Abstract
It has been estimated that one-third of the world’s population lack sufficient Zn for adequate nutrition. This can be alleviated by increasing dietary Zn intakes through Zn biofortification of edible crops. Biofortification strategies include the application of Zn-fertilizers and the development of crop genotypes that acquire more Zn from the soil and accumulate it in edible portions. Zinc concentrations in roots, leaves, and stems can be increased through the application of Zn-fertilizers. Root Zn concentrations of up to 500–5000 mg kg−1 dry matter (DM), and leaf Zn concentrations of up to 100–700 mg kg−1 DM, can be achieved without loss of yield when Zn-fertilizers are applied to the soil. It is possible that greater Zn concentrations in non-woody shoot tissues can be achieved using foliar Zn-fertilizers. By contrast, Zn concentrations in fruits, seeds, and tubers are severely limited by low Zn mobility in the phloem and Zn concentrations higher than 30–100 mg kg−1 DM are rarely observed. However, genetically modified plants with improved abilities to translocate Zn in the phloem might be used to biofortify these phloem-fed tissues. In addition, genetically modified plants with increased tolerance to high tissue Zn concentrations could be used to increase Zn concentrations in all edible produce and, thereby, increase dietary Zn intakes.
Keywords: Arabidopsis, bean, cassava, maize, potato, rice, wheat, zinc
Introduction
Zinc (Zn) is an essential element for human nutrition (White and Broadley, 2005; Graham et al., 2007). Symptoms of Zn-deficiency include stunting, diarrhea, and pneumonia in children, with the latter two contributing significantly to infant mortality (Stein et al., 2005). The US recommended daily allowance (RDA, or adequate intake) of Zn is 8.0–13.0 mg and the UK guidance daily reference nutrient intake (RNI) is 7.0–13.0 mg for adults (Department of Health (UK), 1991; Institute of Medicine (USA), 2001). Unfortunately, the diets of many people across the world lack sufficient Zn for their adequate nutrition (White and Broadley, 2009; Bouis and Welch, 2010; Stein, 2010; Sayre et al., 2011). This has been attributed to sourcing produce from land with low mineral phytoavailability, eating crops with inherently low tissue mineral concentrations, or consuming processed foods. It has been estimated that almost one-third of the world’s population consumes less Zn than the US RDA and that Zn-deficiency contributes 1.9% of the overall burden of disease caused by major health risks worldwide (World Health Organization, 2002; Hotz and Brown, 2004). This has considerable socio-economic impacts (Stein, 2010).
Dietary Zn intakes can be increased through a variety of interventions (Stein, 2010). These include both agronomic and genetic biofortification of edible crops (Graham et al., 1999, 2001, 2007; White and Broadley, 2005, 2009; Cakmak, 2008; Khoshgoftarmanesh et al., 2009; Bouis and Welch, 2010; Martínez-Ballesta et al., 2010). Agronomic biofortification can be achieved by increasing soil Zn phytoavailability or by applying Zn-fertilizers. This requires appropriate infrastructures, but can be very successful in regions where mineral fertilizers are used to increase crop yields and Zn is added to these at the point of manufacture or distribution (Cakmak, 2004, 2009). Genetic biofortification is predicated on increasing Zn acquisition from the soil and its accumulation in edible portions. In most agricultural soils there is sufficient Zn to produce biofortified crops for many years, provided it becomes phytoavailable (Graham et al., 1999). Genetic biofortification strategies are, of course, ineffective if there is insufficient Zn present in the soil. Most economic analyses suggest that genetic strategies toward Zn biofortification are more practical, enduring, and cost effective than dietary diversification, supplementation, or food fortification programs for increasing dietary Zn intakes of vulnerable populations (Horton, 2006; Graham et al., 2007; Stein et al., 2007; Ma et al., 2008; Bouis and Welch, 2010; Meenakshi et al., 2010; Stein, 2010).
Several national and international projects are addressing Zn biofortification of edible crops (Graham et al., 2001, 2007; Cakmak, 2004; Pfeiffer and McClafferty, 2007; White and Broadley, 2009; Bouis and Welch, 2010; Stein, 2010; Sayre et al., 2011). The target Zn concentrations set by the HarvestPlus program are 28 μg g−1 dry matter (DM) in polished rice, 38 mg kg−1 DM in wheat grain, 38 mg kg−1 DM in maize, 66 mg kg−1 DM in pearl millet, 56 mg kg−1 DM in beans, 34 mg kg−1 DM in cassava roots, and 70 mg kg−1 DM in roots of sweet potatoes (Bouis and Welch, 2010). These target concentrations are considered to be conservative, and have been exceeded in breeding lines of rice, wheat, and maize (Bouis and Welch, 2010). This article asks whether higher target Zn concentrations can be achieved and, more broadly, what the physiological limits to Zn biofortification of crops might be. It considers (a) the physiological requirements and tolerance of Zn in crop plants, (b) the uptake and distribution of Zn between and within plant organs, (c) agronomic strategies to increase Zn concentrations of edible tissues, (d) genetic variation in Zn concentrations of edible portions within plant species, and (e) transgenic strategies to increase Zn concentrations of edible produce.
Plant Physiology
Physiological requirements and tolerance of zinc in plants
Plants, like other living organisms, require Zn for the regulation of transcription and translation, the structural stability of proteins, the function of oxidoreductases and hydrolytic enzymes, and the control of enzyme activities (Broadley et al., 2007; Clemens, 2010; White, 2012b). However, excessive tissue Zn concentrations are toxic. Plant species differ in both their Zn requirements and their tolerance of high tissue Zn concentrations (Broadley et al., 2007; Fageria, 2009). Most crop plants require leaf Zn concentrations greater than 15–30 mg kg−1 DM for maximal yield, and their growth is inhibited at leaf Zn concentrations greater than 100–700 mg kg−1 DM (Fageria, 2009; White and Brown, 2010). By contrast plant species that hyperaccumulate Zn, such as Noccaea caerulescens (formerly Thlaspi caerulescens) and Arabidopsis halleri, not only tolerate more Zn in their tissues than congeneric species that do not hyperaccumulate Zn but also require greater leaf Zn concentrations for optimal growth (Hammond et al., 2006; Broadley et al., 2007; White, 2012b). The trait of Zn hyperaccumulation is defined as a leaf Zn concentration exceeding 10,000 mg kg−1 DM when plants are sampled from their natural habitat, although a figure of 3000 mg kg−1 DM might be a more realistic threshold (Reeves and Baker, 2000; Broadley et al., 2007). Only 15–20 species hyperaccumulating Zn have been reported, mostly in the Brassicaceae (Broadley et al., 2007; Verbruggen et al., 2009; Krämer, 2010; White, 2012b). Zinc uptake, delivery to the xylem and tolerance in shoot tissues is maximized in plants that hyperaccumulate Zn (Broadley et al., 2007; White and Broadley, 2009; Krämer, 2010; Hassan and Aarts, 2011; Rascio and Navari-Izzo, 2011; White, 2012b). To achieve this, genes encoding enzymes synthesizing compounds enabling Zn uptake and xylem transport, and proteins catalyzing Zn uptake, vacuolar efflux and xylem loading are constitutively highly expressed in plants hyperaccumulating Zn (Hammond et al., 2006; Broadley et al., 2007; Hanikenne et al., 2008; Roosens et al., 2008; Verbruggen et al., 2009; Hassan and Aarts, 2011; Rascio and Navari-Izzo, 2011; Ó Lochlainn et al., 2011). Shoot Zn concentrations are often an order of magnitude greater than root Zn concentrations in plants that hyperaccumulate Zn, although the exact ratio depends on soil Zn phytoavailability (Frey et al., 2000; Broadley et al., 2007). It is believed that the formation of Zn-complexes and the translocation of Zn from the root to the shoot prevent the accumulation of toxic Zn concentrations in root tissues, thereby enabling plants that hyperaccumulate Zn to tolerate high Zn concentrations in the soil solution (Broadley et al., 2007; White, 2012b).
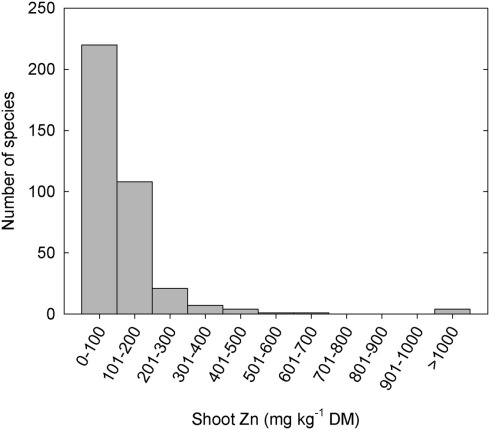
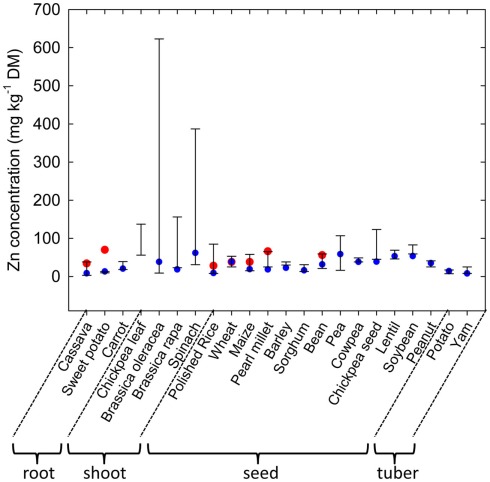
Plant species can differ greatly in their tissue Zn concentrations when grown under comparable conditions (Figure 1; Broadley et al., 2001, 2007). Shoot Zn concentrations are generally lower in the Ericales and commelinoid monocotyledons, and higher in the Brassicales, Caryophyllales, and non-commelinoid monocotyledons. Amongst the well-replicated plant families studied by Broadley et al. (2007), the lowest shoot Zn concentrations were observed in the Linaceae, Poaceae, and Solanaceae, and the highest shoot Zn concentrations were observed in the Brassicaceae, Amaranthaceae, and Salicaceae. These phylogenetic effects on shoot Zn concentration are also observed in surveys of plants growing in their natural habitats (Watanabe et al., 2007). Similarly, seeds of cereals generally have lower Zn concentrations than seeds of legumes (Figure 2). As a consequence, the occurrence of Zn-deficiency disorders has increased in populations changing from traditional diets dominated by pulses, vegetables, and fruits to diets dominated by cereals (Graham et al., 2001).
