Abstract
Aim:
This experimental study was designed to produce ischemia-reperfusion injury in rat kidney by performing partial unilateral ureteral obstruction (PUUO) and investigated the effects of melatonin on the levels of oxidative injury parameters.
Materials and Methods:
Twenty-four adult male rats were randomly divided into three groups as follows; control group (Group 1); only nephrectomy and blood (5 ml) drawn from vena cava inferior, PUUO group (Group 2); PUUO (10 days)+ipsilateral nephrectomy after recovery of PUUO+blood from vena cava inferior VCI, melatonin treated group (Group 3); PUUO (10 days)+melatonin (1/2 hr before release, 50 mg/kg, ip)+ipsilateral nephrectomy after recovery of PUUO+blood from VCI. The left ureter was embedded into the psoas muscle to create PUUO. After 10 days, PUUO was recovered and ipsilateral nephrectomies were performed for biochemical analysis of superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and protein carbonyl (PC) in the tissues and blood was drawn from inferior vena cava to study the same parameters in systemic circulation. The results were compared statistically.
Results:
The blood levels of MDA, NO, and PC were increased in the PUUO group in comparison to the sham-operated group (P<0.05). Melatonin treatment reduced MDA, NO, and PC levels in blood after PUUO recovery, but statistically significance consisted only for MDA and NO (P<0.05). The antioxidant enzyme activities (SOD, GSH-Px) were increased in the PUUO group (P<0.05). Melatonin treatment reduced SOD and GSH-Px activities in comparison with the sham-operated control group (P<0.05). Similarly, renal tissue levels of MDA, NO, and PC were increased in the PUUO group in comparison with the sham-operated group (P<0.05). Melatonin treatment ameliorated MDA, NO, and PC levels in renal tissue after PUUO recovery only MDA was statistically significant (P<0.05). Antioxidant enzyme activities (SOD, CAT, and GSH-Px) were increased in the PUUO group. Melatonin treatment caused reduction in SOD, CAT, and GSH-Px activities in comparison to the sham-operated control group (P<0.05).
Conclusion:
The results of this study showed that experimentally induced PUUO caused oxidative stress in rat kidney and melatonin treatment reduced oxidative stress and therefore may have a preventive effect on PUUO induced oxidative kidney damage in rats.
Keywords: Kidney, melatonin, obstruction, oxidative stress, ureter
INTRODUCTION
Obstructive uropathy occurs at all ages from infancy to elderly subjects and it is often a reversible condition. It is classified according to the degree, duration, and level of the obstruction. It may develop as a consequence of functional or anatomic lesions located in the urinary tract.[1] There are many causes of obstructive uropathy.[2,3] Although partial unilateral ureteral obstruction (PUUO) is one of the most common urologic problems in daily clinical practice, the mechanism of the pathophysiologic changes in PUUO is not well known. Most of the information about the mechanisms by which urinary tract obstruction may affect renal function has been gained from studies in experimental animal models. Renal blood flow impairment, intrapelvic pressure elevation, vasoactive and inflammatory mediators are some of the known factors in pathophysiology of renal obstructive renal parenchymal injury.[2]
As the chief secretory product of the pineal gland, melatonin (N-acetyl-5-methoxytryptamine) functions as synchronizer of the biological clock and has a powerful antioxidant activity.[4] Melatonin's protective role has been reported in various experimental models of tissue damage by reducing oxidative stress and lipid peroxidation. There are very limited numbers of experimental studies about antioxidant and antiinflamatory properties of melatonin in PUUO induced nephropathy in the literature. In this experimental study, we aimed to show oxidant/antioxidant enzyme levels in blood and kidney tissue after PUUO in rat kidney.
MATERIALS AND METHODS
After obtaining nzym ethical committee permission, a total of 24 male 5.5–6 months old Wistar albino rats weighing 250–300 g, were used in the study. The experimental animals were housed at 18-22°C under a 12 h light/12 h dark cycle and had free access to standard pellet diet for rats and tap water ad libitum throughout the study. All surgical procedures were performed under xylazine/ketamine anesthesia in sterile conditions. All rats were sacrificed after the experimental procedures.
