Abstract
A recent proposal called the Scaffolding Theory of Cognitive Aging (STAC) postulates that functional changes with aging are part of a lifespan process of compensatory cognitive scaffolding that is an attempt to alleviate the cognitive declines associated with aging. Indeed, behavioral studies have shown that aging is associated with both decline as well as preservation of selective cognitive abilities. Similarly, neuroimaging studies have revealed selective changes in the aging brain that reflect neural decline as well as compensatory neural recruitment. While aging is associated with reductions in cortical thickness, white-matter integrity, dopaminergic activity, and functional engagement in posterior brain regions such as the hippocampus and occipital areas, there are compensatory increases in frontal functional engagement that correlate with better behavioral performance in older adults. In this review, we discuss these age-related behavioral and brain findings that support the STAC model of cognitive scaffolding and additionally integrate the findings on neuroplasticity as a compensatory response in the aging brain. As such, we also examine the impact of external experiences in facilitating neuroplasticity in older adults. Finally, having laid the foundation for STAC, we briefly describe a proposed intervention trial (The Synapse Program) designed to evaluate the behavioral and neural impact of engagement in lifestyle activities that facilitates successful cognitive scaffolding using a controlled experiment where older adult participants are randomly assigned to different conditions of engagement.
1. Introduction
There is a large literature that documents clearly and in considerable detail the declines that occur in the cognitive system of humans as they age. The speed at which information is processed (Verhaeghen and Cerella, 2008), the capacity of the working memory system (Braver and West, 2008), the ability to learn and recall new information (Old and Naveh-Benjamin, 2008), the clarity and efficiency of reasoning processes (Berg, 2008) – all of these behaviors show age-related declines in healthy adults. In the past decade or so, neuroscientists have also documented, using advanced imaging techniques, reliable decreases in the volume of many brain structures with age, as well as decreases in neuroreceptors and structural integrity of the white matter (Park and Reuter-Lorenz, 2009; Park and Goh, 2009). In light of this plethora of findings, the puzzle that neuroscientists face, in our view, is not so much why cognitive decline occurs with age, but rather why older adults continue to function quite well in both demanding personal and professional situations in light of the declines in neural structures and cognitive processing efficiency that they are experiencing. Increasingly, there is evidence that one of the reasons that people continue to function at a high level in later adulthood is that the brain has the ability to change and adapt to the process of aging. Functional neuroimaging studies have demonstrated quite clearly that the brain has the capacity to increase the breadth of its function with age. That is, as various insults of aging occur (e.g., shrinkage, white matter lesions, etc.), the brain responds to these changes by expanding its activity or by recruiting additional sites of activation to process the information streaming into the system (Dennis and Cabeza, 2008; Hedden and Gabrieli, 2004; Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Cappell, 2008). This ability to expand function and perhaps even to grow additional tissue in response to extensive usage is the essence of neuroplasticity – the brain's ability to respond and adapt to its changing circumstances.
In this review, we will provide a description of the cognitive and structural brain changes that occur with age, as well as patterns of functional activation. We will then relate these findings to a model of aging and neural adaptation – the Scaffolding Theory of Aging and Cognition (STAC). The STAC model, recently proposed by Park and Reuter-Lorenz, (2009), integrates extant cognitive, structural and functional imaging data and makes some predictions about the brain's plasticity and ability to change. We additionally discuss recent animal and human studies that demonstrate neuroplasticity in the aging brain and consider the implications of these studies for the development of cognitive scaffolds. Finally, we close with a description of an ambitious intervention trial we are presently conducting in our lab (The Synapse Program) that is based on the STAC model. “Synapse” is designed to evaluate the behavioral and neural effects of engaging activities on maintaining and even enhancing the adaptive capacity and function of the older adult brain within a controlled experimental context.
2. Theoretical background
2.1. Behavioral theories of cognitive aging
Behavioral performance in aging is characterized by both decline and preservation. Preservation is evidenced by findings in both longitudinal and cross-sectional studies that verbal knowledge, primarily vocabulary, is intact with age (Hultsch et al., 1998; Park et al., 2002). There is also evidence that implicit, procedural memory is intact with age (Howard et al., 2008; Song et al., 2009), and that aspects of memory that rely more on familiarity than active recollection show minimal age differences (Jennings and Jacoby, 1993; MacDaniel et al., 2008).
Despite evidence for these intact abilities, however, the dominant picture with respect to cognitive aging is one of decline. As adults age, they become slower, and this slowing accounts for the majority of age-related variance on many tasks (Salthouse, 1996). Salthouse, (1996) has argued that age-related slowing is a fundamental mechanism in accounting for age-related decline on many cognitive tasks. An even more fundamental mechanism may be that of dedifferentiation of cognitive processes. Baltes and Lindenberger noticed that along with a general decline in behavioral performance in older adults, there was an increasing relationship with age between sensory abilities and many cognitive functions (Baltes and Lindenberger, 1997; Lindenberger et al., 2001). They reported that auditory and visual perceptual abilities are both strong predictors of other cognitive task performance in very old adults. In contrast, this link between sensory and cognitive function is not present in young adults. These findings suggest that cognitive mechanisms in young adults are more specialized, but they become less specific and selective in older adults. Baltes and Lindenberger proposed that this gradual dedifferentiation of cognitive processes with age stems from a reduction in sensory processing fidelity, constituting a common-cause that results in a significant proportion of the decline in behavioral performance observed in older adults.
