Abstract
Small cell lung cancer (SCLC) is one of many types rapidly growing malignant diseases, such as Burkitt’s lymphoma and testicular germ cell cancers. At present, there is no reliable way to screen for SCLC, and imaging modalities tend to be delayed in detecting this type of cancer. The clinical presentation of acutely and rapidly growing SCLC can mimic those of pulmonary inflammatory or infectious disorders, and in some instances, this delays appropriate management and negatively affects patient outcome.
Keywords: small cell, doubling time
Introduction
Lung cancer is the leading cause of cancer deaths in the United States among both men and women.1 Among various types of cancers, small cell lung cancer (SCLC) carries the worst outcomes, and accounts for 10%–20% of all bronchogenic carcinomas in the United States.2,3 Since its early description as “the oat-cell carcinoma of the mediastinum,” the fatality of SCLC has not changed considerably,4 and early diagnosis and initiation of treatment for lung cancer remains a considerable problem plaguing medicine today. SCLC continues to carry a grim prognosis, with median survival of approximately 2–4 months when untreated, and a 5-year survival rate in the range of 4%–5% when treated.3,5
In view of the high mortality associated with this disease, it may be time to examine other ways to detect SCLC in its earlier stages, which will likely result in more favorable outcomes. Given the rapid doubling time, SCLC may present as an acute syndrome that has a decent potential of being misdiagnosed as an infectious or inflammatory condition. Whether this clinical scenario has a significant impact on patient outcomes is yet to be determined, but will probably delay the prompt administration of chemotherapy, which is shown to improve survival in all stages of SCLC.
Methods
A PubMed and Google search was performed and 22 articles were found to be relevant to our topic and were therefore included in this review. We also briefly described a case example of rapidly growing SCLC.
Results
Various imaging modalities are currently ineffective for screening for SCLC, including computed tomography (CT).6,7 Therefore, alternative diagnostic methods are becoming essential in order to identify SCLC at its earliest stages. Chest radiograph was proven to be ineffective after several randomized trials demonstrate no significant effect.8 A number of randomized clinical trials are currently under way in Europe to investigate lung cancer screening using low dose chest CT. Chest X-rays, chest CT scans, bone scans, and magnetic resonance imaging (MRI) of the brain have been proven effective in the staging workup of SCLC. Recently, positron emission tomography has been shown to improve staging accuracy, but none of these imaging modalities have played an effective role in screening for SCLC.
Although modern chest CT scans are able to detect smaller solid nodules, these lesions lack specific radiographic features, which render this method incapable of differentiating the origin of the nodules.9 However, certain nodules escape identification by chest x-ray or CT scan secondary to factors such as the technique used or small size of the lesion.10 The challenge begins with cancers that do not manifest preneoplastic lesions, making their detection at an earlier stage (when therapeutic intervention may affect survival) a difficult, if not impossible, task. In a study comparing the cost effectiveness of CT screening to smoking cessation rates or combined approaches, the cost-effectiveness of chest CT screening was linked to smoking cessation rates.11
In a recently published European study, Maisonneuve et al found that recalibrating the Bach model to include nodule characteristic and size and investigating the presence of emphysema through a chest CT was a suitable way to select the high risk population for CT screening.12
The absence of characteristic changes in bronchial epithelium associated with SCLC creates false negative findings when bronchoscopy is used.13 Wistuba et al studied surgical specimens from patients with different types of primary lung cancers, and significant genetic damage was found in the majority of patients with SCLC who had normal or mildly abnormal bronchial epithelium and preserved histologic examination.13,14 More recently, new means of screening for SCLC are being investigated, such as autoantibody markers15 and multi-variable clinical scores.16 Evidently, in cases of high-risk nodules, histopathologic diagnosis should be considered.