Figure 1.
Frequency distribution of mean zinc (Zn) concentrations in leaf or non-woody shoot tissues of 365 species from 48 angiosperm families grown under controlled conditions at non-toxic substrate Zn concentrations (Broadley et al., 2007). Data from 1108 studies were combined using residual maximum likelihood (REML) procedures.
Figure 2.
Variation in zinc (Zn) concentrations in roots, shoots, seeds and tubers of edible crops. Bars represent maximum and minimum values obtained for large collections of cassava (Chávez et al., 2005), sweet potato (Pfeiffer and McClafferty, 2007), carrot (Nicolle et al., 2004), chickpea (leaves; Ibrikci et al., 2003), Brassica oleracea (Broadley et al., 2010), Brassica rapa (Wu et al., 2007), spinach (Grusak and Cakmak, 2005), rice (Yang et al., 1998), wheat (Graham et al., 1999), maize (Bänziger and Long, 2000), pearl millet (Velu et al., 2007), barley (P. J. White and I. J. Bingham cited in White and Broadley, 2009), sorghum (Reddy et al., 2005), bean (Islam et al., 2002), pea (Grusak and Cakmak, 2005), cowpea (Pfeiffer and McClafferty, 2007), chickpea (seed, M. A. Grusak cited in White and Broadley, 2009), lentil (Pfeiffer and McClafferty, 2007), soybean (Raboy et al., 1984), peanut (Branch and Gaines, 1983), potatoes (White et al., 2009), and yam (Agbor-Egbe and Trèche, 1995) genotypes. Blue circles indicate Zn concentrations in the U.S. Department of Agriculture, Agricultural Research Service (2011). Red circles indicate target Zn concentrations proposed by the HarvestPlus program (Bouis and Welch, 2010).
The uptake and distribution of zinc between and within plant organs
Zinc is unevenly distributed within the plant. When plants are supplied Zn through the rhizosphere, tissue Zn concentrations generally decrease in the order root ≈ shoot > fruit, seed, tuber (Broadley et al., 2012). Consequently Zn concentrations are often greater in root crops and leafy vegetables than in grain, seed, fruit, or tuber crops (Figure 2; White and Broadley, 2005, 2009; Pfeiffer and McClafferty, 2007). Within each organ, Zn is preferentially accumulated by specific cell types. For example Zn is often localized in distinct regions within the root, such as the elongation zone, and is concentrated in endodermal cells of dicotyledonous species and in the pericycle of monocotyledonous species (Van Steveninck et al., 1994). The distribution of Zn within shoots and leaves varies between plant species. For example, in N. caerulescens, leaf epidermal cells, with the exception of guard cells, have greater Zn concentrations than leaf mesophyll cells and leaf Zn concentrations are higher in older leaves (Vázquez et al., 1994; Küpper et al., 1999, 2004; Frey et al., 2000; Ma et al., 2005; Monsant et al., 2010), whereas in A. halleri, and in Arabidopsis murale, Zn is more uniformly distributed across the leaf, although mesophyll cells have higher Zn concentrations than trichomes, which have greater Zn concentrations than other epidermal cells (Küpper et al., 2000; Zhao et al., 2000; Tappero et al., 2007). In seeds of cereals, Zn is preferentially accumulated in the husk, aleurone layers or embryo (Lin et al., 2005; Ozturk et al., 2006; Liu et al., 2007; Hansen et al., 2009; Persson et al., 2009; Cakmak et al., 2010a,b; Lombi et al., 2011; Stomph et al., 2011). In potato tubers about 17% of total tuber Zn is present in the skin (Subramanian et al., 2011). These distribution patterns reflect both local and long-distance transport of Zn within the plant (Broadley et al., 2007; Stomph et al., 2011; Subramanian et al., 2011). The distribution of Zn within cereal seeds and tubers will reduce the potential dietary Zn intakes from these crops when, for example, polished grains or peeled tubers are consumed.
Plant tissues accumulate Zn in both soluble and insoluble forms (Broadley et al., 2007). In crop plants, much of the soluble Zn is complexed with organic compounds. These compounds include carboxylic acids, such as citrate, malate, and oxalate, amino acids, such as histidine and asparagine, glutathione, phytochelatins, nicotianamine (NA), and proteins. In addition, Zn is found as phosphate salts, such as Zn3(PO4)2, and organic Zn-phytates. In the apoplast, Zn2+ binds to negatively charged cell-wall components, organic acids and phytosiderophores if these are present, and can be precipitated as phosphate or phytate salts when its concentration in the apoplast becomes excessive (Van Steveninck et al., 1994; Broadley et al., 2007; Straczek et al., 2008; Terzano et al., 2008; Kopittke et al., 2011). In the cytosol, Zn can be complexed by proteins, glutathione, phytochelatins and NA, and Zn2+ concentrations are vanishingly low (Broadley et al., 2007; Roosens et al., 2008; Clemens, 2010). The vacuoles of root and leaf cells contain Zn largely as Zn2+ and Zn-organic acid complexes (Broadley et al., 2007; Straczek et al., 2008; Sarret et al., 2009). Within the xylem, Zn is also present predominantly as Zn2+ and as complexes with carboxylic acids, such as citrate and malate (Welch, 1995; Broadley et al., 2007; Terzano et al., 2008). By contrast, phloem sap contains little Zn2+, and Zn is thought to be transported complexed with NA or small proteins (Welch, 1995; Curie et al., 2009; Waters and Sankaran, 2011).
The forms of Zn present in tissues of plants that hyperaccumulate Zn depends on the plant species, the tissue studied, and the concentration of Zn in that tissue (Küpper et al., 2004; Sarret et al., 2009; Monsant et al., 2011). More than 30% of the Zn in roots of N. caerulescens is generally associated with cell walls, and much of the remainder is complexed with histidine (Salt et al., 1999; Monsant et al., 2010, 2011). The presence of Zn-phytate is also observed occasionally (Monsant et al., 2011). About 80% of the Zn in the xylem sap of this plant species is Zn2+, with the remainder complexed with carboxylic acids (Salt et al., 1999; Monsant et al., 2011). In shoots of Zn-hyperaccumulator plants, such as N. caerulescens and A. halleri, 20–50% of the Zn is present as Zn2+, 40–99% is associated with vacuolar carboxylic acids, such as citrate, malate, and oxalate, up to 45% can be associated with histidine, and the remainder is largely bound to phosphate-groups and cell-wall components (Salt et al., 1999; Küpper et al., 2004; Sarret et al., 2009; Monsant et al., 2011). No major contributions of phytochelatins, metallothioneins, or other cysteine-rich peptides are observed (Küpper et al., 2004; Sarret et al., 2009).
In general, Zn enters plants from the soil solution and is transported either symplastically, following uptake by root cells, or apoplastically, in regions of the root lacking a Casparian Band, to the stele where it enters the xylem (White et al., 2002b; Broadley et al., 2007). Zinc is taken up by root cells as Zn2+ and, in some plant species, also as Zn-phytosiderophore complexes (Broadley et al., 2007; Palmer and Guerinot, 2009; Puig and Peñarrubia, 2009; Verbruggen et al., 2009; White and Broadley, 2009; Clemens, 2010; Krämer, 2010; White, 2012b). Although some plasma membrane Ca2+ channels are permeable to Zn2+ (Demidchik et al., 2002; White et al., 2002a), it is thought that most Zn2+ influx to the cytoplasm of root cells is mediated by ZRT-, IRT-like proteins (ZIPs), in Arabidopsis thaliana principally AtZIP4 and AtIRT1, and that yellow stripe like proteins (YSLs) catalyze the uptake of Zn-phytosiderophore complexes in cereals and grasses (Broadley et al., 2007; Roosens et al., 2008; Curie et al., 2009; Palmer and Guerinot, 2009; Verbruggen et al., 2009; White and Broadley, 2009; Waters and Sankaran, 2011; White, 2012b). In the cytosol of root cells, Zn2+ is complexed by numerous proteins, including many that modulate enzymic activities or gene transcription, by glutathione, by phytochelatins, and by NA, and the cytosolic Zn2+ concentration is likely to be extremely low (Broadley et al., 2007; Roosens et al., 2008; Clemens, 2010).
Members of the cation diffusion facilitator (CDF) family, such as orthologs of the A. thaliana metal tolerance proteins AtMTP1 and AtMTP3, and the Mg2+/H+ antiporter AtMHX, transport Zn2+ into the vacuole, whilst orthologs of the A. thaliana Zn-induced facilitator 1 (AtZIF1) protein transport Zn2+-complexes into the vacuole (Roosens et al., 2008; Palmer and Guerinot, 2009; Puig and Peñarrubia, 2009; White and Broadley, 2009; Clemens, 2010; Hassan and Aarts, 2011). It is thought that Zn is sequestered in the vacuole as an organic acid complex (Broadley et al., 2007). Zinc is released from the root vacuole through NRAMPs, including orthologs of the AtNRAMP3 and AtNRAMP4 transporters of A. thaliana (Roosens et al., 2008; Verbruggen et al., 2009). Members of the heavy metal P1B-ATPase family, such as AtHMA2 and AtHMA4 in A. thaliana, load Zn2+ into the xylem (Roosens et al., 2008; Palmer and Guerinot, 2009; Puig and Peñarrubia, 2009; Verbruggen et al., 2009; White and Broadley, 2009; Waters and Sankaran, 2011; White, 2012b). Within the xylem, Zn is transported either as Zn2+ or as a complex with organic acids or NA, and it is thought that YSL proteins load Zn-NA complexes into the xylem and orthologs of AtFRD3 load citrate into the xylem to promote Zn transport (Broadley et al., 2007; Roosens et al., 2008; Waters and Grusak, 2008; Curie et al., 2009; Waters and Sankaran, 2011).