Another perspective on cognitive aging declines suggests that age-related decline on a range of cognitive tasks is mediated by deterioration in specific executive processing functions, including working memory and attentional processing. Age-related declines in executive functions include reductions in the ability to select relevant information and to inhibit irrelevant stimuli, decreased ability to perform task switching, or deficits in attentional processing resources (Craik and Byrd, 1982; Hasher and Zacks, 1988; Hasher et al., 2007; Hasher and Zacks, 1979; Zacks et al., 2000). Importantly, the cognitive processes involved in these executive-function accounts typically engage more flexible and individually varied mechanisms.
Despite these declines,there is clear evidence that external guidance on cognitive strategy and environmental support alleviates some age-related declines in cognitive performance, at times increasing performance in old to the level of young adults (Bherer et al., 2005; Castel, 2005; Castel, 2007; Castel et al., 2007; Craik, 2002; Craik, 1977; Dunlosky et al., 2003; Kramer et al., 1999; Kramer et al., 1999; Luo and Craik, 2008; Luo et al., 2007; Madden and Langley, 2003; Meiran et al., 2001; Rendell et al., 2005). For example, when distractors were salient during visual search, older adults were more affected by perceptual load changes than young adults, suggesting poorer inhibition (Madden and Langley, 2003). When the distractor was less salient, however, perceptual load effects were the same in young and older adults. Dual-task training, in which participants practice task switching over extended periods, results in greater improvement for older adults than young adults generally because the latter already perform at a relatively high level (Bherer et al., 2005; Kramer et al., 1999; Kramer et al., 1999; Meiran et al., 2001). Older adults also benefit greatly from instruction and training, such as on the use of mnemonic strategies, for associative learning and other memory tasks (Castel, 2005; Castel et al., 2007; Castel, 2007; Dunlosky et al., 2003; Luo et al., 2007).
2.2. Structural brain changes with aging
Structural studies of the aging brain generally show reductions of brain volume, cortical thickness and white-matter integrity (Davatzikos and Resnick, 2002; Head et al., 2004; Moseley, 2002; Raz et al., 2005; Resnick et al., 2003; Salat et al., 2004; Sullivan and Pfefferbaum, 2006 et al., 2006; Wen and Sachdev, 2004). Raz et al., (2005) obtained longitudinal structural brain images of older adults over a course of five years. They showed both cross-sectional and longitudinal declines in brain volume, with the prefrontal, hippocampal, cau-date, and cerebellar regions showing the greatest reductions, and the entorhinal and visual regions of the brain showing relative stability. Salat et al., (2004) obtained cross-sectional data ranging from young adults in their 20s to older adults in their 90s and measured cortical thickness over the entire cortex. They found that the gray matter of older adults were significantly thinner in the prefrontal cortex, but selective preservation in the parahippocampal and temporal areas. Interestingly, using this measure, they detected significant cortical thinning in the visual regions of older adults as well. To date, however, there is no clear link between reduction in cortical thickness and volume with age and behavioral performance (Raz and Rodrigue, 2006).
Age-related structural decline has also been observed in the white-matter fibers that connect grey-matter regions (Head et al., 2004; Sullivan et al., in press; Sullivan and Pfefferbaum, 2006; Sullivan et al., 2006). For example, using Diffusion Tensor Imaging (DTI), Head et al., (2004) showed deterioration in white-matter diffusivity and anisotropy, suggesting that pathways from one region of the cortex to another are compromised with age. Moreover, these effects were greater in the frontal regions in older adults, the primary site for executive function. Recent studies have also quantified the frequent observation of white-matter hyperintensi-ties in older adults. Wen and Sachdev, (2004) found that normal older adults have more white-matter hyper-intensities than young adults, particularly in the frontal and occipital areas of the brain. There is evidence that such white matter deterioration is related to speed of processing (Bartzokis et al., in press; Sullivan et al., in press) as well as performance on a face perception task (Thomas et al., 2008) and the Trails-Making Test, involving executive processing (O'Sullivan et al., 2001).
2.3. Neurobiological changes in the aging brain
Aging is also associated with the dysregulation of dopaminereceptors in the frontal regions in older adults (Kaasinen et al., 2000). This frontal depletion of dopaminergicreceptors has been hypothesized to cause frontal neural “noise”. Computational modeling of dopamine depletion has been shown to effectively account for and predict much of the observed declines in old adults' behavioral performance in working memory tasks such as the N-back task (Li et al., 2001; Li and Sikström, 2002). This loss of dopamine efficacy in older adults has also been associated with altered reward processing cognitive mechanisms related to the mid-brain regions (Dreher et al., 2008). Interestingly, it has recently been shown that cognitive training increases dopamine receptor binding in frontal and parietal regions of young adults that is in turn associated with improved working memory function (McNab et al., 2009), adding credence to the role of the dopaminergic system in cognitive aging.