The rats were randomly divided into three groups each consisting of eight rats. Group 1 (sham-operated control group) underwent a sham operation to determine basal values for biochemical evaluation. The left nephrectomy was performed out through midline abdominal incision.
Group 2 was designed to study the effects of PUUO. The left ureter was embedded into the psoas muscle to create PUUO which was described first in dogs by Ulm and Miller[5] and was subsequently used by Djurhuus et al.[6] who established PUUO in the adult pig. A midline incision was made and the left ureter was exposed on the left psoas muscle. Ureter embedded into the psoas muscle with 4/0 nonabsorbable silk suture [Figure 1]. Ten days after constitution of PUUO, obstruction was released and left nephrectomy was performed.
Figure 1.
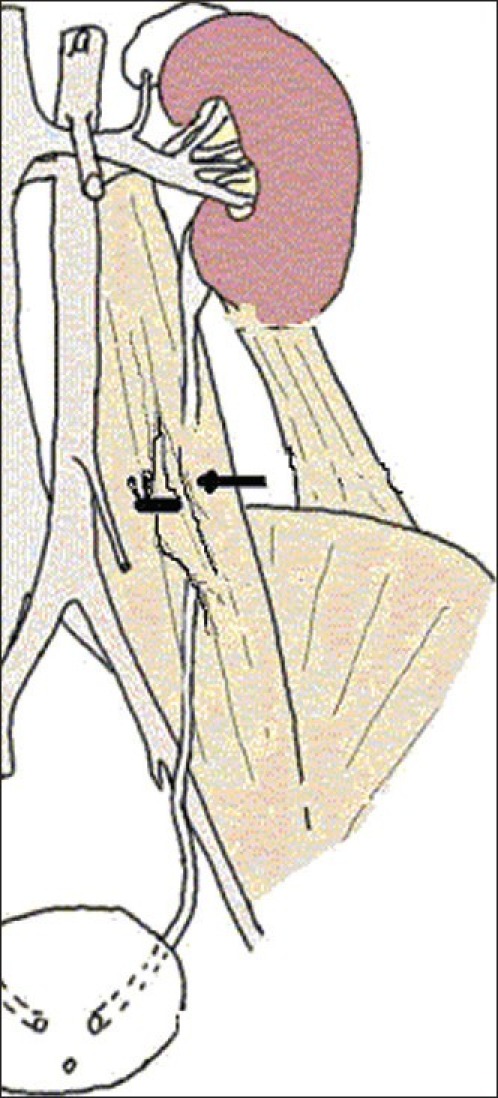
Embedding the ureter into the psoas muscle to create PUUO
Group 3 was designed to determine the effect of melatonin on antioxidant levels after PUUO. After 10 days, PUUO was released and left nephrectomy was performed. In this group, melatonin (50 mg/kg) was administered intraperitoneally (i.p.) in a single dose 30 min before recovery of PUUO. The kidney of each rat was fixed in 10% formaldehyde solution and was stored at –70°C pending biochemical studies. International standarts for the care of laboratory animals were followed and the study protocol was approved by the local ethical committee. Approximately 5 cc blood samples were obtained from the vena cava inferior of the rats to determine the blood levels of malondialdehyde (MDA), superoxide dismutase (SOD), protein carbonyl (PC), glutathione peroxidase (GSH-Px), and nitric oxide (NO). Ipsilateral nephrectomy was performed in all groups to determine biochemically antioxidant enzyme activities (SOD, catalase, GSH-Px) and malondialdehyde (MDA), protein carbonyl (PC), and nitric oxide (NO) levels in renal tissue.
Biochemical analyses
After centrifugation (×2000 g for 15 min at +4°C), serum samples were stored at –70°C. Kidney tissues were homogenized in 5 ml of icecold tris-HCl buffer (50 mmol/l, pH 7.4) containing 0.50 mL/L Triton × 100. The homogenization procedure (IKA Ultra-Turrax t 25 Basic, Staufen, Germany) was carried out for 2 min at ×5000 g. Homogenate, supernatant, and extracted samples were prepared. Determinations of the following parameters were made on the serum samples and kidney homogenates using commercial chemicals (Sigma, St. Louis, MO, USA). All of the procedures were performed at 4°C.