Within the shoot, the uptake of Zn2+ and Zn-complexes by specific cell types are facilitated by members of the ZIP and YSL families, respectively (White and Broadley, 2009; Waters and Sankaran, 2011). Members of these protein families are also thought to load Zn into the phloem (Curie et al., 2009; White and Broadley, 2009), where it is transported as a complex with NA or small proteins (Curie et al., 2009; Puig and Peñarrubia, 2009; White and Broadley, 2009; Waters and Sankaran, 2011). In A. thaliana, AtYSL1, AtYSL3, and AtOPT3 have been implicated in delivering Zn from vascular tissues to developing seeds (Waters and Grusak, 2008; Puig and Peñarrubia, 2009; Waters and Sankaran, 2011). The mobility of Zn in the phloem will determine Zn accumulation by phloem-fed tissues, such as fruits, seeds, and tubers. Although Zn is generally considered to have a low mobility in the phloem (Fageria, 2009; White, 2012a), the translocation of Zn in the phloem of several plant species following the application of foliar Zn-fertilizers has been found to be nutritionally significant for their growth and development especially when cultivated in substrates with low Zn phytoavailability (Haslett et al., 2001; Brown, 2009; Waters and Sankaran, 2011).
The activity of transport proteins catalyzing Zn uptake, and the expression of the genes encoding proteins responsible for the mobilization of transition-metal elements from soil, their uptake by plant roots, and their distribution within the plant are regulated in response to plant Zn status to ensure appropriate tissue Zn concentrations. Thus, ZIPs, YSLs, HMAs, MTPs, ZIF1, FRD3, and enzymes involved in the synthesis of phytosiderophores and NA are upregulated during Zn-deficiency and downregulated when plant tissues have sufficient Zn for their physiological requirements (Broadley et al., 2007; White and Broadley, 2009; Waters and Sankaran, 2011). Recently, two members of the basic region/leucine zipper motif (bZIP) transcription factor gene family, bZIP19 and bZIP23, were shown to coordinate the adaptation of A. thaliana to low Zn phytoavailability (Assunção et al., 2010). This general homeostatic regulation of tissue Zn concentrations through Zn acquisition and distribution within the plant could limit Zn accumulation by edible tissues.
Agronomic Strategies to Increase Zinc Concentrations of Edible Crops
Although the total Zn concentrations in many soils are sufficient to support mineral-dense crops (Graham et al., 1999) Zn uptake by plants is often limited by its phytoavailability and acquisition by roots (Broadley et al., 2007; White and Broadley, 2009). Agronomic strategies seek to improve Zn phytoavailability in the soil, for example by remedying soil alkalinity, adopting appropriate crop rotations or introducing beneficial soil microorganisms (Rengel et al., 1999; He and Nara, 2007; Fageria, 2009; White and Broadley, 2009), or to deliver phytoavailable Zn through the application of Zn-fertilizers to soil or foliage (Cakmak, 2004; Graham et al., 2007; Fageria, 2009; White and Broadley, 2009; Bouis and Welch, 2010). Common inorganic Zn-fertilizers include ZnSO4, ZnO, and synthetic Zn-chelates (Fageria, 2009; White and Broadley, 2009). When Zn-fertilizers are applied to foliage, it is particularly important that the Zn compounds used are readily soluble, enter the leaf apoplast, and can be taken up by plant cells (Haslett et al., 2001; Cakmak, 2008; Brown, 2009). This avoids the accumulation of Zn salts on the surface of leaves or in the leaf apoplast, which can interfere with photosynthesis and cell function, and promotes the translocation of Zn from leaves to phloem-fed tissues.
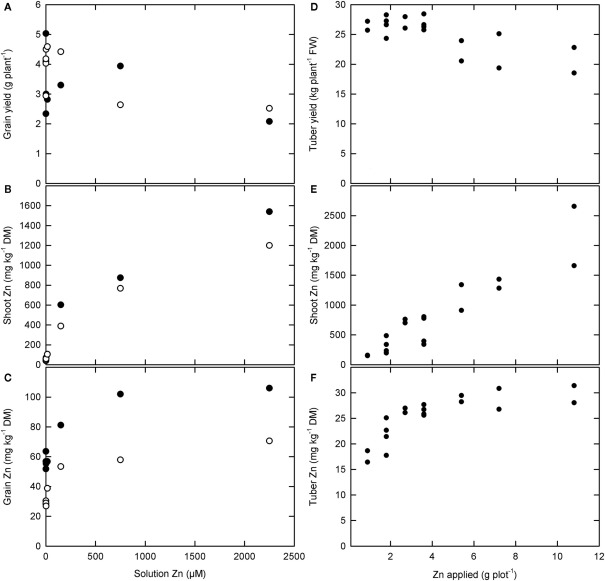
Zinc concentrations in roots, leaves, and stems can be increased readily by applying Zn-fertilizers to the soil in plants growing on most, but not all, soils and by foliar application of Zn-fertilizers (Figure 3; Rengel et al., 1999; Cakmak, 2008; Fageria, 2009; Sagardoy et al., 2009; White and Broadley, 2009; Bouis and Welch, 2010). Thus, Zn concentrations in these tissues will be limited solely by Zn toxicity. When Zn-fertilizers are added to the soil, root tissues often exhibit higher Zn concentrations than shoot tissues, and it is likely that plant Zn accumulation and yield is limited by Zn toxicity to root cells under these conditions. Critical leaf Zn concentrations for most crop plants lie between 100 and 700 mg kg−1 DM when Zn-fertilizers are applied to the soil (MacNicol and Beckett, 1985; Fageria, 2009). It is possible that, when Zn-fertilizers are applied foliarly, higher leaf Zn concentrations might be achieved without loss of yield (Cakmak et al., 1999; White et al., submitted).
Figure 3.
(A–C) The effect of solution zinc (Zn) concentration on (A) grain yield and Zn concentrations in (B) shoots and (C) grain of two rice varieties, Handao297 (filled circles) and K150 (open circles), grown in quartz sand irrigated with a complete nutrient solution (Jiang et al., 2008). (D–F) The effect of foliar Zn fertilizer applications on (D) tuber yield and (E) shoot and (F) tuber Zn concentrations in “Maris Piper” potatoes grown in the field (White et al., submitted).
Soil or foliar applications of Zn-fertilizers can also increase Zn concentrations in phloem-fed tissues, such as fruits, seeds, and tubers (Rengel et al., 1999; Cakmak, 2004, 2008; Fang et al., 2008; White et al., 2009; Cakmak et al., 2010a,b). However, increasing Zn concentrations in these tissues requires adequate Zn mobility in the phloem and, unless Zn-fertilizers are applied directly or they have functional xylem continuity, the mobility of Zn in the phloem will limit their Zn accumulation (Rengel et al., 1999; White and Broadley, 2005, 2009; Broadley et al., 2007; Stomph et al., 2009; Cakmak et al., 2010a,b; White et al., submitted). The relationship between seed Zn concentration and Zn phytoavailability often follows a saturation curve. For example, when rice is grown in quartz sand irrigated with a complete nutrient solution, Zn concentrations in brown rice reach a maximum of about 50–110 mg Zn kg−1 DM depending upon variety (Figure 3; Jiang et al., 2008). Similarly, the relationship between tuber Zn concentration and foliar Zn application in “Maris Piper” potatoes grown in the field followed a saturation curve, reaching a maximum of about 30 mg Zn kg−1 DM (Figure 3; White et al., submitted). In the phloem, Zn is thought to be transported either as Zn–NA or complexed with small proteins (White and Broadley, 2009). In cereals, grain Zn concentration is correlated with grain protein concentration (e.g., Zhao et al., 2009; Cakmak et al., 2010a,b; Gomez-Becerra et al., 2010), and the limit to grain Zn concentration can be increased by higher N-fertilizer applications (Hao et al., 2007; Kutman et al., 2010, 2011a,b; Shi et al., 2010). Similarly, there is a significant relationship between tuber Zn concentration and tuber N concentration among potato genotypes (White et al., submitted) and tuber Zn concentrations can be increased by N-fertilization (Hlusek et al., 1997). Nevertheless, Zn concentrations in phloem-fed tissues rarely exceed 30–100 mg kg−1 DM.
