Abstract
Intracellular pH homeostasis is an essential process in all plant cells. The transport of H+ into intracellular compartments is critical for providing pH regulation. The maintenance of correct luminal pH in the vacuole and in compartments of the secretory/endocytic pathway is important for a variety of cellular functions including protein modification, sorting, and trafficking. It is becoming increasingly evident that coordination between primary H+ pumps, most notably the V-ATPase, and secondary ion/H+ exchangers allows this endomembrane pH maintenance to occur. This article describes some of the recent insights from the studies of plant cation/H+ exchangers and anion/H+ exchangers that demonstrate the fundamental roles of these transporters in pH homeostasis within intracellular compartments.
Keywords: anion/H+ exchanger, cation/H+ exchanger, CHX gene family, pH homeostasis, H+ transport, NHX gene family, secretory pathway, V-ATPase
Introduction
Many metabolic and enzymatic processes are dependent on specific pH conditions, due in part to the regulation of protein structure and function by pH, therefore intracellular pH regulation is an essential process in all organisms (Casey et al., 2010; Orij et al., 2011). In eukaryotes, various cellular processes are compartmentalized within the organelles, each with distinct functions and distinct pH requirements. Most notably, pH has a major role in secretory function where it regulates protein modification and sorting via a gradient of pH along the secretory pathway (Paroutis et al., 2004).The cell must therefore maintain and control the distinct pH environments within the various compartments. pH regulation also allows a cell or organism to tolerate potentially toxic external acidic or alkaline conditions, and to cope with the large amounts of H+ that are produced during metabolic reactions. Furthermore, many essential transport processes depend on the proton motive force which is generated largely by the transmembrane H+ gradient (Gaxiola et al., 2007). There is also speculation that a change in pH may act as a signal (Orij et al., 2011). While cytosolic pH levels are tightly regulated (see below), free H+ may be modulated in a highly localized and transient manner to act as a signal. The ability to quantify cytosolic pH using highly pH-sensitive reporters has demonstrated that dynamic changes in cytosolic pH do occur in many plant cell types and in response to conditions such as salt stress, anoxia, and during growth (Swanson et al., 2011). For example, controlled H+ fluxes have been observed in response to environmental signals, such as pathogen infection and gravitropic stimulation (Felle, 2001; Roos et al., 2006). In addition, pH changes can trigger downstream responses, such as the activation of transporters (Tournaire-Roux et al., 2003; Pittman et al., 2005).
The mechanisms of cytosolic pH regulation can be essentially divided into two types. A metabolic-based regulatory mechanism, referred to as the biochemical pH-stat, is a critical component in cytosolic pH regulation (Sakano, 2001). It relies on metabolites acting as strong pH buffers and pH-dependent metabolic reactions such as the carboxylation and decarboxylation of organic acids like malate to produce or consume H+. Correct partitioning of malate in the cell is therefore likely to be an important determinant of cytosolic pH. Indeed it has been demonstrated that the tonoplast dicarboxylate transporter AttDT which transports malate into the vacuole is critical for pH regulation (Hurth et al., 2005). Furthermore, the amount of organic acids such as citrate or malate that accumulate into the vacuole may also be a determinant of vacuolar acidity and thus vacuolar lumen pH (Muller and Taiz, 2002). The second major regulatory mechanism is the membrane transport of H+ between the cytosol and the two main acidic compartments, the apoplast and vacuole. This is primarily facilitated by directly energized H+ pumps, including the P-type H+-ATPase at the plasma membrane, which pumps H+ into the apoplast, and the V-type H+-ATPase (V-ATPase) at the tonoplast, which in tandem with a second vacuolar H+ pump, the H+-pyrophosphatase (H+-PPase), pumps H+ into the vacuolar lumen (Gaxiola et al., 2007). However, it is becoming increasingly apparent that a larger number of distinct transport pathways are involved in intracellular pH regulation in plant cells, in particular for the regulation of organelle luminal pH. This article will provide an overview of the roles that various endomembrane-localized H+ and ion transporters play in mediating pH regulation within intracellular compartments including the secretory/endocytic pathways and vacuole, and will concentrate on some of the very recent insights from the study of plant ion/H+ exchangers. The mechanisms for the regulation and maintenance of cytosolic pH in plant cells are not the main focus of this review, and the reader is referred to the references provided in this paragraph, and the references therein.
Vacuolar and Endomembrane H+ Pumps: V-ATPase and H+-PPase
The V-ATPase is a large, abundant, multi-subunit protein that is ubiquitous throughout eukaryotes (Sze et al., 2002; Schumacher and Krebs, 2010). It is thought to play a major role in maintaining cytosolic pH at slightly alkaline levels (∼pH 7.2–7.5) and in regulating vacuolar pH. Vacuolar pH can vary markedly between plant species, as low as pH 2.0 in some citrus fruit (Muller and Taiz, 2002), but is generally maintained around pH 5.5, despite the potential of the V-ATPase to decrease the vacuolar pH to 1.0–2.0 (Sze et al., 1999). The V-ATPase is thus regulated to prevent maximal lumen acidification in the vacuoles of most species. The V-ATPase is regulated by cytosolic pH, with maximal activity at neutral pH (Dietz et al., 2001), but less is clear regarding the potential regulation by luminal pH. It may be expected that V-ATPase activity is regulated in part by altered luminal pH, such as increased acidification of the lumen. Several regulatory mechanisms of the V-ATPase have been proposed (Dietz et al., 2001). One potential mechanism by which the V-ATPase could be regulated is by alteration to the coupling ratio which is the number of H+ pumped per ATP hydrolyzed. Studies in red beet have indicated that the V-ATPase may alter this ratio depending on cytosolic and luminal pH (Davies et al., 1994). Regulation of the V-ATPase via protein interactions or phosphorylation have also been indicated (Hong-Hermesdorf et al., 2006) but the specific details of such regulation are not fully clear.