Case description
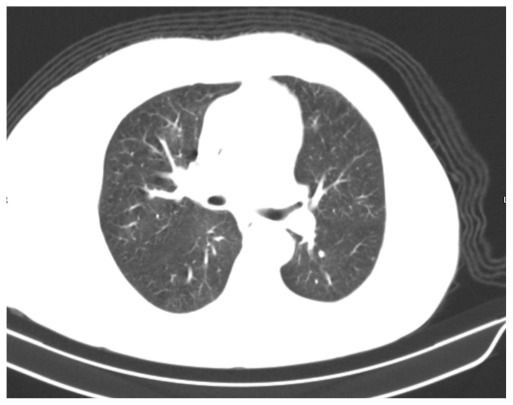
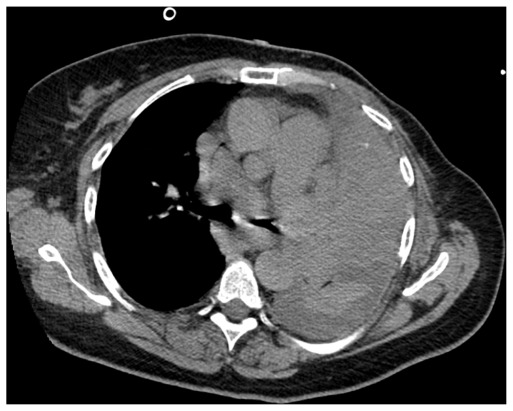
A 66-year-old Caucasian woman who was an exsmoker was being evaluated as an outpatient for exertional shortness of breath that had worsened over the course of several weeks. The performed imaging studies included normal chest radiography and thoracic CT with no evidence of any parenchymal or mediastinal lesions as interpreted by the radiologist (Fig. 1). The spirometry and cardiac evaluation were normal. Three months after the onset of symptoms, the patient presented to the emergency department for worsening dyspnea, which had been occurring at rest for two weeks. It was associated with yellowish productive cough with no chills, fever, or weight loss. The patient denied sick contact and recent travel. The physical examination was unremarkable with the exception of diffuse expiratory wheezing and labored breathing. The oxygen saturation in the room air was normal. A left multilobar opacity was present on the chest radiography, suggesting pneumonia. On the fourth day of hospitalization, the patient developed acute respiratory failure despite intravenous steroids and broad-coverage antibiotics. The patient was intubated and put on mechanical ventilation. Thoracic CT was performed and revealed several abnormalities in addition to the tumorous lesion itself (Fig. 2). There was atelectasis of the left lower lobe associated with small pleural effusion. Part of these findings could be secondary to obstructive pneumonia, so identifying an accurate area of cancerous lesion spreading is difficult. After discussions with the radiologist, we assumed that the left hilar mass was probably between 7 and 8 cm with associated lymphangitic spread. Additionally, an obstructive endobronchial mass in the upper and lingular left lobes was visualized and biopsied using flexible bronchoscopy. Findings from the pathological examination of the biopsied specimen were indicative of SCLC. Given the initial normal chest CT, the doubling time was probably too rapid to preclude early detection and, therefore earlier, more effective chemo-radiation therapy. Given her acute presentation and negative initial chest CT scan, the admission diagnosis was presumed to be severe pneumonia and the patient was started on intravenous antibiotics and other supportive measures. This management approach delayed chemo-radiation therapy for about five days and the patient expired a few days later from multiorgan failure.
Figure 1.
Initial chest CT was interpreted as no acute pathology.
Figure 2.
Chest CT scan 3 months later showing large left hilar mass of about 7.5 cm with mediastinal lymphadenopathy.
Note: Other CT sections showed thickening of the interlobular septa in the aerated portion of the left lung suggestive of lymphangitic spread of tumor.
Doubling Time of Small Cell Lung Cancer
In some cases, SCLC is characterized by significantly rapid doubling time. Therefore, the clinical presentation of these cancers is likely to differ from those experienced with more typical cancers and becomes more like an acute pulmonary manifestation, such as in an inflammatory or infectious disorder. Doubling time is calculated by estimating the volume of the nodule or tumor in two different dimensions.17 The doubling time is usually calculated using the following equation:
where Ti = interval time, Di = initial diameter, Do = final diameter, Vi = initial volume, Vo = final volume.
Given the neuroendocrinological origin of SCLC, it is considered the prototype of rapidly growing malignancies with doubling time in the range of 25 to 217 days according to several studies.10,18–20 A described by Wang et al, the doubling time of SCLC ranges from 54–132 days.9 In a study by Son et al, the doubling time for SCLC was 38 to 217 days and all patients were smokers.20 In addition, Arai, Kuroishi21 used serial chest X-ray films to study the doubling time of lung cancers in relation to prognosis and found that SCLC mean doubling time was 86.3 days. SCLC and large cell lung cancers represented most patients in the rapidly growing cancer group, which was defined as those having a doubling time of 109.6 days. It has been noted that the rate of growth in the size of the lung nodules is much faster for malignant versus benign nodules.22 Many factors affect the rate of growth of these nodules, and hence should be taken into consideration when determining the frequency and type of imaging studies for followup. Generally, among patients who have lung cancers with a rapid doubling time, SCLC had the shortest doubling time, followed by squamous cell carcinoma and adenocarcinoma.9 Eventually, the histological subtype of the tumor as well as the smoking status should be assessed in order to make suggestions concerning follow-up. In instances where SCLC doubling time is exceptionally rapid, the clinical presentation can mimic other acute lung pathologies such as pneumonia and other acute interstitial and inflammatory lung diseases.
Conclusion
Among smokers who present with acute pulmonary manifestations, rapidly growing SCLC can be an over-looked diagnosis. To date, screening for SCLC is still an outreach. Imaging techniques for detecting SCLC at the curative or early stages seems to be ineffective and alternative methods are being investigated.
