Abstract
Background/Aim
To describe a case of invasive orbital aspergillosis and evaluate treatments and outcomes.
Methods
A case report and review of orbital aspergillosis treatment with voriconazole in the English language literature.
Conclusion
Amphotericin B with debridement is the current standard of care for orbital aspergillosis; however, its prognosis is unfavorable. When compared to amphotericin B, voriconazole demonstrates a survival benefit, has less systemic toxicity, and is better tolerated by patients. While a prospective trial comparing amphotericin B to voriconazole in orbital aspergillosis is not feasible, there is evidence to support the use of voriconazole as primary therapy.
Key words: Orbital aspergillosis, Orbital apex syndrome, Voriconazole, Aspergillus fumigatus
Background
Orbital aspergillosis is a rare and often fatal condition in healthy patients. Due to its vague initial symptomatology, it is often misdiagnosed and improperly treated which, in turn, facilitates disease progression. Once proper treatment is initiated, the therapy is often poorly tolerated and the mortality rate remains high.
In this case report, we will discuss a patient whose symptoms began in July 2010 and follow the historical, clinical, radiographic, and histological course until the diagnosis of invasive orbital aspergillosis was made in November 2010. We will then discuss treatment options presented to this patient and summarize treatment options in the context of the available literature.
Case Report
A 68-year-old white man with a past medical history of glaucoma with remote bilateral trabeculectomies, hypertension, depression, and sinusitis presented with a 1-month history of headaches and pain around his right eye. The patient described the pain as a constant ache behind his right eye which, over the course of the month, had intensified to become a sharp, stabbing pain. During the third week, he began to have blurred vision and ptosis of the right lid. He sought treatment at his local hospital where he was admitted and had a head CT, a head MRI, and bilateral temporal artery biopsies. No pathology was seen on imaging, and the temporal artery biopsies were negative. As a result, the patient was presumptively diagnosed with giant cell arteritis (GCA) and treated with corticosteroids. He was subsequently discharged.
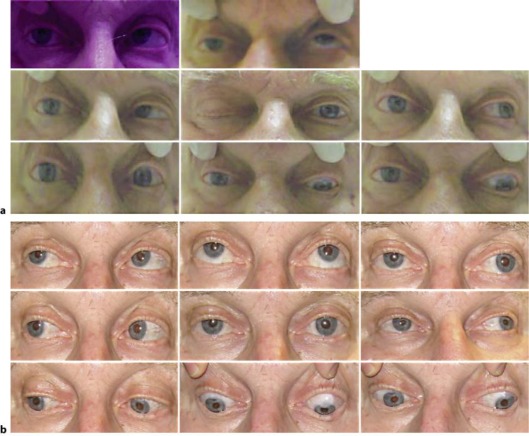
The patient presented in our neuro-ophthalmology clinic 4 weeks after the initial onset of his symptoms. At this time, the patient's medications consisted of Amlodipine, Valsartan, Paroxetine, Omeprazole, and Oxycodone. His best-corrected visual acuity was 20/60 OD and 20/25 OS; his color vision tested with Ishihara plates was 1/11 OD and 11/11 OS. On examination, he had a trace right relative afferent pupillary defect, complete ptosis of the right lid, and a frozen right eye due to paralysis of cranial nerves III, IV, and VI (fig. 1a). The combination of the visual and clinical findings led to the diagnosis of orbital apex syndrome. His trabeculectomy blebs were moderately elevated and avascular bilaterally. Fundus examination demonstrated pink optic nerves with a cup/disc ratio of 0.8 OD and 0.9 OS. The examination was otherwise unremarkable.
Fig. 1.
a Extraocular movements prior to treatment, demonstrating an orbital apex syndrome. b Eye movements after 2 weeks of voriconazole therapy.
