Abstract
The myofibroblast has recently been identified as an important mediator of tumor necrosis factor-α (TNF-α)-associated colitis and cancer, but the mechanism(s) involved remains incompletely understood. Recent evidence suggests that TNF-α is a central regulator of multiple inflammatory signaling cascades. One important target of TNF-α may be the signaling pathway downstream of the epidermal growth factor receptor (EGFR), which has been associated with many human cancers. Here, we show that long-term exposure of 18Co cells, a model of human colonic myofibroblasts, with TNF-α led to a striking increase in cell surface EGFR expression, an effect that was completely inhibited by cycloheximide. Subsequent EGFR binding by EGF and heparin binding (HB)-EGF was associated with enhanced EGFR tyrosine kinase activity, prolonged ERK activation, and a significant increase in cyclooxygenase-2 (COX-2) expression compared with 18Co cells treated with EGF and HB-EGF alone. TNF-α also increased EGFR expression and signaling in primary myofibroblasts isolated from human colon tissue. TNF-α-induced upregulation of EGFR may be a plausible mechanism to explain the exaggerated cellular responsiveness that characterizes inflammatory bowel disease and that may contribute to a microenvironment that predisposes to colitis-associated cancer through enhanced COX-2 expression.
Keywords: tumor necrosis factor-α, epidermal growth factor receptor, cyclooxygenase-2
the inflammatory bowel diseases (IBD; Crohn's disease and ulcerative colitis) are chronic inflammatory conditions of the gastrointestinal (GI) tract that are associated with an increased cancer risk. Although the etiology is unclear, the role of the intestinal stroma on the development of colitis-associated cancer has been gaining considerable attention. Myofibroblasts are an influential subpopulation of stromal cells that are a target of inflammatory mediator signaling (2, 35, 48). Located in the lamina propria just subjacent to the epithelial layer, myofibroblasts interact with neighboring cells in a paracrine fashion to regulate a number of important cellular processes, including epithelial proliferation and differentiation along the crypt-villous axis, mucosal repair, and fibrosis (33). They also participate in immune and inflammatory responses and have been implicated in the pathophysiology underlying both IBD and colorectal cancer (1–2, 9). It is increasingly recognized that myofibroblasts rather than intestinal epithelial cells are the major source of the inducible isoform of cyclooxygenase (COX-2; Refs. 1, 20, 24, 29, 39, 48), the rate-limiting enzyme in the biosynthesis of prostanoids that include prostaglandins (PGs) and thromboxanes (25).
TNF-α, a potent 17-kDa proinflammatory cytokine, is strongly implicated in the pathogenesis of IBD (2, 45) and colitis-associated cancer (4, 32, 46) and is known to regulate myofibroblast function (2, 35, 48). TNF-α initiates signaling events through TNF-α receptor 1 (TNFR1) and TNF-α receptor 2 (TNFR2) binding, leading to the formation of a multiprotein complex (TRADD, RIP, and TRAF-2) that culminates in the activation of MAPK and the transcription factor NF-κB (reviewed in Ref. 43). Medical therapies (e.g., infliximab) that directly target TNF-α are capable of inducing clinical remission in patients with IBD, suggesting that TNF-α is a central regulator of multiple inflammatory signaling cascades (12, 19, 35), although the underlying mechanism remains unclear.
Preclinical and clinical evidence suggest that epidermal growth factor receptor (EGFR) signaling plays a protective role in colonic inflammation and a key role in promoting colonic tumorigenesis. The EGFR is a member of the ErbB family of receptors that consists of four receptor tyrosine kinases (EGFR-ErbB-1, HER2/c-neu-ErbB-2, Her3-ErbB-3, and Her4-ErbB-4) characterized by an extracellular region, a single transmembrane spanning region, and a cytoplasmic tyrosine kinase domain (23). EGFR ligand binding induces receptor dimerization, intracellular tyrosine kinase activity, and binding of adaptor proteins that activate downstream signaling cascades (MAPK, phosphatidylinositol 3-kinase, signal transducer and activator of transcription, and PLCγ1), which have been strongly implicated in intestinal repair (21) and the development of several human cancers (20). Consequently, it was important to elucidate the relationship between TNF-α and EGFR signaling systems in colonic myofibroblasts (34, 40). Recent studies (14, 16) in intestinal epithelial cells demonstrated that TNF-α induces transactivation of members of the ErbB family but the crosstalk between TNF-α and EGFR signaling systems in myofibroblasts remains unknown. Consequently, the purpose of this study was to determine whether TNF-α regulates the expression and/or signaling of EGFR in human colonic myofibroblasts.
Here, we demonstrate, for the first time, that long-term exposure of colonic myofibroblasts (18Co) to TNF-α induced a striking increase in the expression of EGFR protein on the cell surface. The enhanced EGFRs expressed in response to TNF-α in 18Co cells are functionally active, as revealed by augmented EGF-induced EGFR phosphorylation, ERK activation, and COX-2 expression. To substantiate the physiological significance of the results obtained with the 18Co model system, we also demonstrated that TNF-α induced a marked increase in the expression and signaling of EGFRs in primary cultures of myofibroblasts isolated from human colon tissue. Our results support the notion that TNF-α induces EGFR expression and may explain how EGFR ligands are protective in the repair phase of inflammatory injury but may lead to the development of cancer in the setting of dysregulated persistent inflammation.
MATERIALS AND METHODS
Cell culture.
18Co cells (CRL-1459) were purchased from American Type Culture Collection (Rockville, MD). These cells were originally cultured from a mucosal biopsy of human neonatal colon and share many of the structural and functional characteristics of in situ colonic subepithelial myofibroblasts, including a reversible stellate morphology, α-smooth actin expression, and the presence of multiple cell surface receptors (41). 18Co cells provide a model to elucidate physiological and pathophysiological functions of intestinal subepithelial myofibroblasts and, accordingly, have been used extensively to study colonic myofibroblast function in a variety of settings (22, 31, 38). 18Co cells were maintained at 37°C in DMEM supplemented with 10% FBS in a humidified atmosphere containing 10% CO2-90% air. For experimental purposes, cells were plated in 35-mm dishes (1 × 105 cells/dish) and grown in DMEM containing 10% FBS for 5–7 days until confluent and used from passages 8–14.
