cells were grown on four-chamber slides, fixed with formaldehyde, and permeabilized with methanol as described previously (15). After blocking with 5% bovine serum albumin, the cells were incubated overnight with antibodies against Beclin-1 (1:100; ProSci). Next, Alexa Fluor 488 secondary antibodies (1:100; Molecular Probes, Eugene, OR) were applied for 60 min. The slides were counterstained with propidium iodide and coverslipped using VECTASHIELD HardSet Mounting Medium (Vector Laboratories, Burlingame, CA).
Real-time RT-PCR and microarray studies.
To evaluate Beclin-1 mRNA expression in patient samples, total RNA from BE (n = 18) and EAC (n = 6) was isolated using the Qiagen RNeasy Mini Kit (Valencia, CA) according to the manufacturer's instructions. The isolated total RNAs were used to produce a labeled target, hybridized to Affymetrix U133A GeneChips, and read using the Agilent/Affymetrix 2500A scanner according to manufacturer's protocols as described previously (47).
Total RNA was isolated from different cell lines using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentration and purity were evaluated by NanoDrop (Thermo Scientific, Wilmington, DE) at 260/280 nm. Real-time RT-PCR assays were performed to quantify mRNA levels of Beclin-1 as described previously (15). Primers were obtained from Real Time Primers (Elkins Park, PA). The following sequences were used for each of the primers: Beclin-1: forward primer 5′AGGAACTCACAGCTCCATTAC-3′; reverse primer 5′-AATGGCTCCTCTCCTGAGTT-3′; β-actin: forward primer 5′-AGAGGGAAATCGTGCGTCAC-3′, reverse primer 5′-CAATAGTGACCTGGCCGT-3′. Beclin-1 Cp (crossing point) values were normalized to β-actin Cp values for each cell line. The relative mRNA expression of Beclin-1 in different cells was compared with HET-1A cells.
Beclin-1 siRNA experiments.
CP-A were cotransfected with pEGFP-LC3 DNA and Ambion Silencer Select predesigned Beclin-1 short interfering RNA (siRNA; Ambion, Carlsbad, CA) or control siRNA (scrambled siRNA; Ambion) using Lipofectamine 2000 according to manufacturer's instructions. After transfection, the cells were exposed to control medium, medium supplemented with 0.2 mM DCA or HBSS, for 4 h. The slides were fixed with formaldehyde and immunostained using Beclin-1 antibody as described above. Alexa Fluor 594 (1:100; Molecular Probes) was used as a secondary antibody. The percentage of GFP-LC3-positive cells with punctate pattern was counted in at least three independent experiments for each treatment.
Statistical analysis.
Statistical significance was determined by the Student's t-test or by Mann-Whitney test at the 95% confidence level.
RESULTS
Beclin-1 is decreased in patient biopsies from dysplastic BE and EAC.
First, we wanted to know whether Beclin-1 expression changes in the progression from normal squamous epithelium to EAC. Normal colon was used as a control. The expression of Beclin-1 was evaluated altogether in 62 patients' samples by microarray analysis or by immunohistochemistry.
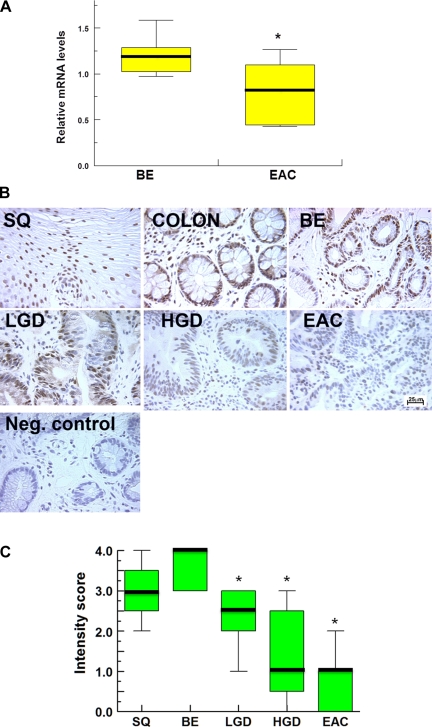
Microarray analysis revealed that Beclin-1 mRNA is significantly decreased in EAC (n = 6) compared with BE (n = 18; P < 0.05; Fig. 1A). Next, we evaluated Beclin-1 expression by immunohistochemistry. In agreement with microarray studies, low signal of Beclin-1 was detected in EAC samples (n = 9). Beclin-1 was expressed in nondysplastic BE (n = 12), whereas a lower signal of Beclin-1 was detected in BE with LGD (n = 8) and in BE with HGD (n = 9; Fig. 1B). As a control, we used normal colonic tissue and squamous epithelium (n = 5) where high Beclin-1 signal was found. No signal of Beclin-1 was detected in immunocontrols (negative control; Fig. 1B).
