Abstract
Transient cytosolic calcium ([Ca2+]cyt) elevation is an ubiquitous denominator of the signaling network when plants are exposed to literally every known abiotic and biotic stress. These stress-induced [Ca2+]cyt elevations vary in magnitude, frequency, and shape, depending on the severity of the stress as well the type of stress experienced. This creates a unique stress-specific calcium “signature” that is then decoded by signal transduction networks. While most published papers have been focused predominantly on the role of Ca2+ influx mechanisms to shaping [Ca2+]cyt signatures, restoration of the basal [Ca2+]cyt levels is impossible without both cytosolic Ca2+ buffering and efficient Ca2+ efflux mechanisms removing excess Ca2+ from cytosol, to reload Ca2+ stores and to terminate Ca2+ signaling. This is the topic of the current review. The molecular identity of two major types of Ca2+ efflux systems, Ca2+-ATPase pumps and Ca2+/H+ exchangers, is described, and their regulatory modes are analyzed in detail. The spatial and temporal organization of calcium signaling networks is described, and the importance of existence of intracellular calcium microdomains is discussed. Experimental evidence for the role of Ca2+ efflux systems in plant responses to a range of abiotic and biotic factors is summarized. Contribution of Ca2+-ATPase pumps and Ca2+/H+ exchangers in shaping [Ca2+]cyt signatures is then modeled by using a four-component model (plasma- and endo-membrane-based Ca2+-permeable channels and efflux systems) taking into account the cytosolic Ca2+ buffering. It is concluded that physiologically relevant variations in the activity of Ca2+-ATPase pumps and Ca2+/H+ exchangers are sufficient to fully describe all the reported experimental evidence and determine the shape of [Ca2+]cyt signatures in response to environmental stimuli, emphasizing the crucial role these active efflux systems play in plant adaptive responses to environment.
Keywords: cytosolic calcium, signatures, oscillations, Ca2+-ATPase, calcium exchanger
Introduction
Cytosolic calcium homeostasis and signaling in plant–environmental interaction
Calcium is an essential plant nutrient which plays a crucial structural role in cell walls and maintains membrane integrity. Calcium can easily interact with proteins, membranes, and organic acids through its ability to form different coordination bonds (from six to nine) which results in a high-affinity for carboxylate oxygen, rapid binding kinetics, and complex geometries (Medvedev, 2005; Case et al., 2007). Because of this, calcium can be a toxic cellular compound at higher concentrations as it would trigger aggregation of proteins and nucleic acids, precipitation of phosphates (present in ATP), and affect the integrity of lipid membranes (Case et al., 2007). As a result, plants have evolved efficient Ca2+ efflux mechanisms that can keep cytosolic free calcium, [Ca2+]cyt, at a constant and very low (submicromolar) level by exporting Ca2+ out of the cell or into the intracellular organelles (Dodd et al., 2010). In a typical plant cell, free Ca2+ concentrations are in the range of 1–10 mM in the apoplasm, 100–200 nM in the cytoplasm, 0.2–10 mM in the vacuole, ∼1 mM in the endoplasmic reticulum (reviewed in Medvedev, 2005), and 2–6 μM in chloroplast stroma (cf. Ettinger et al., 1999). Such extremely low [Ca2+]cyt creates a unique cellular environment in which [Ca2+]cyt concentration can be elevated by a factor of 10 or 20 rapidly (within seconds) upon sensing stress by using large electrochemical potentials either at the plasma or organelle membranes (Sanders et al., 1999). Indeed, [Ca2+]cyt elevation is an ubiquitous denominator of the signaling network when plants are exposed to soil acidity, salinity, anoxia, ozone, drought, osmotic, oxidative, heat and cold stresses, gaseous pollutants, mechanical cues, light, plant hormones, pathogens, bacterial, and fungal signals (reviewed in Sanders et al., 1999; McAinsh and Pittman, 2009). Resulting [Ca2+]cyt elevations are decoded by Ca2+ sensor proteins (e.g., CaM, CMLs, CDPKs, CBL/CIPKs) which regulate downstream targets leading to a stress-specific physiological response (DeFalco et al., 2010).
Stress-specific calcium “signatures”
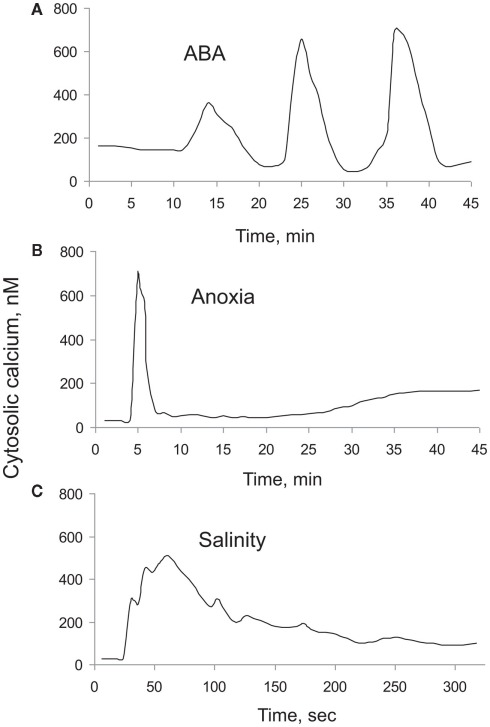
Stress or stimuli induced [Ca2+]cyt elevations vary in magnitude, frequency, and shape. These depend on the severity of the stress as well the type of stress experienced, thus creating a unique stress-specific calcium “signature” that is then decoded by signal transduction networks. An example of such stress-specific “signatures” is shown in Figure 1, which depicts [Ca2+]cyt elevation in response to abscisic acid (ABA), anoxia, and salt stresses. Very often, not one but a series of repetitive spikes (oscillations) is observed in response to environmental stimuli (e.g., for 10 nM ABA in guard cells Figure 1A). In the case of anoxia, [Ca2+]cyt elevation showed two distinctive peaks; one rapid (within a minute), and another one that lasted for hours (Figure 1B), whereas [Ca2+]cyt responses to salt stress did not have a second peak (Figure 1C).
Figure 1.
Typical [Ca2+]cyt “signatures” measured in plant cells in response to a range of external stimuli. (A) Response to 10 nM ABA treatment measured in guard cells of Commelina communis (Staxén et al., 1999); (B) response to anoxia (Sedbrook et al., 1996); and (C) response to salt (Tracy et al., 2008) treatments in Arabidopsis thaliana seedlings. For (B), luminescence ratio values were converted to [Ca2+]cyt values by considering peak [Ca2+]cyt value as 700 nM.
The magnitude of [Ca2+]cyt elevations shows a clear dose-dependency of external stimulus as was shown for salinity (Tracy et al., 2008), ozone (Clayton et al., 1999), hypo-osmotic shock (Goddard et al., 2000), H2O2 (Price et al., 1994), high temperatures (Gong et al., 1998), and apoplastic Ca2+ treatments (McAinsh et al., 1995). In addition to the shape and magnitude of [Ca2+]cyt elevation, repetition of [Ca2+]cyt elevations (spikes) could encode stimuli specific information. A classical example comes from Miwa et al. (2006) where Early Nodulation 11 (ENOD11) inductions were observed only when the [Ca2+]cyt spikes lasted for at least 60 min. Jasmonic acid treatment lengthened the period between spikes but did not alter the number of spikes required for ENOD11 expression, which suggests that indeed the number of spikes carries information required for ENOD11 expression (Miwa et al., 2006). More specific aspects of temporal encoding mechanisms and the role of cytosolic calcium oscillations in plant–environmental information are discussed in Section “Temporal Encoding Mechanisms: Cytosolic Calcium Oscillations.” Altogether, these results demonstrate that information concerning the type and strength of stress are encoded within the magnitude, shape, and frequency of [Ca2+]cyt elevations. While most published papers have focused predominantly on the role of Ca2+ influx mechanisms in shaping [Ca2+]cyt signatures, the latter is impossible without involvement of Ca2+ efflux mechanisms, removing excess of Ca2+ from the cytosol. The purpose of the current review is to emphasize the role of Ca2+ efflux systems in the cytosolic Ca2+ signaling and shaping [Ca2+]cyt signatures.
Encoding Environmental Information by Calcium
Spatial encoding: Intracellular calcium microdomains
Pleiotropic Ca2+ effects on cellular functions are due to a spatio-temporal organization of Ca2+ signal, and it would be a gross oversimplification to assume that [Ca2+]cyt is uniform across the cell. Instead, localized [Ca2+]cyt increases take place. Several factors make such localization possible. First, due to a presence of different buffer components in the cytosol Ca2+ mobility is very low (e.g., diffusion coefficient value of 1 to 5 × 10−11 m2/s as compared to 8 × 10−10 m2/s for pure water; Allbritton et al., 1992; Hille, 2001). The second contributing factor is a very specific configuration of different intracellular Ca2+ stores and their non-random distribution within a cell. Third, as a result of heterogeneous distribution and clustering of various Ca2+ transporters, the contact zones may exist between different organelles and a plasma membrane (PM), creating so-called “Ca2+ microdomains.” Such microdomains may be only a few nanometers in size, as ones which occur at the immediate vicinity of an open Ca2+-permeable channel (Rizzuto and Pozzan, 2006).
There are numerous examples of a specific subcellular localization of a Ca2+ signal for a variety of plant responses, with a preferential involvement of the PM, vacuole, nucleus, mitochondria, chloroplasts, or some combinations of the aforementioned stores (Sanders et al., 1999). The shaping of Ca2+ response is very likely dependent on the non-random distribution of Ca2+ pumps, with the expression of ACA 2 in the ER, ACA 4 and 11 in the tonoplast, and ACA 8–10 in the PM (Sze et al., 2000; Harper, 2001; Boursiac and Harper, 2007; Kudla et al., 2010).
During polar growth, e.g., of root hairs or pollen tubes, Ca2+ increases occur exclusively in the apical zone, which can be explained by a localized expression of some Ca2+ influx components. CNGC18 encoding a putative Ca2+-permeable channel, whose expression is essential for tube growth, is predominantly expressed in the tip of a pollen tube (Frietsch et al., 2007), whereas ACA 9 Ca2+ pump is expressed uniformly over the tube (Schiøtt et al., 2004). Despite this uniform distribution, ACA 9 is believed to be active mainly in the high Ca2+ region in the tip, due to Ca2+–CaM binding; on the contrary, CNGC18 is presumed to be inactivated by Ca2+–CaM (Hepler and Winship, 2010). This pair could obviously form a Ca2+ oscillator, where ACA 9 Ca2+ pump may prevent long-lasting Ca2+ overloads. In addition, in the pollen tube the stimulation of a hyperpolarization-activated Ca2+-influx channel by external apoplastic CaM was demonstrated (Shang et al., 2005). This may add an additional feed-forward loop via Ca2+-activated exocytosis of CaM-containing vesicles (Hepler and Winship, 2010). Another Ca2+ influx channel, a stretch-activated one, was functionally characterized in patches derived from a tip of pollen tube (Dutta and Robinson, 2004), whose activity may be modulated by a periodic growth-related cell wall loosening.
Growth-related local Ca2+ rises are also mediated by PM ROS-activated channels (Foreman et al., 2003), and the activation of ROS-generating enzyme NADPH-oxidase by incoming Ca2+ tends to act as a positive feedback (Takeda et al., 2008). It is not surprising then that local Ca2+ and ROS changes are often coupled (Foreman et al., 2003; Terada, 2006; Cárdenas et al., 2008). Moreover, integration of NADPH-oxidase in sterol-rich lipid rafts in the tip of a pollen tube is very essential for generation of a tip-focused cytosolic Ca2+ gradient, underlying the polarized growth (Liu et al., 2009).
It is widely reported in animal literature that opening of a single Ca2+ permeable channel is capable of generating very high (up to 300 μM) local Ca2+ increases (Llinas et al., 1992; Naraghi and Neher, 1997; Heidelberger, 1998). The peak amplitude and spatial diffusion of the Ca2+ microdomain formed at the mouth of a Ca2+ channel and its immediate neighborhood depends on the conductance and Ca2+ selectivity of the channel, electrochemical gradient for Ca2+ across the membrane, and local intracellular Ca2+ buffering. To the best of our knowledge, no such work has been performed on plant systems.
Multiple roles have been attributed to such Ca2+ microdomains. Presynaptic termini Ca2+ microdomains are believed to control vesicles fusion and exocytosis (Llinas et al., 1992; Heidelberger et al., 1994). In non-excitable cells subplasma membrane Ca2+ microdomains control Ca2+-sensitive adenylate cyclases (Mons et al., 1998) and NO synthase (Lin et al., 2000). Strikingly, a very local Ca2+ increase at the mouth of an open PM channel may initiate the signaling sequence, leading eventually to gene activation far away (for a review see Rizzuto and Pozzan, 2006). Finally, Ca2+ channels themselves are targets for ultra-local Ca2+ signaling. Several types of Ca2+ channels such as L-type Ca2+-selective channels and CRAC have been shown to be inactivated by incoming Ca2+ (Zweifach and Lewis, 1995; Soldatov, 2003). Conversely, opening of several clustered RyR channels required a localized Ca2+ spark to be generated (Franzini-Armstrong and Protasi, 1997). Tight contact zones existing between the PM, ER, and mitochondria are not only important for creating local Ca2+ microdomains, but may result in direct transport of Ca2+ from a translocator in one membrane to its counterpart in another membrane, so-called “linked Ca2+ transport” (Poburko et al., 2004). As mitochondrial Ca2+ uptake may eventually lead to Ca2+ overload followed by the activation of the transition pore and release of apoptotic factors, location of mitochondria in relation of a high Ca2+ microdomain may be crucial for determining a cell’s fate (Spät et al., 2009).
