Abstract
Inflammatory myofibroblastic tumor (IMFT) of the urinary bladder is an unusual spindle cell lesion that exhibits cytologic atypia, infiltrative growth, and mitotic activity mimicking malignant tumors, such as leiomyosarcoma, rhabdomyosarcoma, and sarcomatoid carcinoma. Recently, anaplastic lymphoma kinase (ALK) gene translocations or ALK protein expression in IMFT has been reported, especially in patients of children and young adults. This lesion has been described in numerous locations in addition to the urinary bladder. The detection of ALK protein and ALK gene rearrangements are useful in distinguishing IMFT from spindle cell malignancies in the urinary bladder.
Keywords: Anaplastic lymphoma kinase-1, inflammatory myofibroblastic tumor, sarcomatoid urothelial carcinoma, urinary bladder
INTRODUCTION
Inflammatory myofibroblastic tumor (IMFT) of the urinary bladder is a very uncommon spindle cell tumor, which has undetermined malignant potential. We report a case of IMFT arising from urinary bladder in a young adult female and discuss its clinicoradiologic presentation, histopathologic and immunohistochemical diagnostic criteria, differential diagnosis, behavior, and management.
CASE REPORT
A 27-year-old female presented with painless hematuria, clots in urine, burning micturition, and weakness since 20 days. There was no past family history or tuberculosis. Urine cytology did not suggest a malignancy. The patient underwent cystoscopy, which revealed an anterior wall bladder growth. Abdominal computed tomography (CT) revealed blood clots.
Pathological findings
Gross feature
Transurethral resection of bladder tumor with partial cystectomy as an emergent operation was done due to perforation in bladder wall and infiltration of muscular layer. The cystectomy specimen measuring 8 × 8 × 5 cm along with multiple soft tissue bladder tumor chips measuring 4 × 2 × 2 cm. Outer surface is congested and showed a polypoidal growth in the bladder lumen measuring 6 × 5 cm [Figure 1] and grossly infiltrating the muscularis.
Figure 1.
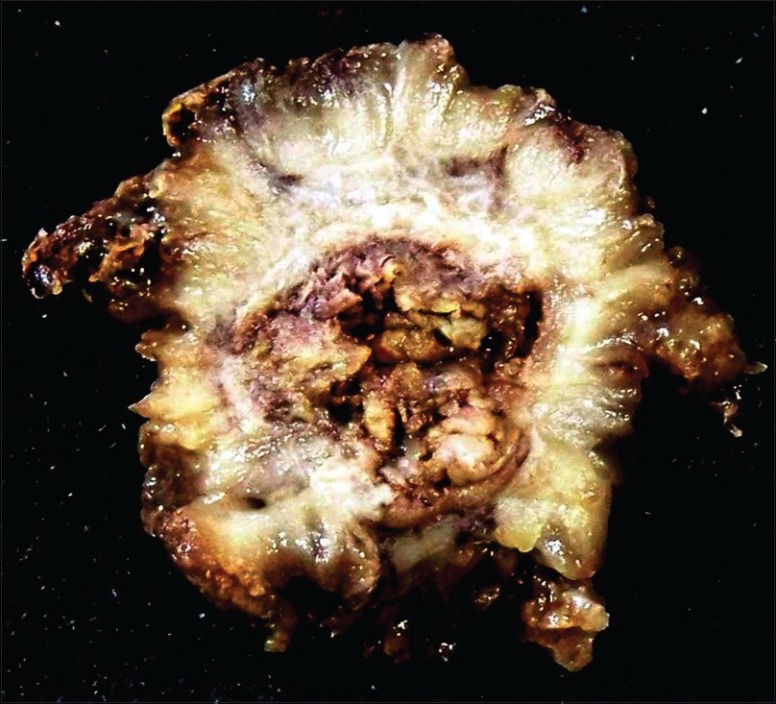
Partial cystectomy specimen with a polypoidal growth in the bladder lumen measuring 6 × 5 cm and grossly infiltrating the muscle layer
Histopathologic examination revealed a normal urothelial lining epithelium with underlying spindle cell tumor composed of spindle-shaped cells accompanied by inflammatory infiltrates comprising lymphocytes and plasma cells [Figure 2] infiltrating the muscularis layer on a myxoid stroma. The spindle-shaped cells have a high n:c ratio, oval to elongated pleomorphic hyperchromatic nuclei, prominent nucleoli, and moderate amount of eosinophilic cytoplasm [Figure 3]. Frequent mitosis and areas of focal necrosis are also seen. Morphologic diagnosis of spindle cell neoplasms was given. Immunohistochemical profile was done in the case, which showed strong cytoplasmic staining in the myofibroblasts by anaplastic lymphoma kinase immunostaining [Figure 4]. These tumors also showed positivity for Vimentin, Cytokeratin, Smooth Muscle Actin (SMA), Muscle- specific actin (MSA) and negative for Cytokeratin 20, Desmin, S100, and CD117. A final confirmative diagnosis of IMFT was made. He has had neither recurrence nor metastasis for 15 months.