Fig. 1.
Beclin-1 expression in human tissues. A represents relative mRNA levels of Beclin-1 in Barrett's esophagus (BE; n = 18) and esophageal adenocarcinoma (EAC; n = 6). B shows the representative images of immunohistochemical staining of Beclin-1 (brown signal) in squamous epithelium (SQ), colon, nondysplastic BE (BE), BE with low-grade dysplasia (LGD), BE with high-grade dysplasia (HGD), EAC, and immunocontrol (Neg. control; magnification ×400). C shows the summary of immunohistochemical experiments for Beclin-1. We used a simple grading system, 0–4. Overall staining was evaluated in SQ (n = 5), BE (n = 12), LGD (n = 8), HGD (n = 9), and EAC (n = 9). Median values are shown as thick lines, asterisks indicate statistically significant difference (P < 0.05), and bar indicates 25 μm.
Two independent investigators used a simple grading system (0–4) to evaluate the intensity of Beclin-1 staining in different tissues. Mann-Whitney test was used to determine statistical significance. We found a significantly lower signal of Beclin-1 in dysplastic BE and EAC compared with nondysplastic BE tissue (Fig. 1C; P < 0.05).
The expression of Beclin-1 is reduced in rat model of BE/EAC and esophageal cancer cells.
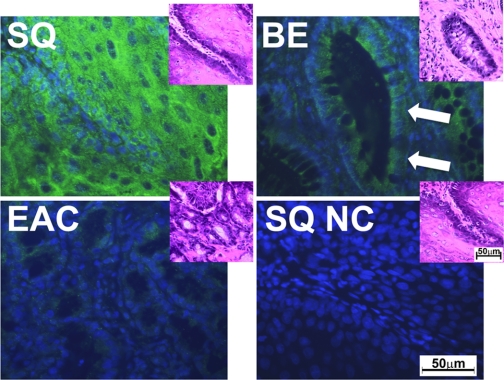
Beclin-1 expression was evaluated in tissues from 12 rats that underwent esophagojejunostomy (Fig. 2). Similarly to human biopsies, we found a high signal of Beclin-1 in squamous epithelium, whereas Beclin-1 expression was lower in BE and adenocarcinoma (Fig. 2).
Fig. 2.
Beclin-1 expression in rat tissues. The fluorescent microscopy images show Beclin-1 expression (green signal) in squamous epithelium, BE, EAC, and negative control (NC). 4′,6′-Diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain (blue signal). Insets show the tissues stained with hematoxylin and eosin (H&E). Bars indicate 50 μm. The white arrows show typical BE glands.
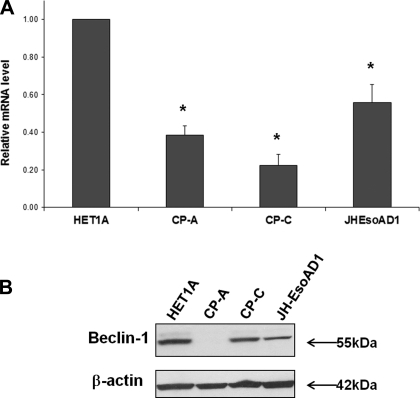
In the next experiment, Beclin-1 expression was evaluated in the esophageal cell lines derived from normal esophagus (HET-1A), nondysplastic BE (CP-A), dysplastic BE (CP-C), and EAC (JH-EsoAD1). Real-time RT-PCR and Western blot analysis were used to detect changes in Beclin-1 levels. High levels of Beclin-1 mRNA were detected in normal HET-1A cells, whereas lower Beclin-1 levels were found in BE-derived cells (CP-A and CP-C). Lower levels of Beclin-1 were also detected in JH-EsoAD1 (Fig. 3).
Fig. 3.
Beclin-1 expression in esophageal cell lines. The relative mRNA amounts of Beclin-1 in different esophageal cell lines are shown in A. Beclin-1 Cp values (crossing point) were normalized to β-actin Cp values for each cell line. Asterisks indicate significant difference compared with HET-1A cells (P < 0.05). The experiment was repeated 4 times. B shows Beclin-1 and β-actin protein expression.
BE is resistant to autophagy induced by amino acid deprivation.