It has been often proposed that the combined action of the V-ATPase and the vacuolar H+-PPase generates the vacuolar proton motive force and regulates vacuolar pH, and that these pumps have partially redundant function (Gaxiola et al., 2007). Indeed Arabidopsis AVP1 encoding the vacuolar H+-PPase is able to complement a yeast V-ATPase vma mutant and recover vacuole acidification (Perez-Castineira et al., 2011). But a number of recent observations suggest that there are clear differences between the two vacuolar pumps. Tonoplast-specific deletion of the Arabidopsis V-ATPase generated by vha-a2 vha-a3 double knockouts leads to an increase in vacuolar pH from pH 5.9 to 6.4 (Krebs et al., 2010). In contrast, two independent AVP1 knockout alleles both display a very minor increase in vacuolar pH by only 0.2–0.3 pH units (Li et al., 2005; Ferjani et al., 2011). Furthermore, H+-PPase activity was found not to increase in the vacuolar V-ATPase mutant (Krebs et al., 2010). A recent study of AVP1 has led to the suggestion that the major role of this protein might be in the hydrolysis and removal of otherwise metabolically toxic inorganic pyrophosphate rather than vacuolar acidification (Ferjani et al., 2011). This has led to the speculation that other processes may also contribute toward vacuolar acidification, such as via the fusion of acidic secretory pathway vesicles (Schumacher and Krebs, 2010).
The vesicular bodies of the secretory and endocytic pathways are also thought to be acidified. The luminal pH of secretory/endocytic compartments are challenging to measure and have not yet been experimentally determined in plants. However, pH values have been measured in animal secretory compartments (Wu et al., 2001; Nakamura et al., 2005) and more recently in yeast Golgi (Braun et al., 2010; Tarsio et al., 2011). These measurements indicate that the pathway becomes increasing acidic from the endoplasmic reticulum (ER) toward the vacuole (Figure 1). In animal cells, the ER has a neutral pH, while the luminal pH drops to ∼pH 6.0 in the trans-Golgi and the trans-Golgi network (TGN), then between pH 6.0 and 5.0 in the late secretory granules (Paroutis et al., 2004). Likewise in the endocytic pathway, luminal pH ranges from pH 6.3 in the early endosome (EE), pH 6.0 in the late endosome to pH 5.5 in the lysosome (equivalent to the plant vacuole). This reduction in pH regulates the proper processing, targeting, and sorting of cargo proteins through the secretory/endocytic pathways, therefore when the components that control luminal pH of these pathways are perturbed, many defects occur. The presence of H+ pumps throughout the plant secretory/endocytic pathways (Figure 1), and the requirement of these pumps for proper function of these pathways, strengthens the concept that plants, like animals and yeast, maintain acidic endomembrane compartments. In plants, as in yeast, the V-ATPase is found at both the vacuole and the TGN/EE, indicating that it is likely to be a key component in causing acidification of these endosomal compartments (Dettmer et al., 2006), and therefore critical for plant protein trafficking. The use of V-ATPase mutants has begun to confirm this critical role (reviewed in Schumacher and Krebs, 2010); for example, a number TGN/EE V-ATPase mutants yield phenotypes such as perturbed cell expansion, abnormal endosomal structure, and cell wall defects (Strompen et al., 2005; Padmanaban et al., 2007; Brux et al., 2008). A K+-independent (Type II) H+-PPase, distinct from the K+-dependent (Type I) vacuolar H+-PPase AVP1, has been shown to be Golgi localized in Arabidopsis (Segami et al., 2010) indicating that it acts as a H+ pump to acidify Golgi vesicles, although this activity has yet to be confirmed.
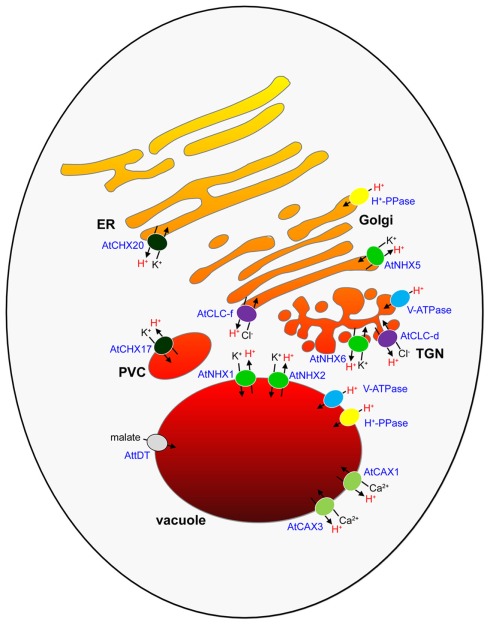
Figure 1.
The endomembrane compartments of a plant cell and their putative pH regulators. The different colors of the endomembrane compartments indicate different luminal pH values of organelles. The secretory compartments are proposed to increase in acidity from the ER (at near neutral pH) to the vacuole (∼pH 5.5), as determined from measurements in secretory compartments of other eukaryotes. V-ATPase and H+-PPase H+ pumps, K+/H+ exchangers, Ca2+/H+ exchangers, Cl−/H+ exchangers, and a malate transporter that are involved or implicated in intracellular pH regulation in Arabidopsis are shown. ER, endoplasmic reticulum; TGN, trans-Golgi network; PVC, pre-vacuolar compartment.
Fine Tuning of Vacuolar and Endomembrane pH by Ion/H+ Exchangers
Although the V-ATPase is clearly an important component in the regulation of intracellular pH, there is increasingly strong evidence from plant studies that other endomembrane-localized H+ transport pathways play important pH regulatory roles. Ion/H+ exchangers directly couple the transport of an ion, either a cation or an anion, across the membrane, and into the endomembrane compartment, with the counter-exchange of H+ and thus are energized by the proton motive force generated by the V-ATPase and H+-PPase. Although such H+ exchanger pathways are considered as “H+ leaks,” they may provide a means to fine tune the action of the H+ pumps to prevent maximal acidification of a compartment lumen. A large number of putative ion/H+ exchanger genes have been identified in the genomes of sequenced plant species like Arabidopsis, which include members of the CPA1, CPA2, and CaCA gene superfamilies (Mäser et al., 2001; Cai and Lytton, 2004; Brett et al., 2005a). Many of these genes have yet to be characterized in any detail, but a few ion/H+ exchangers have begun to be identified that localize at various endomembranes (Figure 1) and which may provide one of the mechanisms by which a range of luminal pH values in different endomembrane compartments can occur.