Genetic Variation in Zinc Concentrations of Edible Crops
Genetic strategies to increase Zn concentrations in edible portions seek to exploit genetic variation in the acquisition of Zn from the soil, Zn accumulation in edible portions and tolerance to high tissue Zn concentrations (Pfeiffer and McClafferty, 2007; White and Broadley, 2009). There is considerable genetic variation in Zn concentration in most edible crops (Figure 2). Edible roots often have low Zn concentrations, but researchers have reported >14-fold variation in root Zn concentrations among 600 cassava genotypes (Chávez et al., 2005) and 2.2-fold variation among 20 carrot genotypes (Nicolle et al., 2004). Zinc concentrations in edible greens are often relatively high and trials have indicated 2.5-fold variation in leaf Zn-concentration among 19 chickpea genotypes (Ibrikci et al., 2003), 6.7-fold variation in leaf Zn-concentration among 111 Brassica rapa genotypes (Wu et al., 2007), 12.3-fold variation in leaf Zn-concentration among 327 spinach genotypes (Grusak and Cakmak, 2005), and over 26-fold variation among 339 Brassica oleracea genotypes (Broadley et al., 2010). Absolute Zn concentrations and genetic variation in Zn concentrations are often lower in seeds than in leaves. Nevertheless, between 2.2- and 11.6-fold variation in seed Zn concentrations have been observed in large core collections of cereal germplasm (e.g., Yang et al., 1998; Graham et al., 1999; Bänziger and Long, 2000; Gregorio et al., 2000; Reddy et al., 2005) and between 1.8- and 6.6-fold variation in seed Zn concentrations in large core collections of legume germplasm (Raboy et al., 1984; Islam et al., 2002; Grusak and Cakmak, 2005; Pfeiffer and McClafferty, 2007; White and Broadley, 2009). Fruits generally have low Zn concentrations, and studies report about twofold variation among three to six cultivars in strawberries (Hakala et al., 2003), apples (Iwane, 1991), and plantains (Davey et al., 2007). Similarly, although tuber Zn concentrations are relatively low, 2.4-fold and 3.1-fold variation has been reported among 26 potato and 23 yam genotypes, respectively (Agbor-Egbe and Trèche, 1995; White et al., 2009). These data indicate that breeding for increased Zn concentrations is, in principle, feasible for most edible crops. Indeed, genetic loci (QTL) affecting Zn concentrations in cereal grain (Distelfeld et al., 2007; Stangoulis et al., 2007; Genc et al., 2009; Lonergan et al., 2009; Peleg et al., 2009), bean seeds (Guzmán-Maldonado et al., 2003; Cichy et al., 2005, 2009; Gelin et al., 2007; Blair et al., 2009, 2010, 2011), brassica leaves (Wu et al., 2008; Broadley et al., 2010), and potato tubers (N. K. Subramanian et al., unpublished data) have been identified.
Genetic Modification Strategies for Zinc Biofortification of Edible Crops
It has been speculated that the constitutive expression of a suite of Zn-deficiency inducible responses through the overexpression of bZIP19 and bZIP23 transcription factors could be used to increase Zn accumulation in edible portions of crop plants (Assunção et al., 2010). Specific targets for the manipulation of root Zn concentrations include transport proteins in the plasma membrane and tonoplast of root cells that facilitate the uptake and sequestration of Zn in the vacuole, together with enzymes involved in the synthesis of compounds that bind Zn2+ in the rhizosphere, cytoplasm, and vacuole. Targets for the manipulation of shoot Zn concentrations include (a) transport proteins in the plasma membrane of root cells that facilitate Zn uptake and delivery to the xylem, or root structural modifications that facilitate apoplastic movement to the xylem, (b) enzymes involved in the synthesis of compounds that facilitate Zn movement through the root symplast or apoplast and in the xylem, (c) transport proteins in the plasma membrane and tonoplast of shoot cells that facilitate Zn uptake and vacuolar Zn sequestration, and (d) enzymes involved in the synthesis of compounds that detoxify Zn2+ within and outside shoot cells. Targets for the manipulation of Zn concentrations in phloem-fed tissues will additionally include transport proteins in the plasma membrane of shoot cells that facilitate the loading of Zn into the phloem and compounds that facilitate Zn movement in the phloem.
Most published studies describing GM strategies that increase Zn concentrations in plant tissues have been performed on “model” plants such as A. thaliana and, even when studies have been performed on crop species, data on commercial yields are rarely presented. Yield per plant can have a substantial effect on the tissue concentrations of mineral elements through dilution effects caused by plant growth (Jarrell and Beverly, 1981; Davis, 2011). Higher-yielding genotypes often have lower Zn concentrations in their edible tissues than lower-yielding genotypes (Monasterio and Graham, 2000; Garvin et al., 2006; Murphy et al., 2008; White et al., 2009; Zhao et al., 2009). Thus, it is important to consider whether any increase in tissue Zn concentration is simply a consequence of slower growth or reduced yields.
Overexpressing genes encoding Zn-transporters catalyzing Zn2+ influx to root cells often increases root Zn concentrations but reduces leaf Zn concentrations. This has been observed when overexpressing OsZIP4 or OsZIP5 in rice (Ishimaru et al., 2007; Lee et al., 2010) and when overexpressing AtZIP1 in cassava (Sayre et al., 2011). Overexpressing a root plasma membrane Zn-transporter, AtZIP1, in barley had no effect on leaf Zn concentration, but reduced seed weight and increased seed Zn concentrations from 31 to 61–85 mg kg−1 DM (Ramesh et al., 2004). Overexpression of the ZIPs TcZNT5 and TcZNT6 in A. thaliana had no consistent effects on root or shoot Zn concentrations (Wu et al., 2009). Overexpressing genes encoding proteins that transport Zn2+ into the vacuole, such as AtMTP1 (AtZAT1), AtMTP3, or TgMTP1, increases Zn uptake and root Zn concentrations, but rarely shoot Zn concentrations, when expressed in roots, and increases Zn uptake and leaf Zn concentrations, but rarely seed Zn concentrations, through systemic induction of Zn-deficiency responses when expressed in the shoot (van der Zaal et al., 1999; Arrivault et al., 2006; Gustin et al., 2009). The expression of AtMTP1 (AtZAT1) increased Zn concentrations in roots of cassava about fourfold to 40 mg kg−1 DM (Sayre et al., 2011). The ferric reductase defective 3/manganese accumulator 1 (frd3 = man1) null mutants of A. thaliana, which constitutively express genes allowing increased rhizosphere Fe(III) reductase activity (AtFRO2), Zn uptake (AtIRT1), and tissue NA concentrations, have greater shoot Zn concentrations, but similar seed Zn concentrations, to wild-type plants (Rogers and Guerinot, 2002). Similarly, a mutant of A. thaliana (opt3.2) with reduced expression of AtOPT3 that shows constitutive expression of AtFRO2 and AtIRT1 has higher Zn concentrations in leaves, stems, and seed than wild-type plants (Stacey et al., 2008). Overexpressing enzymes involved in the synthesis of phytosiderophores, either HvIDS3 (encoding a dioxygenase, referred to as Iron-Deficiency Specific clone 3) alone or both HvNAS1 (encoding nicotianamine synthase) and HvNAAT (encoding nicotianamine aminotransferase) increased concentrations of Zn in seeds of paddy-grown rice from 11.2 mg kg−1 DM to 13.4 and 15.3 mg kg−1 DM, respectively (Suzuki et al., 2008). Leaf Zn concentrations can be increased in A. thaliana by reducing the expression of AtHMA2, which is thought to catalyze Zn2+ efflux across the membranes of root cells (Eren and Argüello, 2004) or by overexpressing the gene encoding AtHMA4, which is thought to load Zn into the xylem (Verret et al., 2004).
The overexpression of genes encoding nicotianamine synthase (NAS) often leads to increased Zn concentrations in leaves and grain. The overexpression of HvNAS1 in tobacco increased leaf Zn concentrations from 16 to 39 mg kg−1 DM and seed Zn concentrations from 20 to 35 mg kg−1 DM (Takahashi et al., 2003). The overexpression of OsNAS3 in rice also increased leaf and seed Zn concentrations (Lee et al., 2009; Johnson et al., 2011). Seed Zn concentrations of glasshouse-grown plants were increased from ∼40 to 79 mg kg−1 DM (Johnson et al., 2011) and those of paddy-grown plants were increased from 16 to 35 mg kg−1 DM (Lee et al., 2009). Constitutive overexpression of OsNAS2 in rice resulted in an increase in seed Zn concentrations from ∼23 to ∼60 mg kg−1 DM (Lee et al., 2011) and from ∼40 to 95 mg kg−1 DM (Johnson et al., 2011) in two independent studies. Constitutive overexpression of OsNAS1 in rice resulted in an increase in seed Zn concentrations from ∼40 to 59 mg kg−1 DM (Johnson et al., 2011). The maximum Zn concentrations in polished grain of transgenic rice expressing Pvferritin in the endosperm and AtNAS1 throughout the plant was 34 mg kg−1 DM, about 50% greater than in wild-type plants (Wirth et al., 2009). Earlier studies of plants expressing only Gmferritin found ∼56 mg Zn kg−1 DM in brown rice (Vasconcelos et al., 2003). The overexpression of genes increasing the production of glutathione, phytochelatins, and total thiols resulted in increased leaf Zn concentrations in Indian mustard (Brassica juncea) grown on soils with high Zn phytoavailability (Bennett et al., 2003). Seed Zn concentrations can be increased in wheat by increasing the expression of a NAC transcription factor (NAM-B1) that accelerate senescence and increase remobilization of mineral elements from leaves to developing grain (Uauy et al., 2006).
Conclusion
Zinc concentrations in roots, leaves, and stems can be increased greatly by applying Zn-fertilizers (Figure 3). The accumulation of Zn in these tissues appears to be limited by Zn toxicity. In some crops, such as cereals and beans, root Zn concentrations of between 500 and 5000 mg kg−1 DM have been reported without loss of yield (Reichman, 2002). Leaf Zn concentrations ≥100 mg kg−1 DM, and perhaps up to 700 mg kg−1 DM, can be achieved without loss of yield in Zn-tolerant crops when Zn-fertilizers are applied to the soil (Fageria, 2009). It is possible that greater Zn concentrations in non-woody shoot tissues might be achieved using foliar Zn-fertilizer applications. These Zn concentrations approach that found in raw beefsteak, which approximates 200–250 mg Zn kg−1 DM (U.S. Department of Agriculture, Agricultural Research Service,2011). By contrast, Zn concentrations in fruits, seeds, and tubers are severely limited by Zn transport in the phloem. This might be a consequence of the need to maintain low Zn2+ concentrations in phloem sap to avoid cellular toxicity. Increased production of compounds that chelate Zn2+, such as NA, can increase Zn concentrations in the phloem and its delivery to phloem-fed tissues. Agrochemicals that increase the concentrations of Zn-complexes in the phloem might be used to increase Zn concentrations in fruits, seeds, and tubers. Thus, application of Zn-fertilizers, especially in combination with nitrogen fertilizers, can increase Zn concentrations in seeds of cereals to about 100 mg kg−1 DM and Zn concentrations in seeds of legumes to about 120 mg kg−1 DM (see Agronomic Strategies to Increase Zinc Concentrations of Edible Crops).