Western blot.
Confluent 18Co cells, treated with different agonists, antagonists, and inhibitors as indicated in the individual experiments, were lysed in 2× SDS-PAGE sample buffer (20 mM Tris·HCl pH 6.8, 6% SDS, 2 mM EDTA, 4% 2-mercaptoethanol, and 10% glycerol) and boiled for 10 min. After SDS-PAGE, proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A at 4°C for 5 h using a Bio-Rad transfer apparatus. The transfer buffer consisted of 200 mM glycine, 25 mM Tris, 0.01% SDS, and 20% CH3OH. For detection of proteins, membranes were blocked using 5% nonfat dried milk in PBS (pH 7.2) and then incubated for 2 h with the desired antibodies diluted in PBS (pH 7.2) containing 3% nonfat dried milk. Primary antibodies bound to immunoreactive bands were visualized by ECL detection with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare, Piscataway, NJ).
Quantum dot live cell imaging.
18Co cells were plated in 35-mm dishes at 80% confluence. Quantum dot (Qdot) 655-streptavidin was first diluted into Ca2+ and Mg2+ free PBS with 1 mg/ml BSA to an 80-nM concentration. Biotinylated-EGF (Invitrogen) was diluted in Ca2+ and Mg2+ free PBS with 1 mg/ml BSA to a 400-nM concentration. Subsequently, equal volumes of Qdot 655-streptavidin (Invitrogen) were added and maintained at 4°C with constant mixing for 30–45 min (1:5 Qdot-to-agonist molar ratio). This results in approximately five peptide molecules per Qdot, on average, with few unconjugated peptide molecules (49). The mixture was diluted in HBSS to a final 10-nM Qdot concentration and then the cells were labeled for 45 min at 4°C. The samples were examined with a laser scanning confocal microscope 5 Pascal (Carl Zeiss MicroImaging), and images of Qdot fluorescence were obtained using a cooled CCD camera (SPOT 2; Diagnostic Instruments, Sterling Heights, MI) and stored on a computer disk for later analysis.
Immunoprecipitation.
Confluent 18Co cells grown on 60-mm dishes were incubated at 37°C and were serum starved in DMEM in the presence or absence of TNF-α for 18 h. The medium was aspirated, and cells were washed two times with PBS. Next, the cells were lysed in 0.3 ml ice-cold NP-40 cell lysis buffer (Invitrogen) supplemented with a protease inhibitor cocktail and 100 μM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride. The cell suspension was then mixed for 30 min at 4°C, and cell lysates were clarified by centrifigation at 15,000 rpm for 10 min. The supernatants were transferred to new microtubes and mixed with 10 μg/ml polyclonal EGFR antibody at 4°C for 4 h. The immunocomplex was captured by addition of 100 μl protein-A-agarose beads and incubated overnight, and then agarose beads were collected by pulse centrifugation (0.5 min at 14,000 rpm). The beads were washed three times with ice-cold lysis buffer and subsequently solubilized in 100 μl 2× SDS-PAGE sample buffer. Samples were boiled for 10 min, and proteins were resolved by 8% SDS-PAGE and then transferred to Immobilon-P membranes. Total EGFR was detected by immunoblotting using a polyclonal anti-EGFR antibody (Santa Cruz).
Myofibroblast isolation.
A protocol to obtain human tissue from surgical patients was approved by the University of California, Los Angeles, Institutional Review Board. A section of normal human colon tissue was immediately taken from surgically resected colon of patients with colorectal cancer and was washed with ice cold sterile PBS and then shaken five times for 15 min in HBSS containing 5 mM EDTA, which deepithelialized the tissue. Next, the tissue was incubated in 20 ml of RPMI-5 (RPMI with 5% FCS, 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, and 100 U/ml Pen-Strep) containing 10.5 mg of dispase (GIBCO-Invitrogen, Carlsbad, CA) and 7.2 mg of collagenase D (Roche Diagnostics, Indianapolis, IN) for 2 h in a shaking 37°C incubator. The digested tissue was treated with ACK lysis buffer for 5 min and then were passed through a 70-μM cell strainer into 100-mm dishes in RPMI-5. After a 3-h incubation, the nonadherent cells were washed away, leaving adherent cells that consisted mainly of macrophages, epithelial cells, and myofibroblasts. After several days, the macrophages and epithelial cells died off leaving cells that were morphologically homogeneous and composed primarily of cells with a myofibroblast-like phenotype that were consistently α-smooth muscle actin and vimentin positive. Primary colonic myofibroblast cultures were used for experiments up to passage 4.
Materials.
TNF-α and heparin binding (HB)-EGF were purchased from R&D Systems (Minneapolis, MN). Qdots were purchased from Invitrogen (Carlsbad, CA). Vimentin antibody and EGFR antibody, which were used for Western blotting and immunoprecipitation studies, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A-smooth muscle actin antibody was purchased from Abcam (Cambridge, MA). Cycloheximide was purchased from MP Biomedicals (Solon, OH). Y1068 antibody, Y845 antibody, and p42/44 MAPK antibody were purchased from Cell Signaling Technology (Danvers, MA). COX-2 antibody was purchased from Caymann Chemical (Ann Arbor, MI). AG1478 was purchased from Calbiochem (Gibbstown, NJ).
RESULTS
TNF-α increases EGFR expression in 18Co cells.