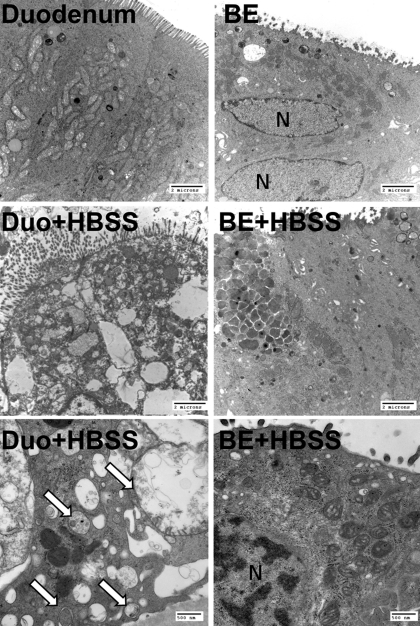
Since Beclin-1 is a critical regulator of autophagy, we tested whether autophagy can be induced in BE tissues in an ex vivo assay. In this experiment, we evaluated samples of dysplastic BE and duodenum because we wanted to compare the response in normal intestinal tissue and dysplastic BE. The tissues were incubated ex vivo with HBSS (positive control) for 4 h to induce autophagy and then evaluated by TEM for the presence of autophagic vacuoles. In BE and duodenal tissues that were incubated in normal medium, no changes typical for autophagy were found (Fig. 4). However, many autophagic vacuoles were found in duodenum incubated in HBSS, whereas no such changes were detected in BE incubated with HBSS (Fig. 4).
Fig. 4.
Transmission electron micrographs of duodenum (Duo) and BE incubated ex vivo for 4 h in control medium or in HBSS (inducer of autophagy). Typical autophagic vacuoles were found in duodenum incubated with HBSS (arrows). No changes consistent with autophagy were seen in BE incubated in control medium or in HBSS. The magnification was ×7,100 (4 top images) and ×25,000, respectively. Bars indicate 2 μm and 500 nm. N, nucleus.
Beclin-1 expression and autophagy are induced in BE cells by acute exposure to DCA.
Next, we evaluated whether bile acids modulate the expression of Beclin-1 and autophagy. For these experiments, we used CP-A cells that were exposed to 0.2 mM DCA or HBSS for 4 h. DCA is a model hydrophobic bile acid that also was shown to activate autophagy in normal colonic cells, or hepatocytes (39, 51).
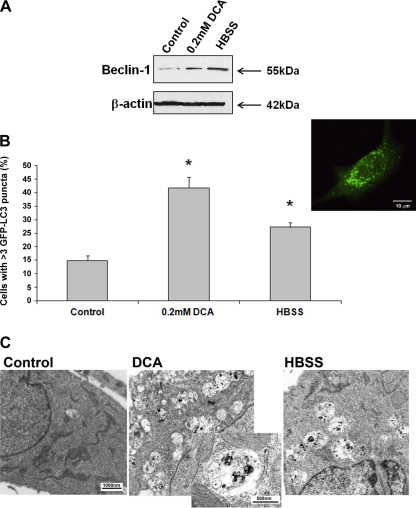
Confocal microscopy and Western blot analysis indicated that Beclin-1 expression was increased after the exposure to DCA (Figs. 5A and 6B). Beclin-1 expression was also increased after the exposure to HBSS (positive control; Fig. 5A).
Fig. 5.
The effect of acute exposure to deoxycholic acid (DCA) on Beclin-1 expression and autophagy. CP-A cells were treated for 4 h with control medium, 0.2 mM DCA or HBSS (positive control). Beclin-1 and β-actin immunoblots of CP-A are shown in A. B summarizes data from 3 different experiments evaluating percentage of GFP-LC3-positive cells with >3 puncta per cell. Asterisks indicate significant difference compared with untreated cells (P < 0.05). Inset shows a fluorescent microscopy image of a CP-A cell transfected with GFP-LC3 with typical puncta. Bar indicates 10 μm. Transmission electron micrographs of CP-A cells are shown in C (magnification ×11,500, inset ×31,000; bars represent 1,000 and 500 nm, respectively).
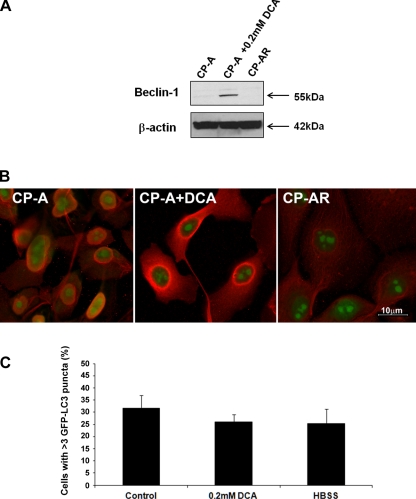
Fig. 6.