Since there is appreciable variation in Zn concentrations of edible tissues of food crops, conventional breeding for increased Zn concentrations appears feasible (see Genetic Variation in Zinc Concentrations of Edible Crops). It is possible that breeding can increase Zn-tolerance in root and leaf crops and increase Zn mobility in the phloem in fruit, seed, and tuber crops. Improving Zn-tolerance in root and leaf crops and increasing phloem Zn mobility in fruit, seed, and tuber crops might also be addressed through GM technologies (see Genetic Modification Strategies for Zinc Biofortification of Edible Crops). Several transgenic crop plants have been created that have greater Zn concentrations in their edible tissues than conventional varieties. These include cassava roots with about 40 mg Zn kg−1 DM (Sayre et al., 2011), brown rice with 56–95 mg Zn kg−1 DM (Vasconcelos et al., 2003; Johnson et al., 2011), and barley grain with 85 mg Zn kg−1 DM (Ramesh et al., 2004). Nevertheless, it is possible that the general homeostatic regulation of tissue Zn concentrations through Zn acquisition and distribution within the plant could be a major constraint to increasing Zn concentrations in edible portions (see Plant Physiology). A critical role of Zn in plant cells is the control of transcription, translation, and protein activity through cytoplasmic Zn2+ concentration (Broadley et al. 2007). The key to Zn-homeostasis in plant cells is likely, therefore, to pivot on cytoplasmic Zn2+ concentration. Increasing tissue Zn concentrations will require increased biosynthesis of Zn-complexes and effective sequestration of Zn in non-vital compartments, such as the vacuole, to avoid unwanted perturbations in cytoplasmic Zn2+ concentration. For this, lessons might be learnt from Zn-hyperaccumulator plants.
To increase Zn concentrations in edible crops, future research should focus on (i) integrating agronomic and genetic strategies to increase Zn transport to phloem-fed tissues and (ii) identifying the mechanisms effecting Zn-homeostasis in plant cells and strategies to manage subcellular Zn compartmentalization.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Rural and Environment Science and Analytical Services Division (RESAS) of the Scottish Government through Workpackage 7.2 (2011–2016).
References
- Agbor-Egbe T., Trèche S. (1995). Evaluation of the chemical composition of Cameroonian yam germplasm. J. Food Compost. Anal. 8, 274–283 10.1006/jfca.1995.1020 [DOI] [Google Scholar]
- Arrivault S., Senger T., Krämer U. (2006). The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 46, 861–879 10.1111/j.1365-313X.2006.02746.x [DOI] [PubMed] [Google Scholar]
- Assunção A. G. L., Herrero E., Lin Y.-F., Huettel B., Talukdar S., Smaczniak C., Immink R. G. H., van Eldik M., Fiers M., Schat H., Aarts M. G. M. (2010). Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 107, 10296–10301 10.1073/pnas.1004788107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänziger M., Long J. (2000). The potential for increasing the iron and zinc density of maize through plant-breeding. Food Nutr. Bull. 21, 397–400 [Google Scholar]
- Bennett L. E., Burkhead J. L., Hale K. L., Terry N., Pilon M., Pilon-Smits E. A. H. (2003). Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J. Environ. Qual. 32, 432–440 10.2134/jeq2003.0432 [DOI] [PubMed] [Google Scholar]
- Blair M., Astudillo C., Grusak M., Graham R., Beebe S. (2009). Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 23, 197–207 10.1007/s11032-008-9225-z [DOI] [Google Scholar]
- Blair M. W., Astudillo C., Rengifo J., Beebe S. E., Graham R. (2011). QTL analyses for seed iron and zinc concentrations in an intra-genepool population of Andean common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 122, 511–521 10.1007/s00122-010-1465-8 [DOI] [PubMed] [Google Scholar]
- Blair M. W., Medina J. I., Astudillo C., Rengifo J., Beebe S. E., Machado G., Graham R. (2010). QTL for seed iron and zinc concentration and content in a Mesoamerican common bean (Phaseolus vulgaris L.) population. Theor. Appl. Genet. 121, 1059–1070 10.1007/s00122-010-1371-0 [DOI] [PubMed] [Google Scholar]
- Bouis H. E., Welch R. M. (2010). Biofortification – a sustainable agricultural strategy for reducing micronutrient malnutrition in the global South. Crop Sci. 50, S20–S32 10.2135/cropsci2009.09.0531 [DOI] [Google Scholar]
- Branch W. D., Gaines T. P. (1983). Seed mineral composition of diverse peanut germplasm. Peanut Sci. 10, 5–8 10.3146/i0095-3679-10-1-3 [DOI] [Google Scholar]
- Broadley M., Brown P., Cakmak I., Rengel Z., Zhao F. (2012). “Function of nutrients: micronutrients,” in Marschner’s Mineral Nutrition of Higher Plants, 3rd Edn, ed. Marschner P. (London: Academic Press; ), 191–248 [Google Scholar]
- Broadley M. R., White P. J., Hammond J. P., Zelko I., Lux A. (2007). Zinc in plants. New Phytol. 173, 677–702 10.1111/j.1469-8137.2007.01996.x [DOI] [PubMed] [Google Scholar]
- Broadley M. R., Willey N. J., Wilkins J. C., Baker A. J. M., Mead A., White P. J. (2001). Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol. 152, 9–27 10.1046/j.0028-646x.2001.00238.x [DOI] [PubMed] [Google Scholar]
- Broadley M. R., O Lochlainn S., Hammond J. P., Bowen H. C., Çakmak I., Eker S., Erdem H., King G. J., White P. J. (2010). Shoot zinc (Zn) concentration varies widely within Brassica oleracea L. and is affected by soil Zn and phosphorus (P) levels. J. Hortic. Sci. Biotechnol. 85, 375–380 [Google Scholar]
- Brown P. (2009). “Development of a model system for testing foliar fertilizers, adjuvants and growth stimulants,” in Proceedings of the California Department of Food and Agriculture Fertilizer Research and Education Program Conference 2008, Visalia, CA, 17–23 [Google Scholar]
- Cakmak I. (2004). “Identification and Correction of Widespread Zinc Deficiency in Turkey – A Success Story,” in Proceedings of the International Fertiliser Society 552 (York: International Fertiliser Society; ). [Google Scholar]
- Cakmak I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302, 1–17 10.1007/s11104-007-9466-3 [DOI] [Google Scholar]
- Cakmak I. (2009). Enrichment of fertilizers with zinc: an excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 23, 281–289 10.1016/j.jtemb.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Kalayci M., Ekiz H., Braun H. J., Kilinc Y., Yilmaz A. (1999). Zinc deficiency as a practical problem in plant and human nutrition in Turkey: a NATO-science for stability project. Field Crops Res. 60, 175–188 10.1016/S0378-4290(98)00139-7 [DOI] [Google Scholar]
- Cakmak I., Kalayci M., Kaya Y., Torun A. A., Aydin N., Wang Y., Arisoy Z., Erdem H., Yazici A., Gokmen O., Ozturk L., Horst W. J. (2010a). Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 58, 9092–9102 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Pfeiffer W. H., McClafferty B. (2010b). Biofortification of durum wheat with zinc and iron. Cereal Chem. 87, 10–20 10.1094/CCHEM-87-1-0010 [DOI] [Google Scholar]
- Chávez A. L., Sánchez T., Jaramillo G., Bedoya J. M., Echeverry J., Bolaños E. A., Ceballos H., Iglesias C. A. (2005). Variation of quality traits in cassava roots evaluated in landraces and improved clones. Euphytica 143, 125–133 10.1007/s10681-005-3057-2 [DOI] [Google Scholar]
- Cichy K. A., Caldas G. V., Snapp S. S., Blair M. W. (2009). QTL analysis of seed iron, zinc, and phosphorus levels in an Andean bean population. Crop Sci. 49, 1742–1750 10.2135/cropsci2008.03.0142 [DOI] [Google Scholar]
- Cichy K. A., Forster S., Grafton K. F., Hosfield G. L. (2005). Inheritance of seed zinc accumulation in navy bean. Crop Sci. 45, 864–870 10.2135/cropsci2004.0104 [DOI] [Google Scholar]
- Clemens S. (2010). “Zn – a versatile player in plant cell biology,” in Plant Cell Monographs 17, Cell Biology of Metals and Nutrients, eds Hell R., Mendel R. R. (Berlin: Springer; ), 281–298 [Google Scholar]
- Curie C., Cassin G., Couch D., Divol F., Higuchi K., Le Jean M., Misson J., Schikora A., Czernic P., Mari S. (2009). Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 103, 1–11 10.1093/aob/mcn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Stals E., Ngoh-Newilah G., Tomekpe K., Lusty C., Markham R., Swennen R., Keulemans J. (2007). Sampling strategies and variability in fruit pulp micronutrient contents of West and Central African bananas and plantains (Musa species). J. Agric. Food Chem. 55, 2633–2644 10.1021/jf063119l [DOI] [PubMed] [Google Scholar]
- Davis D. R. (2011). “Impact of breeding and yield on fruit, vegetable, and grain nutrient content,” in Breeding for Fruit Quality, eds Jenks M. A., Bebeli P. J. (New York: John Wiley & Sons; ), 49–70 [Google Scholar]
- Demidchik V., Bowen H. C., Maathuis F. J. M., Shabala S. N., Tester M. A., White P. J., Davies J. M. (2002). Arabidopsis thaliana root nonselective cation channels mediate calcium uptake and are involved in growth. Plant J. 32, 799–808 10.1046/j.1365-313X.2002.01467.x [DOI] [PubMed] [Google Scholar]
- Department of Health (UK) (1991). Report on Health and Social Subjects 41. Dietary Reference Values for Food Energy and Nutrients. London: HMSO; 10.1046/j.1365-313X.2002.01467.x [DOI] [PubMed] [Google Scholar]