The physical basis for a non-random distribution of membrane elements is due to the existence of lipid rafts, well established for animal cells and becoming more evident also for plants (Zappel and Panstruga, 2008). In animal cells, recruitment of Ca2+ channels, pumps, exchangers into lipids rafts, and their functional consequences are known (Balijepalli and Kamp, 2008). In plants such evidence so far exists only for KAT1 channels (Sutter et al., 2006; Homann et al., 2007), but there is no reason to exclude such possibility also for plant Ca2+ permeable channels. Recently, we obtained some indirect evidence that double-pore Ca2+ (TPC) channels tend to cluster and communicate via local Ca2+ changes, as closed–open transitions of individual channels demonstrated interdependency (Pottosin, unpublished). Such an arrangement seems logical in light of a very high threshold for the TPC channel activation by intracellular Ca2+ (several tens of micromolar for the physiological voltage range (Schulz-Lessdorf and Hedrich, 1995; Pottosin et al., 1997; Pérez et al., 2008). Such high free Ca2+ concentrations occur only in close proximity of the mouth of an open Ca2+-permeable channel. A high threshold protects the cell from a global Ca2+ release from the non-exhaustible store, the central vacuole, which would be fatal. This consideration was obviously overlooked the when original hypothesis for the Ca2+-induced Ca2+ release based on the Ca2+ activation and Ca2+ permeability of vacuolar TPC was formulated (Ward and Schroeder, 1994). Recent studies with tpc-mutants show that TPC channels do not significantly contribute to any type of global Ca2+ response in plants (Ranf et al., 2008). On the other hand, clustering of the tonoplast TPC channels and/or their contacts with PM Ca2+-permeable channels of other organelles and PM would tend to split the large vacuole into multiple local Ca2+ circuits, where local feed-forward-looped Ca2+ rises could take place (Pérez et al., 2008). The fact that the tonoplast Ca2+/H+ (CAX) exchanger also has a relatively low (Kd ∼10–15 μM) affinity for Ca2+ (Hirschi, 2001) indirectly indicates that it may encounter very high local Ca2+ rises.
Temporal encoding mechanisms: cytosolic calcium oscillations
Advantages of oscillatory strategy
most other biological systems, cellular metabolism in general, and membrane transporters in particular, are governed by non-liner mechanisms and include a large number of positive and negative feedback loops (Hansen, 1978; Feijo et al., 2001; Shabala et al., 2006). It is not surprising, therefore, that such systems exhibit oscillatory behavior. Moreover, transient oscillatory responses are the most typical response of every feedback-controlled system to step-wise changes in external parameters. It is hardly surprising, therefore, that [Ca2+]cyt oscillations are widely reported in a range of plant systems (see below). Such a periodic behavior confers several functional advantages for the organism (Rapp, 1987), with precision of control and discrimination of true signals from environmental noise being the most important. Theoretical findings by Rapp et al. (1981) suggest that many biological oscillations reflect the biochemical implementation of analog-digital-analog control strategy; a strategy that provides significant functional advantages for living cells.
Oscillations may also facilitate synchronization of events widely separated in space between subcellular compartments (Lloyd and Stupfel, 1991), and it has been suggested that Ca2+ oscillations can act as cellular timekeepers to coordinate related biochemical reactions and enhance their overall efficiency (Izu and Spangler, 1993). Another advantage of oscillatory strategy is that oscillations may enhance signal efficiency specifically at low levels of stimulation. Experiments carried out on lymphocytes T cells revealed that oscillations in cytosolic free Ca2+ increase the efficiency and specificity of gene expression (Dolmetsch et al., 1998). The authors showed that this effect arises from the highly non-linear dependence of transcription on [Ca2+]cyt, so that oscillations periodically exceed the threshold for activation whereas a small constant [Ca2+]cyt rise of the same average magnitude does not. In other words, oscillatory control optimized sensitivity to weak external stimuli.
Theoretical studies also show that such systems will possess complex dynamics leading to “strange” behavior such as bifurcation and chaos (May, 1989), and both theoretical (Rand et al., 1981) and experimental (Shabala et al., 1997) evidence for the chaotic stomatal behavior were presented. Given the fact that the stomata aperture is controlled by [Ca2+]cyt modulation, evidence for deterministic chaos in [Ca2+]cyt kinetics is waiting to be revealed.
Ca2+ oscillations in plant cells
Two major types of [Ca2+]cyt oscillations are known: (i) a baseline spiking, in which the magnitude of the spike remains the same but the frequency of the spiking is affected by environmental stimulus, and (ii) sinusoidal [Ca2+]cyt oscillations, in which agonist dose regulates the amplitude but has no effect on oscillation frequency. In technical terms this is equivalent to the frequency and amplitude modulation (Berridge, 1997).
Over the last decade, calcium oscillations have been a popular subject of numerous reviews (e.g., McAinsh and Hetherington, 1998; Ng and McAinsh, 2003; McAinsh and Pittman, 2009; Roelfsema and Hedrich, 2010) and, thus, are only briefly covered here. Such oscillations have been found in various plant systems such as guard cells (McAinsh et al., 1995, 1997), pollen tubes (Holdaway-Clarke et al., 1997; Feijo et al., 2001), roots (Kiegle et al., 2000), root hairs (Ehrhardt et al., 1996; Monshausen et al., 2008), and some other systems (Bauer et al., 1998; Schonknecht et al., 1998). Oscillations in [Ca2+]cyt result from a dynamic balance of fluxes of Ca2+ into and out of the cytosol and include release and uptake from both intracellular stores and external media (Ng and McAinsh, 2003). The frequency of [Ca2+]cyt oscillations ranges typically from ca 20 s (Bauer et al., 1998) to 10–15 min (McAinsh et al., 1995) and shows a clear dependence on the magnitude of external stimulus (discussed in the next section). The latter findings lead to the concept of the existence of a frequency encoding mechanism, which conveys information about the severity of environmental fluctuation (e.g., temperature; heavy metal toxicity; hormonal level) by means of [Ca2+]cyt spikes. The functional role of [Ca2+]cyt oscillations was shown in nodulation experiments with alfalfa. The pronounced [Ca2+]cyt spikes were observed only in nodulating wild type plants, but not in non-nodulating alfalfa mutant (Ehrhardt et al., 1996); they were also absent in roots of tomato plants which are not capable of nodulating.
Both plasma and endomembrane Ca2+ channels are considered to be essential for generation of stimulus-induced [Ca2+]cyt oscillations (McAinsh et al., 1995, 1997). Involvement of hyperpolarization-dependent PM Ca2+ channels (HACC) has been shown (Pei et al., 2000), and oscillatory coupling between MP and [Ca2+]cyt has been suggested (Grabov and Blatt, 1998; Blatt, 1999). Indirect control via ABA-induced H2O2 production and a subsequent activation of Ca2+ influx through HACC has also been demonstrated (Pei et al., 2000).
Repetitive transient [Ca2+]cyt spikes were observed in unicellular green alga Eremosphaera viridis upon stimulation with Sr2+ (Bauer et al., 1998). These spikes were inhibited in cells pretreated with either ruthenium red or ryanodine, two known agents affecting activity of ryanodine/cyclic ADP-ribose type of Ca2+ channel, indicating the involvement of ER channels in generation of [Ca2+]cyt oscillations. InsP3-gated Ca2+-release channels have also been implicated (McAinsh and Hetherington, 1998). It was suggested that these channels may form a part of the signal transduction pathway of different stimuli based on difference in distribution, gating properties, and sensitivity.
Ca2+ as a component of encoding/decoding mechanism
Increases in [Ca2+]cyt have been observed in several cell types in response to a number of stimuli (see Stress-Specific Calcium “Signatures”). Most of these treatments resulted in long-lasting [Ca2+]cyt oscillations and showed all signs of frequency encoding. As such, a strong correlation between mechanical signal strength and an amplitude of the resulting [Ca2+]cyt spike has been shown in Nicotiana cotyledons (Knight et al., 1991, 1992). Sr2+-induced [Ca2+]cyt oscillations in Eremosphaera showed an increase in frequency and decrease in amplitude at increasing agonist concentrations (Bauer et al., 1998). In stomata guard cells, the period of [Ca2+]cyt oscillations increased from T = 8.3–13.6 min as external Ca2+ rose from 0.1 to 1.0 mM (McAinsh et al., 1995). This is consistent with animal models (Tang and Othmer, 1995) and points out that the signal specificity may be encoded by the amplitude and frequency of oscillations.
At the other end of the equation is a decoding mechanism. It was suggested that the Ca2+ signature may be decoded by the differential effects of Ca2+ on various downstream Ca2+-regulated proteins, such as calmodulin, phosphoinositide-specific phospholipase C, and Ca2+-dependent phosphatases and kinases (Tang and Othmer, 1995; Leckie et al., 1998; McAinsh and Hetherington, 1998). Direct evidence that calmodulin-dependent protein kinase II can decode the frequency of [Ca2+]cyt spikes into distinct amounts of kinase activity was given by De Koninck and Schulman (1998).
Dolmetsch et al. (1997) demonstrated that differential gene transcription in B lymphocytes is achieved through amplitude modulation of the [Ca2+]cyt signaling system. Low [Ca2+]cyt concentrations activate the nuclear factor of activated T cells (NF-AT), whereas much larger elevations stimulate a different set of transcriptional regulators. In their model, mechanisms of AM-modulation are based on the recruitment of a variable number of elementary events, resulting from the opening of either individual or small group of channels located in the internal stores (organized as a hierarchy). The same group has also shown that [Ca2+]cyt oscillation frequency can discriminate among different transcriptional pathways (Dolmetsch et al., 1998). While low frequency spikes recruited NF-kB alone, higher frequency oscillations (T < 6 min) activated NF-AT, Oct/OAP, and NF-kB, resulting in a differential gene expression. Another work used caged InsP3 to induce forced [Ca2+]cyt oscillations in T lymphocytes (Li et al., 1998). It was shown that [Ca2+]cyt oscillations were more effective in activation of the NF-AT gene expression than a single, prolonged [Ca2+]cyt increase, provided that the period was roughly 1 min; slower (T = 2 min) and faster (T = 0.5 min) oscillations were less efficient (Li et al., 1998).
It was also shown in animal systems that [Ca2+]cyt oscillations were more effective in Ca2+-sensitive mitochondrial dehydrogenase (CSMDH) activation than a sustained [Ca2+]cyt increase of similar amplitude (Hajnoczky et al., 1995). In this work, sustained NADPH elevation was achieved when the [Ca2+]cyt oscillation frequency was 0.5–1/min, while sustained increase in [Ca2+]cyt caused only a transient elevation of NADPH. These results suggest that the pulsating organization and frequency modulation of [Ca2+]cyt signaling are superior to amplitude modulation of [Ca2+]cyt responses in controlling mitochondrial metabolism. Thus, it appears that mitochondria are tuned to the oscillating [Ca2+]cyt signal.
Shaping Cytosolic Calcium Signals
As prolonged [Ca2+]cyt elevation is detrimental to normal cell metabolism, the basal [Ca2+]cyt levels must be restored after the signaling process has been completed. This may be achieved by orchestrated action of the cytosolic buffering system, and by the action of Ca2+ efflux mechanisms present in the PM and endomembranes. As argued below, the buffering capacity of Ca2+-binding proteins inside the cytosol is rather limited, making the Ca2+ efflux system absolutely essential in the above process of restoration of basal [Ca2+]cyt levels.
Cytosolic buffering
Intracellular Ca2+ buffering is an important determinant of the Ca2+ signal specificity, as both the magnitude and kinetics of Ca2+ signatures are critically dependent on Ca2+ buffering (Lew et al., 1984; Koopman et al., 2001). At the same time, there appears to be no correlation between cytosol buffering capacity and steady-state free Ca2+ level (Rizzuto and Pozzan, 2006). In animal cells cytosolic Ca2+ buffering may be described by a simple Michaelis–Menten formalism, with apparent dissociation constant (Kapp) ranging from 0.4 to 0.7 μM and maximal capacity (Bmax) of 0.15–0.3 mM. Assuming for simplicity one (high-affinity)-site binding, the total-to-free Ca2+ ratio in cytosol could be expressed as
Ca(tot)/Cafree = 1 + Bmax/(Cafree + Kapp)
Assuming basal [Ca2+]cyt levels = 0.1 μM, one may calculate that only one of 200–600 Ca2+ ions in cytosol is free while all others are bound.
In plants, a very high (15–45 mM) cytosolic buffering capacity for Ca2+ was estimated by some authors (Plieth et al., 1997). This estimate, however, included vacuolar Ca2+ sequestration, and, more importantly, was made under the assumption that Ca2+ and H+ always bind to the same sites; the assumption was later challenged by other authors (Schönknecht and Bethmann, 1998). Thus, it is generally accepted that the cytosolic buffering capacity in plants is not different from their animal counterparts, and is in a range of Bmax = 0.2–0.5 mM (Trewavas, 1999).