Superoxide dismutase activity determination
Total (Cu–Zn and Mn) superoxide dismutase (SOD) activity was determined according to the method of Sun et al.,[7] including a modification made by Durak et al.[8] The principle of SOD activity determination method was based on the inhibition of nitroblue tetrazolium reduction by the xanthine-xanthine oxidase system as a superoxide radical generator. One unit of SOD was defined as the enzyme activity causing 50% inhibition in the nitroblue tetrazolium reduction rate. The SOD activity was expressed as units per nzymatic tissue protein (U/mg prot).
Catalase activity determination
Catalase (CAT) activity was determined according to Aebi's method.[9] The essentials of CAT activity determination method were based on the determination of the rate constant of the H2O2 decomposition rate at 240 nm. The CAT activity results were expressed as k (rate constant) per gram protein (k/g prot).
Glutathione peroxidase activity determination
Glutathione peroxidase (GSH-Px) activity was measured using the method of Paglia and Valentine.[10] It was measured by the enzymatic reaction which was initiated by addition of H2O2 to the reaction mixture containing reduced glutathione, nicotinamide adenine dinucleotide phosphate (NADPH), and glutathione reductase. The change in the absorbance at 340 nm was monitored using a spectrophotometer and the nzymatic activity was given as international units per gram tissue protein (U/g prot).
Malondialdehyde level determination
Malondialdehyde (MDA) level was determined using Wasowicz's method,[11] which was based on the reaction of MDA with thiobarbituric acid at 95–100°C. MDA or MDA-like substances and thiobarbituric acid react together to produce a pink pigment with an absorbtion maximum of 532 nm. The results were expressed as nanomoles per gram wet tissue protein of testis (nmol/g wet tissue) according to standart graphics which was prepared with serial dilutions of standard 1,1,3,3-tetramethoxypropane.
Protein carbonyl level determination
The protein carbonyl (PC) contents were determined spectrophotometrically (GBC Cintra 10 E UV/Visible spectrophotometry, Melbourne, Australia) with the reaction of the carbonyl group with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazone.[12] The results were given as nanomoles of protein carbonyl per milligram of protein.
Nitric oxide determination
Tissue nitrite (NO2–) and nitrate (NO3–) were estimated as an index of Nitric oxide (NO) production. Quantitations of NO2– and NO3– were based on the Griess reaction in which a chromophore with a strong absorbance at 540 nm was formed by reaction of nitrite with a mixture of naphthlethylenediamine and sulphanilamide.[13] The results were expressed as μmol per gram wet tissue (μmol/g wet tissue).
Statistical analysis
Data were analyzed by a commercially available Statistical Package for Social Sciences (SPSS) program for Windows software. P values of <0.05 were regarded as statistically significant. Distribution of the groups was analyzed with the Kolmogorov–Smirnov one-sample test. One-way Analysis of Variance (ANOVA) test was performed and post hoc multiple comparisons were done with least-squares differences (LSD).
RESULTS
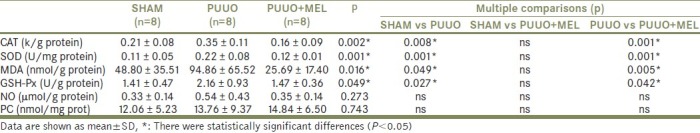
Renal tissue levels of MDA, NO, and PC which are lipid peroxidation products were increased in the PUUO group in comparison with the sham-operated group (P<0.05). Melatonin treatment ameliorated MDA, NO, and PC levels in renal tissue after PUUO recovery, but statistically significance consisted only for MDA (P<0.05). Antioxidant enzyme activities (SOD, CAT and GSH-Px) were increased in the PUUO group. Melatonin treatment caused decreased SOD, CAT, and GSH-Px activities in comparison with the sham-operated control group (P<0.05). The results of renal tissue CAT, MDA, SOD, NO, PC, and GSH-Px values in both groups are shown in Table 1.