The effect of chronic exposure to DCA on Beclin-1 expression and autophagy. A shows an immunoblot of Beclin-1 in CP-A cells treated with normal medium (CP-A), with 0.2 mM DCA for 4 h (CP-A+DCA), and CP-AR cells chronically exposed to 0.2 mM DCA. B shows confocal microscopy images of Beclin-1 immunostaining in these cells. The graph in C shows the percentage of GFP-LC3-positive cells with punctate pattern in CP-AR cells that were allowed to grow in DCA-free medium for 24 h after transfection and treated with either control medium or medium supplemented with 0.2 mM DCA or HBSS for 4 h. Data are from 3 different experiments. Bar indicates 10 μm.
Next, we assessed autophagy by evaluating the punctate pattern in cells transfected with GFP-LC3 and TEM. DCA induces a significant increase in the percentage of cells with punctate pattern, as does HBSS (positive control; Fig. 5B). Whereas we found 14.9 ± 1.9% cells with punctate pattern in untreated cells, after exposure to DCA or HBSS, the percentage of GFP-LC3-positive cells with punctate increased to 41.8 ± 4.0 and 27.2 ± 1.8%, respectively (P < 0.05). In addition, typical autophagic vacuoles were detected in the cells treated with DCA or HBSS by TEM (Fig. 5C).
Chronic exposure to DCA does not induce upregulation of Beclin-1.
Since esophageal tissue is exposed to bile acids during reflux episodes, we speculated that bile acids may be responsible for activation of autophagy after acute exposure. However, after long-term repeated exposure to bile acids, the tissues develop resistance to autophagic stimuli. To study chronic effects of bile acids, we developed CP-AR cells that are resistant to cytotoxic effects of DCA. These cells are able to grow in medium containing 0.2 mM DCA. Our studies show that these cells express increased levels of two antiapoptotic proteins, Mcl-1 and Bcl-xL, compared with parental CP-A cells (data not shown). Using confocal microscopy and immunoblotting, we found that Beclin-1 expression in DCA-resistant cells is not increased after chronic exposure to DCA (Fig. 6, A and B). Furthermore, we counted the percentage of cells with punctate pattern after transfection with GFP-LC3 to evaluate autophagy. During transfection, DCA was not present in the medium. Overall, the number of cells with GFP-LC3 punctate pattern was elevated (31.6 ± 5.3%) in CP-AR cells compared with parental CP-A (14.9 ± 1.9%). However, treatment with 0.2 mM DCA or HBSS for 4 h did not result in any significant increase of the GFP-LC3-positive cells with punctate pattern (26.0 ± 3.0% for DCA and 25.4 ± 5.8% for HBSS; P > 0.05; Fig. 6C). Altogether, these data suggest chronic exposure to bile acids leads to decreased Beclin-1 expression and reduced autophagic response.
Beclin-1 is necessary for autophagy induced by DCA.
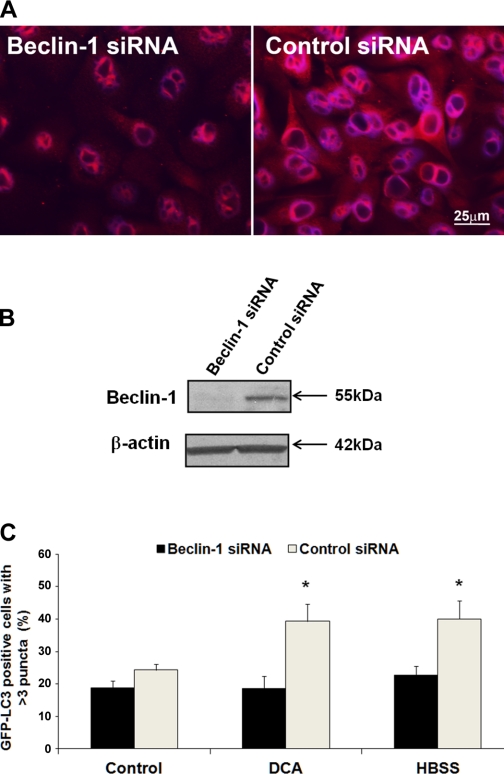
To study whether Beclin-1 is truly important for DCA-induced autophagy, we cotransfected CP-A cells with pGFP-LC3 and Beclin-1 siRNA or control (scrambled) siRNA. First, we tested whether Beclin-1 expression is decreased after treatment with Beclin-1 siRNA compared with control siRNA. Western blot and immunofluorescence showed that siRNA reduced Beclin-1 expression (Fig. 7, A and B). Next, the autophagy was evaluated after exposure to DCA and HBSS. The percentage of GFP-LC3-positive cells with punctate pattern was significantly increased after exposure to DCA and HBSS in the cells transfected with control siRNA (P < 0.05), whereas no significant increase was detected in the cells transfected with Beclin-1 siRNA (Fig. 7C; P > 0.05).