2.4. Functional imaging of the aging brain
Thus far, we present a pattern of age-related declines in cognitive function, brain structure and neurobiology. However, the picture of aging looks quite different when functional imaging studies are examined. There is now a huge literature that demonstrates that older adults show greater breadth of activation across frontal sites in the brain compared to young adults when they are performing cognitive tasks (Davis et al., 2008; Dennis and Cabeza, 2008; Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Cappell, 2008). The pattern is observed in many different tasks and takes various forms. One dominant pattern of particular interest in this review, and of relevance to the STAC model, is that of frontal functional bilaterality in older adults.
In young adults, left lateralized frontal activity is typically engaged for tasks that involve verbal working memory, semantic processing, and recognition memory. In older adults, however, while left frontal activity is also observed, there is additional contralateral recruitment in the homologous region of the right hemisphere that was not present in young (Cabeza et al., 1997; Daselaar et al., 2003; Logan et al., 2002; Madden et al., 1999; Reuter-Lorenz et al., 2000; Rosen et al., 2002; Stebbins et al., 2002). Similarly, while young adults engage right lateralized frontal activity with tasks that involve face processing (Grady et al., 1995), spatial working memory (Reuter-Lorenz et al., 2000), and episodic recall (Cabeza et al., 1997), older adults engage both right and left frontal activity during these tasks. This bilateral frontal recruitment in older adults has been replicated in several studies involving many different types of tests and has been described as hemispheric asymmetry reduction in older adults (HAROLD; Cabeza, 2002).
There is increasing evidence that this additional activation in older adults is facilitative of task performance. Studies involving episodic encoding and retrieval additionally reported that when old adults were separated into high-performing and low-performing groups, high-performing older adults engaged more bilateral activity than the low-performing group (Cabeza et al., 2002; Daselaar et al., 2003; Rosen et al., 2003). In Cabeza et al.'s, (2002) study, older adults who had poorer behavioral performance showed similar left lateralized frontal engagementas youngadults, suggesting that lateralized recruitment was no longer sufficient for effective memory processing in older adults. In contrast, older adults who had better memory performance engaged both left and right frontal regions,suggesting a facilitative role of the additional right frontal activity for memory retrieval in older adults. Faster response time in older adults is also associated with increased bilaterality (Reuter-Lorenz et al., 2000) and greater dorsal prefrontal activity (Rypma and D'Esposito, 2000). Stebbins et al., (2002) showed that greater extent of activation in older adults in the same hemisphere as young adults was associated with better performance in a few behavioral neuropsychological measures. More direct evidence for the compensatory role of extra-frontal recruitment is observed from a Transcranial Magnetic Stimulation (TMS) study, which demonstrated that older adults' behavioral performance was greatly contingent on contralateral frontal recruitment whereas younger adults were less affected by inactivation of the contralateral frontal region (Rossi et al., 2004). Patient studies also support the compensatory role of extra-recruitment in that contralateral hemisphere recruitment after damage to one side of the frontal lobes is often associated with better patient outcomes (see Cabeza, 2002 for brief review).
Thus, the functional imaging literature provides clear evidence that older adults show greater breadth of activation than young adults when performing a cognitive task, particularly in the frontal cortex, and that this additional activation facilitates task performance. While it is possible that increased frontal activity may not be compensatory, we believe the vast majority of evidence supports a compensatory view (see Park and Reuter-Lorenz, 2009 for an extended discussion of this). It should also be noted that while we have focused thus far on additional frontal activation with age as facilitative, there are other relevant brain areas where significant age differences in activation patterns are observed. For example, there is additional evidence suggesting that the additional frontal activation in old may be compensating for decreased hippocampal activation (Gutchess et al., 2005; Park and Gutchess, 2005; Park et al., 2003), or decreased specificity of the ventral visual cortex (Park et al., 2004). Aging is also associated with a decreased ability to suppress irrelevant or “default” activity that normally occurs at rest reflected by less me-dial anterior and posterior deactivation in older adults (Persson et al., 2007). Davis et al., (2008) demonstrated that individual differences in default activity in me-dial frontal regions might have a compensatory association with posterior medial activity as well, although there were some methodological differences between these two studies. In addition, age-related differences in parietal regions have also been observed with older adults showing greater and more bilateral parietal recruitment (Davis et al., 2008; Grady et al., 1994).
3. The scaffolding theory of aging and cognition (STAC)
3.1. A lifespan perspective of neuroplasticity
With the overview of behavioral, structural, neuro-biological, and functional findings in mind, we now consider the STAC model. Park and Reuter-Lorenz, (2009) have proposed an integrative theory (STAC) and argue that the behavioral, structural and functional data about aging can be understood within the framework of a compensatory scaffolding model. They suggest that the brain builds protective “scaffolds” in response to the age-related neural insults of brain shrinkage, decreased white matter integrity, and decreased dopamine receptors. Scaffolding is defined as “the recruitment of additional circuitry that shores up declining structures whose function has become noisy, inefficient, or both. (p. 183).” Thus, increased frontal bilaterality with aging is one reflection of this additional recruitment that is in response to the underlying structural declines.