Table 1.
The histopathological test results in the renal tissues according to groups
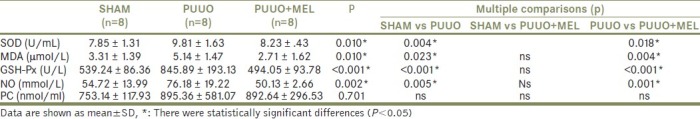
Similarly, the blood levels of MDA, NO, and PC which are lipid peroxidation products were increased in the PUUO group in comparison to the sham-operated group (P<0.05). Melatonin treatment reduced MDA, NO, and PC levels in blood after PUUO recovery, but statistically significance consisted only for MDA and NO (P<0.05). Similarly, antioxidant enzyme activities (SOD, GSH-Px) were increased in the PUUO group. Melatonin treatment reduced SOD and GSH-Px activities in comparison with the sham-operated control group (P<0.05). The results of blood MDA, SOD, NO, PC, and GSH-Px values in both groups are shown in Table 2.
Table 2.
The biochemical test results in the blood according to groups
DISCUSSION
Depending on the duration and severity of obstruction PUUO may cause detrimental changes on glomerular filtration rate (GFR) and renal blood flow (RBF).[14] In antenatal animal models, GFR and RBF were significantly decreased due to PUUO.[15] Also, it is well known that in pediatric clinical practices neonatal PUUO is one of the most frequently encountered abnormality.[16] Gillenwater et al. denoted that 4-28 days after PUUO GFR was reduced to 20-70% of normal levels. They also reported that in PUUO condition, RBF was decreased to 25% of normal levels.[17] Furthermore Yarger et al. found that while outer cortical blood flow was decreased, deep cortical blood flow was relatively increased.[18] The underlying mechanisms of hemodynamic changes have not been precisely established. Many studies proved that monocystic infiltration of the interstitial compartments was associated with renal injury. In obstructive nephropathy, leucocyte infiltration (particularly macrophages) occurs in paranchymal space within 24 hours of the obstruction.[19] These macrophages are capable of releasing different products such as proteolytic enzymes, reactive oxygen species, platelet derivated growth factor, cyclooxygenase, and lipooxygenase products and these products play an active role in establishment of interstitial fibrosis due to PUUO.[20]
Reactive oxygen species (ROS; e.g. superoxide, hydrogen peroxide and hydroxyl radical) are intermediary metabolites that are normally produced in the course of oxygen metabolism. ROS can destroy proteins, lipids, nucleic acids, carbohydrates and other molecules, and cause inflammation, apoptosis, fibrosis and cell proliferation. However, under normal conditions, ROS play a critical role as signal molecules and ROS produced by activated leucocytes and macrophages are essential for defense mechanism against invading microorganisms. ROS are generated by mitochondrial cytochrome oxidase, nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, lipooxygenase, cyclooxygenase, hemeoxygenase, cytochrome P-450 enzymes, nitric oxide synthase (NOS), and various other oxidase enzymes. Additionally, ROS may lead to severe injury to the cell membrane by lipid peroxidation reactions and may have an important role in tubulointerstitial inflammation associated with obstructive nephropathy.[21] Because of the difficulty in measuring ROS in vivo, little is known about the production of ROS in the unilateral ureteral obstruction model. Membrane lipid peroxidation may generate reactive carbonyl compounds such as MDA, one of the reliable indicators of ROS-induced ischemia–reperfusion tissue damage.
Ischemia–reperfusion (I/R) injuries are well known in surgical practice, but I/R injury in obstructive nephropathy has not been extremely studied yet. Recent reports indicate that the release of ureteral obstruction leads to enhanced renal production of ROS.[22] In this study, we observed that recovery of PUOO induced elevations in ROS levels which played role in mechanism of renal paranchymal injury. Antioxidant enzyme levels rised to compensate the ROS simultaneously and this was an indirect indicator of increased ROS levels. Elevated SOD, GSH-Px, and MDA levels in blood and tissue of PUUO group compared to controls (P<0.05) showed that ureteral obstruction produced an ischemic event in renal tissue after PUUO. This may depend on congestion that is emerging due to prevention of urine flow.