- Distelfeld A., Cakmak I., Peleg Z., Ozturk L., Yazici A. M., Budak H., Saranga Y., Fahima T. (2007). Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant 129, 635–643 10.1111/j.1399-3054.2006.00841.x [DOI] [Google Scholar]
- Eren E., Argüello J. M. (2004). Arabidopsis HMA2, a divalent heavy metal-transporting PIB-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol. 136, 3712–3723 10.1104/pp.104.046292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fageria N. K. (2009). The Use of Nutrients in Crop Plants. Boca Raton, FL: CRC Press [Google Scholar]
- Fang Y., Wang L., Xin Z., Zhao L., An X., Hu Q. (2008). Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J. Agric. Food Chem. 56, 2079–2084 10.1021/jf8017436 [DOI] [PubMed] [Google Scholar]
- Frey B., Keller C., Zierold K., Schulin R. (2000). Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 23, 675–687 10.1046/j.1365-3040.2000.00590.x [DOI] [Google Scholar]
- Garvin D. F., Welch R. M., Finley J. W. (2006). Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 86, 2213–2220 10.1002/jsfa.2601 [DOI] [Google Scholar]
- Gelin J. P., Forster S., Grafton K. F., McClean P. E., Kooas-Cifuentes G. A. (2007). Analysis of seed zinc and other minerals in a recombinant inbred population of navy bean (Phaseolus vulgaris L.). Crop Sci. 47, 1361–1366 10.2135/cropsci2006.08.0510 [DOI] [Google Scholar]
- Genc Y., Verbyla A., Torun A., Cakmak I., Willsmore K., Wallwork H., McDonald G. (2009). Quantitative trait loci analysis of zinc efficiency and grain zinc concentration in wheat using whole genome average interval mapping. Plant Soil 314, 49–66 10.1007/s11104-008-9704-3 [DOI] [Google Scholar]
- Gomez-Becerra H. F., Erdem H., Yazici A., Tutus Y., Torun B., Ozturk L., Cakmak I. (2010). Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. J. Cereal Sci. 52, 342–349 10.1016/j.jcs.2010.05.003 [DOI] [Google Scholar]
- Graham R., Senadhira D., Beebe S., Iglesias C., Monasterio I. (1999). Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crops Res. 60, 57–80 10.1016/S0378-4290(98)00133-6 [DOI] [Google Scholar]
- Graham R. D., Welch R. M., Bouis H. E. (2001). Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv. Agron. 70, 77–142 10.1016/S0065-2113(01)70004-1 [DOI] [Google Scholar]
- Graham R. D., Welch R. M., Saunders D. A., Ortiz-Monasterio I., Bouis H. E., Bonierbale M., de Haan S., Burgos G., Thiele G., Liria R., Meisner C. A., Beebe S. E., Potts M. J., Kadian M., Hobbs P. R., Gupta R. K., Twomlow S. (2007). Nutritious subsistence food systems. Adv. Agron. 92, 1–74 10.1016/S0065-2113(04)92001-9 [DOI] [Google Scholar]
- Gregorio G. B., Senadhira D., Htut H., Graham R. D. (2000). Breeding for trace mineral density in rice. Food Nutr. Bull. 21, 382–386 [Google Scholar]
- Grusak M. A., Cakmak I. (2005). “Methods to improve the crop-delivery of minerals to humans and livestock,” in Plant Nutritional Genomics, eds Broadley M. R., White P. J. (Oxford: Blackwell; ), 265–286 [Google Scholar]
- Gustin J. L., Loureiro M. E., Kim D., Na G., Tikhonova M., Salt D. E. (2009). MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 57, 1116–1127 10.1111/j.1365-313X.2008.03754.x [DOI] [PubMed] [Google Scholar]
- Guzmán-Maldonado S. H., Martínez O., Acosta-Gallegos J. A., Guevara-Lara F., Paredes-López O. (2003). Putative quantitative trait loci for physical and chemical components of common bean. Crop Sci. 43, 1029–1035 10.2135/cropsci2003.1029 [DOI] [Google Scholar]
- Hakala M., Lapveteläinen A., Houpalahti R., Kallio H., Tahvonen R. (2003). Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compost. Anal. 16, 67–80 10.1016/S0889-1575(02)00165-5 [DOI] [Google Scholar]
- Hammond J. P., Bowen H. C., White P. J., Mills V., Pyke K. A., Baker A. J. M., Whiting S. N., May S. T., Broadley M. R. (2006). A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol. 170, 239–260 10.1111/j.1469-8137.2006.01662.x [DOI] [PubMed] [Google Scholar]
- Hanikenne M., Talke I. N., Haydon M. J., Lanz C., Nolte A., Motte P., Kroymann J., Weigel D., Kramer U. (2008). Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–395 10.1038/nature06877 [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Laursen K. H., Persson D. P., Pedas P., Husted S., Schoerring J. K. (2009). Micro-scaled high-throughput digestion of plant tissue samples for multi-elemental analysis. Plant Methods 5, 12. 10.1186/1746-4811-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H.-L., Wei Y.-Z., Yang X.-E., Feng Y., Wu C.-Y. (2007). Effects of different nitrogen fertilizer levels on Fe, Mn, Cu and Zn concentrations in shoot and grain quality in rice (Oryza sativa). Rice Sci. 14, 289–294 10.1016/S1672-6308(08)60007-4 [DOI] [Google Scholar]
- Haslett B. S., Reid R. J., Rengel Z. (2001). Zinc mobility in wheat: uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 87, 379–386 10.1006/anbo.2000.1349 [DOI] [Google Scholar]
- Hassan Z., Aarts M. G. M. (2011). Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ. Exp. Bot. 72, 53–63 10.1016/j.envexpbot.2010.04.003 [DOI] [Google Scholar]
- He X., Nara K. (2007). Element biofortification: can mycorrhizas potentially offer a more effective and sustainable pathway to curb human malnutrition? Trends Plant Sci. 12, 331–333 10.1016/j.tplants.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Hlusek J., Juzl M., Zrust J. (1997). Potato yields and cadmium, nickel and zinc contents in tubers. Rostlinna Vyroba 43, 263–267 [Google Scholar]
- Horton S. (2006). The economics of food fortification. J. Nutr. 136, 1068–1071 [DOI] [PubMed] [Google Scholar]
- Hotz C., Brown K. H. (2004). International Zinc Nutrition Consultative Group. Technical Document #1 Assessment of the risk of inc deficiency in populations and options for its control. Food Nutr. Bull. 25, S91–S203 [PubMed] [Google Scholar]
- Ibrikci H., Knewtson S. J. B., Grusak M. A. (2003). Chickpea leaves as a vegetable green for humans: evaluation of mineral composition. J. Sci. Food Agric. 83, 945–950 10.1002/jsfa.1427 [DOI] [Google Scholar]
- Institute of Medicine (USA) (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, (Arsenic), Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- Ishimaru Y., Masuda H., Suzuki M., Bashir K., Takahashi M., Nakanishi H., Mori S., Nishizawa N. K. (2007). Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J. Exp. Bot. 58, 2909–2915 10.1093/jxb/erm147 [DOI] [PubMed] [Google Scholar]
- Islam F. M. A., Basford K. E., Jara C., Redden R. J., Beebe S. (2002). Seed compositional and disease resistance differences among gene pools in cultivated common bean. Genetic Resour. Crop Evol. 49, 285–293 10.1023/A:1015567513005 [DOI] [Google Scholar]
- Iwane A. (1991). Effect of cultivar and year on mineral components of apples. J. Jpn. Soc. Food Sci. Technol. 38, 329–336 10.3136/nskkk1962.38.329 [DOI] [Google Scholar]
- Jarrell W. M., Beverly R. B. (1981). The dilution effect in plant nutrition studies. Adv. Agron. 34, 197–224 10.1016/S0065-2113(08)60887-1 [DOI] [Google Scholar]
- Jiang W., Struik P. C., van Keulen H., Zhao M., Jin L. N., Stomph T. J. (2008) , Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann. Appl. Biol. 153, 135–147 10.1111/j.1744-7348.2008.00243.x [DOI] [Google Scholar]
- Johnson A. A. T., Kyriacou B., Callahan D. L., Carruthers L., Stangoulis J., Lombi E., Tester M. (2011). Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 6, e24476. 10.1371/journal.pone.0024476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshgoftarmanesh A. H., Schulin R., Chaney R. L., Daneshbakhsh B., Afyuni M. (2009). Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 30, 83–107 10.1051/agro/2009017 [DOI] [Google Scholar]
- Kopittke P. M., Menzies N. W., de Jonge M. D., McKenna B. A., Donner E., Webb R. I., Paterson D. J., Howard D. L., Ryan C. G., Glover C. J., Scheckel K. G., Lombi E. (2011). In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiol. 156, 663–673 10.1104/pp.111.173716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U. (2010). Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 61, 517–534 10.1146/annurev-arplant-042809-112156 [DOI] [PubMed] [Google Scholar]
- Küpper H., Lombi E., Zhao F.-J., McGrath S. P. (2000). Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212, 75–84 10.1007/s004250000366 [DOI] [PubMed] [Google Scholar]
- Küpper H., Mijovilovich A., Meyer-Klaucke W., Kroneck P. M. H. (2004). Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 134, 748–757 10.1104/pp.103.032953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Zhao F. J., McGrath S. P. (1999). Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 119, 305–311 10.1104/pp.119.1.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutman U. B., Yildiz B., Cakmak I. (2011a). Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci. 53, 118–125 10.1016/j.jcs.2010.10.006 [DOI] [Google Scholar]
- Kutman U. B., Yildiz B., Cakmak I. (2011b). Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 342, 149–164 10.1007/s11104-010-0679-5 [DOI] [Google Scholar]