Cytosolic Ca2+-buffering is achieved mainly by Ca2+ binding proteins. Most of these proteins also act as Ca2+-sensors (Schwaller, 2009). Arabidopsis genome alone harbors 7 calmodulin (CaM) and 50 calmodulin-like genes (McCormack et al., 2005). The estimated CaM concentration in the cytosol is between 5 and 40 μM (Zielinski, 1998). These and some other proteins contain at least one (and up to six) specific helix–loop–helix structural motifs termed as EF-hand, which can interact each with other and bind Ca2+ in a co-operative manner, resulting in a protein activation due to a relatively small Ca2+ change. Another large group of Ca2+ sensor proteins are calcineurin B-like proteins, bearing three EF hands (10 CBL genes in Arabidopsis, Luan et al., 2002) and Ca2+-dependents protein kinases (CDPKs) with four EF hands (34 genes for CDPK in Arabidopsis, Cheng et al., 2002b). In Ca2+ sensors Ca2+ binding within EF-hand results in a relatively large scale conformational change, more pronounced than in a few “pure” Ca2+ buffering proteins such as calbindin and parvalbumin in animal cells.
The affinity and co-operativity of Ca2+ binding could vary greatly in different EF-proteins, and there are at least two classes of binding sites: one highly selective for Ca2+ against Mg2+, and another with a lower and comparable affinity for Ca2+ and Mg2+ (Gifford et al., 2007). In plants, besides EF-hand proteins, there are several other cytosolic Ca2+ binding (and normally, also Ca2+-regulated) proteins like phospholipase D and annexins (White and Broadley, 2003; Tuteja and Mahajan, 2007). Other Ca2+-binding proteins have been recently discovered (Ide et al., 2007).
Calcium efflux systems
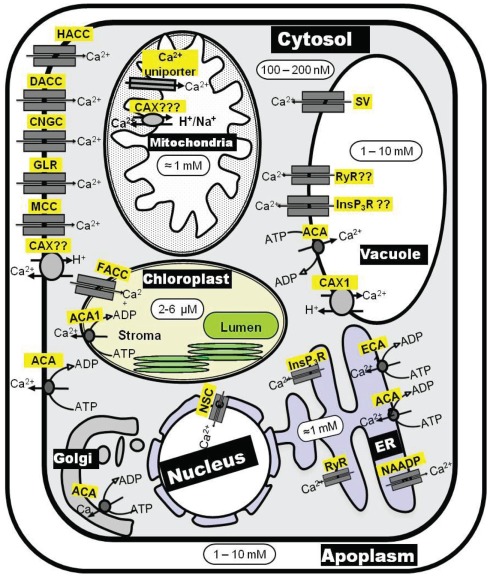
The most potent factor in shaping [Ca2+]cyt signatures is the activity of Ca2+ efflux systems. These are of utmost importance in both keeping [Ca2+]cyt at submicromolar level and in replenishing Ca2+ stores after [Ca2+]cyt signaling is completed. There are two groups of Ca2+ efflux mechanisms, Ca2+-ATPases and Ca2+ exchangers (CAX), both of which operate at the PM and endomembranes (Figure 2). Ca2+-ATPases are high-affinity (Km = 0.1–2 μM) but low-capacity transporters whereas Ca2+ exchangers are low-affinity (Km = 10–15 μM) but high-capacity transporters. This suggest that (i) Ca2+-ATPases may be primarily involved in termination of [Ca2+]cyt signaling, whereas (ii) Ca2+ exchangers may be primarily involved in removal of [Ca2+]cyt when [Ca2+]cyt elevations are higher than normal (Sze et al., 2000). As a result, both Ca2+-ATPases and CAX transporters contribute to shaping the [Ca2+]cyt signal. Ca2+-efflux transport mechanisms originated early on in biological evolution, and there is significant sequence conservation of these transporters in all forms of life (McAinsh and Pittman, 2009). This has facilitated the work to reveal the molecular identity of these systems. Significant knowledge exists about Ca2+-ATPases and CAX transporters in terms of activation kinetics and regulation, expression pattern, cellular locations, and physiological functions (Sze et al., 2000; Pittman and Hirschi, 2003; Shigaki and Hirschi, 2006; Boursiac and Harper, 2007). Despite this fact, the role of specific Ca2+ efflux systems has never been included in any [Ca2+]cyt signaling model.
Figure 2.
Schematic diagram of Ca2+ transporters involved in Ca2+ homeostasis maintenance inside the plant cell. ACA, autoinhibited calcium ATPase; CAX, calcium exchanger; CNGC, cyclic nucleotide gated channel; DACC, depolarization activated cation channel; ECA, ER-type calcium ATPase; FACC, fast-activating cation channel; GLR, glutamate receptor-like channel; ACC, hyperpolarization activated cation channel; InsP3R, inositol 1,4,5-trisphosphate receptor-like channel; MCC, mechanosensitive cation channel; NSC, non-selective cation channel; RyR, cyclic ADP-ribose (cADPR)-activator ryanodine receptor-like channel; SV, slow-activating vacuolar channel.
Molecular Identity of Calcium Efflux Systems
Ca2+-ATPases
Ca2+-ATPases are energized directly by ATP and belong the super family of P-type ATPases, ion pumps that are ubiquitous in all life forms. A hallmark of P-type ATPases is that they form a phosphorylated reaction cycle intermediate during catalysis. Two types of Ca2+-ATPases are known in plants: P2A-ATPase [or ER-type Ca2+-ATPase (ECA)] and P2B-ATPase [or autoinhibited Ca2+-ATPase (ACA); Geisler et al., 2000; Sze et al., 2000]. The structurally most distinctive difference between plant P2A- and P2B-ATPases is the extended N-terminus of P2B-ATPases, which serves a role as an autoinhibitor of pump activity and binds calmodulin. Four members of ECA (ECA 1–4) and 10 members of ACA (ACA1–10) have been identified in Arabidopsis (Sze et al., 2000).
ACAs can be present in the PM as well as in endomembranes whereas ECAs are exclusively localized to endomembranes. The cellular locations of ACAs and ECAs in Arabidopsis are depicted in Figure 2. (i) ACA8 (Bonza et al., 2000), ACA9 (Schiøtt et al., 2004) and ACA10 (George et al., 2008) reside at the PM, (ii) ACA4 (Geisler et al., 2000) and ACA11(Lee et al., 2007) at the tonoplast, (iii) ECA 1 (Liang et al., 1997) and ACA2 (Harper et al., 1998) at the ER, (iv) ECA 3 at the Golgi (Mills et al., 2008) and endosomes (Li et al., 2008), and (v) ACA1 at the plastid envelope (Huang et al., 1993). Apart from ACAs and ECAs, a P1-ATPase (HMA1) has been implicated in acting as a Ca2+/heavy metal pump at the chloroplast envelope (Moreno et al., 2008).
Ca2+ exchangers
Ca2+ exchangers are energized by the counter transport of another cation, usually H+ or Na+. In Arabidopsis, six CAX genes (AtCAX1 to AtCAX6) that encode H+/Ca2+ exchangers plus five CCX (cation/Ca2+ exchangers, previously described as AtCAX7 to AtCAX11) that encode K+-dependent Na+/Ca2+ exchangers, have been identified to date (Mäser et al., 2001; Shigaki et al., 2006). The function of CAX (CAX1 to CAX4) in the tonoplast is widely studied (Hirschi, 1999; Hirschi et al., 2000; Cheng et al., 2002a, 2003, 2005); CAX activity at the PM is also reported (Kasai and Muto, 1990; Luo et al., 2005).
Regulation of Calcium Efflux Systems Activity
Ca2+-ATPase regulation
Ca2+-ATPases are activated by submicromolar concentrations of Ca2+. For this reason they are defined as high-affinity pumps (Møller et al., 2010). Due to tight coupling between ion binding and ATP hydrolysis, ATP hydrolysis will never take place if Ca2+ has not been bound in the membranous region of the Ca2+-ATPase (Morth et al., 2011). Likewise, hydrolysis of ATP is always associated with transport of Ca2+.
As [Ca2+]cyt increases in response to environmental stress, Ca2+-ATPases are immediately activated as a result of Ca2+ binding to their transport sites. P2A-ATPases have two Ca2+ binding sites in their membrane domain both of which have to be occupied before ATP hydrolysis can occur (Møller et al., 2010). In contrast, P2B-ATPases have a single membranous Ca2+ binding site (Brini and Carafoli, 2009) and therefore can proceed to ATP hydrolysis directly after Ca2+ binding. Further, in contrast to P2A Ca2+-ATPases, P2B-ATPases are equipped with a Ca2+ sensor that allows the pump to change its activation state depending on [Ca2+]cyt (see below). These combined features make P2B-ATPases optimal for responding to increased [Ca2+]cyt.
The sensor function of P2B Ca2+-ATPases is achieved by the ability of a terminal autoinhibitory domain to bind calmodulin. A calmodulin binding site was first identified in the C-terminal domain of an animal P2B Ca2+-ATPase (James et al., 1988) and later found in the N-terminal domain of a plant P2B Ca2+-ATPase (Malmström et al., 1997). We now know that the N-terminal localization of an autoinhibitory calmodulin binding domain is a distinctive feature of plant P2B Ca2+-ATPases (Geisler et al., 2000; Boursiac and Harper, 2007). The calmodulin protein binds four Ca2+ ions cooperatively and as a result changes from a loose to a compact conformation when binding to the Ca2+ pump (Ishida and Vogel, 2010). The calmodulin binding site of the N-terminus of P2B Ca2+-ATPases is thought to interact with a cytoplasmic domain of the pump in this way restricting domain movements and pump function (Luoni et al., 2004). The sequence of amino acid residues that serves as a calmodulin binding site also functions as a pump autoinhibitor (Baekgaard et al., 2006). This suggests that, as calmodulin binds Ca2+, its affinity for the N-terminal calmodulin binding site increases and, when fully loaded with Ca2+, calmodulin competes favorably with the intramolecular binding site for the autoinhibitor and, as the autoinhibitory sequence becomes neutralized by calmodulin with bound Ca2+, the N-terminal autoinhibition is relieved. In addition to calmodulin, other cellular components such as acidic phospholipids might influence the autoinhibitory effect of the N-terminal domain (Bonza and De Michelis, 2011).
The presence of a regulatory terminal domain in P2B Ca2+-ATPases is not a unique feature of these pumps. The plant PM H+-ATPase has an extended C-terminal domain with two autoinhibitory sequences (Axelsen et al., 1999) and the PM heavy metal pump HMA4 has an extended C-terminal domain that serves as a Zn2+ and Cd2+ sensor (Baekgaard et al., 2010). Both these pumps belong to the P-type ATPase superfamily. How are P2A Ca2+-ATPases with very short terminal domains then regulated? At least in animal cells, they interact with inhibitory subunits that are small membrane proteins (Palmgren and Nissen, 2011). Whether in a similar way plant P2A-ATPases are post-translationally regulated by associated subunits remains to be shown.
CAX regulation
Plant cation/H+ exchangers, like plant Ca2+-ATPases, appear to be primarily regulated at the post-translational level, although some form of transcriptional regulation may occur (Shigaki et al., 2010; Manohar et al., 2011). CAX1 may be regulated via an N-terminal autoinhibitory domain, which binds to an adjacent region within the N-terminus (Pittman et al., 2002; Mei et al., 2007). Other forms of regulation via (i) formation of a “hetero-CAX” complex through interaction between CAX1 and CAX3 (Zhao et al., 2009); (ii) phosphorylation (Pittman et al., 2002), or (iii) various CAX interacting proteins (CXIP) including CXIP4 and the Ser/Thr Kinase SOS2 (Cheng and Hirschi, 2003; Cheng et al., 2004a,b) and/or (iv) pH homeostasis (Zhao et al., 2008) have also been observed.
Control of Ca2+ATPases by polyamines and ROS
Our recent findings also suggest that PM Ca2+-efflux systems may be regulated by synergistic effects of polyamines (PAs) and hydroxyl-radicals The levels of both PA and are known to increase dramatically under stress conditions. Also, PAs block a variety of K+ and non-selective cation channels in plants (Dobrovinskaya et al., 1999; Liu et al., 2000; Shabala et al., 2007; Zhao et al., 2007), whereas and H2O2 activate different PM Ca2+ influx channels (Pei et al., 2000; Demidchik et al., 2003, 2007), thus affecting cytosolic ionic homeostasis. There is also a cross-talk between ROS and PAs, as several types of plant responses to environmental clues such as salt or drought involve PAs export to apoplast and further oxidation by available diamine- or polyamine-oxidase, resulting in H2O2 and formation and activation of the Ca2+ influx across the PM (An et al., 2008; Moschou et al., 2008).