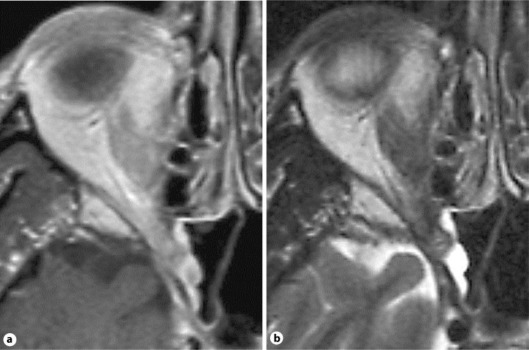
The patient was immediately admitted to the hospital for further workup. We held corticosteroids due to the suspicion of an infectious etiology. Laboratory testing revealed a C-reactive protein level of 7.9 mg/l, erythrocyte sedimentation rate of 46 mm/h, and white blood cell count of 6,900/µl with 93% neutrophils. An MRI and CT of the head were ordered which demonstrated an infiltrative process centered in the right pterygopalatine fossa and involving the soft tissues of the nasal posterolateral recess, right orbital apex, right sphenoid sinus, and dura adjacent to the clinoid process (fig. 2). The infiltrative process was suggestive of a lymphomatous rather than fungal etiology.
Fig. 2.
MRI T1- (a) and T2-weighted (b) images from our patient, demonstrating an infiltrative process centered in the right pterygopalatine fossa and involving the adjacent soft tissues of the nasal posterolateral recess, right orbital apex, right sphenoid sinus, and dura of the clinoid process.
The Department of Otolaryngology was consulted, and a right trans-sphenoid orbital biopsy was performed. Preliminary pathology from the biopsy demonstrated an infiltrate consisting of a mixture of granulocytes, giant cells, and small mature lymphocytes without evidence of infection. The slides were considered suggestive of a nonspecific inflammatory etiology, such as Tolosa-Hunt syndrome, and were sent for further staining. The patient was discharged on prednisone 80 mg/day (1 mg/kg/day). The following morning, a pathological examination revealed numerous septate hyphae branching at 45° angles consistent with Aspergillus. This finding was later confirmed by a culture positive for Aspergillus fumigatus. The patient was immediately notified and readmitted.
Upon admission, the patient's best-corrected visual acuity had deteriorated to 20/100 OD, and his right eye remained frozen. According to recommendations provided by the Division of Infectious Diseases, he was started on voriconazole 4 mg/kg i.v. The patient's ophthalmoplegia and visual acuity improved dramatically within 72 h, and after 6 days of therapy the patient's visual acuity was 20/40 OD. There was resolution of his relative afferent pupillary defect and color vision deficits. He also showed a marked improvement in motility (fig. 1b). He was discharged on voriconazole 350 mg p.o. b.i.d., with close follow-up.
After 6 weeks of treatment, the patient returned to our clinic with full extraocular movements, full color vision, and a visual acuity of 20/30 OD. An interval MRI of the orbits demonstrated an overall improvement. During this visit, the patient complained of a new rash on his trunk and upper extremities. The Divisions of Infectious Diseases and Dermatology were consulted, and it was found that the patient had developed a drug-induced photosensitivity rash secondary to voriconazole. His therapy was changed to daily intravenous micafungin 150 mg, which he has tolerated well for the last month.
Discussion
Orbital aspergillosis is a rare infection in healthy individuals. Aspergillus is a ubiquitous species that resides in the soil. Approximately 5.7 × 107 Aspergillus spores are inhaled daily by the average American [1]. The best-established risk factor for orbital aspergillosis is sinusitis of a paraorbital sinus; however, there is no consensus on which loci confer the greatest risk [1 3]. Other risk factors include trauma, facial surgery, high-exposure professions (demolition, yard work, or working in endemic areas of Aspergillus flavus such as the Sudan), and immunocompromised states including AIDS, absolute neutrophil count <1,000, leukemia, and diabetes mellitus.