- Kutman U. B., Yildiz B., Ozturk L., Cakmak I. (2010). Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 87, 1–9 10.1094/CCHEM-87-1-0001 [DOI] [Google Scholar]
- Lee S., Jeon U. S., Lee S. J., Kim Y. K., Persson D. P., Husted S., Schjorring J. K., Kakei Y., Masuda H., Nishizawa N. K., An G. (2009). Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc. Natl. Acad. Sci. U.S.A. 106, 22014–22019 10.1073/pnas.0809284106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Jeong H. J., Kim S. A., Lee J., Guerinot M., An G. (2010). OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 73, 507–517 10.1007/s11103-010-9629-0 [DOI] [PubMed] [Google Scholar]
- Lee S., Persson D. P., Hansen T. H., Husted S., Schjoerring J. K., Kim Y.-S., Jeon U. S., Kim Y.-K., Kakei Y., Masuda H., Nishizawa N. K., An G. (2011). Bio-available zinc in rice seeds is increased by activation tagging of nicotianamine synthase. Plant Biotechnol. J. 9, 865–873 10.1111/j.1467-7652.2011.00606.x [DOI] [PubMed] [Google Scholar]
- Lin L., Ockenden I., Lott J. N. A. (2005). The concentrations and distribution of phytic acid-phosphorus and other mineral nutrients in wild-type and low phytic acid1-1 (lpa1-1) corn (Zea mays L.) grains and grain parts. Can. J. Bot. 83, 131–141 10.1139/b04-146 [DOI] [Google Scholar]
- Liu K., Peterson K. L., Raboy V. (2007). Comparison of the phosphorus and mineral concentrations in bran and abraded kernel fractions of a normal barley (Hordeum vulgare) cultivar versus four low phytic acid isolines. J. Agric. Food Chem. 55, 4453–4460 10.1021/jf0628072 [DOI] [PubMed] [Google Scholar]
- Lombi E., Smith E., Hansen T. H., Paterson D., de Jonge M. D., Howard D. L., Persson D. P., Husted S., Ryan C., Schjoerring J. K. (2011). Megapixel imaging of (micro)nutrients in mature barley grains. J. Exp. Bot. 62, 273–282 10.1093/jxb/erq270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P. F., Pallotta M. A., Lorimer M., Paull J. G., Barker S. J., Graham R. D. (2009). Multiple genetic loci for zinc uptake and distribution in barley (Hordeum vulgare). New Phytol. 184, 168–179 10.1111/j.1469-8137.2009.02956.x [DOI] [PubMed] [Google Scholar]
- Ma G., Jin Y., Li Y., Zhai F., Kok F. J., Jacobsen E., Yang X. (2008). Iron and zinc deficiencies in China: what is a feasible and cost-effective strategy? Public Health Nutr. 11, 632–638 10.1017/S1368980007001085 [DOI] [PubMed] [Google Scholar]
- Ma J. F., Ueno D., Zhao F.-J., McGrath S. P. (2005). Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220, 731–736 10.1007/s00425-004-1392-5 [DOI] [PubMed] [Google Scholar]
- MacNicol R. D., Beckett P. H. T. (1985). Critical tissue concentrations of potentially toxic elements. Plant Soil 85, 107–129 10.1007/BF02197805 [DOI] [Google Scholar]
- Martínez-Ballesta M. C., Dominguez-Perles R., Moreno D. A., Muries B., Alcaraz-López C., Bastías E., García-Viguera C., Carvajal M. (2010). Minerals in plant food: effect of agricultural practices and role in human health. A review. Agron. Sustain. Dev. 30, 295–309 10.1051/agro/2009022 [DOI] [Google Scholar]
- Meenakshi J. V., Johnson N. L., Manyong V. M., Degroote H., Javelosa J., Yanggen D. R., Naher F., Gonzalez C., García J., Meng E. (2010). How cost-effective is biofortification in combating micronutrient malnutrition? An ex ante assessment. World Dev. 38, 64–75 10.1016/j.worlddev.2009.03.014 [DOI] [Google Scholar]
- Monasterio I., Graham R. D. (2000). Breeding for trace minerals in wheat. Food Nutr. Bull. 21, 392–396 [Google Scholar]
- Monsant A. C., Kappen P., Wang Y., Pigram P. J., Baker A. J. M., Tang C. (2011). In vivo speciation of zinc in Noccaea caerulescens in response to nitrogen form and zinc exposure. Plant Soil 348, 167–183 10.1007/s11104-011-0887-7 [DOI] [Google Scholar]
- Monsant A. C., Wang Y., Tang C. (2010). Nitrate nutrition enhances zinc hyperaccumulation in Noccaea caerulescens (Prayon). Plant Soil 336, 391–404 10.1007/s11104-010-0490-3 [DOI] [Google Scholar]
- Murphy K. M., Reeves P. G., Jones S. S. (2008). Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica 163, 381–390 10.1007/s10681-008-9681-x [DOI] [Google Scholar]
- Nicolle C., Simon G., Rock E., Amouroux P., Rémésy C. (2004). Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Am. Soc. Hortic. Sci. 129, 523–529 [Google Scholar]
- Ó Lochlainn S., Bowen H. C., Fray R. G., Hammond J. P., King G. J., White P. J., Broadley M. R. (2011). Tandem quadruplication of HMA4 in the zinc (Zn) and cadmium (Cd) hyperaccumulator Noccaea caerulescens. PLoS ONE 6, e17814. 10.1371/journal.pone.0017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk L., Yazici M. A., Yucel C., Torun A., Cekic C., Bagci A., Ozkan H., Braun H.-J., Sayers Z., Cakmak I. (2006). Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant 128, 144–152 10.1111/j.1399-3054.2006.00737.x [DOI] [Google Scholar]
- Palmer C. M., Guerinot M. L. (2009). Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 5, 333–340 10.1038/nchembio.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z., Cakmak I., Ozturk L., Yazici A., Jun Y., Budak H., Korol A. B., Fahima T., Saranga Y. (2009). Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat x wild emmer wheat RIL population. Theor. Appl. Genet. 119, 353–369 10.1007/s00122-009-1044-z [DOI] [PubMed] [Google Scholar]
- Persson D. P., Hansen T. H., Laursen K. H., Schjoerring J. K., Husted S. (2009). Simultaneous iron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics 1, 418–426 10.1039/b905688b [DOI] [PubMed] [Google Scholar]
- Pfeiffer W. H., McClafferty B. (2007). HarvestPlus: breeding crops for better nutrition. Crop Sci. 47, S88–S105 10.2135/cropsci2007.09.0020IPBS [DOI] [Google Scholar]
- Puig S., Peñarrubia L. (2009). Placing metal micronutrients in context: transport and distribution in plants. Curr. Opin. Plant Biol. 12, 299–306 10.1016/j.pbi.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Raboy V., Dickinson D. B., Below F. E. (1984). Variation in seed total phosphorus, phytic acid, zinc, calcium, magnesium and protein among lines of Glycine max and G. soja. Crop Sci. 24, 431–434 10.2135/cropsci1984.0011183X002400030001x [DOI] [Google Scholar]
- Ramesh S. A., Choimes S., Schachtman D. P. (2004). Over-expression of an Arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol. Biol. 54, 373–385 10.1023/B:PLAN.0000036370.70912.34 [DOI] [PubMed] [Google Scholar]
- Rascio N., Navari-Izzo F. (2011). Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 180, 169–181 10.1016/j.plantsci.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Reddy B. V. S., Ramesh S., Longvah T. (2005). Prospects of breeding for micronutrients and β-carotene-dense sorghums. Int. Sorghum Millets Newsl. 46, 10–14 [Google Scholar]
- Reeves R. D., Baker A. J. M. (2000). “Metal-accumulating plants,” in Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment, eds Raskin I., Ensley B. D. (New York: John Wiley & Sons; ), 193–229 [Google Scholar]
- Reichman S. M. (2002). The Responses of Plants to Metal Toxicity: A Review Focusing on Copper, Manganese and Zinc. Melbourne: Australian Minerals and Energy Environment Foundation [Google Scholar]
- Rengel Z., Batten G. D., Crowley D. E. (1999). Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res. 60, 27–40 10.1016/S0378-4290(98)00131-2 [DOI] [Google Scholar]
- Rogers E. E., Guerinot M. L. (2002). FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14, 1787–1799 10.1105/tpc.001495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosens N. H. C. J., Willems G., Saumitou-Laprade P. (2008). Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends Plant Sci. 13, 208–215 10.1016/j.tplants.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Sagardoy R., Morales F., López-Millán A. F., Abadía A., Abadía J. (2009). Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol. 11, 339–350 10.1111/j.1438-8677.2008.00153.x [DOI] [PubMed] [Google Scholar]
- Salt D. E., Prince R. C., Baker A. J. M., Raskin I., Pickering I. J. (1999). Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy. Environ. Sci. Technol. 33, 713–717 10.1021/es980825x [DOI] [Google Scholar]
- Sarret G., Willems G., Isaure M.-P., Marcus M. A., Fakra S. C., Frérot H., Pairis S., Geoffroy N., Manceau A., Saumitou-Laprade P. (2009). Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol. 184, 581–595 10.1111/j.1469-8137.2009.02996.x [DOI] [PubMed] [Google Scholar]
- Sayre R., Beeching J. R., Cahoon E. B., Egesi C., Fauquet C., Fellman J., Fregene M., Gruissem W., Mallowa S., Manary M., Maziya-Dixon B., Mbanaso A., Schachtman D. P., Siritunga D., Taylor N., Vanderschuren H., Zhang P. (2011). The BioCassava Plus Program: biofortification of Cassava for Sub-Saharan Africa. Annu. Rev. Plant Biol. 62, 251–272 10.1146/annurev-arplant-042110-103751 [DOI] [PubMed] [Google Scholar]
- Shi R., Zhang Y., Chen X., Sun Q., Zhang F., Römheld V., Zou C. (2010). Influence of long-term nitrogen fertilization on micronutrient density in grain of winter wheat (Triticum aestivum L.). J. Cereal Sci. 51, 165–170 10.1016/j.jcs.2009.11.008 [DOI] [Google Scholar]