Fig. 7.
The effect of Beclin-1 short interfering RNA (siRNA) on autophagy. A shows the fluorescent microscopy images of Beclin-1 in CP-A cells treated with Beclin-1 siRNA or control (scrambled) siRNA (red signal). The nuclei are counterstained with DAPI (blue signal). Bar represents 25 μm. B shows an immunoblot of Beclin-1 in CP-A cells treated with Beclin-1 siRNA and control siRNA. The graph in C shows the percentage of GFP-LC3-positive cells with punctate pattern in CP-A cells cotransfected with Beclin-1 siRNA or control siRNA that were allowed to grow in DCA-free medium for 24 h after transfection and treated with either control medium or medium supplemented with 0.2 mM DCA or HBSS for 4 h. Data are from 3 different experiments. Asterisks indicate significant difference (P < 0.05 compared with control siRNA treated with DCA or HBSS, respectively).
DISCUSSION
In this paper, we show for the first time that during Barrett's carcinogenesis, the expression of Beclin-1, a major autophagic protein, is decreased as the disease progresses from nondysplastic BE to adenocarcinoma. Our studies were focused on BE, a premalignant lesion, and EAC, because BE is an ideal model to study early events in neoplastic progression. In the majority of other cancers, premalignant conditions cannot be studied in detail because they are either not detected early enough or are removed before cancer develops, such as in the case of colonic polyps. Furthermore, we evaluated the effect of bile acids on autophagy and Beclin-1 expression since bile acids play an important role in EAC development. The data suggest that acute exposure to DCA increases Beclin-1 expression and activates autophagy, whereas chronic exposure to DCA leads to decreased expression of Beclin-1 and inhibition of autophagy.
Autophagy is a conserved process involving self-digestion of whole organelles and macromolecules, which promotes the survival of starved and stressed cells including cancer cells, but autophagy may also lead to so-called type II programmed cell death (8). Autophagy plays an important role in many physiological functions, and defects in this process have been linked to many cancers, suggesting that it may function as a barrier for cellular transformation (8, 28, 32).
Beclin-1, a tumor suppressor protein, acts as an initiator of autophagy. Previous studies demonstrated that Beclin-1 plays an important role in tumor biology (26). It is speculated that Beclin-1 protects cells against chromosomal instability and decreases the frequency of additional mutations (31). For example, transgenic mouse models have shown that monoallelic deletion of beclin 1 promotes tumor development (41). The growth of colorectal cancer cells that overexpress Beclin-1 is reduced compared with the mock-transfected cells (26). Beclin-1 deficiency is also associated with increased angiogenesis (27).
The pattern of Beclin-1 expression in gastrointestinal cancers is not consistent. Studies show that Beclin-1 and autophagy were suppressed in hepatocellular carcinoma and pancreatic cancer (13) but were elevated in colon and gastric cancers compared with adjacent normal tissue (1, 29). However, nearly all studies suggest that high Beclin-1 expression is associated with better prognosis in gastrointestinal cancers. For example, Kim et al. (25) evaluated the expression of Beclin-1 in human pancreatic ductal adenocarcinoma and found that increased Beclin-1 expression was associated with a significantly lower rate of distant metastasis. Similarly, a high expression of Beclin-1 was associated with a favorable prognosis in colon and gastric cancers (1, 29). We found that Beclin-1 is decreased in biopsies obtained from patients with dysplastic BE and EAC and is high in normal tissues such as colon and squamous epithelium and nondysplastic BE. In addition, we also observed significantly decreased Beclin-1 mRNA in EAC compared with BE tissues (Fig. 1A). A similar trend of Beclin-1 expression was detected in animal model of EAC and BE (Fig. 2) and esophageal cell lines derived from normal esophagus, nondysplastic BE, dysplastic BE, and EAC (Fig. 3).