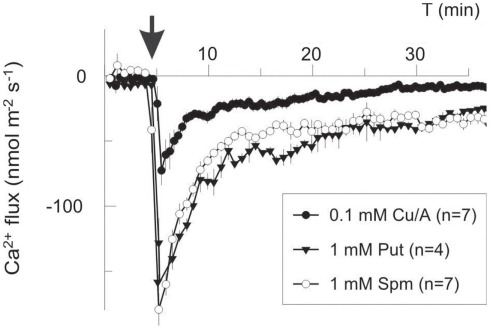
In our work, (1 mM Cu/ascorbate) treatment evoked a long-lasting Ca2+ influx into pea roots due to -induced non-selective passive conductance. However, at shorter times, transient Ca2+ efflux was measured to be sensitive to eosine yellow, a specific Ca2+ pump inhibitor (data not shown). Lowering the amount of (0.1 mM Cu/ascorbate) shifted the balance between Ca2+ uptake and efflux toward net efflux (Figure 3), implying a lower threshold for the -inducible Ca2+ efflux system as compared to the Ca2+ influx one. Addition of either 1 mM Spm4+ or Put2+ provoked a massive net Ca2+ efflux with very similar kinetics and magnitude (Figure 3). Integrating this flux over the period of 30 min and taking into account the root geometry and dimensions, we estimated that the total intracellular Ca2+ loss was equivalent to 0.2 mM for treatment, and ∼0.6 mM for PAs. The latter value may be even in excess of the total cytosolic Ca2+, implying the mobilization of the vacuolar pool.
Figure 3.
Polyamines and ROS induce Ca2+ efflux in the mature zone of pea roots. Polyamines (1 mM of putrescine, Put, or spermine, Spm) and copper-ascorbate mixture to generate (Cu/A, 0.1 mM) were added externally at the moment indicated by the arrow. Negative flux corresponds to Ca2+ efflux from the root to the bath. Data are mean ± SE, with a number of individual roots used as specified (n).
To the best of our knowledge, PAs effects on Ca2+ pumps have never been reported. However, PAs are known to stimulate another P-type pump, H+-ATPase, presumably via interaction of autoinhibitory domain protein with 14-3-3 proteins (Garufi et al., 2007). PAs may also activate the H+-pump via an NO-dependent pathway (Tun et al., 2006; Arasimowicz-Jelonek et al., 2009; Zandonadi et al., 2010). Another interesting possibility is that this comes about due to formation of a complex of Mg2+-ATP-spermine, which seems to present an increased rate for catalysis by ATPases as compared to Mg2+-ATP (Meksuriyen et al., 1998). It may also be possible that PAs stimulate the PM H+ pump, which indirectly affects/stimulates the Ca2+ pump in an H+-coupled mechanism.
There is a large body of data on animal PM Ca2+-pumps (PMCA), showing their inhibition by ROS resulting from protein cross-linking, lipid peroxidation, and concurrent inhibition by an oxidized form of calmodulin (Waring, 2005). However, these effects develop slowly (time scale of hours) and, thus, could not be responsible for the rapid induction of net Ca2+ efflux in our experiments. On the contrary, for plants a rapid activation of Ca2+-ATPase by ROS via the CaM–Ca2+ binding complex, has been described (Romani et al., 2004). However, the observation of a net Ca2+ efflux at lower ROS generated levels (0.1 mM Cu/A; Figure 3) implies that the activation of Ca2+ pumping in this case is an early event, independent on the Ca2+-pump activation via Ca2+–CaM.
It should be also mentioned that PAs are readily catabolized in the apoplast, releasing H2O2, which could be further converted to by apoplastic peroxidases iron and/or diamine oxidases copper (Liszkay et al., 2004; Kukavica et al., 2009). A possible contribution of this mechanism should be validated in direct experiments.
Incorporating Calcium Efflux Systems into Existing Signaling Models
Experimental evidence
Although transient increases in [Ca2+]cyt are essential for plant responses to a variety of environmental stimuli, long-lasting elevations in [Ca2+]cyt are harmful for cells. Hence, the basal conditions must be restored back to resting level after the signal is completed, enabling cells to react to further signals (Sanders et al., 1999; Beffagna et al., 2005). In animal systems, active Ca2+ efflux systems have been widely implicated in a wide range of stress responses (e.g., Jornot et al., 1999; Zaidi and Michaelis, 1999). Much less is known about the involvement of Ca2+ efflux systems in stress responses in plant cells. We believe this is wrong, and argue that active Ca2+ efflux systems must be incorporated in all stress signaling models.
Biotic stresses
Intracellular calcium signaling is universally accepted as a key component of plant biotic stress defense mechanisms (Grant et al., 2000; Lam et al., 2001, Pike et al., 2005). Elicitor-induced elevations in [Ca2+]cyt were reported during hypersensitive (HR) plant–pathogen interactions (Nurnberger et al., 1994; Blume et al., 2000; Lecourieux et al., 2002); these are believed to be essential for the development of the oxidative burst needed to trigger the activation of several plant defense reactions (Blumwald et al., 1998; Lecourieux et al., 2002). It was shown that Ca2+ channel blockers inhibit HR in many species (Atkinson et al., 1990; Levine et al., 1996), suggesting that PM-mediated calcium influx is required for HR initiation. As a result, most preceding reports were focused on the role of calcium cannels in HR induction (Atkinson et al., 1990; Levine et al., 1996, Jabs et al., 1997; Grant et al., 2000, Lam et al., 2001; Balagué et al., 2003, Pike et al., 2005; Hann and Rathjen, 2007). However, recent experiments in our laboratory have suggested that pathogen-induced Ca2+ influx occurs only at the first stages of pathogen–host interaction, during 0–7 h after the challenge (Nemchinov et al., 2008). Using P. syringae-inoculated tobacco plants we have shown that the initial calcium uptake is subsequently followed by the net calcium efflux initiated between 10 and 12 h and continued up to 48 h after the pathogen challenge. This efflux was inhibited by cyclopiazonic acid, a known inhibitor of Ca2+-ATPase, suggesting that active Ca2+ efflux systems play an important role in HR. A new model of a multi-step HR process has been put forward (Nemchinov et al., 2008). According to this model, prolonged Ca2+ uptake, which continues to occur 1–7 h after the challenge, reflects the pathogen’s successful overcoming the initial PAMP-triggered defense reaction, and sustained increases in [Ca2+]cyt at this stage are necessary for generation of ROS, oxidative burst, and induction of HR (Atkinson et al., 1990; Grant et al., 2000). After HR transduction pathway has been initiated with the help of Ca2+ uptake, [Ca2+]cyt levels sharply decline to mediate the last HR phase – an expanded cell death.
We have also recently demonstrated that Ca2+ efflux systems play a crucial role in the phenomenon of acquired cross-tolerance to oxidative stress in plants. Nicotiana benthamiana plants were infected with Potato virus X (PVX) and exposed to oxidative (either UV-C or H2O2) stress. It was shown that virus-infected plants had a better ability to control UV-induced elevations in [Ca2+]cyt free Ca2+ and prevent structural and functional damage of chloroplasts (Shabala et al., 2011a), and that PM Ca2+ efflux systems play a critical role in this process. Several major lines of evidence support this conclusion: (1) significant net Ca2+ efflux was measured from UV-treated leaves 2 h after stress exposure. As passive Ca2+ leak from the cytosol is thermodynamically impossible, such efflux cannot be attributed to the general change in PM permeability and may be explained only by the activation of some Ca2+ efflux (active) system at the PM (e.g., either Ca2+-ATPases or Ca2+/H+ exchanger); (2) PVX-inoculated leaves were actively pumping Ca2+ out (net efflux) while mock controls were still taking up Ca2+; (3) PVX-inoculated cells have a much better capacity to activate PM Ca2+ efflux systems to deal with UV-induced elevation in [Ca2+]cyt thereby preventing damage to chloroplast structure (Shabala et al., 2011a).
To separate the different types of active Ca2+ transport systems, a series of pharmacological experiments was conducted. Neither erythrosine B nor eosin yellow (EY), two known inhibitors of P2B-type Ca2+-ATPases, had a significant impact on the magnitude of net Ca2+ fluxes from tobacco mesophyll segments (Shabala et al., 2011a). Also unaffected was Ca2+-ATPase activity in purified PM vesicles from tobacco leaves. This suggested that the PM Ca2+-ATPases play a limited (if any) role in mediating Ca2+ efflux under oxidative stress conditions and suggested that the latter is mediated by PM Ca2+/H+ exchangers.
Not only plasma but also endomembrane Ca2+ efflux systems mediate the phenomenon of cross-protection in plants. Using biochemical and electrophysiological approaches, it was revealed that both endomembrane P2A and P2B Ca2+-ATPases play significant roles in adaptive responses to oxidative stress by removing excessive Ca2+ from the cytosol, and that their functional expression is significantly altered in PVX-inoculated plants (Shabala et al., 2011b). Taken together, these findings highlight the crucial role of Ca2+ efflux systems in acquired tolerance to oxidative stress and open up prospects for practical applications in agriculture.
Abiotic stresses
The evidence for the importance of Ca2+-ATPase in shaping [Ca2+]cyt signatures came from two independent salinity studies. First, knocking out both AtACA4 and AtACA2 in Saccharomyces cerevisiae can increase NaCl sensitivity, but expression of AtACA4 or AtACA2 can provide tolerance to NaCl. Moreover, [Ca2+]cyt elevations upon salinity stress are brought back to resting levels quickly, by expression of AtACA2 in this yeast mutant (Anil et al., 2008). Secondly, in moss (Physcomitrella patens), salinity stress to a loss-of-function mutant of ACA type ATPase (PCA1), resulted in sustained [Ca2+]cyt elevation and never returned to resting level (Qudeimat et al., 2008). ACAs may also e involved in [Ca2+]cyt signal shaping in response to other stresses. For example, AtACA8 was found to be unregulated, whereas AtACA10 was found be down regulated in response to cold stress (Schiøtt and Palmgren, 2005).
Earlier Romani et al. (2004) showed that submicromolar concentrations of EY (a P2B-type Ca2+-ATPase inhibitor) prevented both the increase in Ca2+ efflux and the transient ROS accumulation in Egeria densa in response to ABA treatment. This result was explained by assuming an important role of PM Ca2+-ATPase in switching off the signal triggering ROS production. Another report from the same group implicated PM Ca2+-ATPase activation in plant adaptation to osmotic stress (Beffagna et al., 2005). Interestingly, knocking out cax1 resulted in increased freezing tolerance (Catalá et al., 2003) but knocking out cax3 resulted in an increased sensitivity to salinity (Zhao et al., 2008), suggesting that each stress targets a particular CAX transporter within the CAX family. Stress-induced [Ca2+]cyt measurements involving cax knock out mutants may provide more insight into the specific role of each CAX transporter in shaping [Ca2+]cyt signals.
Theoretical considerations and modeling
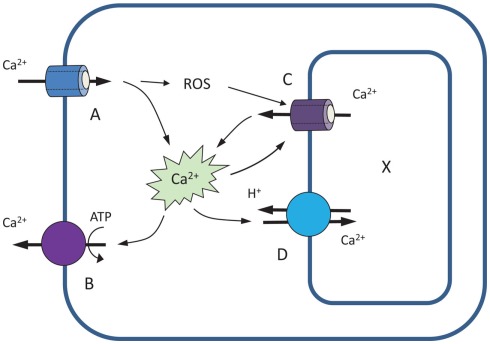
The importance of Ca2+ efflux systems in shaping Ca2+ signatures is further investigated by modeling stimulus-induced changes in [Ca2+]cyt. As a starting point, we use the model of Stucki and Somogyi (1994), as adopted by Bauer et al. (1998). This model includes four major components: two Ca2+-permeable channels (A and C), located respectively at the plasma – and endomembranes, and two active Ca2+ efflux systems: PM-based Ca2+-ATPase pump (B) and endomembrane-based Ca2+/H+ exchanger (D; Figure 4). Unlike the Bauer et al. (1998) model, we assume no leakage from the cytosol. We also assume that activity of endomembrane Ca2+ channel is dependent on ROS accumulation in the cytosol; this is parametrized by factor R.
Figure 4.
A four-component model illustrating the role of Ca2+ efflux systems in shaping up Ca2+ signatures (based on Stucki and Somogyi, 1994; Bauer et al., 1998). See text for explanations.
The amount y of Ca2+ in the cytosol, and x in the intracellular store is then given by (cf. Stucki and Somogyi, 1994):
Here ytotal denotes the total Ca2+ in the cytosol. This includes both bound and free calcium.
The mechanistic (biological) meaning of these equations is as follows. In Eq.(1), the rate of change of Ca2+ in the cytosol dytotal/dt is affected by both channels (A and C) and both active Ca2+-efflux systems (B and D). The latter drive an efflux of Ca2+ from the cytosol (hence the minus sign), and this efflux is proportional to the concentration yfree of free cytosolic Ca2+. Influx of Ca2+ into the cytosol is driven by a concentration gradient between external calcium and the cytosol (assumed to be a constant A, since the external calcium is typically plentiful); and an efflux from the intracellular store, driven by the concentration gradient (x−yfree). The functional form for the multiplicative factor for C is given by the Hill equation, modified to include ROS accumulation (parametrized by R). Physically it corresponds to buffering-type kinetics.
In Eq.(2), the rate of change of intracellular store Ca2+ concentration, dx/dt, is then governed by an efflux due to channel C that is equal in magnitude but opposite in sign to the influx into the cytosol as described in Eq.(1); and an influx proportional to the free cytosolic Ca2+ concentration due to pump D.
The buffering capacity of the cytosol is described by Michaelis–Menten kinetics as
| (2) |
The left-hand side of Eq.(1) can then be expressed in terms of yfree,
and the expression for amount x in the intracellular store remains unchanged.
Model parameters
The equations above are scalable. A has dimensions of (amount/time); B, C, and D have dimension (1/time); K is in [amount(1/n)]; Bmax, Kd and R are in (amount), just as x and y. This means that the scaled quantities can be related to physical ones via the initial conditions, x(t = 0) and y(t = 0) which are given in moles.