The two most common pathogens in humans are A. flavus and A. fumigatus. In the orbit, A. flavus causes an invasive granulomatous infection, whereas A. fumigatus may present as a noninvasive or an invasive necrotizing-fulminate infection. Noninvasive orbital aspergillosis is typically found in diabetics, corticosteroid users, and immunocompetent individuals. There is typically a sparse immune response resulting in a slow indolent course. Patients often present with a chronic, dull retrobulbar pain present for months, headache, red eyes, and proptosis. Over time, these symptoms may progress to include sharp pain, cranial nerve palsies, amaurosis, and orbital apex syndrome. These symptoms can be temporarily ameliorated with steroids. As the disease progresses, Aspergillus may invade the vasculature resulting in necrotizing angiitis, mycotic aneurysms, dissemination, and thrombosis of the cavernous sinus, leading to mortality within days [2, 4]. As a result, aggressive early treatment of noninvasive infections is paramount.
Invasive orbital aspergillosis presents similarly but with a more rapid progression and is uniformly fatal if untreated. Invasive aspergillosis typically manifests in immunocompromised individuals and is characterized by a granulomatous or fulminate immune response with a centrifugal invasion of adjacent tissues, vasculature, and bone. Invasive orbital aspergillosis may result in orbital apex syndrome, necrotizing angiitis, mycotic aneurysms, thrombosis, systemic dissemination, erosion of the orbit, and direct invasion of the central nervous system. When the disease is limited to the orbit, there is a 28% mortality rate for patients receiving standard treatment with amphotericin B [5]. However, once orbital apex syndrome has manifested, the mortality rate increases to 70–80% despite standard treatment, and if the central nervous system is involved, the mortality rate increases to 80–100% [4, 5, 6, 7].
The diagnosis of orbital aspergillosis is difficult to confirm and is often delayed 2–10 months from the initial presentation of symptoms [1]. Orbital aspergillosis should be suspected whenever a patient presents with painful ophthalmalgia. However, since its initial symptoms are vague, similar to more prevalent conditions, and respond to steroids, aspergillosis is often misdiagnosed as orbital pseudotumor or GCA (table 1). Many patients, including ours, are presumptively started on corticosteroids and demonstrate a subsequent improvement resulting in a premature conclusion to the workup. Steroids decrease the inflammation in the orbit and transiently improve symptoms but may hasten the course of aspergillosis.
Table 1.
Differential diagnosis
| Inflammatory | Idiopathic orbital inflammation |
| Tolosa-Hunt syndrome | |
| Sarcoidosis | |
| Infectious | Extension of bacterial sinusitis |
| Tuberculosis | |
| Histoplasmosis | |
| Coccidiomycosis | |
| Other fungi (Mucor, Fusarium) | |
| Vasculitis | Giant cell arteritis |
| Wegener's arteritis | |
| Neoplastic | B cell lymphoma |
| Primary orbital tumors | |
| Neuro-ophthalmic | Optic neuritis |
When a diagnosis of orbital aspergillosis is suspected, an MRI of the orbit is indicated. MRI is useful for evaluating the optic nerve, cerebral dura, paraorbital sinuses, and masses. Any sinusitis (>8-mm thickening of the sinus mucosa) with an enhancement of the optic nerve or erosion of the orbital bones should prompt suspicion for orbital aspergillosis. A CT of the orbit without contrast may aid in the diagnosis by screening for bony erosion and examining for intraluminal calcification, which is pathognomonic. To confirm the diagnosis, a biopsy is required. Fungal cultures of the nares have been shown to be unreliable and should not be used for screening [2]. If imaging reveals a paraorbital sinusitis, an anterior rhinoscopy as well as a biopsy may be performed. While anterior rhinoscopy is the least invasive approach, 50% of cases require a second biopsy [1, 8]. Alternative approaches include orbital fine needle aspiration or trans-sinus orbital biopsy; these approaches have a higher yield but are more invasive than anterior rhinoscopy. The tissue should be sent for both frozen and permanent sections to look for fungi as well as other pathology. In addition, slides should be sent for periodic acid-Schiff stain and Gomori methenamine silver stain to look for haemotoxophilic organisms with 45° branching septate hyphae 2–4 µm in width. Cultures should be incubated for at least 30 days. If pathological testing is initially negative but a high suspicion of aspergillosis is maintained, corticosteroids should be held until cultures are known and empiric treatment for aspergillosis should be initiated.