Figure 2.
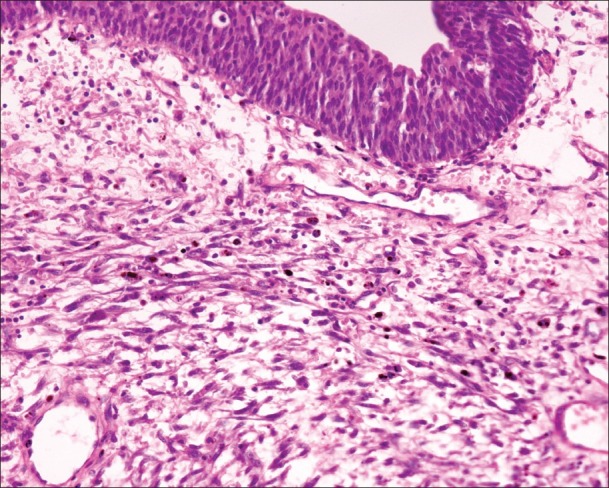
Section showing normal urothelial lining epithelium with underlying spindle cell tumor composed of oval- to spindle shaped cells admixed with lymphocytes and plasma cells on a myxoid stroma (H and E, ×400)
Figure 3.
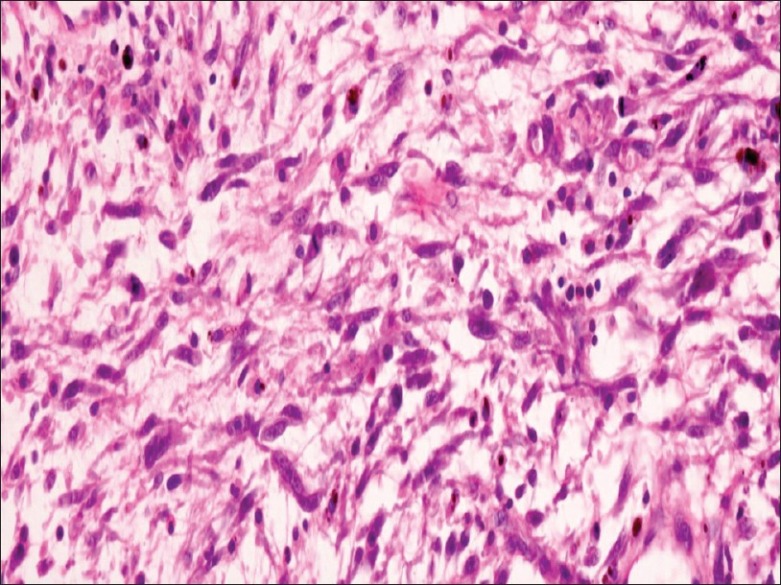
Section showing a tumor composed of spindle-shaped cells having high nucleocytoplasmic ratio, moderately pleomorphic hyperchromatic nuclei, prominent nucleoli, brisk mitosis, and moderate amount of eosinophilic cytoplasm (H and E, ×400)
Figure 4.