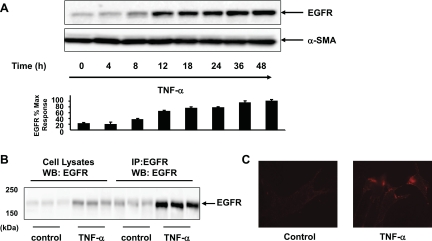
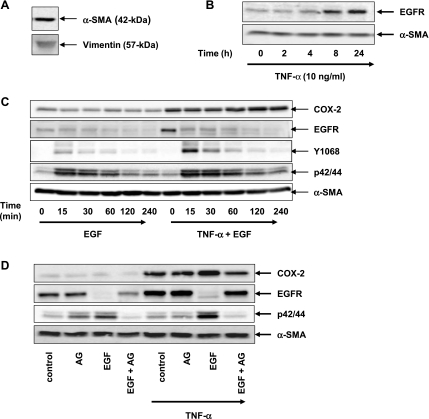
To determine whether TNF-α regulates EGFR expression, 18Co cells were exposed to TNF-α (10 ng/ml) over 48 h and EGFR protein level was analyzed by Western blot analysis. As demonstrated in Fig. 1A, exposure of 18Co cells to TNF-α led to a striking increase in EGFR protein expression, which was evident after 8 h and increased steadily over a 48-h time period. To confirm that the band detected in Western blots of whole cell lysates from 18Co cells treated with TNF-α was EGFR, we first immunoprecipitated cell lysates from cultures of these cells exposed to TNF-α, and then the immunoprecipitates were Western blotted with the EGFR antibody. As shown in Fig. 1B, exposure to TNF-α for 18 h led to an increase in signal intensity of a band that migrated with an apparent molecular mass of ∼170 kDa that corresponded to EGFR protein for both cell lysate and immunoprecipitated samples.
Fig. 1.
TNF-α increases epidermal growth factor receptor (EGFR) expression in 18Co cells. A: confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, followed by treatment with 10 ng/ml TNF-α for various times (4, 8, 12, 18, 24, 36 and 48 h, as indicated). The dose of TNF-α used (10 ng/ml) is consistent with its use in other studies (14, 16, 35, 48) and is also based on the dose-response experiment illustrated in Fig. 4B. Cell lysates were then analyzed by Western blot using an antibody that detects EGFR protein. Autoluminograms were quantified by densitometric scanning. Results are means ± SE (n ≥ 3) and are expressed as percentage of the maximum level of EGFR expression. Equal protein loading was verified using an antibody that detects α-smooth muscle actin (α-SMA). B: cultures of confluent 18Co cells were incubated in serum-free medium with or without 10 ng/ml TNF-α for 18 h. Cell lysates were immunoprecipitated (IP), and then immunoprecipitates were Western-blotted (WB) with EGFR antibody and compared with whole cell lysates. Results represent 3 separate experiments. C: confluent 18Co cells were exposed to 10 ng/ml TNF-α for 18 h, equilibrated at 4°C, and then labeled with Quantum dot-EGF conjugates, as described in materials and methods. Cells were examined by confocal microscopy, and images were recorded. Images represent ≥5 images taken for each condition.
To determine whether the increase in EGFR protein expression was associated with an increase in EGFR present on the cell surface, live cell imaging was performed using Qdot technology (49). 18Co cells were exposed to 10 ng/ml TNF-α for 18 h, equilibrated at 4°C to prevent EGFR internalization, and then exposed to biotinylated EGF that was conjugated to Qdot nanocrystal conjugates coated with streptavidin. As shown in Fig. 1C, the Qdot-EGF conjugate labeled cell surface EGFR in live 18Co cells. The salient feature of these results is that 18Co cells exposed to TNF-α display a marked increase in signal intensity on the cell surface, in line with the notion that the increase in the expression of EGFR protein is associated with an increase in cell surface EGFR.
Induction of EGFR expression in response to TNF-α requires de novo protein synthesis.
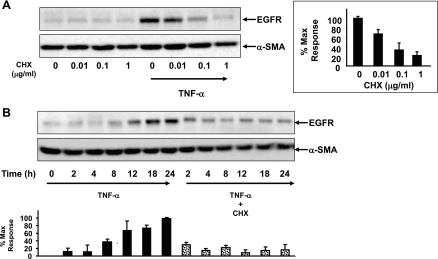
The gradual increase in EGFR expression over time in response to TNF-α suggested a long-term process, such as de novo protein synthesis rather than rapid alterations in preformed receptor trafficking. To determine whether the increase in EGFR expression involved de novo protein synthesis, 18Co cells were exposed to TNF-α in the presence or absence of varying concentrations of cycloheximide (0–1 μg/ml), an inhibitor of protein synthesis (Fig. 2A). Cycloheximide blocked the TNF-α-induced increase in EGFR in a dose-dependent manner, suggesting that the increase in EGFR protein expression induced by TNF-α required de novo protein synthesis. Next, we exposed 18Co cells to TNF-α for various lengths of time in the absence or presence of cycloheximide. As shown in Fig. 2B, the TNF-α-induced increase in EGFR expression was markedly inhibited by cycloheximide at 8, 12, 18, and 24 h, suggesting that this was the major mechanism responsible for the increase in EGFR.
Fig. 2.
Induction of EGFR expression in response to TNF-α requires de novo protein synthesis. A: confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, incubated with cycloheximide at various concentrations (0–1 μg/ml) for 1 h, and then exposed to 10 ng/ml TNF-α for 18 h. Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects EGFR protein. Results are means ± SE (n ≥ 3) and are expressed as a percentage of the maximum level of EGFR expression, displayed in graphical form at right. Equal protein loading was verified using an antibody that detects α-SMA. B: 18Co cells were pretreated with 1 μg/ml cycloheximide (CHX) for 1 h and then were exposed to 10 ng/ml TNF-α for various times (2, 4, 8, 12, 18, and 24 h, as indicated). Cell lysates were analyzed by SDS-PAGE and Western blot using an antibody that detects EGFR protein. Results are means ± SE (n ≥ 3) and are expressed as a percentage of the maximum level of EGFR expression, displayed in graphical form at bottom. Equal protein loading was verified using an antibody that detects α-SMA.