In agreement with our studies conducted in human biopsies and in animal tissues, studies on pancreatic cancer in rats showed that cells from pancreatic adenocarcinoma had decreased autophagic activity compared with cells from premalignant nodules (45). These observations suggest that autophagy is initially increased during premalignant stages of carcinogenesis and then decreased during the adenocarcinoma transition.
Because Beclin-1 expression was low in dysplastic BE, we wanted to determine whether BE tissue is sensitive/resistant to autophagic stimuli. Our data suggest that BE is resistant to autophagy compared with duodenum. Typical changes associated with autophagy (the presence of large autophagic vacuoles containing cellular debris) were observed in the duodenum after incubation for 4 h in HBSS by TEM (Fig. 3A). No autophagic vacuoles were seen in BE incubated in media with HBSS (Fig. 3B) or in duodenum or BE incubated with control medium (Fig. 3).
The mechanism by which autophagy defects lead to accelerated tumorigenesis is not readily apparent. However, it was suggested that autophagy can inhibit tumorigenesis by several different mechanisms. An elegant study showed that the autophagic machinery can limit DNA damage and chromosomal instability (32). Failure to clear damaged mitochondria that produce reactive oxygen species leads to nuclear DNA mutations and, consequently, cancer. Also, impairment of both apoptosis and autophagy promotes necrosis and thus inflammation (48). Importantly, inflammatory response leads to recruitment of the proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 that are associated with cancer development.
Bile acids are important in the etiology of gastrointestinal cancers, including EAC and colon and pancreatic cancer (4, 5, 42). During reflux episodes, the esophageal epithelium is exposed to gastric acid and hydrophobic bile acids (46). Evidence shows that the concentrations of bile acids are increased in the refluxate of patients with BE and are even higher in patients with EAC (42). Thus progression from BE to adenocarcinoma is strongly influenced by bile acid exposure. Bile acid concentrations in the refluxate of BE are in the range of 0.03–0.82 mM (median 0.18 mM) (38). However, bile acid concentrations as high as 6.4 mM have been reported in some BE patients (23). In addition, BE patients also have a significantly greater duration of bile reflux compared with patients with esophagitis (24, 35). In our studies, we used 0.2 mM DCA, which is in the physiological range.
Furthermore, the mainstream therapy for BE patients is treatment with proton pump inhibitors (PPIs). Whereas the reflux of gastric acid is controlled by PPIs, this therapy does not suppress reflux of bile acids (44). Importantly, at normal pH of the stomach (pH ∼2), the majority of bile acids present in the refluxate irreversibly precipitates. At higher pH (∼5–7), bile acids are soluble and interact with esophageal mucosa. Thus bile acids may cause cellular alteration, especially in esophageal tissue of patients treated long-term with PPIs.
Bile acids induce oxidative stress (16), activation of STAT3 signaling (15), cytokine alteration (15, 17), and DNA damage (22) in BE. Hydrophobic bile acids such as DCA (model bile acid) induce apoptosis (11, 40). However, chronic exposure of cells to bile acids leads to the selection of clones with an apoptosis-resistant phenotype (10). Similarly, we show in this study that the short-term exposure to bile acids leads to a normal cellular response, the induction of autophagy and increased Beclin-1 expression. In contrast, chronic exposure to bile acids does not affect Beclin-1 expression and consequently results in defective autophagy.
Beclin-1 expression and autophagy were evaluated in CP-A (nondysplastic BE) cells that were exposed acutely or chronically to DCA. For acute exposure experiments, CP-A cells were treated for 4 h with 0.2 mM DCA. This concentration of DCA is relatively low, and after 4 h the cytotoxic effects of DCA are minimal. However, prolonged exposure to this concentration of DCA leads to cell death. After acute exposure to 0.2 mM DCA, increased expression of Beclin-1 was detected by immunoblotting and immunocytochemical staining in conjunction with confocal microscopy. The cells were undergoing autophagy as indicated by increased GFP-LC3 punctate pattern and electron microscopy. In addition, many autophagic vacuoles were detected in the DCA-exposed cells by electron microscopy. In contrast, after chronic exposure to 0.2 mM DCA Beclin-1 expression was low and autophagic response was not activated. In agreement with these results, we found that DCA-induced autophagy was low in CP-A cells when Beclin-1 expression was decreased by siRNA. These data confirm the importance of Beclin-1 for autophagy (Fig. 7).