pH Regulation by NHX-Type Na+, K+/H+ Exchangers
Cell membrane/plasma membrane Na+/H+ exchangers have long been known to play a key role in regulation of cytosolic pH in bacteria and in animals (reviewed in Krulwich et al., 2011), but there is increasing evidence that internally localized Na+/H+ exchangers are involved in luminal pH regulation in eukaryotes. Four mammalian Na+/H+ exchangers (NHE6–NHE9) localized in the Golgi, TGN, early, and late endosomes appear to be involved in maintenance of specific luminal pH values (Nakamura et al., 2005). A similar role was uncovered for the yeast endosomal Na+/H+ exchanger Nhx1. Deletion of Nhx1 causes slight acidification of the cytosol and significant acidification of the vacuolar lumen, from nearly pH 4.8 in the wild type to below pH 4.0 in the nhx1 mutant when grown in external acidic conditions (pH 2.7; Ali et al., 2004; Brett et al., 2005b). This causes defective vesicular trafficking between the vacuole and the endosome, indicating the essential role of Nhx1 in generating alkalinization of intracellular compartments.
NHX/NHE genes of the CPA1 superfamily are divided phylogenetically into two subgroups, which correspond to plasma membrane and intracellular isoforms (Brett et al., 2005a). A number of plant NHX genes have been identified that fall within the intracellular NHX/NHE subgroup and are related to yeast Nhx1 and mammalian NHE6–NHE9 (Brett et al., 2005a; Pardo et al., 2006). The most extensively characterized of these is the Arabidopsis vacuolar Na+/H+ exchanger AtNHX1 which has been examined predominantly on the basis of vacuolar Na+ sequestration and its ability to provide tolerance to salt stress (Apse et al., 1999, 2003). However, AtNHX1 can also transport K+ and K+ homeostasis is likely to be a major physiological role of this protein under non-stress conditions (Venema et al., 2002; Bassil et al., 2011b). It was studies of NHX1 orthologs from the flowers of the morning glory plant (Ipomoea species) that first clearly demonstrated a function of the plant Na+/H+ exchangers in vacuolar pH regulation and flower coloration.
Vacuolar pH is important for flower coloration since the color that vacuolar-localized anthocyanins give is dependent on pH. A mutant Japanese morning glory (Ipomoea nil) flower which does not show the characteristic petal color change during flower opening from purple to blue was found to have a mutated NHX1-like gene (InNHX1), which was the cause of the color change mutation (Fukada-Tanaka et al., 2000; Yamaguchi et al., 2001). InNHX1, and related ItNHX1 from the Heavenly Blue morning glory (I. tricolor), mediate vacuolar alkalinization from pH 6.6 to 7.7 to allow the color change of the HBA pigment (Yamaguchi et al., 2001; Yoshida et al., 2005; Figure 2). Furthermore, increased activation of the V-ATPase and vacuolar H+-PPase was shown to occur during flower opening and color change (Yoshida et al., 2005), indicating that the exchanger works in concert with the H+ pump presumably to prevent excessive alkalinization. This demonstrates that a balance between H+ pump and cation/H+ exchange activity carefully regulates vacuolar pH. It was also confirmed that it is the flux of H+ in exchange with K+ rather than Na+ that increases the vacuolar pH (Yoshida et al., 2009). These authors also suggested that this increased vacuolar accumulation of K+ may contribute to the increase in vacuolar osmoticum for cell expansion growth and thus drive flower opening in a manner that is directly coordinated with color change. However, cell expansion and flower opening still occurs in the nhx1 mutant plants, therefore NHX1 K+/H+ exchange activity is not essential for this process, suggesting that other regulators of cell expansion such as the uptake of organic compounds or other K+ transport pathways are also involved.
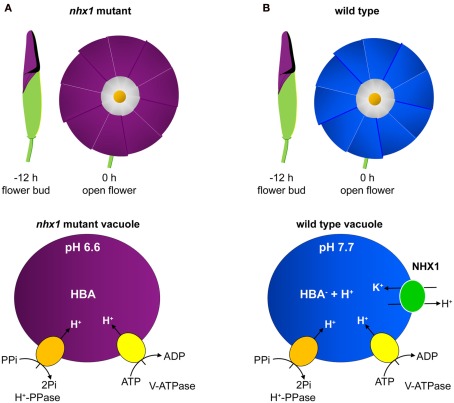
Figure 2.
The effect of NHX1 deletion and altered vacuolar pH on flower petal coloration in morning glory flowers. The contrast between flower coloration and vacuolar H+ fluxes in nhx1 mutant (A) and wild type (B) plants is diagrammatically represented. In wild type plants (B), as the flowers open there is a color change from purple to blue which is dependent on an alkalinization of the vacuolar lumen causing a change in the ionic form of the pigment HBA. When NHX1 is deleted (A) the color change does not occur when the flowers open as the lack of NHX1-dependent K+/H+ exchange activity means that the acidification of the vacuolar lumen by the H+ pumps is not reduced. Figure adapted from Yoshida et al. (2009).
This concept of vacuolar NHX transporters as mediators of K+ homeostasis and vacuolar pH control is not restricted to flower vacuoles. Analysis of Arabidopsis vacuolar NHX knockout mutants has found developmental phenotypes that are unlikely to be just a consequence of altered Na+ transport but appear to be due to altered K+ and pH homeostasis (Apse et al., 2003; Sottosanto et al., 2004; Bassil et al., 2011b). In a nhx1 nhx2 double mutant lacking two of the vacuolar exchangers, vacuolar pH of mature root cells was reduced from pH 6.3 in wild type to pH 5.8, and reduced from pH 5.5 to 5.2 in hypocotyl cells (Bassil et al., 2011b). The particular importance of vacuolar pH in cell expansion and vesicular trafficking is not fully clear, but the observation that the nhx1 knockout has altered expression of genes involved in intravesicular trafficking (Sottosanto et al., 2004), indicates that like yeast Nhx1, via control of vacuolar pH, AtNHX1 is a determinant in protein trafficking.