The scaling comes from available experimental data for Ca2+ flux into the cell. Assuming net Ca2+ influx (through A) into the cell being ∼60 nmol/m2/s (e.g., as in response to ROS treatment; Demidchik et al., 2002, 2007) and cell diameter of 30 μm, then the total amount of Ca2+ influx will be 10−6 mol/L, giving a physical flux Aphys = 2 μM/s. Hence, measuring concentrations in micrometer and time in seconds, we set for our default model A = 2. Assuming [Ca2+]cyt equal 100 nM and Ca2+ concentration inside the internal organelles within 100–1000 μM range (Medvedev, 2005), we adopt y(t = 0) = 0.1 and x(t = 0) = 160.
As in Bauer et al. (1998) we assume n = 4, K = 1. To set the buffering parameters we use the physical values of Bmax,phys = 0.2–0.5 mM (Trewavas, 1999) and Kd,phys = 0.15–0.6 μM (Martinez-Serrano et al., 1992; Kuratomi et al., 2003). This translates to adopted scaled quantities of Bmax = 200–500 and Kd = 0.15–0.6. For the default model, we adopt Bmax = 250 and Kd = 0.6.
The time scaling relates to constants A, B, C, and D. A is simply the uptake rate of Ca2+ from external media, and has dimensions of (amount/time); and units of [μM/s]. B, C, and D are essentially the inverse of decay-like constants, with dimensions of (1/time). This is easy to see from a simplified version of Equation 1, where each of these variables is described by a term like (dy/dt) ∝ By, etc. The solution to such a differential equation is an exponential, y ∝ eBt, and so 1/B is the time it takes for the amount of “stuff” to drop/increase by a factor of e (=2.718).
As discussed above, the time scaling of A is set by the physical parameter describing the influx into the cell (provided no other mechanisms were operating). In our default model A = 2, which corresponds to a flux of 2 μM/s. Setting A in this way means the values of B, C, and D are fixed by what “decay” constants are biologically meaningful.
Qualitative behavior
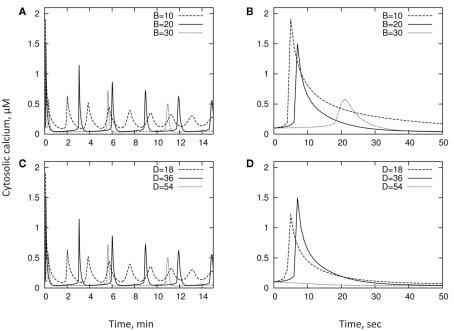
We explore the qualitative behavior of the model and contribution of activities of Ca2+-ATPase pump and Ca2+/H+ exchanger to the kinetics of [Ca2+]cyt. This is done using larger timescales to reflect the oscillatory behavior of [Ca2+]cyt changes. In doing this, we loosely follow the ratios between values adopted by Bauer et al. (1998) and assume the following set parameters: A = 2; B = 20; C = 60; D = 36; R = 0.05; Bmax = 250; Kd = 0.6. We also give the initial conditions y(t = 0) = 0.1; x(t = 0) = 160. As explained below, this set of parameters is motivated by experimental results. We then vary each of the parameters B and D in turn, typically through a dynamic range of a few, around the “default” value. Table 1 summarizes the various parameters and their typical values. Results are shown in Figure 5 and described below.
Table 1.
Model parameters.
| Parameter | Description | Units | Default | Min | Max | Touch | Anoxia | Osmotic | Cold |
|---|---|---|---|---|---|---|---|---|---|
| x | Amount of Ca2+ in cytosol | μM | |||||||
| y | Amount of Ca2+ in intracellular store | μM | |||||||
| B | Plasma membrane-based Ca2+ pump | μM/s | 20 | 10 | 30 | 20 | 2.5 | 8 | 13 |
| D | Endomembrane-based Ca2+/H+ exchanger | μM/s | 36 | 18 | 54 | 36 | 34 | 58 | 50 |
| σB | SD in B for cell population | μM/s | 0 | 0 | 5 | 0.1 | 1.7 | 1 | 3 |
| σD | SD in D for cell population | μM/s | 0 | 0 | 5 | 0.1 | 5 | 4 | 3 |
| A | Ca2+ permeable channel (external → cytosol) | μM/s | 2 | Fixed | |||||
| C | Ca2+ permeable channel (intracellular store → cytosol) | μM/s | 60 | Fixed | |||||
| x(0) | Initial amount of Ca2+ in cytosol | μM | 160 | Fixed | |||||
| y(0) | Initial amount of Ca2+ in intracellular store | μM | 0.1 | Fixed | |||||
| R | ROS concentration | μM | 0.05 | Fixed | |||||
| Kd | Buffering parameter | μM | 0.6 | Fixed | |||||
| Bmax | Buffering parameter | μM | 250 | Fixed | |||||
| K | Half maximal saturating Ca2+ concentration | μM1/n | 1 | Fixed | |||||
| n | Hill coefficient | – | 4 | Fixed |
The “default” model is the reference model. Parameters B, D, σB, and σD are changed in the range minimum – maximum and plotted in Figures 5 and 6. Best fit parameters for touch, cold, anoxia, and osmotic stress correspond to curves in Figure 7. All other model parameters are held constant in this work.
Figure 5.
Qualitative behavior of the model and contribution of activities of Ca2+-ATPase pump and Ca2+/H+ exchanger to the kinetics of [Ca2+]cyt. The exchanger D [shown in panel (C) and zoomed in panel (D)] shifts the location of the first peak, while the pump B [shown in panel (A) and zoomed in panel (B)] alters both the peak location and the speed with which [Ca2+]cyt drops.
Looking at timescales of a few minutes, increase in the activity of either the PM Ca2+-ATPase pump B or the Ca2+/H+ exchanger D, results in a longer oscillation timescale, and sharper peaks in cytosol concentration (Figures 5A,C). Importantly, as one can see, the physiologically relevant set of parameters in our model reproduces oscillation timescales of a few minutes as reported by many experimental studies (reviewed in McAinsh and Hetherington, 1998; Ng and McAinsh, 2003; McAinsh and Pittman, 2009; Roelfsema and Hedrich, 2010).
Shorter-term behavior allows us to decouple the pump/exchanger contributions. The exchanger D only shifts the location of the first peak (Figure 5D), while the pump B both shifts the peak and changes the speed with which the cytosol concentration drops (Figure 5B) – the “decay constant” discussed in the previous section. Thus, pumps and exchangers appear to have a different role in shaping [Ca2+]cyt signatures.
Accounting for spatial heterogeneity
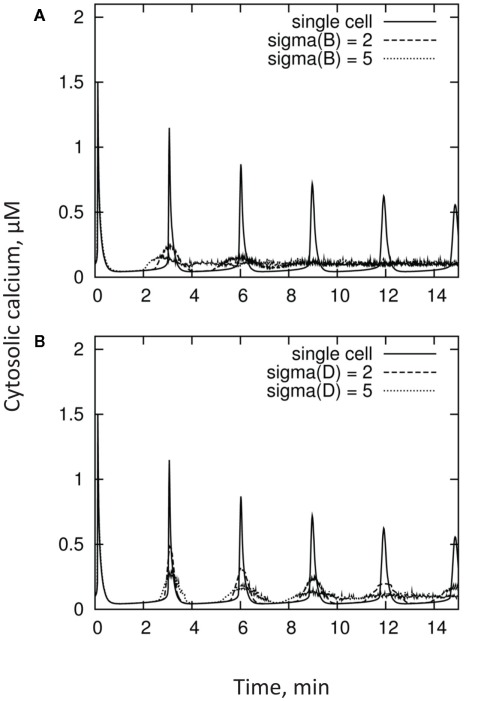
Stimulus-induced elevations in [Ca2+]cyt usually show marked spatial heterogeneities, displaying both “hot-spots” and Ca2+-quiescent regions (Gilroy et al., 1991; McAinsh et al., 1995; Ng and McAinsh, 2003). It was suggested that such spatial heterogeneity could result from either different accessibility of the primary stimulus to only a subset of the signaling machinery, or the non-uniform distribution of the intracellular signaling machinery (Ng and McAinsh, 2003). Regardless of the reason, the kinetics of [Ca2+]cyt reported in the literature reflect a result of integration of these non-uniform [Ca2+]cyt domains within the cell. Moreover, quite often the measured signal reflects the integrated response of many cells and, thus, may combine responses from several populations of cell types (e.g., epidermal and cortical cells in plant roots). It is logical to expect that these cells may have rather different activities of Ca2+ pumps and exchangers and, as a result, display different [Ca2+]cyt kinetics in response to the same stimulus. As a result, the overall measured [Ca2+]cyt signal may be quite different from the response of each individual cell. This is further illustrated in Figure 6, which depicts [Ca2+]cyt kinetics from a population of n = 100 cells having a normal (Gaussian) distribution of parameters B and D.
Figure 6.
Kinetics of [Ca2+]cyt measured from a population of n = 100 cells having a Gaussian (normal) distribution of parameters B panel (A) and D panel (B), as compared with individual cell responses. Distributions in these parameters broaden the first [Ca2+]cyt peak, and result in substantial decorrelation at subsequent maxima.
Unsurprisingly, both lowering of the amplitude and broadening of the oscillations are observed. Moreover, the phase coherence between cells degrades with time, resulting in oscillations becoming more and more “smeared out.” This is qualitatively consistent with results from the literature.
Fitting experimental data
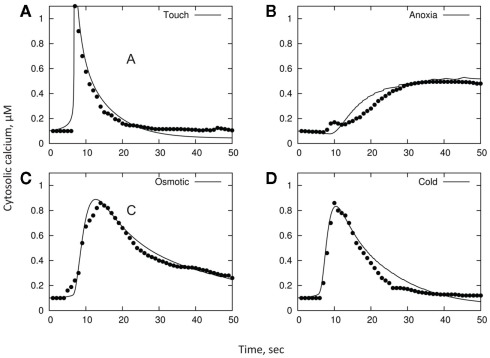
As a final illustration of our model, we consider experimental data reporting changes in [Ca2+]cyt in response to cold, osmotic stress, touch, and H2O2 in Arabidopsis seedlings, as per Logan and Knight (2003). In brief, changes in [Ca2+]cyt were observed in planta using recombinant aequorin Arabidopsis plants. Cold, mannitol, or H2O2 treatments were effected by slowly (to prevent a touch response) injecting 0.5 mL of ice-cold water, 0.7 M mannitol, or 20 mM H2O2, respectively, into a cuvette containing an Arabidopsis seedling floating in 0.5 mL of water at room temperature. Touch treatment was effected by the rapid injection of 0.5 mL of room temperature water into the cuvette. The original data reported in that paper was digitized and is shown in respective panels in Figure 7.
Figure 7.
A qualitative comparison between stress-induced [Ca2+]cyt signatures and model simulation. Unconnected symbols are experimental observations (as per Logan and Knight, 2003); lines are model fits. The basic parameters in all models are: A = 2; C = 60; R = 0.05; Bmax = 250; Kd = 0.6; y(t = 0) = 0.1; x(t = 0) = 160. Only distributions in B and D are varied between treatments. The remaining parameters are: for cold panel (D), B = 13, σB = 3, D = 50, σD = 3; for osmotic panel (C), B = 8, σB = 1, D = 58, σD = 4; for touch panel (A), B = 20, σB = 0.1, D = 36, σD = 0.1; for H2O2 panel (B), B = 2.5, σB = 1.7, D = 74, σD = 5.
As one can see, experimental data (unconnected symbols) can be adequately approximated by the model fits (continuous lines; Figure 7; also summarized in Table 1). Importantly, this is achieved by using a realistic (i.e., physiologically relevant) set of initial characteristics (see above), validating the adequacy of the model. Even more importantly, it appears that specific [Ca2+]cyt signatures observed in response to four different types of stress (cold, osmotic stress, touch, and H2O2 treatments) can be adequately achieved by modifying just the distributions of parameters B and D, i.e., properties of PM-based Ca2+-ATPase pump and endomembrane-based Ca2+/H+ exchanger, respectively.
As such, we use the best fit to touch stress as the reference model. The difference between plant responses to cold and touch may be explained by a 1.5-fold increase in parameter D, a similar decrease in B, and a broadening of their distributions (compared to single-cell responses) by 23 and 6% of the mean, respectively. Much slower rise in [Ca2+]cyt in response to osmotic stress may be explained by still lower values of B (with only a minimal change in D), while both sustained elevation and reduced peak [Ca2+]cyt values in response to H2O2 treatment are given by a further decrease in B and (slightly) higher D values.
The dynamic range spanned by D in these models is only 0.3 dex (i.e., a factor of 2); and the width of the normal distribution relative to the mean is σD/D = 0–0.07. The dynamic range for B is 0.9 dex (factor of 8), and σD/D = 0–0.65. Both these changes are within the physiological range of changes expected under stress conditions. Indeed, the efficiency of ATP production drops 19-fold (from 38 to only two ATP molecules; Gibbs and Greenway, 2003) under anoxic conditions; this is twice as wide as the dynamic range for B in the models. Importantly, oxygen profiles in the root differ dramatically between epidermal, cortical, and stellar tissues (Armstrong et al., 1994), even under normoxic conditions. Thus, differential Ca2+-ATPase activity is expected between these tissues. This special heterogeneity will confer a broad dynamic range for B and may explain the presence of the second peak in [Ca2+]cyt observed 30–40 min after anoxia onset (Figure 1C).