In summary, the decreased Beclin-1 expression and resulting autophagy decrease after chronic exposure to bile acids may lead to increased genomic instability and cancer progression. The incidence of EAC is rapidly rising for unknown reasons. Western-style, high-fat diet is probably one of the major contributing factors to this increase (37). This high-fat diet induces the release of bile acids into the gastrointestinal tract and consequently increases the concentration of bile acids in the refluxate. We suggest that the reflux of bile acids should be controlled in patients with BE to prevent cellular changes associated with chronic exposure to these toxic compounds.
GRANTS
The work was supported by Gastrointestinal (GI) Specialized Program of Research Excellence (SPORE) Grant CA-95060 from National Cancer Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.B.R., M.R.K., H.D.R.C., K.A.H., N.N., G.S.W., X.C., and K.D. performed experiments; H.B.R., K.A.H., G.S.W., and K.D. analyzed data; H.B.R. and K.D. drafted manuscript; H.B.R. and K.D. approved final version of manuscript; M.R.K., K.A.H., G.S.W., and K.D. prepared figures; M.R.K., H.D.R.C., N.N., G.S.W., and K.D. edited and revised manuscript; H.D.R.C. and K.D. interpreted results of experiments; K.D. conception and design of research.
REFERENCES
- 1. Ahn CH, Jeong EG, Lee JW, Kim MS, Kim SH, Kim SS, Yoo NJ, Lee SH. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS 115: 1344– 1349, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez H, Koorstra JB, Hong SM, Boonstra JJ, Dinjens WN, Foratiere AA, Wu TT, Montgomery E, Eshleman JR, Maitra A. Establishment and characterization of a bona fide Barrett esophagus-associated adenocarcinoma cell line. Cancer Biol Ther 7: 1753– 1755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson LA, Murray LJ, Murphy SJ, Fitzpatrick DA, Johnston BT, Watson RG, McCarron P, Gavin AT. Mortality in Barrett's oesophagus: results from a population based study. Gut 52: 1081– 1084, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol 15: 3329– 3340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 589: 47– 65, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis 20: 1801– 1808, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Lu Y, Lu C, Zhang L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res 15: 487– 493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corcelle EA, Puustinen P, Jaattela M. Apoptosis and autophagy: targeting autophagy signalling in cancer cells–‘trick or treats’? FEBS J 276: 6084– 6096, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cronin J, Williams L, McAdam E, Eltahir Z, Griffiths P, Baxter J, Jenkins G. The role of secondary bile acids in neoplastic development in the oesophagus. Biochem Soc Trans 38: 337– 342, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Crowley-Weber CL, Payne CM, Gleason-Guzman M, Watts GS, Futscher B, Waltmire CN, Crowley C, Dvorakova K, Bernstein C, Craven M, Garewal H, Bernstein H. Development and molecular characterization of HCT-116 cell lines resistant to the tumor promoter and multiple stress-inducer, deoxycholate. Carcinogenesis 23: 2063– 2080, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Crowley CL, Payne CM, Bernstein H, Bernstein C, Roe D. The NAD+ precursors, nicotinic acid and nicotinamide protect cells against apoptosis induced by a multiple stress inducer, deoxycholate. Cell Death Differ 7: 314– 326, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dikic I, Johansen T, Kirkin V. Selective autophagy in cancer development and therapy. Cancer Res 70: 3431– 3434, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, Shi GM, Wang XY, Ke AW, Wu B, Fan J. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res 68: 9167– 9175, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 92: 212– 215, 1997 [PubMed] [Google Scholar]
- 15. Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, Dvorak B, Bernstein H, Holubec H, Sampliner RE, Bernstein C, Prasad A, Green SB, Garewal H. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to Barrett's esophagus. Clin Cancer Res 13: 5305– 5313, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A, Bernstein C, Green SB, Prasad A, Garewal HS. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut 56: 763– 771, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, Dvorak B, Bernstein H, Bernstein C, Prasad A, Fass R, Cui H, Garewal H. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res 10: 2020– 2028, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Dvorakova K, Waltmire CN, Payne CM, Tome ME, Briehl MM, Dorr RT. Induction of mitochondrial changes in myeloma cells by imexon. Blood 97: 3544– 3551, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Falk GW. Barrett's esophagus. Gastroenterology 122: 1569– 1591, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am 18: 469– 485, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Galluzzi L, Morselli E, Vicencio JM, Kepp O, Joza N, Tajeddine N, Kroemer G. Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans 36: 786– 790, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Goldman A, Condon A, Adler E, Minnella M, Bernstein C, Bernstein H, Dvorak K. Protective effects of glycoursodeoxycholic acid in Barrett's esophagus cells. Dis Esophagus 23: 83– 93, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Iftikhar SY, Ledingham S, Steele RJ, Evans DF, Lendrum K, Atkinson M, Hardcastle JD. Bile reflux in columnar-lined Barrett's oesophagus. Ann R Coll Surg Engl 75: 411– 416, 1993 [PMC free article] [PubMed] [Google Scholar]