TNF-α enhances EGF-induced EGFR phosphorylation and ERK activation.
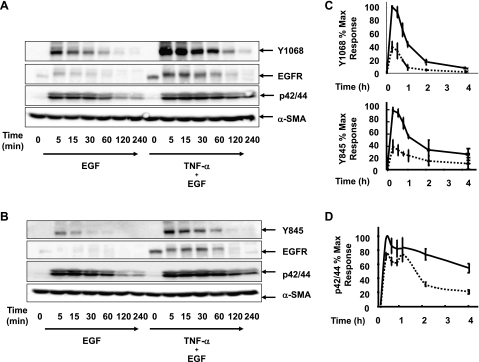
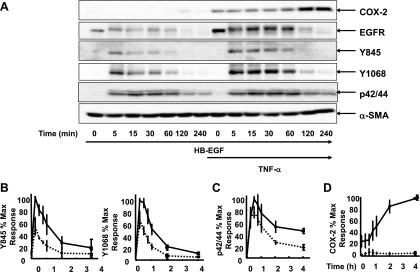
Binding of EGF to its receptor leads to dimerization and activation of intracellular tyrosine kinases, resulting in phosphorylation at several tyrosine residues that trigger subsequent downstream signaling that include the Ras/Raf/MEK/ERK pathway (23). To determine whether the increase in EGFR expression induced by TNF-α was associated with increased signaling activity of the EGFR, 18Co cells were incubated with 5 ng/ml EGF in the presence or absence of exposure to 10 ng/ml TNF-α for 18 h. Src-mediated phosphorylation at the tyrosine residue Y845 (28) as well as autophosphorylation at the tyrosine residue Y1068 (8) were assessed by Western blot using site-specific phospho-tyrosine antibodies. As shown in Fig. 3, A and B, exposure of 18 Co cells to EGF induced a rapid and transient phosphorylation at the residues Y1068 and Y845. In 18Co cells exposed to TNF-α for 18 h before EGF, there was an increase in EGFR protein expression, in agreement with our preceding results. Exposure of these cells with EGF led to a marked increase in the intensity and duration of phosphorylation at Y1068 and Y845 (Fig. 3C). When reprobed with EGFR antibody, the Western blot demonstrates an initial upward shift with EGF treatment that corresponds to receptor phosphorylation, followed by an overall decrease in EGFR protein expression, likely reflecting receptor internalization and degradation.
Fig. 3.
TNF-α enhances EGF-induced EGFR phosphorylation and ERK activation. A and B: 18Co cells were incubated in the presence or absence of 10 ng/ml TNF-α for 18 h, followed by exposure to 5 ng/ml EGF over various times (5, 15, 30, 60, 120, and 240 min, as indicated). Cells were analyzed by Western blotting using antibodies that detects EGFR (A and B), EGFR phosphorylation at tyrosine residue Y1068 (A), EGFR phosphorylation at tyrosine residue Y845 (B), and p42/44 MAPK phosphorylation (A and B). Equal protein loading was verified using an antibody that detects α-SMA. C: band intensity of phosphorylated Y1068 and Y845 was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of phosphorylated Y1068 and Y845. Dotted line depicts 18Co cells exposed to EGF alone. Solid line depicts 18Co cells exposed to both TNF-α and EGF. D: band intensity of p42/44 MAPK phosphorylation was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of p42/44 MAPK phosphorylation. Dotted line depicts 18Co cells exposed to EGF alone. Solid line depicts 18Co cells exposed to both TNF-α and EGF.
As a known downstream target of EGFR signaling, we next tested whether the increase in EGFR expression and EGFR kinase activity was associated with a corresponding increase in EGF-induced ERK activation. In 18Co cells exposed to EGF alone, ERK phosphorylation was evident and paralleled the increase in EGFR tyrosine phosphorylation. In cells exposed to TNF-α, EGF-induced ERK activation was associated with a small increase in early signal intensity but demonstrated sustained activation at 2–4 h compared with cells exposed to EGF alone (Fig. 3D).
TNF-α enhances EGF-mediated COX-2 expression.
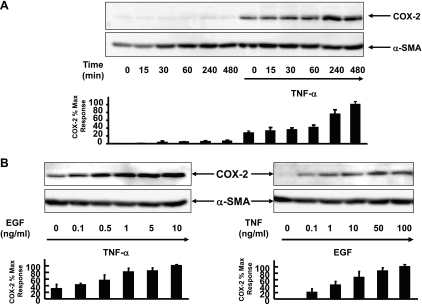
Myofibroblasts are a major source of the inducible isoform of COX-2 (1, 20, 24, 29, 39, 48), the rate-limiting enzyme in arachidonate metabolism that catalyzes the biosynthesis of PGH2, the precursor of prostanoids including PGs and thromboxanes (25). Since EGFR activation is a major pathway leading to the induction of COX-2 expression (7, 44, 47), our previous results led us to hypothesize that prior exposure to TNF-α enhances COX-2 expression in response to subsequent EGF stimulation in human colonic myofibroblasts. To characterize the effects of TNF-α and EGF on the expression of COX-2 in these cells, 18Co cells were stimulated with 5 ng/ml EGF over 8 h, with or without prior exposure to 10 ng/ml TNF-α for 18 h, and the level of COX-2 protein expression was assessed by Western blot analysis. In agreement with our previous results (48), there was no detectable COX-2 protein in the lysates of unstimulated 18Co cells. Exposure of 18Co cells to EGF alone induced minimal COX-2 protein expression over the 8-h time period studied (Fig. 4A). Low levels of COX-2 expression were induced in response to TNF-α for 18 h. In contrast, stimulation of 18Co cells with EGF after exposure to TNF-α led to a synergistic time-dependent increase in COX-2 protein expression that was evident after 4 h and continued to increase at 8 h. Since the enhanced expression of COX-2 protein was evident after 4 h, 18Co cells were exposed to EGF for 4 h at varying concentrations (0–10 ng/ml) following exposure to 10 ng/ml TNF-α for 18 h. The increase in COX-2 expression was dose dependent, as shown in Fig. 4B. A dose-dependent increase in COX-2 expression was also seen when 18Co cells were incubated for 18 h with varying concentrations of TNF-α (0–100 ng/ml) followed by exposure to a fixed concentration of EGF for 4 h.