Anaplastic lymphoma kinase immunohistopathologic studies revealed strong cytoplasmic staining of the myofibroblasts (H and E, ×400)
DISCUSSION
IMFT is a rare spindle cell neoplasm of the urinary bladder, characterized by atypical spindle cell proliferation accompanied by inflammatory cell infiltrate comprised primarily of lymphocytes and plasma cells. The first case was reported by Roth in 1980.[1] It is also known as peudosarcoma, atypical fibromyxoid tumor, atypical myofibroblastic and plasma cell granuloma.[2] IMFT may arise at any anatomical site, including lung, soft tissues, retroperitoneum, and bladder. IMFT exhibits morphologic and immunophenotypic overlap with malignant spindle cell tumors of the urinary bladder and diagnostic distinction from these tumors can be problematic.[3–8] Both epithelial and myogenic markers can be expressed in IMFT and may lead to a misdiagnosis of sarcomatoid carcinoma, leiomyosarcoma, and rhabdomyosarcoma.[7]
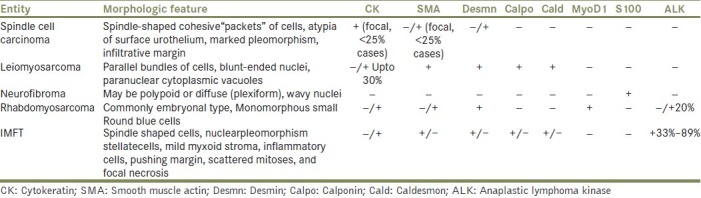
The ALK-1 reactivity correlates with local recurrence[3] and muscle invasion.[5] Originally identified as a protein overexpressed in anaplastic large-cell lymphoma, ALK-1 has subsequently been shown to be overexpressed in a substantial proportion of IMFTs of various anatomic locations,[9] including the urinary bladder. In IMFT of the urinary bladder, positivity for ALK-1 by immunohistochemistry ranges from 33% to 89%, whereas ALK-1 protein expression in leiomyosarcoma and sarcomatoid urothelial carcinoma has not been reported, suggesting that ALK-1 immunohistochemical studies may be useful in the differentiation of IMFT from other spindle cell lesions in the urinary bladder [Table 1].[10]
Table 1.
Differential diagnosis, morphological features, and “classical” immunohistochemical profile of spindle cell neoplasms of urinary bladder
Although necrosis is described in 30%[7] or more[6] of IMFTs, the presence of necrosis at the tumor–detrusor muscle interface in muscle-invasive cases was one criterion present in sarcoma that distinguished it from inflammatory pseudotumor.
It may affect any age group, but it is more common in children and young adults with slight female preponderance (F:M ratio 3:4). The origin of IMFT is controversial, but a recent report suggests that it is neoplastic because of aggressive behavior, involvement of chromosome 2p23, and congenital clonality. Definite diagnostic criteria of IMFT are spindle cell proliferation, presence of stellate cells, lymphoplasmacytic infiltrates, and scattered mitoses in myxoid stroma. Immunohistochemical staining may demonstrate positivity for Anaplastic lymphoma kinase, Vimentin, SMA, and Cytokeratin. Anaplastic Lymphoma Kinase has been described as a good marker for IMFT. Myogenin, a potent marker for rhabdomyosarcoma, helped in exclusion of this tumors.[11] Because of its highly cellular nature and aggressive behavior, it can be confused with malignancy.[12] Initial biopsy and complete histopatholgic examination is recommended where complete resections are problematic. Whole surgical resection is performed to avoid local recurrence.
The results of a preliminary study using nine vesical IMFT support these findings, with ALK-1 protein immunocytochemically identifiable in eight vesical IMFT (89%), but negative in all 11 sarcomatoid carcinoma, leiomyosarcoma, rhabdomyosarcoma, and neurofibromas examined from the bladder.[13]
These above results agree with a recent earlier abstract that showed cytoplasmic ALK-1 staining in 12/16 (75%) IMFT, but not in 15 control cases, including leiomyosarcoma, stromal tumors, and carcinosarcomas.[14] Although a number of cases of rhabdomyosarcoma may show expression of ALK-1, particularly those of alveolar subtype, these lesions usually also express desmin and myogenin or myoD1, and show characteristic morphologic features that allow identification and distinction from IMFT.