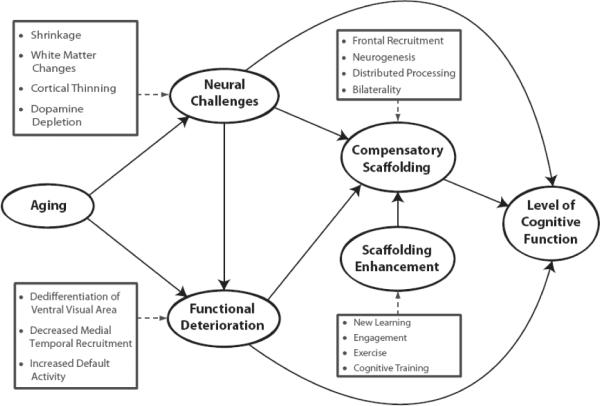
Park and Reuter-Lorenz,(2009) suggest that the scaffolding process is not unique to aging, but rather occurs under conditions of cognitive challenge across the entire lifespan. Thus, when a child or young adult is engaged in active learning, new neural circuitry is recruited and honed for acquisition and performance of the desired task (Petersen et al., 1998). What is unique about scaffolding in older adults is that scaffolding occurs not only under conditions of new learning, but may be invoked even for less novel or practiced behaviors because the existing neural circuitry for performing the task has degraded. These secondary networks may be less efficient than the primary circuitry, but nevertheless result in task performance that is better than could be achieved by using only the primary but degraded network. Park and Reuter-Lorenz, (2009) suggest that the frontal cortex – the most cognitively flexible, strategic component of the brain – is the primary site for cognitive scaffolding. Additional sites for scaffolding may be in the penumbra (surrounding or adjacent regions) of primary activation sites for a given task. The STAC model appears in Fig. 1. As can be seen in the model, the process of aging causes deterioration in functional activation networks, reflected in the process of age-related increases in neural dedifferentiation (Park et al., 2004), decreased medial temporal recruitment (Gutchess et al., 2005; Park and Gutchess, 2005; Park et al., 2003) and the ability to shift efficiently from default activity to task-based activities when under cognitive challenge (Persson et al., 2007). In addition, aging also causes structural deterioration of the brain or “neural challenges,” due to structural shrinkage, decreased white matter integrity, cortical thinning, and depletion of dopamine receptors. As depicted in Fig. 1, the brain responds to this deterioration by engaging in “compensatory scaffolding,” that is, in an effort to maintain cognitive behavior prior to age-related degradation, the brain shows enhanced recruitment of frontal structures, growth and integration of new neural tissue in the hippocampus (see next section), and processing is distributed across more neural sites (which is frequently evidenced in frontal and/or parietal bilaterality). The level of participants' cognitive function is therefore a combination of the mag nitude of the degradation and the effectiveness of the compensatory scaffolding present.
Fig. 1.
Conceptual model of the Scaffolding Theory of Cognitive Aging (STAC), with permission from Park and Reuter-Lorenz, (2009).
A key, moderating factor for the efficiency of compensatory activity, according to the model portrayed in Fig. 1, is the engagement in activities that enhance the ability to engage in scaffolding. The model suggests that individuals will improve their ability to scaffold and develop effective new neural circuitry if they maintain high levels of engagement in novel activities including learning new things, engage in exercise, or possibly participate in cognitive training. Some evidence for this has already been reviewed above regarding the facilitative role of external guidance on behavioral performance in older adults. As such, we now turn to consider and integrate the notion of neuroplasticity, the evidence that it occurs in advanced aging, the impact of external experiences and how it relates to STAC.
3.2. Evidence for neuroplasticity in animals: Neurogenesis
As noted above, the STAC model is predicated on evidence that the aging brain has the ability to adapt and change in response to experiences and environmental demands. There is clear evidence from animal studies that such neuroplasticity in older animals occurs in the form of neurogenesis and synaptic modulation and can result from both increased cognitive and physical engagement (Jessberger and Gage, 2008). We first consider the findings regarding neurogenesis in older animals.
Neurogenesis refers to the proliferation, survival, and differentiation of neural precursor cells into mature neurons or glia that are integrated into the rest of the brain structure (Kempermann et al., 1998). A central site for neurogenesis in adult mammals is in the dentate gyrus of the hippocampus. Although the STAC model postulates that cognitive scaffolding occurs primarily in the frontal regions in older adults, the hippocampus is of particular interest to cognitive aging because most commonly reported behavioral declines in older human adults involves hippocampal-dependent processes such as learning, encoding and retrieving episodic, associative memories, and flexibility in processing (Cohen et al., 1997; Gould et al., 1999; Henke et al., 1999; Hultsch et al., 1998; Kempermann et al., 2004; Park et al., 2002). Moreover, there is tight functional coupling between frontal regions and the hippocampus (Gutchess et al., 2005) such that structural and functional changes in either region are likely to affect the other.