Fig. 4.
TNF-α enhances EGF-mediated cyclooxygenase-2 (COX-2) expression. A: 18Co cells were incubated with 10 ng/ml TNF-α for 18 h and then were exposed to 5 ng/ml EGF at various times (15, 30, 60, 240, and 480 min, as indicated). Cell lysates were analyzed by SDS-PAGE and Western blot using a polyclonal antibody that detects COX-2 protein. Band intensity was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form at bottom, presented as means ± SE, and expressed as a percentage of the maximum level of COX-2. Equal protein loading was verified using an antibody that detects α-SMA. B, left: 18Co cells were incubated overnight for 18 h with 10 ng/ml TNF-α and then were exposed to EGF at various concentrations (0–10 ng/ml, as indicated) for 4 h. Cells were lysed, and the extracts were analyzed by SDS-PAGE and Western blot using a polyclonal antibody that detects COX-2 protein. Band intensity was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form at bottom, presented as means ± SE, and expressed as a percentage of the maximum level of COX-2. Equal protein loading was verified using an antibody that detects α-SMA. B, right: 18Co cells were incubated overnight for 18 h with TNF-α at varying concentrations (0–100 ng/ml) and then were exposed to 5 ng/ml EGF for 4 h. Cell lysates were analyzed by SDS-PAGE and Western blot using a polyclonal antibody that detects COX-2 protein. Band intensity was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of COX-2. Equal protein loading was verified using an antibody that detects α-SMA.
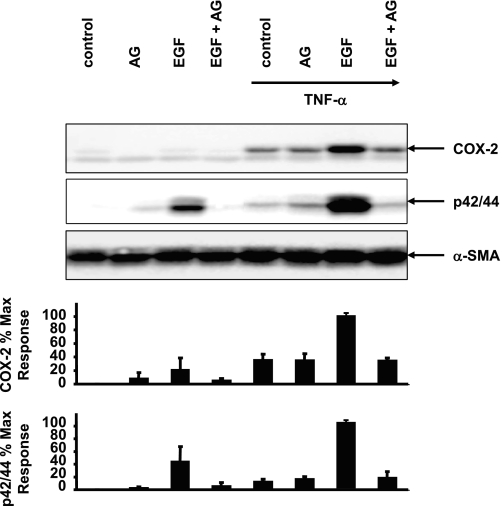
To confirm that the striking increase in the expression of COX-2 in response to EGF in cells exposed to TNF-α depended on EGFR tyrosine kinase activity, confluent 18Co cells were preincubated with the pharmacologic EGFR inhibitor AG1478 for 1 h before stimulation with EGF for 4 h in the presence or absence of TNF-α (Fig. 5). Under these experimental conditions, 18Co cells treated with EGF alone demonstrated low levels of COX-2 expression and moderate ERK phosphorylation, effects that were inhibited by pretreatment with AG1478. 18Co cells exposed to TNF-α for 18 h exhibited an increase in COX-2 expression that was unaffected by cell pretreatment with AG1478, implying that TNF-α-induced COX-2 expression was not mediated by EGFR in myofibroblasts. However, subsequent exposure to EGF led to enhanced ERK phosphorylation and a striking increase in COX-2 expression, effects that were both markedly inhibited by AG1478. The results indicate that the tyrosine kinase activity of the EGFR is required for mediating sustained ERK activation and COX-2 expression in response to EGF in 18Co cells exposed to TNF-α.
Fig. 5.
TNF-α and EGF-induced COX-2 expression requires EGFR tyrosine kinase activity. Cultures of 18Co cells were pretreated for 1 h with the EGFR inhibitor 1 μM AG1478 for 1 h before exposure to 5 ng/ml EGF for 4 h in the presence or absence of an 18-h incubation with 10 ng/ml TNF-α. Cell lysates were analyzed by SDS-PAGE and Western blot using anti-COX-2 antibody and an antibody that detects p/42/44 MAPK phosphorylation. Similar results were obtained in ≥3 independent experiments for each condition. Results are means ± SE and are expressed as percentage of the maximum level of COX-2 expression and as a percentage of the maximum level of p42/44 MAPK phosphorylation, depicted in graphical form at bottom. Equal protein loading was verified using an antibody that detects α-SMA.
HB-EGF triggers signaling through the enhanced EGFR expressed in response to TNF-α.
HB-EGF, a member of the EGF family of growth factors, exerts its biological activity through activation of the EGFR and other ErbB receptors. HB-EGF plays a key role in the acquisition of malignant phenotypes, such as tumorigenicity, invasion, metastasis, and resistance to chemotherapy in a variety of cell types (27). To determine whether the EGFR induced by TNF-α is responsive to other agonists of this receptor that are upregulated in cancer cells, 18Co cells were incubated with or without TNF-α for 18 h and then challenged with HB-EGF. As shown in Fig. 6A, 18Co cells stimulated with HB-EGF responded in a nearly identical fashion to cells treated with EGF. There was an enhanced intensity and duration of EGFR phosphorylation at Y845 and Y1068 (Fig. 6B), prolonged ERK phosphorylation (Fig. 6C), and a corresponding increase in COX-2 expression (Fig. 6D). Taken together, the results indicate that the EGFR expressed in response to TNF-α is functionally responsive to EGF and other EGFR ligands, including HB-EGF.