Unlike vacuolar AtNHX1 and AtNHX2, some of the Arabidopsis NHX proteins are endosomal and may play a role more analogous to the mammalian endosomal NHE proteins. AtNHX5 and AtNHX6 co-localize with known Golgi and TGN markers, and with the secretory pathway V-ATPase (VHA-a1), and protein trafficking defects were observed in an nhx5 nhx6 mutant (Bassil et al., 2011a). An alteration in luminal pH was not measured in this study, but the data infers a role of these exchangers in allowing H+ leak to counter V-ATPase-mediated acidification and maintain the required luminal pH.
Na+ gradients are established in animal cells through the action of Na+ pumps to drive secondary active transport, and thus can be utilized by Na+/H+ exchangers for pH regulation. However, higher plant cells do not generate Na+ gradients. As described above, plant and yeast NHX proteins are now known to be able to transport K+ in addition to Na+, and therefore it is likely that K+/H+ exchange is the predominant means by which NHX-mediated endomembrane and vacuolar alkalinization occurs (Brett et al., 2005b; Martinez-Munoz and Pena, 2005; Yoshida et al., 2009; Bassil et al., 2011b). However, this requirement of K+ in pH regulation has consequence for cellular K+ homeostasis as K+/H+ exchange activity will potentially lead to a significant reduction in cytosolic K+ concentration. K+ is an ion of central importance in plant cells. In addition to mediating osmotic adjustment, cellular K+ flux acts as a counterbalance for the fluxes of other ions, particularly protons (Amtmann and Blatt, 2009). In order to allow K+ to play such a role, cellular K+ transport is carefully regulated through the action of multiple plasma membrane and tonoplast K+ permeable channels and transporters (Maathuis, 2007; Karley and White, 2009). Despite a high concentration of K+ within plant cells there is a strong driving force for K+ influx into the cytosol across the plasma membrane, and therefore cytosolic K+ concentration is unlikely to significantly reduce through endomembrane K+/H+ exchange activity. Furthermore, there are many endomembrane-localized K+ release channels that will prevent excessive intracellular K+ accumulation (Maathuis, 2007).
It is less clear whether plant NHX-type exchangers are involved in cytosolic pH regulation. This is thought to be unlikely as cytosolic pH is very tightly controlled by the H+ pumps and the biochemical pH-stat (see above). However, it has been observed that in poppy, the product (lysophosphatidylcholine) of microbial elicitor-activator phospholipase A2 induces a cytosolic acidification via activation of a vacuolar Na+/H+ exchanger, giving rise to an estimated cytosolic pH shift from pH 7.3 to 6.7 (Viehweger et al., 2002). Such a cytosolic pH change may be the signal to induce the biosynthesis of phytoalexins. This study implicates a vacuolar ion/H+ exchanger not only in the modulation of cytosolic pH, but in the generation of a pH signal.
pH Regulation by CHX-Type Transporters
CHX genes belonging to the CPA2 superfamily of ion exchangers encode putative K+, Na+/H+ exchangers (Brett et al., 2005a). A CHX transporter from yeast, Kha1, is an endomembrane K+/H+ exchanger that has a growth defect to high external pH when deleted, suggestive of a role in pH control (Maresova and Sychrova, 2005). Plants possess a very high number of CHX genes; for example, there are 28 in Arabidopsis and 17 in rice, which are localized in different tissues and probably at different cellular membranes (Sze et al., 2004; Pardo et al., 2006) but the transport characteristics and roles of most of these proteins are unknown. Some of the CHX proteins have been shown to be localized at endomembrane compartments. Five related CHXs (AtCHX16–AtCHX20) and Kha1 were shown to be able to rescue the alkaline pH (pH 7.5) sensitivity of a yeast mutant, implicating them as possible pH regulators (Chanroj et al., 2011). AtCHX17, which has been previously shown to function as a K+ transporter and be important in plant K+ homeostasis (Cellier et al., 2004; Maresova and Sychrova, 2006), was shown to co-localize with pre-vacuolar compartment (PVC) markers, and from a yeast-based protein sorting assay, AtCHX17 was shown to affect protein sorting at alkaline pH but did not alter cytosolic or vacuolar pH in yeast (Chanroj et al., 2011). In contrast, AtCHX20, which is also involved in K+ homeostasis (Padmanaban et al., 2007), was found to induce vacuolar alkalinization when expressed in yeast (Chanroj et al., 2011). AtCHX20 co-localized with an ER marker and was also able to affect protein sorting. Some CHX transporters are therefore clearly important in regulating endosomal protein sorting and trafficking, but only specific CHX isoforms modulate endomembrane pH.
The Role of Anion/H+ Exchangers for Counter-Ion Balance and pH Regulation
The transport of H+ into the lumen of acidic endomembrane compartments by the V-ATPase generates an inside positive electrical potential that will increase the electrochemical gradient and therefore inhibit further inward movement of H+ and promote outward H+ leak. The luminal influx of anions acting as counter-ions will prevent the build up of an electrical potential, maintain H+ accumulation, and thus lumen acidification. Anion transport pathways therefore also have an important role in intracellular pH regulation. Studies in a variety of eukaryotic systems have identified Cl− channels (CLC) and exchangers, in particular those that are members of the CLC family, as key players in secretory/endocytic pathway pH regulation (Jentsch, 2007; Zifarelli and Pusch, 2010). Yeast possess a single CLC called Gef1 which is a late Golgi/PVC-localized Cl−/H+ exchanger and exhibits phenotypes when deleted which are consistent with a role in maintaining Golgi lumen acidification (Gaxiola et al., 1998; Schwappach et al., 1998; Braun et al., 2010). In order to properly regulate the membrane potential, the CLC exchanger requires a Cl−:H+ stoichiometry of at least 2:1, which is the ratio that has been experimentally confirmed for an algal CLC (Feng et al., 2010). Arabidopsis has seven CLC genes, four of which encode vacuolar proteins and function predominantly as exchangers (Zifarelli and Pusch, 2010). However, two of the Arabidopsis CLCs AtCLC-d and AtCLC-f are analogous to Gef1, are able to complement the pH-dependent growth phenotype of the gef1 yeast mutant and are Golgi-localized (Gaxiola et al., 1998; Marmagne et al., 2007; von der Fecht-Bartenbach et al., 2007), suggesting that they also have Cl−/H+ exchange activity, although instead could conceivably act as a counter-ion if it was the substrate. More specifically, AtCLC-d is present at the TGN where it co-localizes with the VHA-a1 V-ATPase subunit (von der Fecht-Bartenbach et al., 2007). Furthermore, this study found that a clcd-1 mutant had reduced root growth when grown on acidic pH conditions compared to wild type. Defective TGN acidification due to a lack of anion accumulation may therefore impair vesicular trafficking and subsequently root growth.