Conclusion and Prospects
Restoration of the basal [Ca2+]cyt levels is essential for removing excess Ca2+ from the cytosol, to reload Ca2+ stores and to terminate Ca2+ signaling. (As shown in this paper, it is impossible to achieve this without having efficient Ca2+ efflux mechanisms in place). It also appears that physiologically relevant variations in the activity of Ca2+-ATPase pumps and Ca2+/H+ exchangers are sufficient to fully describe all the reported experimental evidence and determine the shape of [Ca2+]cyt signatures in response to environmental stimuli. This emphases the crucial role these active efflux systems play in plant adaptive responses to environment and suggests that more attention has to be given to elucidation of the spatio-temporal properties and control modes of Ca2+-ATPase pumps and Ca2+/H+ exchangers in plants.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the ARC Discovery grant DP1094663 and GRDC grant UT0022 to Sergey Shabala and CONACyT grant 82913 to Igor Pottosin.
References
- Allbritton N. L., Meyer T., Stryer L. (1992). Range of messenger action of calcium ion and inositol 1, 4, 5-trisphosphate. Science 258, 1812–1815 10.1126/science.1465619 [DOI] [PubMed] [Google Scholar]
- An Z., Jing W., Liu Y., Zhang W. (2008). Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825 10.1093/jxb/erm370 [DOI] [PubMed] [Google Scholar]
- Anil V. S., Rajkumar P., Kumar P., Mathew M. (2008). A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast. J. Biol. Chem. 283, 3497–3506 10.1074/jbc.M700766200 [DOI] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M., Floryszak-Wieczorek J., Kubi J. (2009). Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J. Plant Growth Regul. 28, 177–186 10.1007/s00344-009-9086-7 [DOI] [Google Scholar]
- Armstrong W., Strange M. E., Cringle S., Beckett P. M. (1994). Microelectrode and modeling study of oxygen distribution in roots. Ann. Bot. 74, 287–299 10.1006/anbo.1994.1120 [DOI] [Google Scholar]
- Atkinson M. M., Keppler L. D., Orlandi E. W., Baker C. J., Mischke C. F. (1990). Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ and hypersensitive responses in tobacco. Plant Physiol. 92, 215–221 10.1104/pp.92.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen K., Venema K., Jahn T., Baunsgaard L., Palmgren M. (1999). Molecular dissection of the C-terminal regulatory domain of the plant plasma membrane H+-ATPase AHA2: mapping of residues that when altered give rise to an activated enzyme. Biochemistry 38, 7227–7234 10.1021/bi982482l [DOI] [PubMed] [Google Scholar]
- Balagué C., Lin B., Alcon C., Flottes G., Malmström S., Köhler C., Neuhaus G., Pelletier G., Gaymard F., Roby D. (2003). HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide–gated channel ion channel family. Plant Cell 15, 365–379 10.1105/tpc.006999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli R. C., Kamp T. J. (2008). Caveolae, ion channels and cardiac arrhythmias. Prog. Biophys. Mol. Biol. 98, 149–160 10.1016/j.pbiomolbio.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. S., Plieth C., Bethmann B., Popescu O., Hansen U.-P., Simonis W., Schonknecht G. (1998). Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol. 117, 545–557 10.1104/pp.117.2.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffagna N., Buffoli B., Busi C. (2005). Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol. 46, 1326–1339 10.1093/pcp/pci142 [DOI] [PubMed] [Google Scholar]
- Berridge M. J. (1997). The AM and FM of calcium signalling. Nature 386, 759–760 10.1038/386759a0 [DOI] [PubMed] [Google Scholar]
- Blatt M. R. (1999). Reassessing roles for Ca2+ in guard cell signalling. J. Exp. Bot. 50, 989–999 10.1093/jexbot/50.suppl_1.989 [DOI] [Google Scholar]
- Blume B., Nürnberger T., Nass N., Scheel D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12, 1425–1440 10.2307/3871140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Aharon G. S., Lam C. H. (1998). Early signal transduction pathways in plant-pathogen interactions. Trends Plant Sci. 3, 342–346 10.1016/S1360-1385(98)01289-8 [DOI] [Google Scholar]
- Bonza M. C., De Michelis M. (2011). The plant Ca2+ ATPase repertoire: biochemical features and physiological functions. Plant Biol. 13, 421–430 10.1111/j.1438-8677.2010.00405.x [DOI] [PubMed] [Google Scholar]
- Bonza M. C., Morandini P., Luoni L., Geisler M., Palmgren M. G., De Michelis M. I. (2000). At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol. 123, 1495–1506 10.1104/pp.123.4.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y., Harper J. F. (2007). The origin and function of calmodulin regulated Ca2+ pumps in plants. J. Bioenerg. Biomembr. 39, 409–414 10.1007/s10863-007-9104-z [DOI] [PubMed] [Google Scholar]
- Brini M., Carafoli E. (2009). Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 10.1152/physrev.00032.2008 [DOI] [PubMed] [Google Scholar]
- Baekgaard L., Luoni L., De Michelis M. I., Palmgren M. G. (2006). The plant plasma membrane Ca2+ pump ACA8 contains overlapping as well as physically separated autoinhibitory and calmodulin-binding domains. J. Biol. Chem. 281, 1058–1065 10.1074/jbc.M508299200 [DOI] [PubMed] [Google Scholar]
- Baekgaard L., Mikkelsen M. D., Sørensen D. M., Hegelund J. N., Persson D. P., Mills R. F., Yang Z., Husted S., Andersen J. P., Buch-Pedersen M. J. (2010). A combined zinc/cadmium sensor and zinc/cadmium export regulator in a heavy metal pump. J. Biol. Chem. 285, 31243–31252 10.1074/jbc.M110.111260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L., Martínez A., Sánchez F., Quinto C. (2008). Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J. 56, 802–813 10.1111/j.1365-313X.2008.03644.x [DOI] [PubMed] [Google Scholar]
- Case R. M., Eisner D., Gurney A., Jones O., Muallem S., Verkhratsky A. (2007). Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42, 345–350 10.1016/j.ceca.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Catalá R., Santos E., Alonso J. M., Ecker J. R., Martínez-Zapater J. M., Salinas J. (2003). Mutations in the Ca2+/+Htransporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15, 2940–2951 10.1105/tpc.015248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Hirschi K. D. (2003). Cloning and characterization of CXIP1, a novel PICOT domain-containing Arabidopsis protein that associates with CAX1. J. Biol. Chem. 278, 6503–6509 10.1074/jbc.M207232200 [DOI] [PubMed] [Google Scholar]
- Cheng N. H., Liu J. Z., Nelson R. S., Hirschi K. D. (2004a). Characterization of CXIP4, a novel Arabidopsis protein that activates the H+/ Ca2+ antiporter, CAX1. FEBS Lett. 559, 99–106 10.1016/S0014-5793(04)00036-5 [DOI] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Zhu J. K., Hirschi K. D. (2004b). The protein kinase SOS2 activates the Arabidopsis H+/ Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 279, 2922–2926 10.1074/jbc.M402828200 [DOI] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Barkla B. J., Shigaki T., Hirschi K. D. (2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15, 347–364 10.1105/tpc.013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Shigaki T., Hirschi K. D. (2002a). Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol. 128, 1245–1254 10.1104/pp.010857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Willmann M. R., Chen H. C., Sheen J. (2002b). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129, 469–485 10.1104/pp.005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Shigaki T., Lachmansingh J., LeClere S., Lahner B., Salt D. E., Hirschi K. D. (2005). Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 138, 2048–2060 10.1104/pp.105.061218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton H., Knight M. R., Knight H., McAinsh M. R., Hetherington A. M. (1999). Dissection of the ozone induced calcium signature. Plant J. 17, 575–579 10.1046/j.1365-313X.1999.00411.x [DOI] [PubMed] [Google Scholar]
- De Koninck P., Schulman H. (1998). Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 10.1126/science.279.5348.227 [DOI] [PubMed] [Google Scholar]
- DeFalco T., Bender K., Snedden W. (2010). Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 425, 27–40 10.1042/BJ20091147 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Bowen H. C., Maathuis F. J. M., Shabala S. N., Tester M. A., White P. J., Davies J. M. (2002). Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J. 32, 799–808 10.1046/j.1365-313X.2002.01467.x [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shabala S. N., Coutts K. B., Tester M. A., Davies J. M. (2003). Free oxygen radicals regulate plasma membrane Ca2+ and K+-permeable channels in plant root cells. J. Cell Sci. 116, 81–88 10.1242/jcs.00201 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shabala S. N., Davies J. M. (2007). Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 49, 377–386 10.1111/j.1365-313X.2006.02971.x [DOI] [PubMed] [Google Scholar]
- Dobrovinskaya O. R., Muniz J., Pottosin II. (1999). Inhibition of vacuolar ion channels by polyamines. J. Membr. Biol. 167, 127–140 10.1007/s002329900477 [DOI] [PubMed] [Google Scholar]
- Dodd A. N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620 10.1146/annurev-arplant-070109-104628 [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. E., Lewis R. S., Goodnow C. C., Healy J. I. (1997). Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 10.1038/386855a0 [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. E., Xu K., Lewis R. S. (1998). Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- Dutta R., Robinson K. R. (2004). Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 135, 1398–1406 10.1104/pp.104.041483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt D. W., Wais R., Long S. R. (1996). Calcium spiking in plant root hairs responding to rhizobium nodulation signals. Cell 85, 673–681 10.1016/S0092-8674(00)81234-9 [DOI] [PubMed] [Google Scholar]
- Ettinger W., Clear A., Fanning K., Peck M. (1999). Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol. 119, 1379–1385 10.1104/pp.119.4.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijo J. A., Sainhas J., Holdaway-Clarke T., Cordeiro M. S., Kunkel J. G., Hepler P. K. (2001). Cellular oscillations and the regulation of growth: the pollen tube paradigm. Bioessays 23, 86–94 [DOI] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J. H. F., Mylona P., Miedema H., Torres M. A., Linstead P., Costa S., Brownlee C., Jones J. D. G., Davies J. M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446 10.1038/nature01485 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F. (1997). Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 77, 699–729 [DOI] [PubMed] [Google Scholar]
- Frietsch S., Wang Y. F., Sladek C., Poulsen L. R., Romanowsky S. M., Schroeder J. I., Harper J. F. (2007). A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. U.S.A. 104, 14531–14536 10.1073/pnas.0701781104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garufi A., Visconti S., Camoni L., Aducci P. (2007). Polyamines as physiological regulators of 14-3-3 interaction with the plant plasma membrane H+-ATPase. Plant Cell Physiol. 48, 434–440 10.1093/pcp/pcm010 [DOI] [PubMed] [Google Scholar]
- Geisler M., Frangne N., Gomès E., Martinoia E., Palmgren M. G. (2000). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 124, 1814–1827 10.1104/pp.124.4.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L., Romanowsky S. M., Harper J. F., Sharrock R. A. (2008). The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol. 146, 716–728 10.1104/pp.107.108118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J., Greenway H. (2003). Review: mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 30, 353–353 10.1071/PP98095_ER [DOI] [PubMed] [Google Scholar]
- Gifford J., Walsh M., Vogel H. (2007). Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 405, 199–221 10.1042/BJ20070255 [DOI] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. (1991). Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3, 333–344 10.1105/tpc.3.4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard H., Manison N., Tomos D., Brownlee C. (2000). Elemental propagation of calcium signals in response-specific patterns determined by environmental stimulus strength. Proc. Natl. Acad. Sci. U.S.A. 97, 1932–1937 10.1073/pnas.020516397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., van der Luit A. H., Knight M. R., Trewavas A. J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437 10.1104/pp.116.1.429 [DOI] [Google Scholar]
- Grabov A., Blatt M. R. (1998). Co-ordination of signalling elements in guard cell ion channel control. J. Exp. Bot. 49, 351–360 10.1093/jexbot/49.suppl_1.351 [DOI] [Google Scholar]
- Grant M., Brown I., Adams S., Knight M., Ainslie A., Mansfield J. (2000). The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23, 441–450 10.1046/j.1365-313x.2000.00804.x [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Robb-Gaspers L. D., Seitz M., Thomas A. P. (1995). Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- Hann D. R., Rathjen J. P. (2007). Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J. 49, 607–618 10.1111/j.1365-313X.2006.02981.x [DOI] [PubMed] [Google Scholar]
- Hansen U.-P. (1978). Do light-induced changes in the membrane potential of Nitella reflect the feed-back regulation of a cytoplasmic parameter? J. Membr. Biol. 41, 197–224 10.1007/BF01870429 [DOI] [Google Scholar]