Fig. 6.
Heparin binding (HB)-EGF triggers signaling through the enhanced EGFR expressed in response to TNF-α. A: 18Co cells were incubated with 10 ng/ml TNF-α for 18 h and then were exposed to 5 ng/ml HB-EGF at various times (5, 15, 30, 60, 140, and 240 min, as indicated). Cell lysates were analyzed by SDS-PAGE and Western blot using antibodies that detect COX-2, EGFR, EGFR phosphorylation at Y1068 and Y845, and p42/44 MAPK phosphorylation. Equal protein loading was verified using an antibody that detects α-SMA. B: band intensity of phosphorylated Y1068 and Y845 was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of phosphorylated Y1068 and Y845. Dotted line depicts 18Co cells exposed to HB-EGF alone. Solid line depicts 18Co cells exposed to both TNF-α and HB-EGF. C: band intensity of p42/44 MAPK phosphorylation was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of p42/44 MAPK phosphorylation. Dotted line depicts 18Co cells exposed to HB-EGF alone. Solid line depicts 18Co cells exposed to both TNF-α and HB-EGF. D: band intensity of COX-2 expression was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphical form, presented as means ± SE, and expressed as a percentage of the maximum level of COX-2. Dotted line depicts 18Co cells exposed to HB-EGF alone. Solid line depicts 18Co cells exposed to both TNF-α and HB-EGF.
TNF-α increases EGFR expression and enhances EGF-mediated COX-2 expression in human colonic myofibroblasts.
To confirm that our experimental findings obtained with the 18Co cell model system were reproducible in early passaged cultures of human cells, colonic myofibroblasts were isolated from surgically resected human colon tissue using a well-established protocol for myofibroblast isolation (15). Isolated cells demonstrated a myofibroblast-like phenotype that was consistently α-smooth muscle actin and vimentin positive (Fig. 7A). Colonic myofibroblasts were incubated with TNF-α over 24 h, and EGFR protein expression was analyzed by Western blotting. As shown in Fig. 7B, there was a time-dependent increase in EGFR that mirrored the previously reported results with 18Co cells. Similarly, human colonic myofibroblasts exposed to TNF-α for 18 h displayed enhanced EGF-induced Y1068 phosphorylation, ERK activation, and COX-2 expression (Fig. 7C), effects that were inhibited by pretreatment with the EGFR inhibitor AG1478 (Fig. 7D). Compared with 18Co cells, there appeared to be some constitutive COX-2 expression in unstimulated primary human colonic myofibroblasts.
Fig. 7.
TNF-α increases EGFR expression and enhances EGF-mediated COX-2 expression in human colonic myofibroblasts. A: cell lysates of primary colonic myofibroblasts isolated from human colon tissue were analyzed by Western blotting using antibodies that detect vimentin and α-SMA. Results represent n ≥ 3 experiments. B: confluent primary colonic myofibroblasts were washed and equilibrated in serum-free media for 30 min and then incubated with 10 ng/ml TNF-α over various times (2, 4, 8, and 24 h, as indicated). Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects EGFR protein. Results represent n ≥ 3 experiments. Equal protein loading was verified using an antibody that detects α-SMA. C: primary colonic myofibroblasts were incubated with 10 ng/ml TNF-α for 18 h and then were exposed to 5 ng/ml EGF at various times (15, 30, 60, 120, and 240 min, as indicated). Cell lysates were analyzed by SDS-PAGE and Western blot using antibodies that detect COX-2, EGFR, EGFR phosphorylation at Y1068, and p42/44 MAPK phosphorylation. Equal protein loading was verified using an antibody that detects α-SMA. Results represent n ≥ 3 experiments. D: cultures of primary colonic myofibroblasts were pretreated for 1 h with the EGFR inhibitor 1 μM AG1478 before exposure to 5 ng/ml EGF for 4 h in the presence or absence of an 18-h incubation with 10 ng/ml TNF-α. Cell lysates were analyzed by SDS-PAGE and Western blot using anti-COX-2 antibody and an antibody that detects EGFR and p/42/44 MAPK phosphorylation. Similar results were obtained in ≥3 independent experiments for each condition. Equal protein loading was verified using an antibody that detects α-SMA.
DISCUSSION
The response to mucosal injury involves dynamic interactions between neighboring cells to facilitate epithelial repair. In patients with IBD, stromal myofibroblasts provide an abundant source of COX-2 derived by-products through complex signaling mechanisms that remain incompletely understood. The local production of prostaglandins may have dual effects, representing a cytoprotective response to mucosal injury that, when dysregulated, may promote epithelial transformation to an invasive phenotype and the development of cancer (42).
Myofibroblasts, TNF-α, EGF, and COX-2 have all been strongly and independently implicated in the development of colorectal cancer (5, 20, 32, 47). This study describes a novel interaction that links myofibroblasts with this trio of mediators and may partially explain how chronic inflammation increases the risk of developing cancer. We demonstrate that long-term exposure to TNF-α induces marked upregulation of EGFR protein expression in the myofibroblast cell line 18Co, leading to enhanced EGFR tyrosine kinase activity, downstream ERK activation, and synergistic induction of COX-2. Furthermore, these findings were replicated in primary colonic myofibroblasts harvested from human colon tissue. Cross talk interactions initiated by TNF-α may explain the exaggerated signaling and amplified physiologic responses that characterize IBD and that may predispose to colitis-associated cancer.