Treatment for orbital aspergillosis in the literature is based upon previous case reports. There are no prospective studies evaluating the efficacy of treatments in orbital aspergillosis. The current mainstay of therapy for orbital aspergillosis is amphotericin B i.v. 0.5 mg/kg/day with debridement of focal abscesses [1, 2, 4]. In total, 40–60% of patients show a response to amphotericin B, including liposomal preparations [1]. Despite treatment with amphotericin B, once the infection has manifested as an orbital apex syndrome, the mortality rate remains 70–80% [1, 2, 4, 5, 6]. Due to a high rate of relapse, prolonged treatment for 2 years to life is suggested [1, 2]. Amphotericin B has numerous side effects, including irreversible nephrotoxicity, and as a result is often poorly tolerated. There is a case report of a patient who died as a direct result of amphotericin B therapy for invasive orbital aspergillosis [8].
Alternative therapies in the literature include itraconazole 200–400 mg/day. Itraconazole is better tolerated than amphotericin B, but has similar response, relapse, and mortality rates [1, 4]. This drug should not be used if there is pulmonary involvement because a higher mortality has been demonstrated in these cases. Concomitant induction of amphotericin B and itraconazole may increase response, and maintenance with itraconazole alone is significantly better tolerated than with amphotericin B [4]. Fluconazole 100–200 mg/kg/day and rifampin 600 mg/day have also been shown to be effective, but there are too few reports to evaluate their role in therapy [4, 9]. Ketoconazole on the other hand has been shown to be ineffective in treating orbital aspergillosis [4].
Voriconazole is a second-generation triazole antifungal that has become the usual treatment of extraorbital invasive aspergillosis after demonstrating a 22% survival benefit over amphotericin B in a comparative evaluation [10, 11]. Voriconazole 6 mg/kg for 1 day, then 4 mg/kg/day, has less systemic side effects than amphotericin B, favorable bone penetration, and is better tolerated for maintenance therapy [10, 11, 12]. Despite its favorable profile, we could only find one case report in the English literature and two abstracts in the Japanese literature on voriconazole treatment of invasive orbital aspergillosis [10, 13, 14]. In each of these case reports, the patients demonstrated a dramatic improvement within several days of voriconazole therapy.
In the English literature, Sasindran et al. [10] presented a case of an 8-year-old boy who developed an orbital apex syndrome after a trauma. He was initially treated with steroids; however, his symptoms worsened. A biopsy was obtained revealing A. flavus infection. He was successfully treated with 150 mg voriconazole b.i.d. for 12 months. The Japanese literature presented 2 cases of patients in their 70s being successfully treated for orbital aspergillosis, but the treatment protocols were not discussed in the abstracts [13, 14].
Conclusion
Despite being the current standard of treatment for invasive orbital aspergillosis, amphotericin B preparations offer an unfavorable prognosis. With optimized amphotericin B therapy, the mortality rate in orbital apex syndrome patients remains 70–80%. Additionally, only 40–60% of patients with orbital aspergillosis will respond to treatment with amphotericin B, and patients poorly tolerate prolonged therapy. Most patients develop serious complications such as nephrotoxicity, and 1 patient is reported to have died as a direct result of the therapy [8].
While the role of voriconazole as the mainstay of therapy for extraorbital invasive aspergillosis is well defined, its role in orbital aspergillosis has not yet been documented. The use of voriconazole seems intuitive for orbital aspergillosis since it confers a survival benefit, provides better bone penetration, and is better tolerated than amphotericin B preparations in extraorbital invasive aspergillosis. The efficacy of voriconazole has been demonstrated in our patient and the 3 above-mentioned case reports found in the literature. Each patient reported a quick response, dramatic symptom improvement, and no relapses. Out of the 4 patients, only our patient reported a side effect (photosensitive drug rash) due to voriconazole.