While the rate of neurogenesis is significantly lower in older animals, environmental and experiential factors effectively modulate this decline (Kempermann et al., 2002; Kempermann et al., 1997; Kempermann et al., 1998; Keuker et al., 2003; Kuhn et al., 1996; Nottebohm, 2002). Studies on the hippocampus of senescent mice show that older mice raised in larger cages that allow for social interaction with other mice, cognitive stimulation, and physical exercise (enriched environment) evinced more neurogenesis in the hippocampus compared to mice raised in a standard or impoverished environment where the opportunity for engagement is reduced (Kempermann et al., 2002; Kempermann et al., 1997; Kempermann et al., 1998; Kronenberg et al., 2006; van Praag et al., 1999). A recent study also demonstrated that physical exercise results in both increases in neurogenesis as well as cerebral blood volume in mice dentate gyrus, providing an in vivo brain-imaging index of improvement in cognitive function related to neurogenesis and experience (Pereira et al., 2007).
Interestingly, Kempermann and colleagues suggest that physical exercise and cognitive stimulation act on different stages of neurogenesis in mice (Kempermann, 2008; Kronenberg et al., 2003; Kronenberg et al., 2006; van Praag et al., 1999). Physical exercise increases proliferation of neural precursor cells whereas cognitive stimulation promotes survival of the new precursor cells. If stimulation is halted, or not novel, or lacking in complexity, the new precursor cells may fail to differentiate into mature neurons and may even die, however, the surviving ones may be maintained for further development when stimulation is present later on (Kempermann and Gage, 1999). Kempermann, (2008) further suggests an interesting role of new neurons in tagging temporal information for episodic or associative memories in the hippocampus. He postulated that as newly born hippocampal neurons mature, they form connections with the existing brain network that carry on through time. In this manner, each new neuron may act as a temporal tag for the state of the brain at the time it is born.
3.3. Evidence for neuroplasticity in animals: Synaptic modulation
Synaptic modulation in adult mammals is also altered with aging and likewise modulated by external experiences. Burke and Barnes, (2006) and colleagues suggest that aging results in the dysregulation of neuronal long-term potentiation (LTP), long-term depression (LTD), and other forms of synaptic signaling important for Hebbian learning and memory, specially in the hippocampus (Geinisman et al., 1992; Hattiangady et al., 2005; Nieto-Sampedro and Nieto-Díaz, 2005; Rosenzweig and Barnes, 2003). Compared to young animals, the synaptic connections of aged animals require higher-intensity stimulations to induce LTP. At the same time, LTD is more easily induced at low-intensity stimulations in older than younger animal synapses. These altered hippocampal neuronal learning responses in older rats lead to signal processing infidelity and has been shown to affect the ability to retrieve correct spatial maps resulting in the animal displaying behavior indicative of being “lost” (Rosenzweig and Barnes, 2003). These age-associated changes in the required stimulation intensity for hippocampal function may be an underlying mechanism for increased frontal activity in older adult humans as a form of compensatory processing or scaffolding. That is, frontal increases in older adults may be an effort to boost hippocampal activity through top-down modulation so that LTP can be induced for learning.
A myriad of studies demonstrate that aged animals that were raised in enriched environments show improved synaptic modulation. Experience and training induces neurochemical changes and increases the number of dendritic spines and complexity of dendritic branching, the number and size of synapses and synaptic contact points, cortical volume, neuronal cell body and nuclei size (Bennett et al., 1996; Diamond et al., 1964; Diamond et al., 1967; Globus et al., 1973; Greenough and Volkmar, 1973; Krech et al., 1960; Rosenzweig et al., 1962; Turner and Greenough, 1985; West and Greenough, 1972). Importantly, these changes also occur in older rats (Bennett et al., 1996; Riege, 1971; Rosenzweig et al., 1962; Zolman and Morimoto,1962), indicating that aged animal brains still maintain the capacity for neuroplasticity in response to environmental experiences.
3.4. Evidence for neuroplasticity in humans
Although direct histological and neurochemical evidence in humans is more limited, the general findings also support the influence of enriched environments on promoting neuroplasticity in older adult humans. Neurogenesis in humans has been reported for postmortem data (Eriksson et al., 1998) as well as in an in vitro study (Roy et al., 2000). There is also broad imaging evidence suggesting that healthy aging, under more controlled circumstances where health complications are avoided, is associated with less drastic structural brain declines not only in the hippocampus but for frontal regions as well (Raz and Rodrigue, 2006; Raz et al., 2005; West et al., 1994). Exercise and training is associated with increases in brain volume as well as functional responses in selective regions such as the frontal and parietal cortices (Colcombe and Kramer, 2003; Colcombe et al., 2004; Colcombe et al., 2006). A famous study shows that the hippocampal cortex of London taxi drivers were larger than control subjects, presumably due to their constant experience in navigation (Maguire et al., 2003). A more recent study showed that training with juggling was associated with increased grey matter integrity in area MT/V5 of older adults probably due to the requirement for visual movement monitoring (Boyke et al., 2008). In terms of neurochemistry, Valenzuela et al., (2003) investigated neurotransmitter activity in older adults who were trained to use the method-of-loci to encode lists of words by visualizing and tagging the items to a physical environment. Using magnetic resonance spectroscopy, they found that training was associated with improvements in both memory performance as well as hippocampal neurochemistry activity in older adults.