An Indirect Role for Ca2+/H+ Exchangers in pH Regulation
While some alkali cation Na+, K+/H+ exchangers are implicated in direct intracellular pH regulation, there is no clear evidence to suggest that divalent ion/H+ exchangers such as Ca2+/H+ exchangers likewise play a pH regulatory role. NHX- or CHX-mediated K+/H+ exchange can be maintained by a high cytosolic K+ concentration, but the low (sub-micromolar) resting cytosolic Ca2+ concentration suggests against Ca2+/H+ exchange as a significant component for pH modulation. As the Ca2+/H+ exchanger requires 3H+ to drive the vacuolar accumulation of 1 Ca2+ ion, as calculated from stoichiometric analysis (Blackford et al., 1990), large elevations in cytosolic Ca2+ during a stimulus-induced Ca2+ signaling event leading to activation of Ca2+/H+ exchange may have a significant, although probably transient effect on intracellular pH. There is no evidence that Ca2+/H+ exchange activity can alter cytosolic pH, but it has been observed in isolated Catharanthus roseus vacuoles that sustained Ca2+/H+ exchange activity at the tonoplast can cause a long-lasting increase in vacuolar pH, from pH 5.6 to 6.2 (Guern et al., 1989). However, it is unknown whether this Ca2+-mediated disturbance in vacuolar pH also occurs within intact cells or whether it has any physiological relevance.
The vacuolar membrane has Ca2+/H+ exchangers encoded by CAX genes that play a major role in cellular Ca2+ homeostasis and are implicated in Ca2+ signaling function (Cai and Lytton, 2004; McAinsh and Pittman, 2009; Manohar et al., 2011). However, by modulating Ca2+ signal generation, these exchangers may indirectly be involved in pH regulation. In addition, the activity of Ca2+/H+ exchangers can themselves be regulated by pH change (Pittman et al., 2005). It is well known that Ca2+ plays an indirect role in pH regulation through Ca2+ signaling (Felle, 2001), such as through the modulation of H+ pumps (Fuglsang et al., 2007). Indeed Arabidopsis knockout lines mutated in CAX genes display phenotypes that suggest impaired pH control. For example, the cax3 mutant shows hypersensitivity to low pH, displayed by reduced root growth on acidic medium (pH 4.5) which is not seen in the wild type (Zhao et al., 2008). However, it is unclear whether this pH sensitivity is due directly to altered CAX3 Ca2+ transport activity or an indirect affect, as P-type H+-ATPase activity is also reduced in the cax3 mutant. Other CAX mutants display morphological and developmental phenotypes: cax1 plants have a reduction in root growth and inflorescence stem growth (Cheng et al., 2003), while a cax1 cax3 double mutant has a dramatic reduction in plant growth including impaired cell wall extensibility (Cheng et al., 2005; Conn et al., 2011). Although these phenotypes are correlated with impaired vacuolar Ca2+ sequestration, it is unknown whether perturbed pH maintenance may also contribute to some of the phenotypes.
Potential of Coordination between H+ Pumps and Ion/H+ Exchangers
Endomembrane ion/H+ exchangers are dependent on the proton motive force generated by the primary V-ATPase and H+-PPase H+ pumps. But it is still unclear from many of these gene knockout and heterologous expression studies described in this article, whether the ion/H+ exchangers play an active role in mediating endomembrane pH homeostasis or whether they effect luminal pH in a passive manner by merely acting as a H+ leak to reduce luminal pH. Furthermore, it is unknown whether there is direct coordination between the H+ pump and ion/H+ exchanger to generate the required luminal pH, such as whether both sets of transporters directly interact to allow dynamic coordination. There is no clear-cut evidence of direct interaction between an ion/H+ exchanger and a V-ATPase. However, such coordination might be indirect. For example, the Arabidopsis CIPK24/SOS2 kinase regulates NHX Na+/H+ exchange activity (Qiu et al., 2004) and also regulates and interacts with the V-ATPase, through interaction with VHA-B subunits (Batelli et al., 2007).
Ca2+/H+ exchangers appear to modulate V-ATPase activity by an as yet unknown mechanism (Barkla et al., 2008). In cax1, cax2, and cax3 mutants a significant decrease in V-ATPase activity was measured and this reduction in V-ATPase activity was exacerbated in the cax1 cax3 double mutant (Cheng et al., 2003, 2005; Pittman et al., 2004). In contrast, over-expression of AtCAX1 or AtCAX2 increased V-ATPase activity (Barkla et al., 2008). These results suggest that there may be a feedback mechanism to dampen H+ pump activity when vacuolar H+ leak by the cation/H+ antiporter is reduced, possibly to prevent excessive acidification. This feedback may involve direct interaction between the cation/H+ antiporter and the V-ATPase although as yet there is no evidence to support this. However, the V-ATPase-interacting protein CIPK24, also interacts with AtCAX1 (Cheng et al., 2004) so possibly this feedback might also be indirect. There was no significant alteration to vacuolar H+-PPase activity following CAX gene manipulation (Cheng et al., 2003; Pittman et al., 2004). It is possible that only the V-ATPase is normally involved in providing the H+ gradient to energize CAX-mediated Ca2+/H+ exchange activity, although under some circumstances H+-PPase activity can energize Ca2+/H+ exchange such as when AVP1 is over-expressed leading to enhanced Ca2+ transport (Park et al., 2005).