Because of its liphophilic properties, melatonin can easily cross cell membranes and the blood-brain barrier and plays a direct scavenger activity against reactive oxygen species (ROS) such as OH, O2-, and NO.[23,24] Many biological effects of melatonin are produced through activation of melatonin receptors while others are due to its role as a pervasive and powerful antioxidant with a particular role in the protection of nuclear and mitochondrial DNA.[25–27] There are high-affinity melatonin receptors located primarily in the kidney, blood vessels, eye, brain, and gastrointestinal tract.[27] Depending on its antioxidant properties, melatonin helps antioxidant enzymes to scavange ROS. As a result of this activities, the antioxidant enzyme levels brought to similar levels in control group in this study (P>0.05). Melatonin also reduced lipid peroxidation, therefore MDA levels in melatonin treated group was not then control group (P>0.05). In terms of blood and tissue PC levels, there was not statistically significant difference among groups. This may depend on the assumption that protein structures are more resistant to ROS effects than lipid structures. NO status has not been clarified during ischemic event yet, but probably inflammation and migration of mediators to the ischemic area during ischemic condition increases NO production from inflammatory cells. Ischemic condition also affects vascular endothelial cells and this may increase endothelial NO production. On the basis of this mechanism, in PUUO group NO level was higher and it was reduced to same levels with control group after melatonin administration (P>0.05). So, these results may arise a thought that melatonin has beneficial effects on inflammation that reduces neuromediators migration to the ischemic region.
There are very few number of studies about the effects of melatonin on PUUO in the literature. Nava et al. noted that nearly complete correction of oxidative stress parameters (MDA, superoxide-positive cells) was accompanied by significant but incomplete (50%) reduction of inflammatory infiltrate and angiotensin II-positive cells by melatonin in spontaneously hypertensive rats.[28] Rodriguez-Reynoso et al. found that exogenous melatonin preserved renal function, increased GSH-Px levels, reduced lipid peroxidation, and prevented the rise in NO levels induced by renal injury. As a result of their study, they concluded that melatonin treatment reduced kidney injury.[29] In a recent study, Ozbek et al. reported the protective effects of melatonin on UUO-induced kidney injury in rats and they added that melatonin exerts a preventive effect on UUO-induced kidney damage in rats by reducing oxidative stress. The mechanism of this action was likely mediated via reduction in the expression of iNOS, p38-MAPK, and NFkB, since melatonin reduced the activation of these pathways and melatonin administration can be used for kidney protection in patients with UUO.[30] Similar to the literature, in the present study, we showed that administiration of melatonin before recovery of PUUO attenuated ROS levels and minimized oxidative stress induced by PUUO in the kidney.