In summary, there is clear evidence that external experience and training serve to improve brain neuroplasticity. The human findings mirror the animal studies and demonstrate that a) physical exercise and cognitive training has impact on neural structural development in older adults throughout the brain and including the hippocampus, and b) cognitive training increases neural resources and improves function in a more specific manner that is localized to the regions that sub-serve the cognitive processes involved. Consistent with the notion of cognitive scaffolding, is it suggested that the frontal functional increases observed in older adults may be a compensatory response to these neurobiological changes observed in the hippocampus, although this remains to be clearly investigated.
4. The synapse program: A lab/life test of the STAC model
In view of the compelling body of evidence for compensatory frontal engagement and experience-dependent neuroplasticity in older adults, we now discuss a proposed interventiontrial in our lab that is based on the STAC model and that engages older adult participants in an effort to enrich their cognitive development during late adulthood. The Park laboratory at the Center for Brain Health in Dallas, funded by the National Institute on Aging, has developed an ambitious project, entitled “Synapse,” designed to assess many of the tenets of the STAC model. One of the problems with understanding conditions that promote cognitive health in older adults, particularly lifestyle factors, is that it is difficult, if not impossible, to randomly assign individuals to conditions that involve major behavioral changes. Other large-scale studies have shown demographically that lifestyle factors such as education, occupation, and leisure-activities affect cognitive aging. Two well-known examples of such studies are the Seattle Longitudinal Study (http://geron.psu.edu/sls/index.htm) and the Berlin Aging Study (http://www.base-berlin.mpg.de/Introduction.html). In Synapse, we propose to further this work by evaluating the effect of engaging activities on cognitive behavioral performance in older adults using a controlled experimental study and relating them to neural structure and function. Older adults will be randomly assigned to conditions that should promote neural scaffolding and the impact of these various conditions will be assessed. Given that neural structures deteriorate with age, cognitive enrichment should serve to improve the existing scaffolding via compensatory frontal engagement and neuroplasticity and thus improve cognitive function compared to when less or no enrichment is experienced.
To do this, we needed to come up with deeply engaging tasks that would appeal to a broad spectrum of older adults and that would be sufficiently interesting that participants would be willing to work on the tasks 20 hours or more per week for many weeks. Moreover, we needed tasks of sufficient complexity and novelty that subjects could continuously keep learning (building scaffolds) across the weeks and adding to their skills sets. The tasks also needed to be sufficiently fun that people would not drop out once randomized into a condition because they did not enjoy participation. After a lot of thought, we decided to try and engage older adults in learning to quilt or learning digital photography over a period of 14 weeks, with a minimum participation of 15 hours per week. We considered many other tasks including piano playing (rejected because we felt too many people would drop out, realizing that they did not enjoy this), bridge-playing (too competitive with a clear hierarchy of who was good or not good at the task), dancing (confounds exercise with cognitive engagement and has potential for falling and injury). Once we settled on the tasks, we developed a detailed cognitive, neuroimaging, and psychosocial battery to assess subjects prior to enrollment, and then this battery is used again at the end of 12 weeks of participation to see whether we have changed cognitive function, brain structure, or patterns of neural recruitment. In order to enroll in Synapse, volunteers had to agree to be randomized into all of six conditions, with participation of 15 hours a week or more in all conditions, except the wait-list control. These conditions include the following:
Quilting Engagement Group: Novice subjects learn all aspects of quilting under the direction of a master quilting instructor.
Digital Photography Group: Novice participants learn all aspects of photocomposition and photo processing, using PhotoShop, under the direction of a master photographer.
Quilting/Photography Group: Participants spend the first six weeks learning photography and the second six weeks learning quilting (counterbalanced across groups).
Social Control: Participants engage in many social activities, game playing, and field trips, but do not actively acquire new skills, under the direction of a social facilitator.
Placebo Control: Participants work at home on tasks that are engaging but have relatively little likelihood of inducing scaffolding, and include things like listening to classical music and National Public Radio (NPR), playing simple computer games of chance, watching movies, and reading non-challenging materials that contains information that they likely already know, such as prominent current events.
No Treatment Control: This group involves participants who agreed to be randomized to all conditions, but do not receive any treatment except pre and post-testing.
In summary, the design of the Synapse study allows us to address the following hypotheses:
-
(a)
Cognitive engagement improves cognition. The three quilting/photography engagement groups will each show more positive change on cognitive constructs compared to the No Treatment Control and the Placebo Control.
-
(b)
Social engagement is just as effective as cognitive engagement. We will contrast the cognitive engagement conditions with the social engagement condition.
-
(c)
What is the effect of general socializing with no new skills learned? There will be a small, significant effect associated with participation in the social engagement group relative to the no treatment control.
-
(d)
What role do beliefs of change play in cognitive intervention effects? We do not expect that a belief that one will improve will impact performance and predict the Placebo Control will perform like the No Treatment Control.
-
(e)
Are there differences in facilitation as a function of a greater breadth of new activities? The scaffolding model predicts that there would be and we believe that the quilting/photography combination condition will show the greatest impact, if any of the three cognitive engagement groups differ from one another.