Our patient's risk factors for orbital aspergillosis included type 2 diabetes and a history of previous sinusitis. These risk factors are usually associated with noninvasive orbital aspergillosis rather than with the invasive disease as confirmed by MRI in our case. Our patient may initially have had a noninvasive orbital aspergillosis that was exacerbated by the use of corticosteroids for the treatment of probable GCA. The reversible immunosuppression conferred by the steroids may explain the rapid invasive progression and the favorable response to voriconazole.
While 4 patients do not demonstrate a statistical benefit, voriconazole can be considered an efficacious alternative to amphotericin B for the treatment of orbital aspergillosis that is better tolerated by patients.
Disclosure Statement
All authors are affiliated with University of Florida and have no other disclosures.
References
- 1.Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, Rootman J. Localized invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol. 2004;88:681–687. doi: 10.1136/bjo.2003.021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard D, deShazo R, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337:254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 3.Bray WH, Giangiacomo J, Ide CH. Orbital apex syndrome. Surv Ophthalmol. 1987;32:136–140. doi: 10.1016/0039-6257(87)90106-8. [DOI] [PubMed] [Google Scholar]
- 4.Levin LA, Avery R, Shore JW, Woog JJ, Baker AS. The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol. 1996;41:142–154. doi: 10.1016/s0039-6257(96)80004-x. [DOI] [PubMed] [Google Scholar]
- 5.Heidegger S, Mattfeldt T, Rieber A, Wikstroem M, Kern P, Kern W, Schreiber H. Orbito-sphenoidal Aspergillus infection mimicking cluster headache: a case report. Cephalalgia. 1997;17:676–679. doi: 10.1046/j.1468-2982.1997.1706676.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo T, Notohara K, Yamadori I. Aspergillosis causing bilateral optic neuritis and later orbital apex syndrome. Jpn J Ophthalmol. 2005;49:423–433. doi: 10.1007/s10384-004-0217-1. [DOI] [PubMed] [Google Scholar]
- 7.Turgut M, Ozsunar Y, Oncü S, Akyüz O, Ertugrul MB, Tekin C, Gültekin B, Sakarya S. Invasive fungal granuloma of the brain caused by Aspergillus fumigatus: a case report and review of the literature. Surg Neurol. 2008;69:169–174. doi: 10.1016/j.surneu.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes YB, Ramina R, Borges G, Queiroz LS, Maldaun MV, Maciel JA., Jr Orbital apex syndrome due to aspergillosis. Arq Neuropsiquiatr. 2001;59:806–808. doi: 10.1590/s0004-282x2001000500029. [DOI] [PubMed] [Google Scholar]
- 9.Rowed DW, Kassel EE, Lewis AJ. Transorbital intracavernous needle biopsy in painful ophthalmoplegia. J Neurosurg. 1985;62:776–780. doi: 10.3171/jns.1985.62.5.0776. [DOI] [PubMed] [Google Scholar]
- 10.Sasindran V, Ravikumar A Senthil. Orbital apex syndrome in a child. Indian J Otolaryngol Head Neck Surg. 2008;60:62–65. doi: 10.1007/s12070-008-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B, Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 12.Parize P, Chandesris MO, Lanternier F, Poirée S, Viard JP, Bienvenu B, Mimoun M, Méchai F, Mamzer MF, Herman P, Bougnoux ME, Lecuit M, Lortholary O. Antifungal therapy of Aspergillus invasive otitis externa: efficacy of voriconazole and review. Antimicrob Agents Chemother. 2009;53:1048–1053. doi: 10.1128/AAC.01220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugai A, Oyake M, Umeda M, Umeda Y, Fujita N. Case of orbital apex syndrome caused by invasive aspergillosis successfully treated during the diagnostic procedure by the use of voriconazole (in Japanese) Rinsho Shinkeigaku. 2008;48:746–749. doi: 10.5692/clinicalneurol.48.746. [DOI] [PubMed] [Google Scholar]
- 14.Kuga A, Oishi K, Ishida H, Kanda F. A case of orbital apex syndrome caused by localized invasive aspergillosis successfully treated with voriconazole (in Japanese) Rinsho Shinkeigaku. 2007;47:207–210. [PubMed] [Google Scholar]