- Harper J. F. (2001). Dissecting calcium oscillators in plant cells. Trends Plant Sci. 6, 395–397 10.1016/S1360-1385(01)02023-4 [DOI] [PubMed] [Google Scholar]
- Harper J. F., Hong B., Hwang I., Guo H. Q., Stoddard R., Huang J. F., Palmgren M. G., Sze H. (1998). A novel calmodulin-regulated Ca2+-ATPase (A Ca2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 273, 1099–1106 10.1074/jbc.273.2.1099 [DOI] [PubMed] [Google Scholar]
- Heidelberger R. (1998). Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J. Gen. Physiol. 111, 225–241 10.1085/jgp.111.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. (1994). Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371, 513–515 10.1038/371513a0 [DOI] [PubMed] [Google Scholar]
- Hepler P. K., Winship L. J. (2010). Calcium at the cell wall cytoplast interface. J. Integr. Plant Biol. 52, 147–160 10.1111/j.1744-7909.2010.00923.x [DOI] [PubMed] [Google Scholar]
- Hille B. (2001). Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates [Google Scholar]
- Hirschi K. (2001). Vacuolar H+/ Ca2+ transport: who’s directing the traffic? Trends Plant Sci. 6, 100–104 10.1016/S1360-1385(00)01863-X [DOI] [PubMed] [Google Scholar]
- Hirschi K. D. (1999). Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11, 2113–2122 10.2307/3871013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K. D., Korenkov V. D., Wilganowski N. L., Wagner G. J. (2000). Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 124, 125–133 10.1104/pp.124.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T. L., Feijo J. A., Hacket G. R., Kunkel J. G., Hepler P. K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9, 1999–2010 10.2307/3870560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann U., Meckel T., Hewing J., Hütt M. T., Hurst A. C. (2007). Distinct fluorescent pattern of KAT1: GFP in the plasma membrane of Vicia faba guard cells. Eur. J. Cell Biol. 86, 489–500 10.1016/j.ejcb.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Huang L., Berkelman T., Franklin A. E., Hoffman N. E. (1993). Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope. Proc. Natl. Acad. Sci. U.S.A. 90, 10066–10070 10.1073/pnas.90.21.10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide Y., Tomioka R., Ouchi Y., Kamiya T., Maeshima M. (2007). Transcriptional induction of two genes for CCaPs, novel cytosolic proteins, in Arabidopsis thaliana in the dark. Plant Cell Physiol. 48, 54–65 10.1093/pcp/pcl042 [DOI] [PubMed] [Google Scholar]
- Ishida H., Vogel H. J. (2010). The solution structure of a plant calmodulin and the CaM-binding domain of the vacuolar calcium-ATPase BCA1 reveals a new binding and activation mechanism. J. Biol. Chem. 285, 38502–38510 10.1074/jbc.M110.131201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu L. T., Spangler R. A. (1993). Mathematical-analysis of a model for calcium oscillations based on calcium-induced calcium release and diffusion. FASEB J. 7, A239–A239 [Google Scholar]
- Jabs T., Tschope M., Colling C., Hahlbrock K., Scheel D. (1997). Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. U.S.A. 94, 4800–4805 10.1073/pnas.94.9.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P., Maeda M., Fischer R., Verma A., Krebs J., Penniston J., Carafoli E. (1988). Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J. Biol. Chem. 263, 2905–2910 [PubMed] [Google Scholar]
- Jornot L., Maechler P., Wollheim C. B., Junod A. F. (1999). Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger. J. Cell Sci. 112, 1013–1022 [DOI] [PubMed] [Google Scholar]
- Kasai M., Muto S. (1990). Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J. Membr. Biol. 114, 133–142 10.1007/BF01869094 [DOI] [PubMed] [Google Scholar]
- Kiegle E., Moore C. A., Haseloff J., Tester M. A., Knight M. R. (2000). Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 23, 267–278 10.1046/j.1365-313x.2000.00786.x [DOI] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526 10.1038/352524a0 [DOI] [PubMed] [Google Scholar]
- Knight M. R., Smith S. M., Trewavas A. J. (1992). Wind-induced plant motion immediately increases cytosolic calcium. Proc. Natl. Acad. Sci. U.S.A. 89, 4967–4971 10.1073/pnas.89.11.4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W., Scheenen W., Schoolderman L., Cruijsen P., Roubos E., Jenks B. (2001). Intracellular calcium buffering shapes calcium oscillations in Xenopus melanotropes. Pflugers Arch. 443, 250–256 10.1007/s004240100680 [DOI] [PubMed] [Google Scholar]
- Kudla J., Batisti O., Hashimoto K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell 22, 541–563 10.1105/tpc.109.072686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukavica B., Mojovi M., Vuc ini Ž., Maksimovi V., Takahama U., Jovanovi S. V. (2009). Generation of hydroxyl radical in isolated pea root cell wall, and the role of cell wall-bound peroxidase, Mn-SOD and phenolics in their production. Plant Cell Physiol. 50, 304–317 10.1093/pcp/pcn199 [DOI] [PubMed] [Google Scholar]
- Kuratomi S., Matsuoka S., Sarai N., Powell T., Noma A. (2003). Involvement of Ca2+ buffering and Na+/ Ca2+ exchange in the positive staircase of contraction in guinea-pig ventricular myocytes. Pflugers Arch. 446, 347–355 [DOI] [PubMed] [Google Scholar]
- Lam E., Kato N., Lawton M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 848–853 10.1038/35081184 [DOI] [PubMed] [Google Scholar]
- Leckie C. P., McAinsh M. R., Montgomery L., Priestley A. J., Staxen I., Webb A. A. R., Hetherington A. M. (1998). Second messengers in guard cells. J. Exp. Bot. 49, 339–349 10.1093/jexbot/49.suppl_1.339 [DOI] [Google Scholar]
- Lecourieux D., Mazars C., Pauly N., Ranjeva R., Pugin A. (2002). Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14, 2627–2641 10.1105/tpc.005579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Kim H. S., Han H. J., Moon B. C., Kim C. Y., Harper J. F., Chung W. S. (2007). Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett. 581, 3943–3949 10.1016/j.febslet.2007.04.069 [DOI] [PubMed] [Google Scholar]
- Levine A., Pennell R. I., Alvarez M. E., Palmer R. (1996). Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6, 427–437 10.1016/S0960-9822(02)00510-9 [DOI] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. (1984). Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J. Cell. Biol. 99, 1212–1220 10.1083/jcb.99.4.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H., Llopis J., Whitney M., Zlokarnik G., Tsien R. Y. (1998). Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941 10.1038/31965 [DOI] [PubMed] [Google Scholar]
- Li X., Chanroj S., Wu Z., Romanowsky S. M., Harper J. F., Sze H. (2008). A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol. 147, 1675–1689 10.1104/pp.108.119909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Cunningham K. W., Harper J. F., Sze H. (1997). ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 94, 8579–8584 10.1073/pnas.94.7.2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Fagan K. A., Li K. X., Shaul P. W., Cooper D. M. F., Rodman D. M. (2000). Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 275, 17979–17985 10.1074/jbc.M002814200 [DOI] [PubMed] [Google Scholar]
- Liszkay A., Van Der Zalm E., Schopfer P. (2004). Production of reactive oxygen intermediates (O2−, H2O2, and OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 136, 3114–3123 10.1104/pp.104.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fu H. H., Bei Q. X., Luan S. (2000). Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 124, 1315–1325 10.1104/pp.124.3.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Li R. L., Zhang L., Wang Q. L., Niehaus K., Baluška F., Šamaj J., Lin J. X. (2009). Lipid microdomain polarization is required for NADPH oxidase dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 60, 303–313 10.1111/j.1365-313X.2009.03955.x [DOI] [PubMed] [Google Scholar]
- Llinas R., Sugimori M., Silver R. (1992). Microdomains of high calcium concentration in a presynaptic terminal. Science 256, 677–679 10.1126/science.1350109 [DOI] [PubMed] [Google Scholar]
- Lloyd D., Stupfel M. (1991). The occurrence and functions of ultradian rhythms. Biol. Rev. Camb. Philos. Soc. 66, 275–299 10.1111/j.1469-185X.1991.tb01143.x [DOI] [PubMed] [Google Scholar]
- Logan D. C., Knight M. R. (2003). Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol. 133, 21–24 10.1104/pp.103.026047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Kudla J., Rodriguez-Concepcion M., Yalovsky S., Gruissem W. (2002). Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. Z., Wang H. W., Huang J., Tian A. G., Wang Y. J., Zhang J. S., Chen S. Y. (2005). A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol. Biol. 59, 809–820 10.1007/s11103-005-1386-0 [DOI] [PubMed] [Google Scholar]
- Luoni L., Meneghelli S., Bonza M. C., DeMichelis M. I. (2004). Auto-inhibition of Arabidopsis thaliana plasma membrane Ca2+-ATPase involves an interaction of the N-terminus with the small cytoplasmic loop. FEBS Lett. 574, 20–24 10.1016/j.febslet.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Malmström S., Askerlund P., Palmgren M. G. (1997). A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Lett. 400, 324–328 10.1016/S0014-5793(96)01448-2 [DOI] [PubMed] [Google Scholar]
- Manohar M., Shigaki T., Hirschi K. (2011). Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol. 13, 561–569 10.1111/j.1438-8677.2011.00466.x [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A., Blanco P., Satrustegui J. (1992). Calcium-binding to the cytosol and calcium extrusion mechanisms in intact synaptosomes and their alterations with aging. J. Biol. Chem. 267, 4672–4679 [PubMed] [Google Scholar]
- Mäser P., Thomine S., Schroeder J. I., Ward J. M., Hirschi K., Sze H., Talke I. N., Amtmann A., Maathuis F. J. M., Sanders D. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667 10.1104/pp.126.4.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. (1989). The chaotic rhythms of life. New Sci. 21–2511659167 [Google Scholar]
- McAinsh M. R., Brownlee C., Hetherington A. M. (1997). Calcium ions as second messengers in guard cell signal transduction. Physiol. Plant 100, 16–29 10.1111/j.1399-3054.1997.tb03451.x [DOI] [Google Scholar]
- McAinsh M. R., Hetherington A. M. (1998). Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 3, 32–36 10.1016/S1360-1385(97)01150-3 [DOI] [Google Scholar]
- McAinsh M. R., Pittman J. K. (2009). Shaping the calcium signature. New Phytol. 181, 275–294 10.1111/j.1469-8137.2008.02682.x [DOI] [PubMed] [Google Scholar]
- McAinsh M. R., Webb A. A. R., Taylor J. E., Hetherington A. M. (1995). Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7, 1207–1219 10.1105/tpc.7.8.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E., Tsai Y. C., Braam J. (2005). Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 10, 383–389 10.1016/j.tplants.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Medvedev S. S. (2005). Calcium signaling system in plants. Russ. J. Plant Physiol. 52, 249–270 10.1007/s11183-005-0038-1 [DOI] [Google Scholar]
- Mei H., Zhao J., Pittman J. K., Lachmansingh J., Park S., Hirschi K. D. (2007). In planta regulation of the Arabidopsis Ca2+/H+ antiporter CAX1. J. Exp. Bot. 58, 3419–3427 10.1093/jxb/erm190 [DOI] [PubMed] [Google Scholar]
- Meksuriyen D., Fukuchi-Shimogori T., Tomitori H., Kashiwagi K., Toida T., Imanari T., Kawai G., Igarashi K. (1998). Formation of a complex containing ATP, Mg2+ and spermine. J. Biol. Chem. 273, 30939–30944 10.1074/jbc.273.47.30939 [DOI] [PubMed] [Google Scholar]
- Mills R. F., Doherty M. L., López-Marqués R. L., Weimar T., Dupree P., Palmgren M. G., Pittman J. K., Williams L. E. (2008). ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol. 146, 116–128 10.1104/pp.107.110817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Sun J., Oldroyd G. E. D., Allan Downie J. (2006). Analysis of calcium spiking using a cameleon calcium sensor reveals that nodulation gene expression is regulated by calcium spike number and the developmental status of the cell. Plant J. 48, 883–894 10.1111/j.1365-313X.2006.02926.x [DOI] [PubMed] [Google Scholar]
- Møller J. V., Olesen C., Winther A. M. L., Nissen P. (2010). The sarcoplasmic Ca2+ -ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 10.1017/S003358351000017X [DOI] [PubMed] [Google Scholar]
- Mons N., Decorte L., Jaffard R., Cooper D. (1998). Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci. 62, 1647–1652 10.1016/S0024-3205(98)00122-2 [DOI] [PubMed] [Google Scholar]
- Monshausen G. B., Messerli M. A., Gilroy S. (2008). Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 147, 1690–1698 10.1104/pp.108.123638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I., Norambuena L., Maturana D., Toro M., Vergara C., Orellana A., Zurita-Silva A., Ordenes V. R. (2008). AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. J. Biol. Chem. 283, 9633–9641 10.1074/jbc.M800736200 [DOI] [PubMed] [Google Scholar]
- Morth J. P., Pedersen B. P., Buch-Pedersen M. J., Andersen J. P., Vilsen B., Palmgren M. G., Nissen P. (2011). A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 12, 60–70 10.1038/nrm3031 [DOI] [PubMed] [Google Scholar]