Interactions between the stromal microenvironment and the epithelium are regulated by a complex network of signaling pathways that become activated in a particular context and combination depending on the cell type. Exposure to TNF-α elicits responses that differ between epithelial and stromal cell populations. Recent reports (14, 16) suggest that the intestinal epithelium responds to TNF-α by stimulating COX-2 expression through transactivation of EGFR as a cytoprotective response. While this mechanism may exist, the stromal compartment has been identified as the major reservoir of COX-2 (1, 3, 30), making stromal cells the more likely source for COX-2-derived by-products that then initiate a paracrine-mediated cytoprotective response to the overlying epithelium. In our present study, we have identified a plausible mechanism for enhanced stromal COX-2 expression in colonic myofibroblasts that also involves TNF-α and EGFR. However, we distinguish the response by epithelial cells from the response by stromal myofibroblasts, which are also capable of enhancing EGFR signaling but do so through an entirely different mechanism involving a striking upregulation of EGFR expression on the cell surface. As shown here, in contrast to epithelial cells, the increase in COX-2 expression in myofibroblasts did not involve TNF-α-induced EGFR transactivation, since pretreatment with the EGFR inhibitor AG1478, as well as with the matrix metalloproteinase inhibitors GM6001 (36) and BB-94 (data not shown), had no effect on TNF-α-induced COX-2 expression. This mechanistic difference may explain how, under the same conditions, the stroma is capable of expressing higher levels of COX-2 compared with the overlying epithelia. We propose that differential mechanisms of cytoprotection by epithelial and stromal cells contribute to an overall response to restore intestinal integrity.
TNF-α appears to increase EGFR through stimulation of de novo protein synthesis, as has been described in pancreatic cancer cells (17–18, 37). Although we cannot rule out the possibility that TNF-α also regulates EGFR recycling as a means of increasing EGFR number, this seems less likely given the dramatic inhibition by cycloheximide, along with the rapid and sustained downregulation of EGFR following ligand binding that was seen in our cells. Although the increase in EGFR following chronic exposure to TNF-α is dramatic, the synergistic increase in COX-2 following subsequent exposure to EGF may not be completely explained by an increase in EGFR alone. Functional differences may exist between native EGFR and the EGFR induced by TNF-α, which has yet to be fully characterized. Functional differences may involve preferential dimerization with other members of the ErbB family of receptors or activation of alternative downstream targets that may enhance COX-2 transcription, stabilize COX-2 mRNA, or lead to posttranscriptional modulation through microRNAs (47).
Medical therapies targeting TNF-α, COX-2, and EGF are all currently in use for the management of IBD (45), the prevention of colonic adenomas (47), and the treatment of metastatic colorectal cancer (10), respectively. While EGFR upregulation on cancer cells has been well documented (6, 11, 13, 26), EGFR upregulation on stromal cells has not been studied and may deserve closer attention as a potential target of anti-cancer therapies. The present study supports the notion that TNF-α utilizes EGFR signaling to mediate physiologic effects and raises the possibility that targeted inhibition of one pathway may influence the biologic function of the other. Future studies may explore how therapies targeting EGFR signaling could be used in combination with anti-TNF-α therapies for the treatment of IBD and for the prevention of colitis-associated cancer.
GRANTS
This work was supported by National Institutes of Health Grants R0-1DK-55003, R01-DK-56930, R21-CA-137292, and P30-DK41301 (to E. Rozengurt) and 5KO8-DK-085136-02 (to J. Yoo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Y., C.E.R.P., R.A.E., J.S.-S., and E.R. conception and design of research; J.Y. and E.R. analyzed data; J.Y., C.E.R.P., J.S.-S., and E.R. interpreted results of experiments; J.Y. prepared figures; J.Y. drafted manuscript; J.Y. and E.R. edited and revised manuscript; J.Y. and E.R. approved final version of manuscript; C.E.R.P. and W.N. performed experiments.
ACKNOWLEDGMENTS
We thank Steve Young and the Morphology/Cell Imaging Core of CURE for technical assistance with live cell imaging experiments.
REFERENCES
- 1.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res 10: 5870–5879, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205: 331–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benamouzig R, Uzzan B, Martin A, Deyra J, Little J, Girard B, Chaussade S. Cyclooxygenase-2 expression and recurrence of colorectal adenomas: effect of aspirin chemoprevention. Gut 59: 622–629, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Burstein E, Fearon ER. Colitis and cancer: a tale of inflammatory cells and their cytokines. J Clin Invest 118: 464–467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen G, Mustafi R, Chumsangsri A, Little N, Nathanson J, Cerda S, Jagadeeswaran S, Dougherty U, Joseph L, Hart J, Yerian L, Tretiakova M, Yuan W, Obara P, Khare S, Sinicrope FA, Fichera A, Boss GR, Carroll R, Bissonnette M. Epidermal growth factor receptor signaling is upregulated in human colonic aberrant crypt foci. Cancer Res 66: 5656–5664, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cronin J, McAdam E, Danikas A, Tselepis C, Griffiths P, Baxter J, Thomas L, Manson J, Jenkins G. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett's esophagus (BE). Am J Gastroenterol 106: 46–56, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Dougherty U, Sehdev A, Cerda S, Mustafi R, Little N, Yuan W, Jagadeeswaran S, Chumsangsri A, Delgado J, Tretiakova M, Joseph L, Hart J, Cohen EE, Aluri L, Fichera A, Bissonnette M. Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model. Clin Cancer Res 14: 2253–2262, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature 311: 483–485, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17: 135–147, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Francoual M, Etienne-Grimaldi MC, Formento JL, Benchimol D, Bourgeon A, Chazal M, Letoublon C, Andre T, Gilly N, Delpero JR, Lasser P, Spano JP, Milano G. EGFR in colorectal cancer: more than a simple receptor. Ann Oncol 17: 962–967, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Ohuchida K, Mizumoto K, Itaba S, Ito T, Nakata K, Yu J, Kayashima T, Hayashi A, Souzaki R, Tajiri T, Onimaru M, Manabe T, Ohtsuka T, Tanaka M. High EGFR mRNA expression is a prognostic factor for reduced survival in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Int J Oncol 38: 629–641, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Grounds MD, Radley HG, Gebski BL, Bogoyevitch MA, Shavlakadze T. Implications of cross-talk between tumour necrosis factor and insulin-like growth factor-1 signalling in skeletal muscle. Clin Exp Pharmacol Physiol 35: 846–851, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Hemming AW, Davis NL, Kluftinger A, Robinson B, Quenville NF, Liseman B, LeRiche J. Prognostic markers of colorectal cancer: an evaluation of DNA content, epidermal growth factor receptor, and Ki-67. J Surg Oncol 51: 147–152, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Hilliard VC, Frey MR, Dempsey PJ, Peek RM, Jr, Polk DB. TNF-alpha converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival. Am J Physiol Gastrointest Liver Physiol 301: G338–G346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang B, Trinh A, Birnbaumer L, Edwards RA. Decreased MAPK PGE2-dependent IL-11 production in Giα2−/− colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 292: G1511–G1519, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hobbs SS, Goettel JA, Liang D, Yan F, Edelblum KL, Frey MR, Mullane MT, Polk DB. TNF transactivation of EGFR stimulates cytoprotective COX-2 expression in gastrointestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G220–G229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalthoff H, Roeder C, Brockhaus M, Thiele HG, Schmiegel W. Tumor necrosis factor (TNF) upregulates the expression of p75 but not p55 TNF receptors, and both receptors mediate, independently of each other, upregulation of transforming growth factor alpha and epidermal growth factor receptor mRNA. J Biol Chem 268: 2762–2766, 1993 [PubMed] [Google Scholar]
- 18.Kalthoff H, Roeder C, Gieseking J, Humburg I, Schmiegel W. Inverse regulation of human ERBB2 and epidermal growth factor receptors by tumor necrosis factor alpha. Proc Natl Acad Sci USA 90: 8972–8976, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoa ND, Postow M, Danielsson J, Cronstein BN. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol 69: 1311–1319, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Konstantinopoulos PA, Vandoros GP, Karamouzis MV, Gkermpesi M, Sotiropoulou-Bonikou G, Papavassiliou AG. EGF-R is expressed and AP-1 and NF-kappaB are activated in stromal myofibroblasts surrounding colon adenocarcinomas paralleling expression of COX-2 and VEGF. Cell Oncol 29: 477–482, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan K, Arnone B, Buchman A. Intestinal growth factors: potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm Bowel Dis 17: 410–422, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Laurens K, Thomas TM, Jane EC, Sylvia LFP, Ian RS. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant cxcl7 from intestinal epithelial cells. Gastroenterology 130: 127, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Liebmann C. EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol 331: 222–231, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol Gastrointest Liver Physiol 273: G1341–G1348, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol 42: 55–80, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer 71: 2454–2460, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci 97: 341–347, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaghan-Benson E, McKeown-Longo PJ. Urokinase-type plasminogen activator receptor regulates a novel pathway of fibronectin matrix assembly requiring Src-dependent transactivation of epidermal growth factor receptor. J Biol Chem 281: 9450–9459, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ohama T, Okada M, Murata T, Brautigan DL, Hori M, Ozaki H. Sphingosine-1-phosphate enhances IL-1β-induced COX-2 expression in mouse intestinal subepithelial myofibroblasts. Am J Physiol Gastrointest Liver Physiol 295: G766–G775, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Omura N, Griffith M, Vincent A, Li A, Hong SM, Walter K, Borges M, Goggins M. Cyclooxygenase-deficient pancreatic cancer cells use exogenous sources of prostaglandins. Mol Cancer Res 8: 821–832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacheco II, MacLeod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 295: G748–G759, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118: 560–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB Myofibroblasts I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology 107: 12–17, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez Perez CE, Nie W, Sinnett-Smith J, Rozengurt E, Yoo J. TNF-α potentiates lysophosphatidic acid-induced COX-2 expression via PKD in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 300: G637–G646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santiskulvong C, Rozengurt E. Galardin (GM 6001), a broad-spectrum matrix metalloproteinase inhibitor, blocks bombesin- and LPA-induced EGF receptor transactivation and DNA synthesis in rat-1 cells. Exp Cell Res 290: 437–446, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci USA 90: 863–867, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res 66: 846–855, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10(−/−) mice. Gastroenterology 118: 337–345, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 349: 350–357, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Valentich JD, Popov V, Saada JI, Powell DW. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol Cell Physiol 272: C1513–C1524, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Vandoros GP, Konstantinopoulos PA, Sotiropoulou-Bonikou G, Kominea A, Papachristou GI, Karamouzis MV, Gkermpesi M, Varakis I, Papavassiliou AG. PPAR-gamma is expressed and NF-kB pathway is activated and correlates positively with COX-2 expression in stromal myofibroblasts surrounding colon adenocarcinomas. J Cancer Res Clin Oncol 132: 76–84, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Wang JY, Chen BK, Wang YS, Tsai YT, Chen WC, Chang WC, Hou MF, Wu YC. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell Signal 24: 162–169, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm SM, McKenney KA, Rivait KN, Kale-Pradhan PB. A review of infliximab use in ulcerative colitis. Clin Ther 30: 223–230, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Wilson JA. Tumor necrosis factor alpha and colitis-associated colon cancer. N Engl J Med 358: 2733–2734, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett 295: 7–16, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Yoo J, Chung C, Slice L, Sinnett-Smith J, Rozengurt E. Protein kinase D mediates synergistic expression of COX-2 induced by TNF-α and bradykinin in human colonic myofibroblasts. Am J Physiol Cell Physiol 297: C1576–C1587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young SH, Rozengurt E. Qdot nanocrystal conjugates conjugated to bombesin or ANG II label the cognate G protein-coupled receptor in living cells. Am J Physiol Cell Physiol 290: C728–C732, 2006 [DOI] [PubMed] [Google Scholar]