-
(f)
We hypothesize that effects may be moderated by personality variables or self-reported levels of lifetime engagement. For example, subjects high in neuroticism may have sustained more hippocampal deterioration with age than subjects low in neuroticism, due to increased cortisol, a substance damaging to the hippocampus (Sapolsky, 2001), and may respond more strongly to the intervention. Alternatively, subjects who are conscientious may be more responsive to the engagement manipulations. We also hypothesize that subjects high in lifetime engagement or in engagement at the time of enrollment may respond less to the manipulation than subjects low in engagement, as it will be more stimulating to low engagement subjects. A similar effect might be predicted for subjects with a low need for cognition, as the manipulation may prove to be more stimulating for subjects who have generally sought less stimulation.
The Synapse study is being conducted as a blind clinical trial, so the results will not be known until early 2011. Thus far, participants have been extraordinarily enthusiastic about the project and dropout has been minimal. The Synapse study provides a strong experimental test of the role of lifestyle manipulations on cognitive abilities and neural structure and function. We are particularly interested in following up Synapse participants in the future, a year or more after they complete the project, as it may be that the effects of stimulation may be even more pronounced at later dates than immediately at the close of the trial.
5. Conclusion
There has been tremendous evolution in our view of the aging mind and brain. The advent of neuroimaging has resulted in scientist's recognition that the brain is much more active and dynamic than was previously suspected from basic behavioral data that showed relentless decline across many different domains. The STAC model posits that the brain adapts and reorganizes in response to both the neural insults associated with aging, but also in response to stimulating activities and new learning. Behavioral and brain imaging findings, as well as studies on neuroplasticity provide converging evidence about the adaptive brain and the role of external engagement. The Synapse project is an attempt to experimentally test these new views of the aging mind. There is a need for much more research on the role of a lifestyle of active engagement and its impact on cognitive health. Moreover, it is of particular importance to explore the possible interactive relationship between cognitive stimulation and physical exercise, as there could be a synergistic or potentiating relationship between the two. Determining how to protect the mind from serious age-related declines in late adulthood is one of the most critical public health issues of the 21st century. The growing evidence that there is plasticity in even the aged neural system adds to the hope that both pharmacologic and behavioral solutions will be available to slow down the rate of cognitive aging.
Acknowledgments
The Synapse program proposed in this article is funded by the NIA grant no. 5R01AG026589 awarded to Denise Park.
References
- Baltes P, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.08.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. J Neuropsychiatry Clin Neurosci. 1996;8(4):459–470. doi: 10.1176/jnp.8.4.459. [DOI] [PubMed] [Google Scholar]
- Berg C. Everyday problem solving in context. In: Hofer S, Alwin DF, editors. Handbook of Cognitive Aging: Interdisciplinary Perspectives. SAGE Publications; Los Angeles, CA: 2008. pp. 207–223. [Google Scholar]
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Training effects on dual-task performance: Are there age-related differences in plasticity of attentional control? Psychol Aging. 2005;20(4):695–709. doi: 10.1037/0882-7974.20.4.695. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, West R. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3rd ed. Psychology Press; New York, USA: 2008. pp. 311–372. [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nature Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J Neurosci. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD. Memory for grocery prices in younger and older adults: The role of schematic support. Psychol Aging. 2005;20(4):718–721. doi: 10.1037/0882-7974.20.4.718. [DOI] [PubMed] [Google Scholar]
- Castel AD. Aging and memory for numerical information: The role of specificity and expertise in associative memory. J Gerontol B Psychol Sci Soc Sci. 2007;62(3):194–196. doi: 10.1093/geronb/62.3.p194. [DOI] [PubMed] [Google Scholar]
- Castel AD, Farb NAS, Craik FIM. Memory for general and specific value information in younger and older adults: Measuring the limits of strategic control. Mem Cognition. 2007;35(4):689–700. doi: 10.3758/bf03193307. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5(1):131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Human memory and aging. In: Bäckman L, von Hofsten C, editors. Psychology at the Turn of the Millennium. Psychology Press; Hove, U.K.: 2002. pp. 261–280. [Google Scholar]
- Craik FIM. Age differences in human memory. In: Birren JE, Schaie KW, editors. The Handbook of Psychology of Aging. Van Nostrand Reinhold; New York, USA: 1977. pp. 384–420. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FI, Trehub S, editors. Aging and Cognitive Processes. Plenum; New York, USA: 1982. pp. 191–211. [Google Scholar]
- Daselaar S, Veltman D, Rombouts S, Raaijmakers J, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126(1):43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cerebral Cortex. 2002;12(7):767–771. doi: 10.1093/cercor/12.7.767. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior Anterior Shift in Aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3rd ed. Psychology Press; New York, USA: 2008. pp. 1–54. [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Lindner B, Raymond A. Extensive cortical depth measurements and neuron size increases in the cortex of environmentally enriched rats. J Comp Neurol. 1967;131(3):357–364. [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci USA. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlosky J, Kubat-Silman AK, Hertzog C. Training monitoring skills improves older adults' self-paced associative learning. Psychol Aging. 2003;18(2):340–345. doi: 10.1037/0882-7974.18.2.340. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82(2):175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: A possible role in learning. Trends Cogn Sci. 1999;3(5):186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14(3):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40(2):491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks R. Inhibitory mechanisms and the control of attention. In: Conway ARA, Jarrold C, Kane MJ, Towse JN, editors. Variation in Working Memory. Oxford University Press; US: 2007. pp. 227–249. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. Psychol Learn Motiv. 1988;22:193–225. [Google Scholar]