Perspectives
It is becoming clear that pH regulation of endomembrane secretory compartments and the vacuole is essential for a range of critical processes, including osmoregulation, membrane trafficking, and fusion, which subsequently control plant growth and development. The V-ATPase has a significant role in endomembrane acidification, but much less is known regarding how the V-ATPase is regulated or the role of other components in pH regulation. There is now strong evidence that a number of ion/H+ exchangers notably K+/H+ exchangers, and anion/H+ exchangers are equally important for intracellular pH regulation, yet there are many exchanger isoforms that remain to be assessed. The availability of genomic resources to systematically examine all ion/H+ exchangers in plants, and the development of sensitive and dynamic reporters for intracellular pH measurements, should allow us to determine in full the components required for intracellular pH regulation. This should then allow us to understand the importance of cellular pH homeostasis in better detail.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ali R., Brett C. L., Mukherjee S., Rao R. (2004). Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279, 4498–4506 10.1074/jbc.M313314200 [DOI] [PubMed] [Google Scholar]
- Amtmann A., Blatt M. R. (2009). Regulation of macronutrient transport. New Phytol. 181, 35–52 10.1111/j.1469-8137.2008.02666.x [DOI] [PubMed] [Google Scholar]
- Apse M. P., Aharon G. S., Snedden W. A., Blumwald E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285, 1256–1258 10.1126/science.285.5431.1256 [DOI] [PubMed] [Google Scholar]
- Apse M. P., Sottosanto J. B., Blumwald E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36, 229–239 10.1046/j.1365-313X.2003.01871.x [DOI] [PubMed] [Google Scholar]
- Barkla B. J., Hirschi K. D., Pittman J. K. (2008). Exchangers man the pumps: functional interplay between proton pumps and proton-coupled Ca2+ exchangers. Plant Signal. Behav. 3, 354–356 10.4161/psb.3.5.5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Ohto M. A., Esumi T., Tajima H., Zhu Z., Cagnac O., Belmonte M., Peleg Z., Yamaguchi T., Blumwald E. (2011a). The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23, 224–239 10.1105/tpc.111.089581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Tajima H., Liang Y.-C., Ohto M.-A., Ushijima K., Nakano R., Esumi T., Coku A., Belmonte M., Blumwald E. (2011b). The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23, 3482–3497 10.1105/tpc.111.089581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelli G., Verslues P. E., Agius F., Qiu Q., Fujii H., Pan S., Schumaker K. S., Grillo S., Zhu J. K. (2007). SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 27, 7781–7790 10.1128/MCB.00430-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford S., Rea P. A., Sanders D. (1990). Voltage sensitivity of H+/Ca2+ antiport in higher-plant tonoplast suggests a role in vacuolar calcium accumulation. J. Biol. Chem. 265, 9617–9620 [PubMed] [Google Scholar]
- Braun N. A., Morgan B., Dick T. P., Schwappach B. (2010). The yeast CLC protein counteracts vesicular acidification during iron starvation. J. Cell Sci. 123, 2342–2350 10.1242/jcs.068403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C. L., Donowitz M., Rao R. (2005a). Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 288, C223–C239 10.1152/ajpcell.00360.2004 [DOI] [PubMed] [Google Scholar]
- Brett C. L., Tukaye D. N., Mukherjee S., Rao R. J. (2005b). The yeast endosomal Na+(K+)/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16, 1396–1405 10.1091/mbc.E04-11-0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brux A., Liu T. Y., Krebs M., Stierhof Y. D., Lohmann J. U., Miersch O., Wastermack C., Schumacher K. (2008). Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 20, 1088–1100 10.1105/tpc.108.058362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. J., Lytton J. (2004). The cation/Ca2+ exchanger superfamily: phylogenetic analysis and structural implications. Mol. Biol. Evol. 21, 1692–1703 10.1093/molbev/msh177 [DOI] [PubMed] [Google Scholar]
- Casey J. R., Grinstein S., Orlowski J. (2010). Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- Cellier F., Conejero G., Ricaud L., Luu D. T., Lepetit M., Gosti F., Casse F. (2004). Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 39, 834–846 10.1111/j.1365-313X.2004.02177.x [DOI] [PubMed] [Google Scholar]
- Chanroj S., Lu Y. X., Padmanaban S., Nanatani K., Uozumi N., Rao R., Sze H. (2011). Plant-specific cation/H+ exchanger 17 and its homologs are endomembrane K+ transporters with roles in protein sorting. J. Biol. Chem. 286, 33931–33941 10.1074/jbc.M111.252650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Barkla B. J., Shigaki T., Hirschi K. D. (2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15, 347–364 10.1105/tpc.013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Shigaki T., Lachmansingh J., LeClere S., Lahner B., Salt D. E., Hirschi K. D. (2005). Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 138, 2048–2060 10.1104/pp.105.061218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N. H., Pittman J. K., Zhu J. K., Hirschi K. D. (2004). The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 279, 2922–2926 10.1074/jbc.M402828200 [DOI] [PubMed] [Google Scholar]
- Conn S. J., Gilliham M., Athman A., Schreiber A. W., Baumann U., Moller I., Cheng N. H., Stancombe M. A., Hirschi K. D., Webb A. A. R., Burton R., Kaiser B. N., Tyerman S. D., Leigh R. A. (2011). Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23, 240–257 10.1105/tpc.109.072769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. M., Hunt I., Sanders D. (1994). Vacuolar H+-pumping ATPase variable transport coupling ratio controlled by pH. Proc. Natl. Acad. Sci. U.S.A. 91, 8547–8551 10.1073/pnas.91.18.8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y. D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18, 715–730 10.1105/tpc.105.037978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Tavakoli N., Kluge C., Mimura T., Sharma S. S., Harris G. C., Chardonnens A. N., Golldack D. (2001). Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J. Exp. Bot. 52, 1969–1980 10.1093/jexbot/52.363.1969 [DOI] [PubMed] [Google Scholar]