- Stacey M. G., Patel A., McClain W. E., Mathieu M., Remley M., Rogers E. E., Gassmann W., Blevins D. G., Stacey G. (2008). The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol. 146, 589–601 10.1104/pp.107.108183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangoulis J. C. R., Huynh B. L., Welch R. M., Choi E. Y., Graham R. D. (2007). Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica 154, 289–294 10.1007/s10681-006-9211-7 [DOI] [Google Scholar]
- Stein A. J. (2010). Global impacts of human mineral malnutrition. Plant Soil 335, 133–154 10.1007/s11104-009-0228-2 [DOI] [Google Scholar]
- Stein A. J., Meenakshi J. V., Qaim M., Nestel P., Sachdev H. P. S., Bhutta Z. A. (2005). Technical Monograph 4. Analysing the Health Benefits of Biofortified Staple Crops by Means of the Disability-Adjusted Life Years Approach: A Handbook Focusing on Iron, Zinc and Vitamin A. Washington, WA: HarvestPlus [Google Scholar]
- Stein A. J., Nestel P., Meenakshi J. V., Qaim M., Sachdev H. P. S., Bhutta Z. A. (2007). Plant breeding to control zinc deficiency in India: how cost-effective is biofortification? Public Health Nutr. 10, 492–501 10.1017/S1368980007223857 [DOI] [PubMed] [Google Scholar]
- Stomph T. J., Choi E. Y., Stangoulis J. C. R. (2011). Temporal dynamics in wheat grain zinc distribution: is sink limitation the key? Ann. Bot. 107, 927–937 10.1093/aob/mcr040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomph T. J., Jiang W., Struik P. C. (2009). Zinc biofortification of cereals, rice differs from wheat and barley. Trends Plant Sci. 14, 123–124 10.1016/j.tplants.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Straczek A., Sarret G., Manceau A., Hinsinger P., Geoffroy N., Jaillard B. (2008). Zinc distribution and speciation in roots of various genotypes of tobacco exposed to Zn. Environ. Exp. Bot. 63, 80–90 10.1016/j.envexpbot.2007.10.034 [DOI] [Google Scholar]
- Subramanian N. K., White P. J., Broadley M. R., Ramsay G. (2011). The three-dimensional distribution of minerals in potato tubers. Ann. Bot. 107, 681–691 10.1093/aob/mcr009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Morikawa K. C., Nakanishi H., Takahashi M., Saigusa M., Mori S., Nishizawa N. K. (2008). Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci. Plant Nutr. 54, 77–85 10.1111/j.1747-0765.2008.00248.x [DOI] [Google Scholar]
- Takahashi M., Terada Y., Nakai I., Nakanishi H., Yoshimura E., Mori S., Nishizawa N. K. (2003). Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15, 1263–1280 10.1105/tpc.010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappero R., Peltier E., Gräfe M., Heidel K., Ginder-Vogel M., Livi K. J. T., Rivers M. L., Marcus M. A., Chaney R. L., Sparks D. L. (2007). Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol. 175, 641–634 10.1111/j.1469-8137.2007.02134.x [DOI] [PubMed] [Google Scholar]
- Terzano R., Chami Z. A., Vekemans B., Janssens K., Miano T., Ruggiero P. (2008). Zinc distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. J. Agric. Food Chem. 56, 3222–3231 10.1021/jf073304e [DOI] [PubMed] [Google Scholar]
- Uauy C., Distelfeld A., Fahima T., Blechl A., Dubcovsky J. (2006). A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314, 1298–1301 10.1126/science.1133649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service (USDA-ARS) (2011). USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory. Available at: http://www.ars.usda.gov/ba/bhnrc/ndl [accessed October 10, 2011].
- van der Zaal B. J., Neuteboom L. W., Pinas J. E., Chardonnens A. N., Schat H., Verkleij J. A. C., Hooykaas P. J. J. (1999). Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 119, 1047–1056 10.1104/pp.119.3.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steveninck R. F. M., Babare A., Fernando D. R., Van Steveninck M. E. (1994). The binding of zinc, but not cadmium, by phytic acid in roots of crop plants. Plant Soil 167, 157–164 10.1007/BF01587611 [DOI] [Google Scholar]
- Vasconcelos M., Datta K., Oliva N., Khalekuzzaman M., Torrizo L., Krishnan S., Oliveira M., Goto F., Datta S. K. (2003). Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 164, 371–378 10.1016/S0168-9452(02)00421-1 [DOI] [Google Scholar]
- Vázquez M. D., Poschenrieder C., Barceló J., Baker A. J. M., Hatton P., Cope G. L. (1994). Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens J & C Presl. Bot. Acta 107, 243–250 [Google Scholar]
- Velu G., Rai K. N., Muralidharan V., Kulkarni V. N., Longvah T., Raveendran T. S. (2007). Prospects of breeding biofortified pearl millet with high grain iron and zinc content. Plant Breed. 126, 182–185 10.1111/j.1439-0523.2007.01322.x [DOI] [Google Scholar]
- Verbruggen N., Hermans C., Schat H. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 181, 759–776 10.1111/j.1469-8137.2008.02748.x [DOI] [PubMed] [Google Scholar]
- Verret F., Gravot A., Auroy P., Leonhardt N., David P., Nussaume L., Vavasseur A., Richaud P. (2004). Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 576, 306–312 10.1016/j.febslet.2004.09.023 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Broadley M. R., Jansen S., White P. J., Takada J., Satake K., Takamatsu T., Tuah S. J., Osaki M. (2007). Evolutionary control of leaf element composition in plants. New Phytol. 174, 516–523 10.1111/j.1469-8137.2007.02078.x [DOI] [PubMed] [Google Scholar]
- Waters B. M., Grusak M. A. (2008). Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol. 177, 389–405 [DOI] [PubMed] [Google Scholar]
- Waters B. M., Sankaran R. P. (2011). Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Sci. 180, 562–574 10.1016/j.plantsci.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Welch R. M. (1995). Micronutrient nutrition of plants. CRC Crit. Rev. Plant Sci. 14, 49–82 10.1080/713608066 [DOI] [Google Scholar]
- White P. J. (2012a). “Long-distance transport in the xylem and phloem,” in Marschner’s Mineral Nutrition of Higher Plants, 3rd Edn, ed. Marschner P. (London: Academic Press; ), 49–70 [Google Scholar]
- White P. J. (2012b). “Heavy metal toxicity in plants,” in Plant Stress Physiology, ed. Shabala S. (Wallingford: CABI; ), (in press). [Google Scholar]
- White P. J., Bowen H. C., Demidchik V., Nichols C., Davies J. M. (2002a). Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta 1564, 299–309 10.1016/S0005-2736(02)00509-6 [DOI] [PubMed] [Google Scholar]
- White P. J., Whiting S. N., Baker A. J. M., Broadley M. R. (2002b). Does zinc move apoplastically to the xylem in roots of Thlaspi caerulescens? New Phytol. 153, 201–207 10.1046/j.0028-646X.2001.00325.x [DOI] [Google Scholar]
- White P. J., Bradshaw J. E., Dale M. F. B., Ramsay G., Hammond J. P., Broadley M. R. (2009). Relationships between yield and mineral concentrations in potato tubers. HortScience 44, 6–11 [Google Scholar]
- White P. J., Broadley M. R. (2005). Biofortifying crops with essential mineral elements. Trends Plant Sci. 10, 586–593 10.1016/j.tplants.2005.10.001 [DOI] [PubMed] [Google Scholar]
- White P. J., Broadley M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84 10.1111/j.1469-8137.2008.02738.x [DOI] [PubMed] [Google Scholar]
- White P. J., Brown P. H. (2010). Plant nutrition for sustainable development and global health. Ann. Bot. 105, 1073–1080 10.1093/aob/mcq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J., Poletti S., Aeschlimann B., Yakandawala N., Drosse B., Osorio S., Tohge T., Fernie A. R., Gunther D., Gruissem W., Sautter C. (2009). Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol. J. 7, 631–644 10.1111/j.1467-7652.2009.00430.x [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2002). The World Health Report 2002. Geneva: World Health Organization [Google Scholar]
- Wu J., Schat H., Sun R., Koornneef M., Wang X., Aarts M. G. M. (2007). Characterization of natural variation for zinc, iron and manganese accumulation and zinc exposure response in Brassica rapa L. Plant Soil 291, 167–180 10.1007/s11104-006-9184-2 [DOI] [Google Scholar]
- Wu J., Yuan Y.-X., Zhang X.-W., Zhao J., Song X., Li Y., Li X., Sun R., Koornneef M., Aarts M. G. M., Wang X. W. (2008). Mapping QTL for mineral accumulation and shoot dry biomass under different Zn nutritional conditions in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Soil 310, 25–40 10.1007/s11104-008-9625-1 [DOI] [Google Scholar]
- Wu J., Zhao F.-J., Ghandilyan A., Logoteta B., Guzman M. O., Schat H., Wang X., Aarts M. G. M. (2009). Identification and functional analysis of two ZIP metal transporters of the hyperaccumulator Thlaspi caerulescens. Plant Soil 325, 79–95 10.1007/s11104-009-0151-6 [DOI] [Google Scholar]
- Yang X., Ye Z. Q., Shi C. H., Zhu M. L., Graham R. D. (1998). Genotypic differences in concentrations of iron, manganese, copper, and zinc in polished rice grains. J. Plant Nutr. 21, 1453–1462 10.1080/01904169809365463 [DOI] [Google Scholar]
- Zhao F. J., Lombi E., Breedon T., McGrath S. P. (2000). Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 23, 507–514 10.1046/j.1365-3040.2000.00569.x [DOI] [Google Scholar]
- Zhao F. J., Su Y. H., Dunham S. J., Rakszegi M., Bedo Z., Mcgrath S. P., Shewry P. R. (2009). Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 49, 290–295 10.1016/j.jcs.2008.10.006 [DOI] [Google Scholar]