- Hasher L, Zacks RT. Automatic and efforful processes in memory. J Exp Psychol Gen. 1979;108(3):356–388. [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci USA. 1999;96(10):5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Dennis NA, LaVine S, Valentino K. Aging and implicit learning of an invariant association. J Gerontol B Psychol Sci Soc Sci. 2008;63(2):100–105. doi: 10.1093/geronb/63.2.p100. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory Change in the Aged. Cambridge University Press; New York, USA: 1998. [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychol Aging. 1993;8(2):283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23(4):684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nägren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21(5):683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52(2):135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31(4):163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14(2):186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: Effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9(3):321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Keuker JIH, Luiten PGM, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Larish J, Weber TA, Bardell L. Training for executive control: Task coordination strategies and aging. Attention and Performance. 1999;XVII:617–652. [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol. 1999;101(2–3):339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J Comp Physiol Psychol. 1960;53:509–519. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467(4):455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends Cogn Sci. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Li SC, Sikström S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002;26(7):795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging. 2001;16(2):196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Luo L, Craik FIM. Aging and memory: A cognitive approach. Can J Psychiatry. 2008;53(6):346–353. doi: 10.1177/070674370805300603. [DOI] [PubMed] [Google Scholar]
- Luo L, Hendriks T, Craik FI. Age differences in recollection: Three patterns of enhanced encoding. Psychol Aging. 2007;22(2):269–280. doi: 10.1037/0882-7974.22.2.269. [DOI] [PubMed] [Google Scholar]
- MacDaniel MA, Einstein GO, Jacoby LL. New considerations in aging and memory: The glass may be half full. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3rd ed. Psychology Press; New York, USA: 2008. pp. 251–310. [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7(2):115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Langley LK. Age-related changes in selective attention and perceptual load during visual search. Psychol Aging. 2003;18(1):54–67. doi: 10.1037/0882-7974.18.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RSJ, Burgess N. Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus. 2003;13(2):250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. J Gerontol B Psychol Sci Soc Sci. 2001;56(2):88–102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15(7–8):553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M, Nieto-Díaz M. Neural plasticity: Changes with age. J Neural Transm. 2005;112(1):3–27. doi: 10.1007/s00702-004-0146-7. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57(6):737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychol Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical disconnection as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess A. Long-term memory and aging: A cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; New York, USA: 2005. pp. 218–245. [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101(35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Goh JOS. Successful aging. In: Cacioppo J, Berntson G, editors. Handbook of Neuroscience for the Behavioral Sciences. John Wiley & Sons; Hoboken, NJ: 2009. [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Ann Rev Psychol. 2009;60(1):173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, et al. Working memory for complex scenes: Age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15(8):1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? J Cogn Neurosci. 2007;19(6):1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell P, Castel A, Craik F. Memory for proper names in old age: A disproportionate impairment? Q J Exp Psychol A. 2005;58(1):54–71. doi: 10.1080/02724980443000188. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17(3):177–182. [Google Scholar]
- Riege WH. Environmental influences on brain and behavior of year-old rats. Dev Psychobiol. 1971;4(2):157–167. doi: 10.1002/dev.420040207. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13(18):2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O'Hara R, Friedman L, et al. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav Neurosci. 2003;117(6):1150–1160. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of pre-frontal cortex in long-term memory: A repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24(36):7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6(3):271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3(5):509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98(22):12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Marks B, Howard JH, Howard DV. Evidence for parallel explicit and implicit sequence learning systems in older adults. Behav Brain Res. 2009;196(2):328–332. doi: 10.1016/j.bbr.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, et al. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17(1):44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.007. in press. Epub ahead of print, retrieved August 31 2009 from http://www.ncbi.nlm.nih.gov/pubmed/18495300. [DOI] [PMC free article] [PubMed]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16(7):1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, et al. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20(2):268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329(1–2):195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, et al. Memory training alters hippocampal neuro-chemistry in healthy elderly. Neuroreport. 2003;14(10):1333–1337. doi: 10.1097/01.wnr.0000077548.91466.05. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Everything we know about aging and response times: A meta-analytic integration. In: Hofer S, Alwin DF, editors. Handbook of Cognitive Aging: Interdisciplinary Perspectives. SAGE Publications; Los Angeles, CA: 2008. pp. 134–150. [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60-to 64-year-old individuals. NeuroImage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and alzheimer's disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- West RW, Greenough WT. Effect of environmental complexity on cortical synapses of rats: Preliminary results. Behav Biol. 1972;7(2):279–284. doi: 10.1016/s0091-6773(72)80207-4. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2nd ed. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 293–357. [Google Scholar]
- Zolman JF, Morimoto H. Effects of age of training on cholinesterase activity in the brains of maze-bright rats. J Comp Physiol Psychol. 1962;55:794–800. [Google Scholar]