- Felle H. H. (2001). pH: signal and messenger in plant cells. Plant Biol. 3, 577–591 10.1055/s-2001-19372 [DOI] [Google Scholar]
- Feng L., Campbell E. B., Hsiung Y., MacKinnon R. (2010). Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science 330, 635–641 10.1126/science.1190614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjani A., Segami S., Horiguchi G., Muto Y., Maeshima M., Tsukaya H. (2011). Keep an eye on PPi: the vacuolar-type H+ pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23, 2895–2908 10.1105/tpc.111.085415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang A. T., Guo Y., Cuin T. A., Qiu Q. S., Song C. P., Kristiansen K. A., Bych K., Schulz A., Shabala S., Schumaker K. S., Palmgren M. G., Zhu J. K. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19, 1617–1634 10.1105/tpc.105.035626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada-Tanaka S., Inagaki Y., Yamaguchi T., Saito N., Iida S. (2000). Colour-enhancing protein in blue petals – spectacular morning glory blooms rely on a behind-the-scenes proton exchanger. Nature 407, 581–581 10.1038/35036686 [DOI] [PubMed] [Google Scholar]
- Gaxiola R. A., Palmgren M. G., Schumacher K. (2007). Plant proton pumps. FEBS Lett. 581, 2204–2214 10.1016/j.febslet.2007.03.050 [DOI] [PubMed] [Google Scholar]
- Gaxiola R. A., Yuan D. S., Klausner R. D., Fink G. R. (1998). The yeast CLC chloride channel functions in cation homeostasis. Proc. Natl. Acad. Sci. U.S.A. 95, 4046–4050 10.1073/pnas.95.7.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Kurkdjian A., Manigault P., Manigault J., Gillet B., Beloeil J. C., Lallemand J. Y. (1989). Regulation of vacuolar pH of plant cells. II. A 31P NMR-study of the modifications of vacuolar pH in isolated vacuoles induced by proton pumping and cation/H+ exchanges. Plant Physiol. 89, 27–36 10.1104/pp.89.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Hermesdorf A., Brux A., Gruber A., Gruber G., Schumacher K. (2006). A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett. 580, 932–939 10.1016/j.febslet.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Hurth M. A., Suh S. J., Kretzschmar T., Geis T., Bregante M., Gambale F., Martinoia E., Neuhaus H. E. (2005). Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol. 137, 901–910 10.1104/pp.104.058453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J. (2007). Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J. Physiol. 578, 633–640 10.1113/jphysiol.2006.124719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karley A. J., White P. J. (2009). Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Curr. Opin. Plant Biol. 12, 291–298 10.1016/j.pbi.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Krebs M., Beyhl D., Gorlich E., Al-Rasheid K. A. S., Marten I., Stierhof Y.-D., Hedrich R., Schumacher K. (2010). Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. U.S.A. 107, 3251–3256 10.1073/pnas.0910993107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Sachs G., Padan E. (2011). Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343 10.1038/nrmicro2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. S., Yang H. B., Peer W. A., Richter G., Blakeslee J., Bandyopadhyay A., Titapiwantakun B., Undurraga S., Khodakovskaya M., Richards E. L., Krizek B., Murphy A. S., Gilroy S., Gaxiola R. (2005). Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310, 121–125 10.1126/science.1118391 [DOI] [PubMed] [Google Scholar]
- Maathuis F. J. M. (2007). Monovalent cation transporters; establishing a link between bioinformatics and physiology. Plant Soil 301, 1–15 10.1007/s11104-007-9429-8 [DOI] [Google Scholar]
- Manohar M., Shigaki T., Hirschi K. D. (2011). Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol. 13, 561–569 10.1111/j.1438-8677.2011.00466.x [DOI] [PubMed] [Google Scholar]
- Maresova L., Sychrova H. (2005). Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol. Microbiol. 55, 588–600 10.1111/j.1365-2958.2004.04410.x [DOI] [PubMed] [Google Scholar]
- Maresova L., Sychrova H. (2006). Arabidopsis thaliana CHX17 gene complements the kha1 deletion phenotypes in Saccharomyces cerevisiae. Yeast 23, 1167–1171 10.1002/yea.1424 [DOI] [PubMed] [Google Scholar]
- Marmagne A., Vinauger-Douard M., Monachello D., de Longevialle A. F., Charon C., Allot M., Rappaport F., Wollman F. A., Barbier-Brygoo H., Ephritikhine G. (2007). Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J. Exp. Bot. 58, 3385–3393 10.1093/jxb/erm187 [DOI] [PubMed] [Google Scholar]
- Martinez-Munoz G. A., Pena A. (2005). In situ study of K+ transport into the vacuole of Saccharomyces cerevisiae. Yeast 22, 689–704 10.1002/yea.1238 [DOI] [PubMed] [Google Scholar]
- Mäser P., Thomine S., Schroeder J. I., Ward J. M., Hirschi K., Sze H., Talke I. N., Amtmann A., Maathuis F. J. M., Sanders D., Harper J. F., Tchieu J., Gribskov M., Persans M. W., Salt D. E., Kim S. A., Guerinot M. L. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667 10.1104/pp.126.4.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh M. R., Pittman J. K. (2009). Shaping the calcium signature. New Phytol. 181, 275–294 10.1111/j.1469-8137.2008.02682.x [DOI] [PubMed] [Google Scholar]
- Muller M. L., Taiz L. (2002). Regulation of the lemon-fruit V-ATPase by variable stoichiometry and organic acids. J. Membr. Biol. 185, 209–220 10.1007/s00232-001-0124-z [DOI] [PubMed] [Google Scholar]
- Nakamura N., Tanaka S., Teko Y., Mitsui K., Kanazawa H. (2005). Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 280, 1561–1572 10.1074/jbc.M410041200 [DOI] [PubMed] [Google Scholar]
- Orij R., Brul S., Smits G. J. (2011). Intracellular pH is a tightly controlled signal in yeast. Biochim. Biophys. Acta 1810, 933–944 10.1016/j.bbagen.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Padmanaban S., Chanroj S., Kwak J. M., Li X. Y., Ward J. M., Sze H. (2007). Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol. 144, 82–93 10.1104/pp.106.092155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. M., Cubero B., Leidi E. O., Quintero F. J. (2006). Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 57, 1181–1199 10.1093/jxb/erj114 [DOI] [PubMed] [Google Scholar]