We concluded that IMFT of urinary bladder is a very rare but distinctive neoplasm with intermediate malignant potential. Optimal treatment when arising in the bladder is transurethral resection and excellent long-term prognosis. The detection of ALK protein expression is useful in distinguishing IMFT from spindle cell malignancies in the urinary bladder.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980;16:635–7. doi: 10.1016/0090-4295(80)90578-6. [DOI] [PubMed] [Google Scholar]
- 2.Zones EC, Clement PB, Young RH. Inflammatory pseudotumour of the urinary bladders.a clinicopathologic, immunohistochemical, ultrstructural and flowcytometric study of 13 cases. Am J Surg Pathol. 1993;17:264–74. doi: 10.1097/00000478-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. In: Fletcher CD, Unni KK, Mertens F, editors. World Health Organization Classification of Tumours: Tumours of Soft Tissue and Bone. Lyons, France: IARC Press; 2002. pp. 91–3. [Google Scholar]
- 4.Mergan F, Jaubert F, Sauvat F, Hartmann O, Lortat-Jacob S, Révillon Y, et al. Inflammatory my ofibroblastic tumor in children: Clinical review with anaplastic lymphoma kinase, Epstein–Barr virus, and human herpesvirus 8 detection analysis. J Pediatr Surg. 2005;40:1581–6. doi: 10.1016/j.jpedsurg.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, Elwood L, et al. Inflammatory myofibroblastic tumors of the urinary tract: A clinicopathologic study of 46 cases, including a malignant example of inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502–12. doi: 10.1097/01.pas.0000213280.35413.1b. [DOI] [PubMed] [Google Scholar]
- 6.Harik LR Merino C, Coindre JM, Amin MB, Pedeutour F, Weiss SW. Pseudosarcomatous myofibroblastic proliferations of the bladder: A clinicopathologic study of 42 cases. Am J Surg Pathol. 2006;30:787–94. doi: 10.1097/01.pas.0000208903.46354.6f. [DOI] [PubMed] [Google Scholar]
- 7.Iczkowski KA, Shanks JH, Gadaleanu V, Cheng L, Jones EC, Neumann R, et al. Inflammatory pseudotumor and sarcoma of urinary bladder: Differential diagnosis and outcome in thirty eight spindle cell neoplasms. Mod Pathol. 2001;14:1043–051. doi: 10.1038/modpathol.3880434. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Wick MR, Mills SE. Sarcomatoid carcinoma of the urinary bladder: A clinicopathologic analysis of 12 cases and review of the literature. Am J Clin Pathol. 1988;90:651–3. doi: 10.1093/ajcp/90.6.653. [DOI] [PubMed] [Google Scholar]
- 9.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: A comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–71. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Sukov WR, Cheville JC, Carlson AW, Shearer BM, Piatigorsky EJ, Grogg KL, et al. Utility of ALK-1 protein expression and ALK rearrangements in distinguishing inflammatory myofibroblastic tumor from malignant spindle cell lesions of the urinary bladder. Mod Pathol. 2007;20:592–603. doi: 10.1038/modpathol.3800776. [DOI] [PubMed] [Google Scholar]
- 11.Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, et al. Are myogenin and myo-D1 expression specific for rhabdomyosarcomas.A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol. 2001;25:1150–7. doi: 10.1097/00000478-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Yagnik V, Chadha A, Chaudhari S, Patel K. Inflammatory myofibroblastic tumor of the urinary bladder-A case report. Urol Ann. 2010;2:78–9. doi: 10.4103/0974-7796.65106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman A, Geddes N, Munson P, Joseph J, Ramani P, Sandison A, et al. Anaplastic lymphoma kinase (ALK 1) staining and molecular analysis in inflammatory myofibroblastic tumours of the bladder: A preliminary clinicopathological study of nine cases and review of the literature. Mod Pathol. 2004;17:765–71. doi: 10.1038/modpathol.3800078. [DOI] [PubMed] [Google Scholar]
- 14.Tsuzuki T, Magi-Galluzzi C, Epstein JI. ALK-1 expression in inflammatory myofibroblastic tumor of the urinary bladder. Am J Surg Pathol. 2004;28:1609–14. doi: 10.1097/00000478-200412000-00009. [DOI] [PubMed] [Google Scholar]