- 24. Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg 222: 525– 531; discussion 531–523, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Lee SH, Do SI, Lim SJ, Park YK, Kim YW. Clinicopathologic correlation of beclin-1 expression in pancreatic ductal adenocarcinoma. Pathol Res Pract 207: 247– 252, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Koneri K, Goi T, Hirono Y, Katayama K, Yamaguchi A. Beclin 1 gene inhibits tumor growth in colon cancer cell lines. Anticancer Res 27: 1453– 1457, 2007 [PubMed] [Google Scholar]
- 27. Lee SJ, Kim HP, Jin Y, Choi AM, Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy 7: 829– 839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27– 42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li BX, Li CY, Peng RQ, Wu XJ, Wang HY, Wan DS, Zhu XF, Zhang XS. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 5: 303– 306, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ 16: 87– 93, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Mathew R, Karantza-Wadsworth V, White E. Assessing metabolic stress and autophagy status in epithelial tumors. Methods Enzymol 453: 53– 81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer 7: 961– 967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev 21: 113– 119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G614– G622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menges M, Müller M, Zeitz M. Increased acid and bile reflux in Barrett's esophagus compared to reflux esophagitis, and effect of proton pump inhibitor therapy. Am J Gastroenterol 96: 331– 337, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol 30: 429– 436, 2007 [PubMed] [Google Scholar]
- 37. Murray L, Romero Y. Role of obesity in Barrett's esophagus and cancer. Surg Oncol Clin N Am 18: 439– 452, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44: 598– 602, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Payne CM, Crowley-Skillicorn C, Holubec H, Dvorak K, Bernstein C, Moyer MP, Garewal H, Bernstein H. Deoxycholate, an endogenous cytotoxin/genotoxin, induces the autophagic stress-survival pathway: implications for colon carcinogenesis. J Toxicol 2009: 785907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne CM, Waltmire CN, Crowley C, Crowley-Weber CL, Dvorakova K, Bernstein H, Bernstein C, Holubec H, Garewal H. Caspase-6 mediated cleavage of guanylate cyclase alpha 1 during deoxycholate-induced apoptosis: protective role of the nitric oxide signaling module. Cell Biol Toxicol 19: 373– 392, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112: 1809– 1820, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and nissen fundoplication. J Gastrointest Surg 2: 333– 341, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Stoner GD, Kaighn ME, Reddel RR, Resau JH, Bowman D, Naito Z, Matsukura N, You M, Galati AJ, Harris CC. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res 51: 365– 371, 1991 [PubMed] [Google Scholar]
- 44. Todd JA, Basu KK, de Caestecker JS. Normalization of oesophageal pH does not guarantee control of duodenogastro-oesophageal reflux in Barrett's oesophagus. Aliment Pharmacol Ther 21: 969– 975, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Toth S, Nagy K, Palfia Z, Rez G. Cellular autophagic capacity changes during azaserine-induced tumour progression in the rat pancreas. Up-regulation in all premalignant stages and down-regulation with loss of cycloheximide sensitivity of segregation along with malignant transformation. Cell Tissue Res 309: 409– 416, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 111: 1192– 1199, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Watts GS, Tran NL, Berens ME, Bhattacharyya AK, Nelson MA, Montgomery EA, Sampliner RE. Identification of Fn14/TWEAK receptor as a potential therapeutic target in esophageal adenocarcinoma. Int J Cancer 121: 2132– 2139, 2007 [DOI] [PubMed] [Google Scholar]
- 48. White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol 22: 212– 217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. White EJ, Martin V, Liu JL, Klein SR, Piya S, Gomez-Manzano C, Fueyo J, Jiang H. Autophagy regulation in cancer development and therapy. Am J Cancer Res 1: 362– 372, 2011 [PMC free article] [PubMed] [Google Scholar]
- 50. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9: 1102– 1109, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E, Graf M, Rahmani M, Gupta S, Hylemon PB, Fisher PB, Grant S, Dent P. Multiple cyclin kinase inhibitors promote bile acid-induced apoptosis and autophagy in primary hepatocytes via p53-CD95-dependent signaling. J Biol Chem 283: 24343– 24358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]