In conclusion, our results showed that melatonin treatment reduces oxidative stress and therefore may have a preventive effect on PUUO-induced kidney damage in rats. In this regard, melatonin administration before PUUO recovery can be used for protection of kidney in patients with PUUO. However, further comprehensive animal and clinical studies are needed to confirm our suggestion.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Klahr S. Urinary tract obstruction. Semin Nephrol. 2001;21:133–45. doi: 10.1053/snep.2001.20942. [DOI] [PubMed] [Google Scholar]
- 2.Wen JG, Frokiaer J, Jorgensen TM, Djurhuus JC. Obstructive nephropathy: An update of the experimental research. Urol Res. 1999;27:29–39. doi: 10.1007/s002400050086. [DOI] [PubMed] [Google Scholar]
- 3.Gulmi FA, Felsen D, Vaughan ED. Pathophysiology of urinary tract obstruction. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell's Urology. 8th ed. Philadelphia: W.B. Saunders Company; 2002. pp. 411–56. [Google Scholar]
- 4.Tan DX. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 5.Ulm AH, Miller F. An operation to produce experimental reversible hydronephrosis in dogs. J Urol. 1962;88:337–9. doi: 10.1016/S0022-5347(17)64796-7. [DOI] [PubMed] [Google Scholar]
- 6.Djurhuus JC, Nerstrom B, Gyrd-Hansen N, Rask-Anderson H. Experimental hydronephrosis.An electrophysiologic investigation before and after release of obstruction. Acta Chir Scand. 1976;427:17–28. [PubMed] [Google Scholar]
- 7.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 8.Durak I, Yurtarslani Z, Canbolat O, Akyol O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta. 1993;214:101–3. doi: 10.1016/0009-8981(93)90307-p. [DOI] [PubMed] [Google Scholar]
- 9.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 10.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 11.Wasowicz W, Nève J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: İmportance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–6. [PubMed] [Google Scholar]
- 12.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG. Methods in Enzimology and Oxygen Radicals in Biological Systems. Vol. 186. New York: Academic Press; 2000. Determination of carbonyl content in oxidatively modified proteins; pp. 464–78. [DOI] [PubMed] [Google Scholar]
- 13.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3. [PubMed] [Google Scholar]
- 14.Fulop M, Brazeau P. Increased ureteral back pressure enhances renal tubular sodium reabsorption. J Clin Invest. 1970;49:2315–23. doi: 10.1172/JCI106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhardt G, Salinas-Madrigal L, Farber R, Lynch R, Vogler G. Experimental ureteral obstruction in the fetal opossum.Renal functional assessment. J Urol. 1990;144:564–66. doi: 10.1016/s0022-5347(17)39524-1. [DOI] [PubMed] [Google Scholar]
- 16.Peters CA. Urinary tract obstruction in children. J Urol. 1995;154:1874–83. doi: 10.1016/s0022-5347(01)66815-0. [DOI] [PubMed] [Google Scholar]
- 17.Gillenwater JY. The pathophysiology of urinary tract obstruction. In: Walsh PC, Retik AB, Stamey TA, Vaughn ED Jr, editors. Campbell's Urology. 8th ed. Philadelphia: WB Saunders; 2002. pp. 499–505. [Google Scholar]
- 18.YargerWE , Schocken DD, Harris RH. Obstructive nephropathy in the rat: Possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J Clin Invest. 1980;65:400–12. doi: 10.1172/JCI109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris KP, Schreiner GF, Klahr S. Effect of leukocyte depletion on the function of the postobstructed kidney in the rat. Kidney Int. 1989;36:210–15. doi: 10.1038/ki.1989.181. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MR, Young IS, Jhonston SR, Rowlands BJ. Lipid peroxidation assessment of free radical production following release of obstructive uropathy. J Urol. 1996;156:1828–32. [PubMed] [Google Scholar]
- 22.Hardeland R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–30. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 23.Poeggeler B, Saarela S, Reiter RJ. Melatonin a highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann NY Acad Sci. 1994;738:419–20. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- 24.Tan DX, Manchester LC, Reiter RJ, Qi W, Karbownik M, Calvo JR. Significance of melatonin in anti oxidative defense system: Reactions and products. Biol Signals Recept. 2000;9:137–59. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 25.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–9. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci. 2001;939:200–15. [PubMed] [Google Scholar]
- 27.Beyer CE, Steketee JD, Saphier D. Antioxidant properties of melatonin—an emerging mystery. Biochem Pharmacol. 1998;56:265–72. doi: 10.1016/s0006-2952(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 28.Nava M, Quiroz Y, Vaziri N, Rodriguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284:447–54. doi: 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Reynoso S, Leal C, Portilla-de Buen E. Melatonin ameliorates renal ischemia-reperfusion injury. J Surg Res. 2004;116:242–7. doi: 10.1016/j.jss.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Ozbek E, Ilbey YO, Ozbek M, Simsek A, Cemken M, Somay A. Melatonin attenuates unilateral ureteral obstruction-induced renal injury by reducing oxidative stress, iNOS, MAPK, and NF-kB expression. J Endourology. 2009;23:1165–73. doi: 10.1089/end.2009.0035. [DOI] [PubMed] [Google Scholar]