- Moschou P. N., Paschalidis K. A., Roubelakis-Angelakis K. A. (2008). Plant polyamine catabolism: the state of the art. Plant Signal. Behav. 3, 1061–1066 10.4161/psb.3.12.7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M., Neher E. (1997). Linearized buffered Ca2(diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 17, 6961–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemchinov L. G., Shabala L., Shabala S. (2008). Calcium efflux as a component of the hypersensitive response of Nicotiana benthamiana to Pseudomonas syringae. Plant Cell Physiol. 49, 40–46 10.1093/pcp/pcm163 [DOI] [PubMed] [Google Scholar]
- Ng C. K. Y., McAinsh M. R. (2003). Encoding specificity in plant calcium signalling: hot-spotting the ups and downs and waves. Ann. Bot. 92, 477–485 10.1093/aob/mcg173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger T., Nennsteil D., Jabs T., Sacks W. R., Hahlbrock K., Scheel D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460 10.1016/0092-8674(94)90423-5 [DOI] [PubMed] [Google Scholar]
- Palmgren M., Nissen P. (2011). P-type ATPases. Annu. Rev. Biophys. 40, 243–266 10.1146/annurev.biophys.093008.131331 [DOI] [PubMed] [Google Scholar]
- Pei Z. M., Murata Y., Benning G., Thomine S., Klusener B., Allen G. J., Grill E., Schroeder J. I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- Pérez V., Wherrett T., Shabala S., Muniz J., Dobrovinskaya O., Pottosin I. (2008). Homeostatic control of slow vacuolar channels by luminal cations and evaluation of the channel-mediated tonoplast Ca2+ fluxes in situ. J. Exp. Bot. 59, 3845–3855 10.1093/jxb/ern225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike S. M., Zhang X. C., Gassmann W. (2005). Electrophysiological characterization of the Arabidopsis avrRpt2-specific hypersensitive response in the absence of other bacterial signals. Plant Physiol. 138, 1009–1017 10.1104/pp.104.047142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman J. K., Hirschi K. D. (2003). Don’t shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Curr. Opin. Plant Biol. 6, 257–262 10.1016/S1369-5266(03)00036-0 [DOI] [PubMed] [Google Scholar]
- Pittman J. K., Shigaki T., Cheng N.-H., Hirschi K. D. (2002). Mechanism of N-terminal autoinhibition in the Arabidopsis Ca2+/H+ Antiporter CAX1. J. Biol. Chem. 277, 26452–26459 10.1074/jbc.M205615200 [DOI] [PubMed] [Google Scholar]
- Plieth C., Sattelmacher B., Hansen U.-P. (1997). Cytoplasmic Ca2+-H+-exchange buffers in green algae. Protoplasma 198, 107–124 10.1007/BF01282136 [DOI] [Google Scholar]
- Poburko D., Kuo K. H., Dai J., Lee C. H., van Breemen C. (2004). Organellar junctions promote targeted Ca2+ signaling in smooth muscle: why two membranes are better than one. Trends Pharmacol. Sci. 25, 8–15 10.1016/j.tips.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Pottosin II., Tikhonova L. I., Hedrich R., Schonknecht G. (1997). Slowly activating vacuolar channels can not mediate Ca2+- induced Ca2+ release. Plant J. 12, 1387–1398 10.1046/j.1365-313x.1997.12061387.x [DOI] [Google Scholar]
- Price A. H., Taylor A., Ripley S. J., Griffiths A., Trewavas A. J., Knight M. R. (1994). Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6, 1301–1310 10.2307/3869827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qudeimat E., Faltusz A. M. C., Wheeler G., Lang D., Brownlee C., Reski R., Frank W. (2008). A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc. Natl. Acad. Sci. U.S.A. 105, 19555–19560 10.1073/pnas.0800864105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. H., Upadhyaya S. K., Cooke J. R., Storti D. W. (1981). Hopf bifurcation in a stomatal oscillator. J. Math. Biol. 12, 1–11 10.1007/BF00275199 [DOI] [Google Scholar]
- Ranf S., Wunnenberg P., Lee J., Becker D., Dunkel M., Hedrich R., Scheel D., Dietrich P. (2008). Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 53, 287–299 10.1111/j.1365-313X.2007.03342.x [DOI] [PubMed] [Google Scholar]
- Rapp P. E. (1987). Why are so many biological systems periodic? Prog. Neurobiol. 29, 261–273 10.1016/0301-0082(87)90023-2 [DOI] [PubMed] [Google Scholar]
- Rapp P. E., Mees A. I., Sparrow C. T. (1981). Frequency encoded biochemical regulation is more accurate than amplitude dependent control. J. Theor. Biol. 90, 531–544 10.1016/0022-5193(81)90304-0 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pozzan T. (2006). Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 10.1152/physrev.00004.2005 [DOI] [PubMed] [Google Scholar]
- Roelfsema M. R. G., Hedrich R. (2010). Making sense out of Ca2+ signals: their role in regulating stomatal movements. Plant Cell Environ. 33, 305–321 10.1111/j.1365-3040.2009.02075.x [DOI] [PubMed] [Google Scholar]
- Romani G., Bonza M. C., Filippini I., Cerana M., Beffagna N., De Michelis M. I. (2004). Involvement of the plasma membrane Ca2+-ATPase in the short-term response of Arabidopsis thaliana cultured cells to oligogalacturonides. Plant Biol. 6, 192–200 10.1055/s-2004-817848 [DOI] [PubMed] [Google Scholar]
- Sanders D., Brownlee C., Harper J. F. (1999). Communicating with calcium. Plant Cell 11, 691–706 10.2307/3870893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiøtt M., Palmgren M. G. (2005). Two plant Ca2+ pumps expressed in stomatal guard cells show opposite expression patterns during cold stress. Physiol. Plant 124, 278–283 10.1111/j.1399-3054.2005.00512.x [DOI] [Google Scholar]
- Schiøtt M., Romanowsky S. M., Bækgaard L., Jakobsen M. K., Palmgren M. G., Harper J. F. (2004). A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. U.S.A. 101, 9502–9507 10.1073/pnas.0401542101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonknecht G., Bauer C. S., Simonis W. (1998). Light-dependent signal transduction and transient changes in cytosolic Ca2+ in a unicellular green alga. J. Exp. Bot. 49, 1–11 10.1093/jexbot/49.318.1 [DOI] [Google Scholar]
- Schönknecht G., Bethmann B. (1998). Cytosolic Ca2+ and H+ buffers in green algae: a comment. Protoplasma 203, 206–209 10.1007/BF01279477 [DOI] [Google Scholar]
- Schulz-Lessdorf B., Hedrich R. (1995). Protons and calcium modulate SV-type channels in the vacuolar- lysosomal compartment – channel interaction with calmodulin inhibitors. Planta 197, 655–671 10.1007/BF00191574 [DOI] [Google Scholar]
- Schwaller B. (2009). The continuing disappearance of “pure” Ca2+ buffers. Cell. Mol. Life Sci. 66, 275–300 10.1007/s00018-008-8564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook J. C., Kronebusch P. J., Borisy C. G., Trewavas A. J., Masson P. H. (1996). Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 111, 243–257 10.1104/pp.111.1.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S., Bækgaard L., Shabala L., Fuglsang A., Babourina O., Palmgren M. G., Cuin T. A., Rengel Z., Nemchinov L. G. (2011a). Plasma membrane Ca2+ transporters mediate virus induced acquired resistance to oxidative stress. Plant Cell Environ. 34, 406–417 10.1111/j.1365-3040.2010.02251.x [DOI] [PubMed] [Google Scholar]
- Shabala S., Bækgaard L., Shabala L., Fuglsang A. T., Cuin T. A., Nemchinov L. G., Palmgren M. G. (2011b). Endomembrane Ca2+-ATPases play a significant role in virus-induced adaptation to oxidative stress. Plant Signal. Behav. 6, 1053–1056 10.4161/psb.6.7.15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S., Cuin T. A., Pottosin I. (2007). Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 581, 1993–1999 10.1016/j.febslet.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Shabala S., Delbourgo R., Newman I. (1997). Observations of bifurcation and chaos in plant physiological responses to light. Aust. J. Plant Physiol. 24, 91–96 10.1071/PP97055 [DOI] [Google Scholar]
- Shabala S., Shabala L., Gradmann D., Chen Z. H., Newman I., Mancuso S. (2006). Oscillations in plant membrane transport: model predictions, experimental validation, and physiological implications. J. Exp. Bot. 57, 171–184 10.1093/jxb/erj022 [DOI] [PubMed] [Google Scholar]
- Shang Z., Ma L., Zhang H., He R., Wang X., Cui S., Sun D. (2005). Ca2+ influx into lily pollen grains through a hyperpolarization-activated Ca2+-permeable channel which can be regulated by extracellular CaM. Plant Cell Physiol. 46, 598–608 10.1093/pcp/pci063 [DOI] [PubMed] [Google Scholar]
- Shigaki T., Hirschi K. (2006). Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol. 8, 419–429 10.1055/s-2006-923950 [DOI] [PubMed] [Google Scholar]
- Shigaki T., Mei H., Marshall J., Li X., Manohar M., Hirschi K. (2010). The expression of the open reading frame of Arabidopsis CAX1, but not its cDNA, confers metal tolerance in yeast. Plant Biol. 12, 935–939 10.1111/j.1438-8677.2010.00368.x [DOI] [PubMed] [Google Scholar]
- Shigaki T., Rees I., Nakhleh L., Hirschi K. (2006). Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J. Mol. Evol. 63, 815–825 10.1007/s00239-006-0048-4 [DOI] [PubMed] [Google Scholar]
- Soldatov N. M. (2003). Ca2+ channel moving tail: link between Ca2+-induced inactivation and Ca2+ signal transduction. Trends Pharmacol. Sci. 24, 167–171 10.1016/S0165-6147(03)00065-8 [DOI] [PubMed] [Google Scholar]
- Spät A., Fülöp L., Koncz P., Szanda G. (2009). When is high Ca2+ microdomain required for mitochondrial Ca2+ uptake? Acta Physiol. (Oxf.) 195, 139–147 10.1111/j.1748-1716.2008.01928.x [DOI] [PubMed] [Google Scholar]
- Staxén I., Pical C., Montgomery L. T., Gray J. E., Hetherington A. M., McAinsh M. R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. U.S.A. 96, 1779–1784 10.1073/pnas.96.4.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki J. W., Somogyi R. (1994). A dialog on Ca2+ oscillations: an attempt to understand the essentials of mechanisms leading to hormone-induced intracellular Ca2+ oscillations in various kinds of cell on a theoretical level. Biochim. Biophys. Acta 1183, 453–472 10.1016/0005-2728(94)90073-6 [DOI] [PubMed] [Google Scholar]
- Sutter J. U., Campanoni P., Tyrrell M., Blatt M. R. (2006). Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18, 935–954 10.1105/tpc.105.038950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Liang F., Hwang I., Curran A. C., Harper J. F. (2000). Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 433–462 10.1146/annurev.arplant.51.1.433 [DOI] [PubMed] [Google Scholar]
- Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244 10.1126/science.1152505 [DOI] [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. (1995). Frequency encoding in excitable systems with application to calcium oscillations. Proc. Natl. Acad. Sci. U.S.A. 92, 7869–7873 10.1073/pnas.92.17.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada L. S. (2006). Specificity in reactive oxidant signaling: think globally, act locally. J. Cell Biol. 174, 615–623 10.1083/jcb.200605036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy F. E., Gilliham M., Dodd A. N., Webb A. A. R., Tester M. (2008). NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ. 31, 1063–1073 10.1111/j.1365-3040.2008.01817.x [DOI] [PubMed] [Google Scholar]
- Trewavas A. (1999). Le calcium, c’est la vie: calcium makes waves. Plant Physiol. 120, 1–6 10.1104/pp.120.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun N. N., Santa-Catarina C., Begum T., Silveira V., Handro W., Floh E. I. S., Scherer G. F. E. (2006). Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47, 346–354 10.1093/pcp/pci252 [DOI] [PubMed] [Google Scholar]
- Tuteja N., Mahajan S. (2007). Calcium signaling network in plants: an overview. Plant Signal. Behav. 2, 79–85 10.4161/psb.2.2.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Schroeder J. I. (1994). Calcium-activated K+ channels and calcium-induced calcium-release by slow vacuolar ion channels in guard-cell vacuoles implicated in the control of stomatal closure. Plant Cell 6, 669–683 10.1105/tpc.6.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P. (2005). Redox active calcium ion channels and cell death. Arch. Biochem. Biophys. 434, 33–42 10.1016/j.abb.2004.08.001 [DOI] [PubMed] [Google Scholar]
- White P. J., Broadley M. R. (2003). Calcium in plants. Ann. Bot. 92, 487–511 10.1093/aob/mcg164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A., Michaelis M. L. (1999). Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+-ATPase. Free Radic. Biol. Med. 27, 810–821 10.1016/S0891-5849(99)00128-8 [DOI] [PubMed] [Google Scholar]
- Zandonadi D. B., Santos M. P., Dobbss L. B., Olivares F. L., Canellas L. P., Binzel M. L., Okorokova-Façanha A. L., Façanha A. R. (2010). Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 231, 1025–1036 10.1007/s00425-010-1106-0 [DOI] [PubMed] [Google Scholar]
- Zappel N. F., Panstruga R. (2008). Heterogeneity and lateral compartmentalization of plant plasma membranes. Curr. Opin. Plant Biol. 11, 632–640 10.1016/j.pbi.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Zhao F., Song C. P., He J., Zhu H. (2007). Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 145, 1061–1072 10.1104/pp.107.105882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Barkla B., Marshall J., Pittman J., Hirschi K. (2008). The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227, 659–669 10.1007/s00425-008-0711-7 [DOI] [PubMed] [Google Scholar]
- Zhao J., Shigaki T., Mei H., Guo Y., Cheng N. H., Hirschi K. D. (2009). Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J. Biol. Chem. 284, 4605–4615 10.1074/jbc.M109.028399 [DOI] [PubMed] [Google Scholar]
- Zielinski R. E. (1998). Calmodulin and calmodulin-binding proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 697–725 10.1146/annurev.arplant.49.1.697 [DOI] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. (1995). Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol. 105, 209–226 10.1085/jgp.105.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]