- Park S., Li J. S., Pittman J. K., Berkowitz G. A., Yang H. B., Undurraga S., Morris J., Hirschi K. D., Gaxiola R. A. (2005). Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc. Natl. Acad. Sci. U.S.A. 102, 18830–18835 10.1073/pnas.0502051102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P., Touret N., Grinstein S. (2004). The pH of the secretory pathway: measurement, determinants, and regulation. Physiology 19, 207–215 10.1152/physiol.00005.2004 [DOI] [PubMed] [Google Scholar]
- Perez-Castineira J. R., Hernandez A., Drake R., Serrano A. (2011). A plant proton-pumping inorganic pyrophosphatase functionally complements the vacuolar ATPase transport activity and confers bafilomycin resistance in yeast. Biochem. J. 437, 269–278 10.1042/BJ20110447 [DOI] [PubMed] [Google Scholar]
- Pittman J. K., Shigaki T., Hirschi K. D. (2005). Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett. 579, 2648–2656 10.1016/j.febslet.2005.03.085 [DOI] [PubMed] [Google Scholar]
- Pittman J. K., Shigaki T., Marshall J. L., Morris J. L., Cheng N. H., Hirschi K. D. (2004). Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol. Biol. 56, 959–971 10.1007/s11103-004-6446-3 [DOI] [PubMed] [Google Scholar]
- Qiu Q. S., Guo Y., Quintero F. J., Pardo J. M., Schumaker K. S., Zhu J. K. (2004). Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 279, 207–215 10.1074/jbc.M401464200 [DOI] [PubMed] [Google Scholar]
- Roos W., Viehweger K., Dordschbal B., Schumann B., Evers S., Steighardt J., Schwartze W. (2006). Intracellular pH signals in the induction of secondary pathways – the case of Eschscholzia californica. J. Plant Physiol. 163, 369–381 10.1016/j.jplph.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Sakano K. (2001). Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defense reaction and secondary metabolism. Int. Rev. Cytol. 206, 1–44 10.1016/S0074-7696(01)06018-1 [DOI] [PubMed] [Google Scholar]
- Schumacher K., Krebs M. (2010). The V-ATPase: small cargo, large effects. Curr. Opin. Plant Biol. 13, 724–730 10.1016/j.pbi.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Schwappach B., Stobrawa S., Hechenberger M., Steinmeyer K., Jentsch T. J. (1998). Golgi localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J. Biol. Chem. 273, 15110–15118 10.1074/jbc.273.24.15110 [DOI] [PubMed] [Google Scholar]
- Segami S., Nakanishi Y., Sato M. H., Maeshima M. (2010). Quantification, organ-specific accumulation and intracellular localization of type II H+-pyrophosphatase in Arabidopsis thaliana. Plant Cell Physiol. 51, 1350–1360 10.1093/pcp/pcq096 [DOI] [PubMed] [Google Scholar]
- Sottosanto J. B., Gelli A., Blumwald E. (2004). DNA array analyses of Arabidopsis thaliana lacking a vacuolar Na+/H+ antiporter: impact of AtNHX1 on gene expression. Plant J. 40, 752–771 10.1111/j.1365-313X.2004.02253.x [DOI] [PubMed] [Google Scholar]
- Strompen G., Dettmer J., Stierhof Y. D., Schumacher K., Jurgens G., Mayer U. (2005). Arabidopsis vacuolar H+ – ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J. 41, 125–132 10.1111/j.1365-313X.2004.02283.x [DOI] [PubMed] [Google Scholar]
- Swanson S. J., Choi W.-G., Chanoca A., Gilroy S. (2011). In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant Biol. 62, 273–297 10.1146/annurev-arplant-042110-103832 [DOI] [PubMed] [Google Scholar]
- Sze H., Li X. H., Palmgren M. G. (1999). Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11, 677–689 10.1105/tpc.11.4.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Padmanaban S., Cellier F., Honys D., Cheng N. H., Bock K. W., Conejero G., Li X. Y., Twell D., Ward J. M., Hirschi K. D. (2004). Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol. 136, 2532–2547 10.1104/pp.104.046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Schumacher K., Muller M. L., Padmanaban S., Taiz L. (2002). A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci. 7, 157–161 10.1016/S1360-1385(02)02240-9 [DOI] [PubMed] [Google Scholar]
- Tarsio M., Zheng H., Smardon A. M., Martinez-Munoz G. A., Kane P. M. (2011). Consequences of loss of Vph1 protein-containing vacuolar ATPases (V-ATPases) for overall cellular pH homeostasis. J. Biol. Chem. 286, 28089–28096 10.1074/jbc.M111.251363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C., Sutka M., Javot H., Gout E., Gerbeau P., Luu D. T., Bligny R., Maurel C. (2003). Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425, 393–397 10.1038/nature01853 [DOI] [PubMed] [Google Scholar]
- Venema K., Quintero F. J., Pardo J. M., Donaire J. P. (2002). The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J. Biol. Chem. 277, 2413–2418 10.1074/jbc.M105043200 [DOI] [PubMed] [Google Scholar]
- Viehweger K., Dordschbal B., Roos W. (2002). Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell 14, 1509–1525 10.1105/tpc.002329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J., Bogner M., Krebs M., Stierhof Y. D., Schumacher K., Ludewig U. (2007). Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 50, 466–474 10.1111/j.1365-313X.2007.03061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. M., Grabe M., Adams S., Tsien R. Y., Moore H. P. H., Machen T. E. (2001). Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276, 33027–33035 10.1074/jbc.M004401200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Fukada-Tanaka S., Inagaki Y., Saito N., Yonekura-Sakakibara K., Tanaka Y., Kusumi T., Iida S. (2001). Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol. 42, 451–461 10.1093/pcp/pce152 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kawachi M., Mori M., Maeshima M., Kondo M., Nishimura M., Kondo T. (2005). The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. Heavenly blue. Plant Cell Physiol. 46, 407–415 10.1093/pcp/pci217 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Miki N., Mononoi K., Kawachi M., Katou K., Okazaki Y., Uozumi N., Maeshima M., Kondo T. (2009). Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly blue. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85, 187–197 10.2183/pjab.85.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Barkla B. J., Marshall J., Pittman J. K., Hirschi K. D. (2008). The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227, 659–669 10.1007/s00425-008-0711-7 [DOI] [PubMed] [Google Scholar]
- Zifarelli G., Pusch M. (2010). CLC transport proteins in plants. FEBS Lett. 584, 2122–2127 10.1016/j.febslet.2009.12.042 [DOI] [PubMed] [Google Scholar]