Footnotes
Author Contributions
Conceived and designed the experiments: KH. Analysed the data: KH, IK. Wrote the first draft of the manuscript: KH. Contributed to the writing of the manuscript: KH, IK, SM, BA. Agree with manuscript results and conclusions: KH, HA, HH, TK, RM, DA, MC, TM. Jointly developed the structure and arguments for the paper: KH, HA. Made critical revisions and approved final version: KH. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA: A Cancer Journal for Clinicians. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. Epub February 6, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Riaz SP, Luchtenborg M, Coupland VH, Spicer J, Peake MD, Moller H. Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer (Amsterdam, Netherlands) 2012;75(3):280–4. doi: 10.1016/j.lungcan.2011.08.004. Epub September 7, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Ihde DC. Small cell lung cancer. State-of-the-art therapy 1994. Chest. 1995;107(Suppl 6):243S–8S. doi: 10.1378/chest.107.6_supplement.243s. Epub June 1, 1995. [DOI] [PubMed] [Google Scholar]
- 4.WGB The nature of the oat-celled sarcoma of the mediastinum. J Pathol Bacteriol. 1926;29:241–4. [Google Scholar]
- 5.Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77(12):2464–70. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. Epub June 15, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England Journal of Medicine. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. Epub July 1, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuffe S, Moua T, Summerfield R, Roberts H, Jett J, Shepherd FA. Characteristics and outcomes of small cell lung cancer patients diagnosed during two lung cancer computed tomographic screening programs in heavy smokers. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2011;6(4):818–22. doi: 10.1097/JTO.0b013e31820c2f2e. Epub May 31, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Sox HC. Screening for lung cancer with chest radiographs. JAMA: The Journal of the American Medical Association. 2011;306(17):1916–8. doi: 10.1001/jama.2011.1609. Epub October 28, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Wang JC, Sone S, Feng L, Yang ZG, Takashima S, Maruyama Y, et al. Rapidly growing small peripheral lung cancers detected by screening CT: correlation between radiological appearance and pathological features. The British Journal of Radiology. 2000;73(873):930–7. doi: 10.1259/bjr.73.873.11064644. Epub November 7, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ajam M, Seymour A, Mooty M, Leaf A. Ten years of disease-free survival between two diagnoses of small-cell lung cancer: a case report and a literature review. Medical Oncology (Northwood, London, England) 2005;22(1):89–97. doi: 10.1385/MO:22:1:089. Epub March 8, 2005. [DOI] [PubMed] [Google Scholar]
- 11.McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2011;6(11):1841–8. doi: 10.1097/JTO.0b013e31822e59b3. Epub September 6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maisonneuve P, Bagnardi V, Bellomi M, Spaggiari L, Pelosi G, Rampinelli C, et al. Lung cancer risk prediction to select smokers for screening CT—a model based on the Italian COSMOS trial. Cancer Prevention Research (Philadelphia, Pa) 2011;4(11):1778–89. doi: 10.1158/1940-6207.CAPR-11-0026. Epub August 5, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Wistuba II, Berry J, Behrens C, Maitra A, Shivapurkar N, Milchgrub S, et al. Molecular changes in the bronchial epithelium of patients with small cell lung cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2000;6(7):2604–10. Epub July 29, 2000. [PMC free article] [PubMed] [Google Scholar]
- 14.Colby TV, Wistuba II, Gazdar A. Precursors to pulmonary neoplasia. Advances in Anatomic Pathology. 1998;5(4):205–15. doi: 10.1097/00125480-199807000-00001. Epub December 22, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kazarian M, Laird-Offringa IA. Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Molecular Cancer. 2011;10:33. doi: 10.1186/1476-4598-10-33. Epub April 1, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titulaer MJ, Maddison P, Sont JK, Wirtz PW, Hilton-Jones D, Klooster R, et al. Clinical Dutch-English Lambert-Eaton Myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29(7):902–8. doi: 10.1200/JCO.2010.32.0440. Epub January 20, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, et al. Growth rate of small lung cancers detected on mass CT screening. The British Journal of Radiology. 2000;73(876):1252–9. doi: 10.1259/bjr.73.876.11205667. Epub February 24, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. The New England Journal of Medicine. 2003;348(25):2535–42. doi: 10.1056/NEJMcp012290. Epub June 20, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kerr KM, Lamb D. Actual growth rate and tumour cell proliferation in human pulmonary neoplasms. British Journal of Cancer. 1984;50(3):343–9. doi: 10.1038/bjc.1984.181. Epub September 1, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, et al. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme. Lung Cancer (Amsterdam, Netherlands) 2007;56(2):207–15. doi: 10.1016/j.lungcan.2006.12.014. Epub January 30, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Arai T, Kuroishi T, Saito Y, Kurita Y, Naruke T, Kaneko M. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Japanese Journal of Clinical Oncology. 1994;24(4):199–204. Epub August 1, 1994. [PubMed] [Google Scholar]
- 22.Webb WR. Radiologic evaluation of the solitary pulmonary nodule. AJR American Journal of Roentgenology. 1990;154(4):701–8. doi: 10.2214/ajr.154.4.2107661. Epub April 1, 1990. [DOI] [PubMed] [Google Scholar]